Abstract
Patients with chronic pain experience spontaneous or ongoing pain as well as enhanced sensitivity to evoked stimuli. Spontaneous or ongoing pain is rarely evaluated in preclinical studies. In fact, it remains controversial whether ongoing or spontaneous pain even develops in mice after tissue or nerve injury. This study tested a hypothesis that negative reinforcement can be used to unmask the presence of pain in mice with tissue or nerve injury. We found that spinal administration of clonidine or lidocaine did not elicit CPP in uninjured or sham-operated mice. However, these agents produced CPP in mice with chronic inflammation induced by complete Freund’s adjuvant (CFA) or following L5/L6 spinal nerve ligation (SNL). These data indicate the presence of non-evoked (i.e., stimulus-independent) ongoing pain in mice with chronic inflammation (CFA) or following nerve injury (SNL). In addition, this study validates the use of negative reinforcement to unmask non-evoked ongoing pain in mice. Given the existence of a large collection of transgenic and knockout mice, our data show the application of this approach to elucidate molecular mechanisms underlying non-evoked pain and to contribute to drug discovery for pain.
Perspective
We demonstrated the presence of non-evoked ongoing pain in mice with chronic inflammation or following nerve injury. The study also validates the use of negative reinforcement to unmask non-evoked pain in mice. We propose to apply this approach to identify molecular mechanisms and effective drugs for chronic pain.
Keywords: spontaneous pain, conditioned place preference, neuropathic pain, inflammatory pain, negative reinforcement
Introduction
Spontaneous pain is a common complaint of patients with chronic pain 1, 28, 32. Although spontaneous pain, either paroxysmal or ongoing, is consistently identified as the major clinical complaint in many pain states, its detection and mechanistic evaluation presents a major challenge preclinically. Current research has relied almost exclusively on reflexes from an evoked stimulus, a threshold response, while patients suffer from non-evoked pain that is evaluated clinically on the basis of self-reported intensity. Evoked hypersensitivity is unquestionably a concern in some clinical settings; however, most clinical trials rely on evaluation of tonic pain intensity, suggesting the possibility that measures of evoked thresholds may not accurately predict efficacy of therapies in development for the treatment of pain 9, 14, 27, 31. This limitation may be a major obstacle in basic and translational research to identify effective drugs for chronic pain.
Recently, negative reinforcement was employed to reveal the presence of non-evoked ongoing pain in rats 19, 29, 30. This approach employed conditioned place pairing to unmask the presence of an aversive state as the result of non-evoked ongoing pain. Importantly, drugs that were not rewarding in the absence of chronic pain elicited conditioned place preference (CPP) in rats with ongoing pain by alleviation of ongoing pain 19. This approach has also been applied to rats with lesions of the spinal cord representing central pain 7.
Whether mice might experience spontaneous or ongoing pain after tissue or nerve injuries remains controversial. A recent study monitored home cage activity and behaviors that are thought to reflect the affective state following chronic inflammation or peripheral nerve injuries and concluded that there was no change in “quality of life measures” in mice 34. The study failed to demonstrate behavioral evidence for ongoing pain in male mice after intraplantar complete Freund’s adjuvant (CFA), spared nerve injury (SNI), or chronic constriction injury (CCI) 34. The current study was designed to test the hypothesis that injuries to tissues or to peripheral nerves were accompanied by spontaneous or ongoing pain in mice. We propose that negative reinforcement can be used to unmask the presence of non-evoked ongoing pain in mice with tissue or nerve injury. Specifically, we determined whether spinal administration of drugs that do not produce CPP in naïve mice would do so in the presence of injury, revealing the presence of an aversive state reflecting ongoing pain in mice. To date, whether this approach can be successfully applied to mice has not been established. If successful, it would not only offer a powerful approach to directly test our hypothesis, but also open the opportunity of genetic advantages conferred by mouse models.
Materials and methods
Animals
Male ICR mice (20-25g; Harlan, Indianapolis, IN) were maintained on a 14/10 h light/dark cycle (light on 5:00 A.M. -- 7:00 P.M.; a standard light/dark schedule that is used by the university animal care facility) with food and water provided ad libitum before experimental procedures. All experiments were performed during the light cycle. Mice were randomly divided into experimental groups according to a computer generated randomization list. All procedures were carried out in accordance with the International Association for the Study of Pain (IASP) and the NIH Guide for the Care and Use of Laboratory Animals after approval by the University of Illinois Institutional Animal Care and Use Committee.
Materials
Complete Freund’s adjuvant (CFA, 0.5 mg/ml Mycobacterium tuberculosis (H 37RA, ATCC 25177), suspended in an oil:saline (1:1) emulsion), lidocaine, clonidine and adenosine were purchased from Sigma (St. Louis, MO). All other reagents were of analytical grade or better from commercial sources.
Drug administration
Intrathecal injection (i.t.) was given in a volume of 5μl by percutaneous puncture through an intervertebral space at the level of the 5th or 6th lumbar vertebra, as described previously 5, 15, 33.
Conditioned Place Preference (CPP)
The CPP apparatus (San Diego Instruments, San Diego, CA) consists of 3 Plexiglas chambers separated by manual doors. A center chamber (6 1/4” W × 8 1/8” D × 13 1/8” H) connects the two end-chambers that are identical in size (10 3/8” W × 8 1/8” D × 13 1/8” H), but can be distinguished by texture of floor (rough vs. smooth) and wall pattern (vertical vs. horizontal stripes). Movement of mice and time spent in each chamber were monitored by 4 × 16 photobeam arrays and automatically recorded in SDI CPP software.
Preconditioning was performed across 3 days for 30 min each day when mice were exposed to the environment with full access to all chambers. On day 3, a pre-conditioning bias test was performed to determine whether a preexisting chamber bias exists. In this test, mice were placed into the middle chamber and allowed to explore open field with access to all chambers for 15 min. Data were collected and analyzed for duration spent in each chamber. Animals spending more than 80% or less than 20% of the total time in an end-chamber were eliminated (~10% of total animals) from further testing.
We used a single trial conditioning protocol in the experiments 19. On conditioning day (day 4), mice first received vehicle control (saline, i.t.) paired with a randomly chosen chamber in the morning and, 4 h later, either clonidine (1 μg in 5 μL saline, i.t.), lidocaine (0.04 % in 5 μL saline, i.t.), or adenosine (3 μg in 5 μL saline, i.t.) paired with the other chamber in the afternoon. During the conditioning, mice were allowed to stay only in the paired chamber without access to other chambers for 15 min immediately following saline or drug injection. On the test day, 20 h after the afternoon pairing, mice were placed in the middle chamber of the CPP box with all doors open so animals can have free access to all chambers. Movement and duration of each mouse spent in each chamber were recorded for 15 min for analysis of chamber preference. Difference scores were calculated as (test time-preconditioning time) spent in the drug chamber.
Complete Freund’s adjuvant (CFA)-induced inflammatory pain model
Unilateral inflammation was induced by injecting 20 μl CFA into the dorsal surface of the left hindpaw (i.pl.), as we have previously described 26, 33. The treatment is known to induce thermal hyperalgesia and mechanical allodynia. Control mice received 20 μl of saline. Sensitivity to thermal and mechanical stimuli was tested before and after CFA injection. CPP preconditioning started 3 days before CFA injection.
Spinal nerve ligation (SNL)-induced neuropathic pain model
Spinal nerve ligation operation was carried out as previously published 4, 18. Separate groups of eight mice had the left L5 and L6 spinal nerves tightly ligated distal to the dorsal root ganglion but before the fibers join to form the sciatic nerve; the sham operation consisted of the same surgery but without nerve ligations. Sensitivity to thermal and mechanical stimuli was tested before and after SNL operation. Preconditioning started 10 d after SNL.
Assessment of mechanical and thermal sensitivity
Sensitivity to mechanical stimulus was assessed as previously described 4, 26. Mice were allowed to acclimate for 30 min before probing with calibrated von Frey filaments (Stoelting Co, Wood dale, IL) by pressing the midplantar surface of the left hindpaw for 5 s or until a withdrawal response happened. Paw withdrawal threshold was determined by the “up-down” algorithm 3, 5, 8.
Thermal sensitivity was determined by paw withdrawal latency to radiant heat applied to the center of the planter surface of the left hindpaw using a plantar tester (model 7372, UGO BASILE) 4, 5, 13. Latency to paw withdrawal was measured. A cut-off time of 20s was applied to avoid tissue damage.
Statistical analysis
Data are expressed as Mean±S.E.M. Comparisons between groups were analyzed using ANOVA followed by a post hoc test. When analyzing evoked pain behavior data, one-way ANOVA followed by Tukey post hoc test wass used to compare unmatched groups at each time point. When analyzing CPP data, two-way ANOVA (pairing vs. treatment) was applied followed by Bonferroni post hoc test Difference scores were analyzed using Student’s paired t test by comparing the differences between test time and preconditioning time in each chamber for each mouse. Statistical significance was established at the 95% confidence limit.
Results
CFA-induced evoked hypersensitivity and ongoing pain in mice
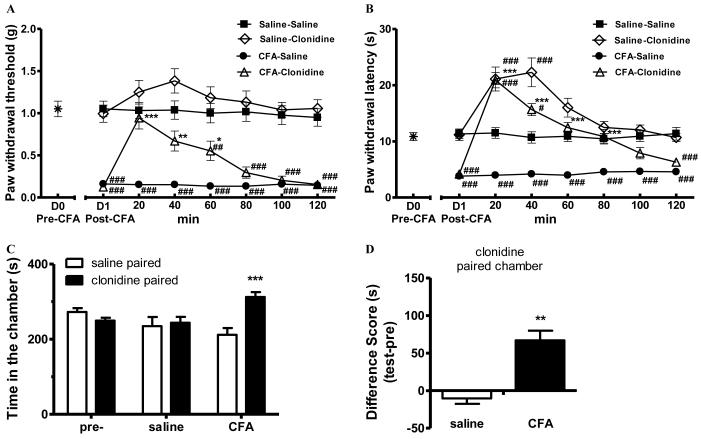
Intraplantar CFA is known to cause evoked hypersensitivity (i.e., hyperalgesia) of the injured paw. When tested 24 h post CFA treatment, mice exhibited hypersensitivity to a normally innocuous mechanical von Frey stimulus (0.16±0.04 g in CFA-mice vs 1.05±0.10 g in saline-mice, p<0.001 one-way ANOVA followed by Tukey post hoc test; Fig. 1 A) as well as to a noxious thermal stimulus (3.80±0.41s in CFA-mice vs 11.31±0.87s in saline-mice, p<0.001 oneway ANOVA followed by Tukey post hoc test; Fig. 1 B) applied to the hindpaw. Within 20 min after administration, clonidine (1 μg, i.t.) reversed CFA-mechanical hypersensitivity, restoring the paw withdrawal threshold (0.94±0.14g) to a level that was indistinguishable from that in saline-mice (1.03±0.10g, p>0.05) and was significantly different from the CFA-saline mice (0.15±0.04, p<0.001, one-way ANOVA followed by Tukey post hoc test). This effect of clonidine persisted for about 60 min. In saline-treated mice, spinal clonidine elicited a slight increase in paw withdrawal threshold that did not achieve significance. CFA-induced thermal hyperalgesia was also reversed by spinal clonidine within 20 min. Spinal clonidine reversed thermal thresholds from 3.80±0.41s in CFA-mice to 20.89 ± 1.37s in CFA-clonidine mice (p<0.001, one-way ANOVA followed by Tukey post hoc test; Fig. 1B). These data indicate that this dose of spinal clonidine is effective in transiently reversing CFA-induced evoked hypersensitivity. Clonidine elicited antinociception in both CFA- and saline-mice in the radiant heat assay (Fig. 1B), which has been previously studied under similar conditions 10.
Figure 1.
Clonidine induced conditioned place preference (CPP) in CFA-, but not saline-, pretreated mice.
(A-B). Complete Freund’s adjuvant (CFA) produced mechanical (A) and thermal hypersensitivity (B), which were transiently attenuated by clonidine (1 μg in 5 μL saline, i.t.). The sensory baseline (D0) was obtained before the injection of CFA on day 0. Post-CFA data were obtained 24 h later (D1). After the administration of clonidine, mechanical and thermal sensitivity were monitored for 120 min. * p<0.05, ** p<0.01, *** p < 0.001, compared between CFA-clonidine and CFA-saline groups;. # p<0.05, ## p<0.01, ### p < 0.001, compared with the saline-saline group, one-way ANOVA followed by Tukey post hoc test; n = 8 for each group. (C). Clonidine induced CPP in CFA-pretreated mice. After 3 d pre-conditioning, mice were tested to ensure the absence of chamber bias (“pre”). CFA-pretreated mice (“CFA”) spent significantly longer time in clonidine-paired chamber, whereas saline-pretreated mice (“saline”) showed no chamber preference. *** p < 0.001, compared between the saline paired and clonidine paired group, two-way ANOVA followed by Bonferroni post hoc test; n = 8 for each group.
(D) Difference scores (test time-preconditioning time spent in the clonidine chamber) confirmed that CFA-, but not saline-, pretreated mice showed CPP to clonidine. ** p < 0.01, paired t test; n = 8 for each group.
We next determined whether CFA produced non-evoked ongoing pain in the mouse. Twenty eight hours post CFA, separate groups of saline- or CFA-treated mice received clonidine (1 μg, i.t.) and were immediately paired with a randomly chosen chamber for 15 min. Chamber preference was tested 20 h later without any additional drug administration. CFA-pretreated mice spent a significantly longer time in the clonidine-paired chamber (313±13s) than in the saline-paired chamber (212±18s, p<0.001, two-way ANOVA followed by Bonferroni post hoc test; Fig 1C), indicative of clonidine-induced CPP in CFA-mice. In contrast, spinal clonidine did not produce CPP in saline-pretreated mice, as mice spent equal amount of time in saline (237±20s) and clonidine (254±17s) chambers (p>0.05, two-way ANOVA followed by Bonferroni post hoc test). It is important to note that mice used in the experiment had no preexisting chamber preference (268±11s in saline chambers vs 254±8s in clonidine chambers, p>0.05; Fig. 1C). Analysis of “difference scores” indicated that clonidine produced CPP only in CFA-, but not saline-, mice. Therefore, spinal clonidine induced CPP selectively in mice with ongoing tissue inflammation (p<0.01, paired t test; Fig. 1D).
CFA-induced evoked and non-evoked pain is blocked by lidocaine
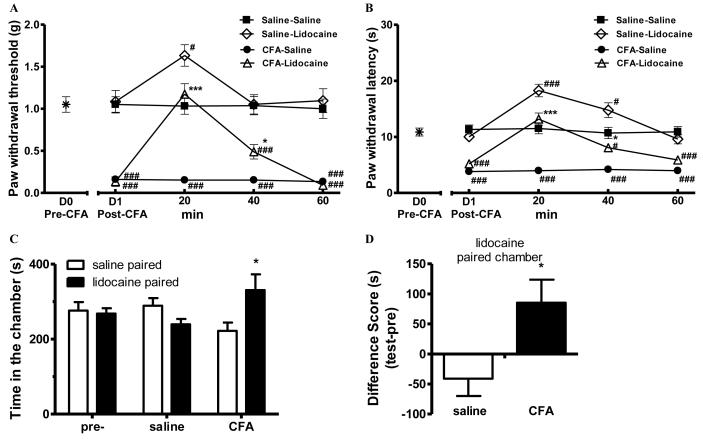
In order to further test our hypothesis, we studied the effects of spinal lidocaine. As expected, lidocaine transiently blocked CFA-induced mechanical and thermal hypersensitivity (Fig. 2 A-B). These effects of lidocaine were most significant at 20 min after its administration, bringing both the paw withdrawal threshold (1.17±0.13g) and the paw withdrawal latency (13.16±1.15s) to levels that were not different from those of saline-saline mice (p>0.05, one-way ANOVA followed by Tukey post hoc test, Fig. 2 A-B). These effects lasted for about 40 min. Similar to what was reported previously 25, 29, lidocaine also raised thresholds above baseline in saline-mice.
Figure 2.
Lidocaine induced CPP in CFA-, but not saline-, pretreated mice.
(A-B). Complete Freund’s adjuvant (CFA) produced mechanical (A) and thermal hypersensitivity (B), which were transiently attenuated by lidocaine (0.04 % in 5 μL saline, i.t.). The sensory baseline (D0) was obtained before the injection of CFA on day 0. Post-CFA data were obtained 24 h later (D1). After the administration of lidocaine, mechanical and thermal sensitivity were monitored for 60 min. * p<0.05, *** p < 0.001, compared between CFA-clonidine and CFA-saline groups;. # p<0.05, ### p < 0.001, compared with the saline-saline group, one-way ANOVA followed by Tukey post hoc test; n = 8 for each group.
(C). Lidocaine induced CPP in CFA-pretreated mice. After 3 d pre-conditioning, mice were tested to ensure the absence of chamber bias (“pre”). CFA-pretreated mice (“CFA”) spent significantly longer time in lidocaine-paired chamber, whereas saline-pretreated mice (“saline”) showed no chamber preference. * p < 0.05, compared between the saline paired and lidocaine paired group, two-way ANOVA followed by Bonferroni post hoc test; n = 8 for each group. (D) Difference scores (test time-preconditioning time spent in the lidocaine chamber) confirmed that CFA-, but not saline-, pretreated CPP to lidocaine. * p < 0.05, paired t test; n = 8 for each group.
Twenty eight hours after intraplantar CFA or saline injection, mice received spinal lidocaine and were placed within a randomly chosen conditioning chamber. Mice treated with intraplantar saline did not show place preference to either spinal saline or lidocaine; these animals spent similar amount of time in saline (289±20s) and lidocaine (239±14s) chambers (p>0.05, Fig 2C-D), confirming that lidocaine did not elicit CPP in saline-mice without ongoing pain. In contrast, CFA-treated mice spent significantly more time in lidocaine chambers (331±42) than in saline chambers (222±22, p<0.05, two-way ANOVA followed by Bonferroni post hoc test; Fig. 2 C-D), revealing the presence of tonic pain in CFA-mice with ongoing tissue injury.
CFA-induced evoked, but not non-evoked, pain behavior is blocked by adenosine
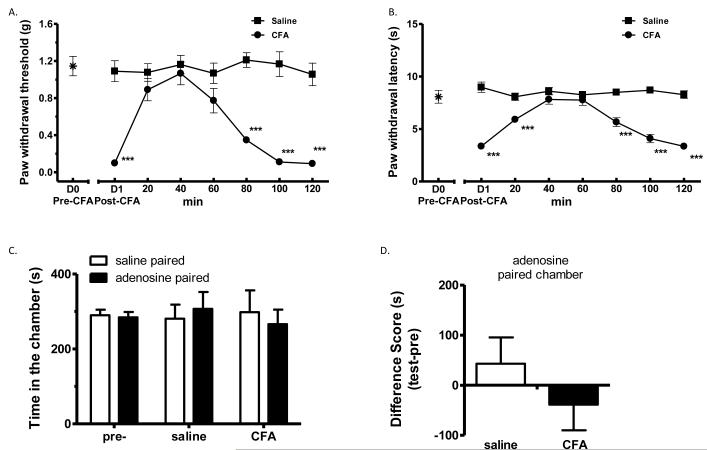
Adenosine is known to block evoked secondary hyperalgesia, but does not affect ongoing pain, in humans 9. We, therefore, applied this drug one day after CFA. Spinal adenosine (3 μg in 5 μl saline, i.t.) effectively reversed CFA-induced mechanical allodynia (Fig. 3A) and thermal hyperalgesia (Fig. 3B), while not altering mechanical and thermal sensitivity in saline-pretreated control mice (Fig. 3 A-B). In the CPP test, adenosine did not produce CPP in either CFA- or saline-pretreated mice (p>0.05, two-way ANOVA followed by Bonferroni post hoc test, Fig. 3 C-D). These data are consistent with findings in humans, suggesting that different mechanisms can be involved in evoked and non-evoked pain.
Figure 3.
Adenosine blocked CFA-induced evoked hypersensitivity, but did not induce CPP in CFA-mice.
(A-B). Complete Freund’s adjuvant (CFA) produced mechanical (A) and thermal hypersensitivity (B), which were transiently attenuated by adenosine (3 μg in 5 μL saline, i.t.). The sensory baseline (D0) was obtained before the injection of CFA on day 0. Post-CFA data were obtained 24 h later (D1). After the administration of adenosine, mechanical and thermal sensitivity were monitored for 120 min in saline- or CFA-mice. *** p < 0.001, compared with the saline-adenosine group, one-way ANOVA followed by Tukey post hoc test; n = 8 for each group.
(C). Adenosine did not produce CPP in CFA- nor saline-pretreated mice. After 3 d preconditioning, mice were tested to ensure the absence of chamber bias (“pre”). CFA-pretreated mice (“CFA”) and saline-pretreated mice (“saline”) showed no chamber preference, by spending similar amount of time in saline- or adenosine-paired chambers, p > 0.05, two-way ANOVA; n = 8 for each group.
(D) Difference scores (test time-preconditioning time spent in the lidocaine chamber) confirmed the absence of chamber preference. p > 0.05, paired t test; n = 8 for each group.
CPP unmasks ongoing neuropathic pain in SNL-mice
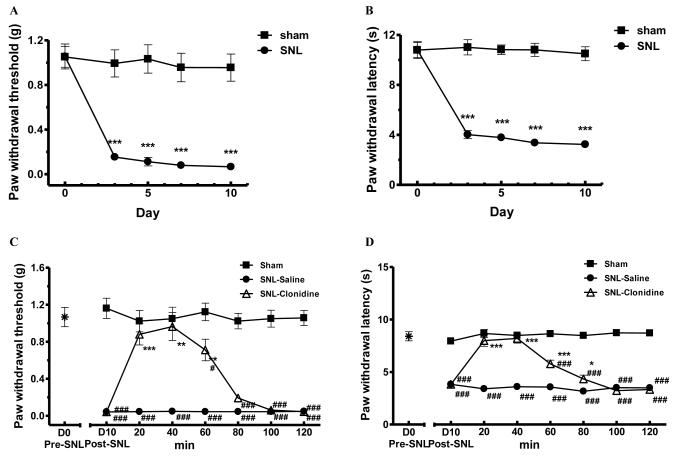
Spinal nerve ligation of lumbar L5/L6 nerves (SNL) is a commonly used experimental model of neuropathic pain. We produced SNL in mice to determine if nerve injury was accompanied by non-evoked pain. Mice exhibited long-lasting evoked pain behaviors to probing by von Frey filaments (0.96±0.13g in sham- vs 0.07±0.03g in SNL-mice, p<0.001 on day 10, one-way ANOVA followed by Tukey post hoc test; Fig. 4A) and to radiant heat challenge (10.50±0.60s in sham-mice vs 3.24±0.27s in SNL-mice, p<0.001 on day 10, one-way ANOVA followed by Tukey post hoc test; Fig. 4B). In our previous studies, we have found that SNL-induced evoked thermal and mechanical hypersensitivity lasted for at least 10 weeks in mice (Chen and Wang, unpublished data).
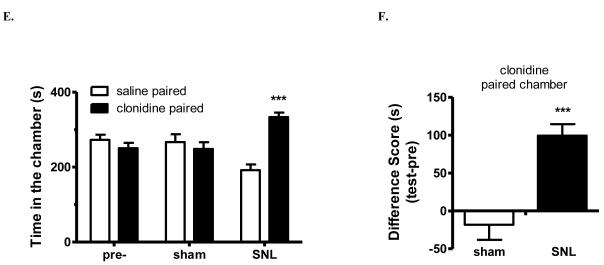
Figure 4.
Clonidine induced conditioned place preference (CPP) in SNL mice.
(A-B). L5/L6 spinal nerve ligation (SNL) produced long-lasting mechanical (A) and thermal hypersensitivity. *** p < 0.001, compared with the sham-operated group, one-way ANOVA followed by Tukey post hoc test; n = 6 for each group.
(C-D). SNL-mechanical (C) and thermal hypersensitivity (D) were transiently attenuated by clonidine (1 μg in 5 μL saline, i.t.). The sensory baseline (D0) was obtained before SNL on day 0. After the administration of clonidine or saline in SNL mice on day 10, mechanical and thermal sensitivity were monitored for 120 min. Sham mice were not treated and were tested at the same time as additional controls for baseline. * p < 0.5, ** p < 0.01, *** p < 0.001, compared between SNL-clonidine and SNL-saline groups; # p<0.05, ### p < 0.001, compared with the sham group, one-way ANOVA followed by Tukey post hoc test; n = 6 for each group.
(E). Clonidine induced CPP in SNL mice. After 3 d pre-conditioning, mice were tested to ensure the absence of chamber bias (“pre”). SNL-mice spent significantly longer time in clonidine-paired chamber, whereas sham-operated mice showed no chamber preference. *** p < 0.001, compared between the saline paired and clonidine paired group, two-way ANOVA followed by Bonferroni post hoc test; n = 6 for each group.
(F) Difference scores (test time-preconditioning time spent in the clonidine chamber) confirmed that SNL-, but not sham-, mice exhibited CPP to clonidine. *** p < 0.001 paired t test; n = 6.
Clonidine (1 μg, i.t.) effectively attenuated both mechanical allodynia and thermal hyperalgesia in SNL mice, with a rapid onset (< 20 min) and duration of action for at least 60 min, whereas SNL mice treated with saline (i.t.) maintained hypersensitivity to mechanical and thermal stimuli (Fig. 4C-D). Sham- or SNL-operated mice were paired with a randomly chosen chamber for 15 min after clonidine (i.t.) and tested 20 h later for chamber preference. SNL-mice showed a robust preference for chambers paired with spinal clonidine (334±13s) than saline chambers (192±17s, p<0.001, two-way ANOVA followed by Bonferroni post hoc test; Fig. 3C). In contrast, sham-mice did not increase time spent in clonidine chambers (249±20s) when compared with time in saline-chambers (267±23s; p>0.05, Fig. 4E). Analysis of “difference scores” indicated that clonidine produced CPP only in SNL-mice (p<0.001, paired t test; Fig. 4F).
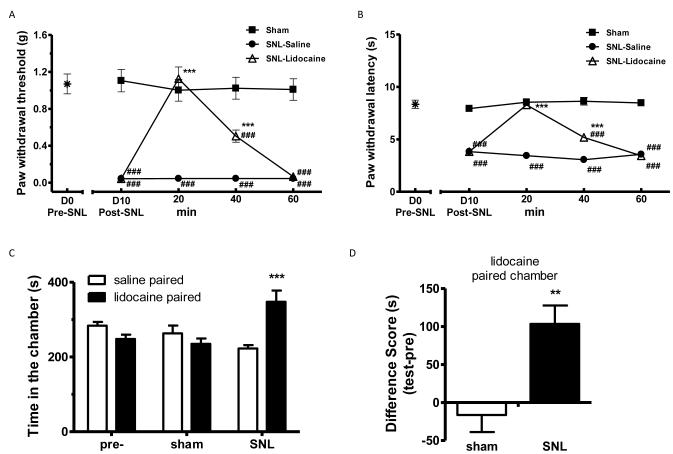
Similarly, lidocaine (0.04 % in 5 μL saline, i.t.) also transiently, but effectively, blocked SNL-induced hypersensitivity to probing by von Frey filaments (Fig. 5A) and radiant heat (Fig. 5B). When sham- or SNL-mice were paired with spinal lidocaine, sham-mice spent similar duration of time in saline-chambers (263±23s) and lidocaine-chambers (235±15s) (p>0.05). On the contrary, SNL-mice showed a strong preference for lidocaine-paired chambers (348±33s) over saline-chambers (223±10s, p<0.001, two-way ANOVA followed by Bonferroni post hoc test; Fig. 5C). These data were supported by the difference scores, which revealed lidocaine-induced pain relief can elicit CPP in SNL-, but not sham-, mice (p<0.01, paired t test, Fig. 5D).
Figure 5.
Lidocaine induced conditioned place preference (CPP) in SNL mice.
(A-B). SNL-induced mechanical (A) and thermal hypersensitivity (B) were transiently attenuated by lidocaine (0.04% in 5 μL saline, i.t.). The sensory baseline (D0) was obtained before SNL on day 0. After the administration of lidocaine or saline in SNL mice on day 10, mechanical and thermal sensitivity were monitored for 60 min. Sham mice were not treated and were tested at the same time as additional controls for baseline. *** p < 0.001, compared between SNL-lidocaine and SNL-saline groups; ### p < 0.001, compared with the sham group, one-way ANOVA followed by Tukey post hoc test; n = 6 for each group.
(C). Lidocaine induced CPP in SNL mice. After 3 d pre-conditioning, mice were tested to ensure the absence of chamber bias (“pre”). SNL-mice spent significantly longer time in lidocaine-paired chamber, whereas sham-operated mice showed no chamber preference. *** p < 0.001, compared between the saline paired and lidocaine paired group, two-way ANOVA followed by Bonferroni post hoc test; n = 6 for each group.
(D) Difference scores (test time-preconditioning time spent in the lidocaine chamber) confirmed that SNL-, but not sham-, mice exhibited CPP to lidocaine. ** p < 0.01 paired t test; n = 6.
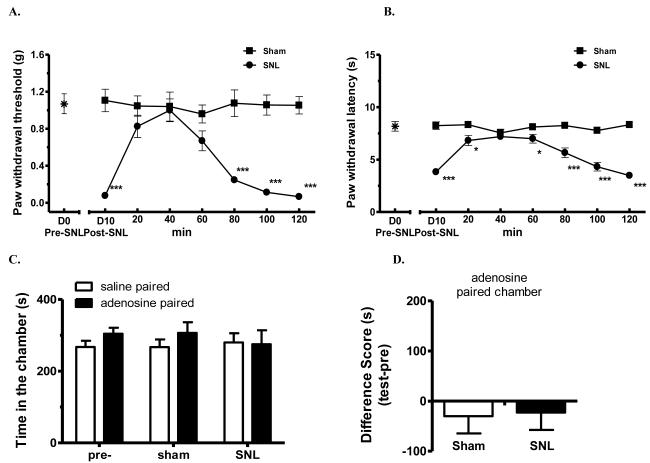
Adenosine also effectively reversed SNL-induced tactile allodynia (Fig. 6A) and thermal hyperalgesia (Fig. 6B) after a single bolus i.t. injection (3 μg). The anti-allodynic and anti-hyperalgesic effects lasted for at least 60 min. Importantly, the drug’s peak anti-allodynic/anti-hyperalgesic effect was rapidly achieved within 20 min. However, when sham- or SNL-mice were paired with spinal adenosine and tested for chamber-preference 20 h later, all mice spent about equal amount of time in saline-paired or adenosine-paired chambers (Fig. 6C, p>0.05). Therefore, adenosine that blocked evoked hypersensitivity did not produce CPP in SNL mice (Fig. 6D).
Figure 6.
Adenosine blocked SNL-induced evoked mechanical allodynia and thermal hyperalgesia, but did not induce CPP in SNL-mice.
(A-B). Spinal nerve ligation (SNL) produced mechanical (A) and thermal hypersensitivity (B), which were transiently attenuated by adenosine (3 μg in 5 μL saline, i.t.). After the administration of adenosine, mechanical and thermal sensitivity were monitored for 120 min in saline- or SNL-mice. * p < 0.05, *** p < 0.001, compared with the sham-adenosine group, oneway ANOVA followed by Tukey post hoc test; n = 8 for each group.
(C). Adenosine did not produce CPP in SNL- or sham-operated mice. After 3 d preconditioning, mice were tested to ensure the absence of chamber bias (“pre”). SNL- and sham-operated mice showed no chamber preference, by spending similar amount of time in saline- or adenosine-paired chambers, p > 0.05, two-way ANOVA; n = 8 for each group.
(D) Difference scores (test time-preconditioning time spent in the lidocaine chamber) confirmed the absence of chamber preference, p > 0.05, paired t test; n = 8 for each group.
Discussion
In this study, we tested the hypothesis whether injuries to tissues or to peripheral nerves were accompanied by spontaneous or ongoing pain in mice. Since ongoing or spontaneous pain can represent a tonic aversive state, relief of pain can produce CPP selectively in mice with tissue or nerve injury. Critical to the study paradigm is the selection of drugs that do not possess rewarding properties in naive animals and their administration at sites that are not a part of the reward circuit. In our study, we employed spinal administration of relatively low, but effective doses of clonidine, lidocaine, and adenosine. These drugs are not known to be rewarding in naïve (i.e., uninjured) mice, for which we verified in our experiments that these agents did not produce CPP in saline-treated, sham-operated, or untreated (data not shown) naïve mice. However, in mice with ongoing tissue injury (by CFA) or after nerve injury (by SNL), both clonidine and lidocaine produced robust CPP after a single conditioning trial, which is consistent with the presence of ongoing spontaneous pain in these mouse models of chronic pain. As expected, these drugs given spinally were able to block evoked hypersensitivity in injured mice. As no place preference was observed with these agents in uninjured mice, the data suggest the requirement for an aversive state that may represent ongoing spontaneous pain.
Adenosine is known in humans to block evoked secondary hyperalgesia, but does not alter ongoing pain 9. Indeed, adenosine attenuated evoked hypersensitivity to mechanical and thermal stimuli in both CFA- and SNL-mice; however, it was not effective in eliciting CPP, suggesting different mechanisms are involved in evoked and non-evoked pain in mice.
In addition to confirming our hypothesis, these data further validated the applicability of the CPP method to unmask non-evoked ongoing pain in mice. Our results are in agreement with data from recent reports using this approach in rats to detect non-evoked ongoing pain in multiple pain states including peripheral nerve injury (SNL, SNI and partial or complete axotomy) 19, 30, intraplantar CFA 29, osteoarthritis pain 25 as well as in central neuropathic pain 7.
Many studies have attempted to evaluate the presence of spontaneous or ongoing pain using a variety of approaches (e.g.,16, 21-23, 34) with inconsistent results. Rats with chronic CCI or SNI did not exhibit facial patterns that would be considered as a “pain face” by experimenters 22. A more recent studied employed an automated home cage monitoring system for feeding and drinking, locomotion and circadian patterns 12 and a wide battery of behavioral tests for anxiety, depression, anhedonia, and social interaction 34. It found no significant changes in these behavioral tests in mice with CFA or SNI when compared with control mice 34. Importantly, mice with CCI showed decreased activity in the early time period; however, the differences disappeared 2 weeks after CCI, a finding that was inconsistent with persistent nature of nerve injury pain. Critically, this study concluded that mice with tissue inflammation and nerve injury did not have significantly altered “quality of life” or signs of ongoing pain in mice. This conclusion is opposite from the clinical experience 2, 11, 17, 24 and appears to cast doubt on the validity of animal models employing nerve injury, the current standard in pain research for neuropathic states. Collectively, these findings underscore the difficulty of detecting ongoing or spontaneous pain preclincally using behavioral measurements that are not specific for pain.
The data presented here reveal the presence of non-evoked ongoing pain in mice elicited by either an inflammatory state or by peripheral nerve injury. Here, relief of pain can be interpreted as eliciting a reward from negative reinforcement. The conclusions are consistent with the human experience that pain is aversive and produces strong motivational drive to seek relief 6. This approach has already produced insights into the central and peripheral mechanisms leading to non-evoked pain in the rat 20, 29, 30. Furthermore, it was found that ongoing pain may be mechanistically and temporally distinctive from evoked pain 29. For these reasons, validation in mice of the use of negative reinforcement as a strategy to detect non-evoked ongoing pain may allow future use of genetic strategies to explore mechanisms.
In summary, we report here the presence of non-evoked ongoing pain in mice with chronic tissue inflammation (CFA) or following nerve injury (SNL). In addition, this study validates the use of negative reinforcement to unmask non-evoked ongoing pain in mice. Considering the existence of a large collection of transgenic and knockout mouse models, this approach can now be employed to study molecular mechanisms that may underlie non-evoked ongoing pain. In turn, it will help facilitate discovery of analgesic drugs for spontaneous or ongoing pain that is the most relevant for patients with chronic pain.
Abbreviations
- CCI
chronic constriction injury
- CFA
complete Freund’s adjuvant
- CPP
conditioned place preference
- SNI
spared nerve injury
- SNL
L5/L6 spinal nerve ligation
Footnotes
Disclosures
This work was supported in part by the National Institutes of Health grant HL098141. Authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Bruce J, Krukowski ZH. Quality of life and chronic pain four years after gastrointestinal surgery. Diseases of the colon and rectum. 2006;49:1362–1370. doi: 10.1007/s10350-006-0575-5. [DOI] [PubMed] [Google Scholar]
- 3.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Luo F, Yang C, Kirkmire C, Wang ZJ. Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.152165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010;30:38–46. doi: 10.1523/JNEUROSCI.4346-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 7.Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. J Pain. 2011;12:868–874. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 9.Eisenach JC, Rauck RL, Curry R. Intrathecal, but not intravenous adenosine reduces allodynia in patients with neuropathic pain. Pain. 2003;105:65–70. doi: 10.1016/s0304-3959(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 10.Fairbanks CA, Kitto KF, Nguyen HO, Stone LS, Wilcox GL. Clonidine and dexmedetomidine produce antinociceptive synergy in mouse spinal cord. Anesthesiology. 2009;110:638–647. doi: 10.1097/ALN.0b013e318195b51d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract. 2000;47:123–128. doi: 10.1016/s0168-8227(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 12.Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH. A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci U S A. 2008;105:20575–20582. doi: 10.1073/pnas.0809053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 14.Hill R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21:244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 15.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 16.Jourdan D, Ardid D, Eschalier A. Analysis of ultrasonic vocalisation does not allow chronic pain to be evaluated in rats. Pain. 2002;95:165–173. doi: 10.1016/s0304-3959(01)00394-3. [DOI] [PubMed] [Google Scholar]
- 17.Kerba M, Wu JS, Duan Q, Hagen NA, Bennett MI. Neuropathic pain features in patients with bone metastases referred for palliative radiotherapy. J Clin Oncol. 2010;28:4892–4897. doi: 10.1200/JCO.2010.28.6559. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 19.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kupers RC, Nuytten D, De Castro-Costa M, Gybels JM. A time course analysis of the changes in spontaneous and evoked behaviour in a rat model of neuropathic pain. Pain. 1992;50:101–111. doi: 10.1016/0304-3959(92)90117-T. [DOI] [PubMed] [Google Scholar]
- 22.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 23.Leite-Almeida H, Almeida-Torres L, Mesquita AR, Pertovaara A, Sousa N, Cerqueira JJ, Almeida A. The impact of age on emotional and cognitive behaviours triggered by experimental neuropathy in rats. Pain. 2009;144:57–65. doi: 10.1016/j.pain.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Liedberg GM, Vrethem M. Polyneuropathy, with and without neurogenic pain, and its impact on daily life activities--a descriptive study. Disabil Rehabil. 2009;31:1402–1408. doi: 10.1080/09638280802621382. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Okun A, Ren J, Guo RC, Ossipov MH, Xie J, King T, Porreca F. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett. 2011;493:72–75. doi: 10.1016/j.neulet.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo F, Yang C, Chen Y, Shukla P, Tang L, Wang LX, Wang ZJ. Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther. 2008;325:267–275. doi: 10.1124/jpet.107.132167. [DOI] [PubMed] [Google Scholar]
- 27.Morgan D, Carter CS, DuPree JP, Yezierski RP, Vierck CJ., Jr. Evaluation of prescription opioids using operant-based pain measures in rats. Exp Clin Psychopharmacol. 2008;16:367–375. doi: 10.1037/a0013520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moryl N, Coyle N, Essandoh S, Glare P. Chronic pain management in cancer survivors. J Natl Compr Canc Netw. 2010;8:1104–1110. doi: 10.6004/jnccn.2010.0079. [DOI] [PubMed] [Google Scholar]
- 29.Okun A, DeFelice M, Eyde N, Ren J, Mercado R, King T, Porreca F. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stohr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Rowbotham MC. Mechanisms of neuropathic pain and their implications for the design of clinical trials. Neurology. 2005;65:S66–73. doi: 10.1212/wnl.65.12_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- 33.Tang L, Chen Y, Chen Z, Blumberg PM, Kozikowski AP, Wang ZJ. Antinociceptive pharmacology of N-(4-chlorobenzyl)-N’-(4-hydroxy-3-iodo-5-methoxybenzyl) thiourea, a high-affinity competitive antagonist of the transient receptor potential vanilloid 1 receptor. J Pharmacol Exp Ther. 2007;321:791–798. doi: 10.1124/jpet.106.117572. [DOI] [PubMed] [Google Scholar]
- 34.Urban R, Scherrer G, Goulding EH, Tecott LH, Basbaum AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain. 2011;152:990–1000. doi: 10.1016/j.pain.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]