Abstract
Background
Among people with asthma, the clinical impact and relative contribution of maternal smoking during pregnancy (in utero smoking) and current secondhand smoke exposure on asthma control is poorly documented, and there is a paucity of research involving minority populations.
Objectives
To examine the association between poor asthma control and in utero smoking and current secondhand smoke exposure among Latino and Black children with asthma.
Methods
Case-only analysis of 2 multi-center case-control studies conducted from 2008–2010 using similar protocols. We recruited 2,481 Latinos and Blacks with asthma (ages 8–17) from the mainland United States and Puerto Rico. Ordinal logistic regression was used to estimate the effect of in utero smoking and current secondhand smoke exposures on National Heart Lung and Blood Institute-defined asthma control.
Results
Poor asthma control among children 8–17 years of age was independently associated with in utero smoking (odds ratio; 95% confidence interval = 1.5; 1.1–2.0). In utero smoking via the mother was also associated with secondary asthma outcomes, including early onset asthma (1.7; 1.1–2.4), daytime symptoms (1.6; 1.1–2.1), and asthma-related limitation of activities (1.6; 1.2–2.2).
Conclusions
Maternal smoking while in utero is associated with poor asthma control in Black and Latino subjects assessed at 8–17 years of age.
Keywords: Secondhand smoke, prenatal exposure delayed effects, asthma, health status disparities
INTRODUCTION
Tobacco smoke exposure is unsafe at any level.1 While the percentage of Americans exposed to secondhand smoke (SHS) has markedly decreased over the last several decades, the decline has been unequal across demographic groups.1, 2 In particular, children are the most likely to be exposed to SHS,2 primarily through their caregivers.3
Negative outcomes attributed to tobacco smoke exposure in utero (i.e., maternal smoking during pregnancy) and in early life include stillbirth, sudden infant death syndrome, acute respiratory infections, decreased lung function, and childhood wheezing.1, 4–11 Secondhand smoke is a major risk factor for developing asthma and a key aspect for successful asthma management.12 The National Heart, Lung, and Blood Institute (NHLBI) defines asthma control as the “extent to which the various manifestations of asthma are reduced or removed by treatment”.12 Uncontrolled asthma significantly affects quality of life and incurs substantial medical expenses and opportunity costs in missed days of work and school, and premature deaths, estimated at $56 billion in the U.S. in 2007.13, 14 Among people with asthma, SHS exposure is a risk factor for asthma exacerbations and the development of severe asthma.15, 16 Avoidance of SHS exposure, therefore, is an important component of asthma prevention and control.
While extensive research has demonstrated the impact of smoking on asthma risk in young children, the clinical impact and relative contribution of in utero smoking and current SHS exposure on asthma control is poorly documented, and there is a paucity of research involving minority populations.6, 17 The objective of the current study was to investigate the contribution of in utero smoking and current SHS exposure toward poor asthma control among 2,481 Latino and Black children.
METHODS
Study design and recruitment
Subjects were recruited from the Study of African Americans, Asthma, Genes, & Environments (SAGE II) and the Gene-Environments and Admixture in Latino Asthmatics (GALA II) Study. Both studies began in 2008 and are parallel, ongoing case-control studies using similar protocols and questionnaires. Subjects are recruited from five urban study centers across the mainland U.S. and Puerto Rico (see Table E1 in the Online Repository). Target sample sizes (cases and controls) for GALA II and SAGE II are 4,000 and 2,000 subjects, respectively. Subjects recruited into the GALA II and SAGE II studies were 8–21 years old with physician-diagnosed asthma and no history of other lung or chronic illnesses; active smokers were excluded. Parents and grandparents self-identified as Latino (GALA II) or Black (SAGE II); self-identification of race/ethnicity was required of study participants. The study population for the current analysis was limited to children 8–17 years old with no history of smoking, representing 1,858 cases from GALA II and 623 cases from SAGE II who were recruited through November 2011. Inclusion/exclusion criteria are detailed in Table E2.
We ascertained demographic, environmental, and medical histories using in-person questionnaires with the children’s parents/caretakers; selected questions are reproduced in Table E3. The primary exposures for our analysis were in utero smoking and current SHS exposure. Current SHS exposure was most correlated with exposure occurring after age 6 (Pearson’s r = 0.55) and least with exposure in the first 2 years of life (0.37). Additionally, postnatal SHS exposure was most correlated with exposure at adjacent time points (e.g., correlation between ages 0–2 and ages 3–6 = 0.75). Our final regression models therefore included postnatal SHS terms for ages 0–2 and current SHS exposure in order to maximize exposure assessment and minimize multicollinearity. Race/ethnicity was categorized as: Black, Mexican, Puerto Rican, and other Latino (Latino subgroups representing <10% of the study population). Socioeconomic status indicators included family income and the child’s father’s employment status.
To assess and account for asthma control medications children might have been using, we asked subjects’ parents to identify their child’s asthma control medication(s) from a picture library of asthma control medications. We grouped their responses into one of four categories: none, monotherapy, combination therapy, and oral corticosteroids. Children using either leukotriene modifiers or inhaled corticosteroids were classified as monotherapy; combination therapy was used to describe the concomitant use two or more medications (except for oral corticosteroids); children using oral corticosteroids were classified into a separate category.
Clinical outcomes
The NHLBI measure of asthma control is a composite score and an accepted standard for measuring asthma control.12 We used NHLBI-defined criteria to classify children with asthma as controlled, partly controlled, or uncontrolled (see Table E4 in the Online Repository for a more detailed description of criteria and cut-points). The component measures of asthma control, assessed retrospectively over the week preceding subject recruitment and interview, included daytime and nighttime symptoms; asthma-related limitation of activities; use of rescue medication; and spirometric lung function measures. The ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) <85% was used as our lung function criterion because it is more sensitive than FEV1 in children.12 Estimates using either criterion produced similar results. Secondary outcomes included early onset (<age 4) asthma; presence of daytime/nocturnal symptoms; asthma-related activity limitations; FEV1 <80%; FEV1/FVC <85%.
Statistical analysis
Only children with asthma were included in the analyses. Statistical methods are detailed in the online data supplement. After verifying the proportional odds assumption, we used ordinal logistic regression to calculate the odds ratio (OR) and 95% confidence interval (CI) to estimate the association of in utero smoking and current SHS with asthma control while controlling for eczema; use of asthma control medication; exposure to home indoor allergens; IgE; socioeconomic status; race/ethnicity; age; gender; and study center. To account for potential differences between mainland and island Puerto Rican subjects, we classified Puerto Rican subjects based on their recruitment site (i.e., mainland versus island Puerto Ricans). Because our outcome variable (asthma control) was a 3-level ordinal variable (controlled asthma; partially controlled asthma; uncontrolled asthma), we used ordinal logistic regression to compare the odds of exposure between one level of asthma control and a worse level of asthma control (i.e., a single odds ratio was used to compare controlled asthma versus partially controlled, or partially controlled versus uncontrolled). All analyses were conducted using SAS v9.2 (Cary, NC).
This study was approved by the institutional review boards at each study center. All subjects (or their parents) provided written informed consent.
RESULTS
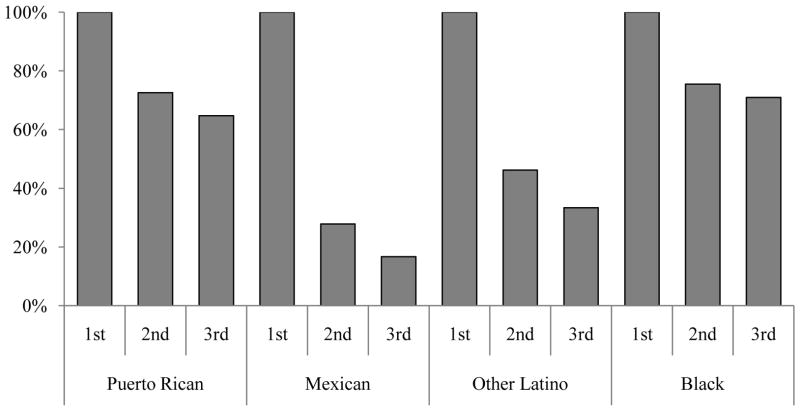
Characteristics of children with asthma are presented in Table I. Nearly half of GALA II subjects were Puerto Rican (47.2%), followed by Mexican (31.8%) and other Latino (21.0%). All SAGE II subjects were non-Hispanic Black. In utero smoke exposure during the first trimester was slightly higher among Puerto Ricans (5.8%) compared to Mexican (3.2%) and other Latino (3.3%) children, and substantially higher among Black children (17.7%). Smoking cessation during pregnancy among Puerto Rican and Black mothers was uncommon (65% and 71% respectively reported smoking during their third trimester) (Figure E1). In contrast, smoking cessation during pregnancy was greater for Mexican mothers and other Latino mothers (72% and 54% respectively stopped smoking by the second trimester), and more mothers continued to stop smoking as pregnancy progressed (83% of Mexican and 67% of other Latino mothers had stopped smoking by their third trimester). We examined potential interactions between race/ethnicity with in utero and current smoking but did not find them to be significant.
Table I.
Characteristics of study sample.
| Characteristic† | GALA II* (1,858) | SAGE II* (623) |
|---|---|---|
| Race/Ethnicity | ||
| Latino | ||
| Puerto Rican | 877 (47.2) | |
| Mexican | 590 (31.8) | |
| Other Latino | 391 (21.0) | |
| Black | 623 (100.0) | |
| Median age (25th, 75th percentile) | 11 (9, 14) | 13 (10, 16) |
| Gender | ||
| Male | 1,061 (57.1) | 340 (54.6) |
| Exposure to tobacco smoke | ||
| In utero, mother | 102 (5.5) | 117 (18.8) |
| In utero, other home exposure | 436 (23.5) | 159 (25.5) |
| First 2 years of life | 449 (24.2) | 187 (30.0) |
| Currently lives with smokers | 380 (20.5) | 163 (26.2) |
| Asthma onset <4years | 985 (53.0) | 264 (42.4) |
| Median age of onset (25th, 75th percentile) | ||
| Black | 3 (1, 6) | |
| Puerto Rican | 1 (0, 3) | |
| Mexican | 4 (2, 7) | |
| Other Latino | 3 (1, 6) | |
| Asthma control‡ | ||
| Controlled | 456 (24.5) | 120 (19.3) |
| Partly controlled | 856 (46.1) | 268 (43.0) |
| Uncontrolled | 546 (29.4) | 235 (37.7) |
| Secondary outcomes | ||
| Wheeze or shortness of breath in past week | 559 (30.1) | 277 (44.5) |
| Woken by asthma in past week | 581 (31.3) | 202 (32.4) |
| Activities limited by asthma in past week | 555 (29.9) | 219 (35.2) |
| Lung function | ||
| FEV1/FVC<85% | 937 (50.4) | 336 (53.9) |
| FEV1<80% predicted | 409 (22.0) | 248 (39.8) |
| FEV1<80% or FEV1/FVC<85% | 1,077 (58.0) | 407 (65.3) |
The GALA II (Latinos) and SAGE II (Blacks) studies were conducted between 2008 and 2010
Reported as N (%) unless otherwise specified
Asthma control was defined using criteria from the National Heart, Lung, and Blood Institute’s Third Expert Panel on the Management of Asthma
Asthma control
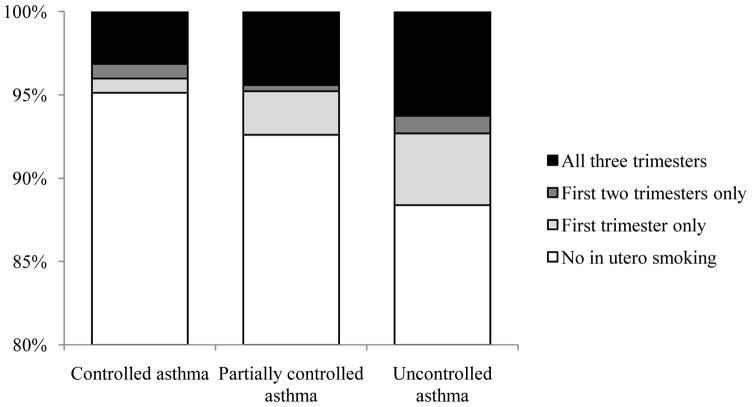
Poor asthma control was positively associated with IgE. Compared to Mexican children, asthma control was worse for Black children (OR: 2.3, 95% CI: 1.6–3.2) but not significantly different from Puerto Ricans (OR: 1.3, 95% CI: 0.8–2.1) and other Latinos (OR: 1.2, 95% CI: 0.9–1.7). After accounting for these factors, in utero smoking remained a significant predictor of poor asthma control. Children with poor asthma control were more likely to have been exposed to in utero smoking (OR: 1.5, 95% CI: 1.1–2.1) (see Table II), and a greater percentage of children were exposed to in utero smoking with worsening asthma control (Figure 1). The association between poor asthma control and in utero smoking was greater among Latino children (OR: 1.7, 95% CI: 1.1–2.7) than among Black children (OR: 1.2, 95% CI: 0.7–1.8), though the latter group may have been underpowered. The odds of poor asthma control were greater among children exposed to in utero smoking only during the first trimester (OR: 2.2, 95% CI: 1.3–3.7) compared to children exposed for all three (OR: 1.2, 95% CI: 0.7–1.8), but the estimates did not suggest a significant difference between children exposed only during the first trimester versus children exposed for all three (P-heterogeneity = 0.09). We did not find significant evidence of an independent association between exposure to current household smokers and poor asthma control (OR = 1.1), but the majority of the 95% confidence interval (95% CI: 0.9–1.3) suggested that current SHS exposure was associated with worse asthma control. In utero smoking was also independently associated with secondary asthma outcomes: early onset asthma, daytime symptoms, and limitation of activities (see Table III).
Table II.
Association odds ratios between asthma control and tobacco smoke exposure, by exposure type.
| Study | N | In utero | P Value | Current SHS | P Value |
|---|---|---|---|---|---|
| GALA II + SAGE II | 2,481 | 1.5 (1.1–2.0) | 0.02 | 1.1 (0.9–1.3) | 0.48 |
| GALA II (Latino) | 1,858 | 1.7 (1.1–2.7) | 0.02 | 1.1 (0.9–1.5) | 0.35 |
| SAGE II (Black) | 623 | 1.2 (0.7–1.8) | 0.62 | 1.0 (0.6–1.4) | 0.80 |
| Period of in utero exposure† | |||||
| No in utero exposure | 2,248 | Referent | |||
| First trimester only | 67 | 2.2 (1.3–3.7) | 0.005 | ||
| All three trimesters | 115 | 1.2 (0.7–1.8) | 0.54 | ||
Odds ratios for asthma control adjusted for eczema; use of asthma control medication; exposure to home indoor allergens; SHS exposure during first 2 years of life; IgE; socioeconomic status; race/ethnicity; age; gender; and study center. P-heterogeneity = 0.21 for in utero smoking, 0.24 for current SHS.
Not calculated for exposure during first two trimesters given that only 17 subjects were exposed during this period.
Figure 1.
Proportion of children with asthma exposed to 0, 1, 2, or 3 trimesters of in utero smoking by category of asthma control (P value for trend < 0.001).
Table III.
Association odds ratios* for secondary outcomes in the combined GALA II/SAGE II sample.
| Characteristic | Early onset | Symptoms | Nocturnal | Activities | FEV1 <80% | FEV1/FVC<85% |
|---|---|---|---|---|---|---|
| In utero SHS (No = reference) | ||||||
| Mother | 1.7 (1.1–2.4) | 1.6 (1.1–2.1) | 1.2 (0.9–1.7) | 1.6 (1.2–2.2) | 1.1 (0.8–1.6) | 1.3 (0.9–1.9) |
| Other home exposure | 1.0 (0.7–1.3) | 1.1 (0.8–1.4) | 1.1 (0.8–1.4) | 1.1 (0.8–1.4) | 1.1 (0.8–1.4) | 0.9 (0.7–1.2) |
| Postnatal SHS (No = reference) | ||||||
| Birth-2 years | 1.0 (0.8–1.3) | 1.1 (0.9–1.4) | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) | 0.9 (0.7–1.2) | 1.2 (0.9–1.5) |
| Currently lives with smokers | 0.8 (0.6–1.0) | 0.9 (0.7–1.2) | 1.0 (0.8–1.3) | 1.3 (0.9–1.6) | 1.2 (0.9–1.5) | 1.0 (0.8–1.3) |
| Family history of asthma | ||||||
| Yes (No = reference) | 1.4 (1.2–1.8) | 1.2 (0.9–1.5) | 1.1 (0.9–1.3) | 1.4 (1.1–1.7) | 1.0. (0.8–1.3) | 1.1 (0.8–1.2) |
| Eczema (No = reference) | 1.5 (1.2–2.0) | 1.2 (0.9–1.5) | 1.3 (1.0–1.6) | 1.4 (1.1–1.7) | 0.9 (0.7–1.1) | 1.0 (0.8–1.3) |
| IgE (log[IU/mL]) | 1.0 (0.9–1.0) | 1.1 (1.1–1.2) | 1.1 (1.0–1.2) | 1.1 (1.1–1.2) | 1.1 (1.0–1.2) | 1.2 (1.1–1.3) |
| Male gender | 1.5 (1.3–1.9) | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 0.9 (0.7–1.1) | 1.3 (1.0–1.5) | 1.7 (1.5–2.1) |
| Ethnicity | ||||||
| Mexican | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Puerto Rican | 1.5 (0.9–2.6) | 2.4 (1.4–4.1) | 1.8 (1.1–3.0) | 0.9 (0.5–1.6) | 1.5 (0.8–2.8) | 1.0 (0.6–1.6) |
| Other Latino | 1.3 (0.9–1.8) | 1.7 (1.2–2.6) | 1.6 (1.1–2.4) | 1.0 (0.7–1.5) | 1.3 (0.8–2.1) | 0.9 (0.7–1.3) |
| Black | 1.0 (0.7–1.4) | 3.5 (2.4–5.2) | 1.6 (1.1–2.4) | 1.8 (1.2–2.7) | 9.6 (5.6–16.5) | 1.2 (0.9–1.7) |
Odds ratios represent multivariable associations between a given characteristic and secondary outcomes, adjusted for the other covariates in table as well as for study center. SHS: secondhand smoke. Early onset: asthma onset <4 years; symptoms: wheeze or shortness of breath in past week; nocturnal: woken by asthma in past week; activities: activities limited by asthma in past week. FEV1: forced expiratory volume in 1 second. FVC: forced vital capacity.
The independent and joint contributions of in utero smoking and current SHS exposure on asthma control are summarized in Table IV. Compared to children with neither in utero smoking nor current SHS exposure, the association between poor asthma control among children with both in utero smoking and current SHS exposure was 1.3 (95% CI: 0.8–2.0), which was not substantially different from estimates for children with only current SHS exposure (OR: 1.1, 95% CI: 0.9–1.4) or in utero smoking (OR: 1.8, 95% CI: 1.2–2.8), though the only statistically significant odds ratio was for children exposed only to in utero smoking.
Table IV.
Independent and joint contributions of in utero and current secondhand smoke exposure on asthma control.
| In utero | Current | GALA II + SAGE II | GALA II (Latino) | SAGE II (Black) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Odds ratio | P Value | N | Odds ratio | P Value | N | Odds ratio | P Value | ||
| No | No | 1805 | 1.0 | 1422 | 1.0 | 383 | 1.0 | |||
| Yes | No | 113 | 1.8 (1.2–2.8) | 0.008 | 44 | 2.0 (1.0–4.0) | 0.04 | 69 | 1.5 (0.8–2.7) | 0.17 |
| No | Yes | 464 | 1.1 (0.9–1.4) | 0.25 | 320 | 1.2 (0.9–1.6) | 0.28 | 114 | 1.1 (0.7–1.7) | 0.62 |
| Yes | Yes | 106 | 1.3 (0.8–2.0) | 0.24 | 58 | 1.8 (0.9–3.2) | 0.06 | 48 | 0.8 (0.4–1.6) | 0.45 |
Odds ratios adjusted for eczema status; use of asthma control medication; log-transformed serum IgE; father’s employment status; annual income; presence of mold or cockroaches in home; SHS exposure during first 2 years of life; race/ethnicity; age; gender; and study center.
DISCUSSION
The association between SHS and asthma control has previously been investigated but the independent effects of in utero smoking and current SHS exposure on asthma control have not been well documented, particularly among minority populations. In our sample of 2,481 Latino and Black children with asthma, we demonstrate that in utero smoking negatively affects asthma control. Compared to children with controlled asthma, in utero smoking is 50% more common among children with poor asthma control, independent of other risk factors for poorly controlled asthma.
Smoking during pregnancy is particularly insidious not only for harming the developing fetus, but also for its effects manifested in later life.6 In our study, children with poor asthma control were more likely to have been exposed to tobacco smoke while they were in utero, suggesting that prenatal exposures have lingering effects more than 8 years post-exposure (children in our study were 8–17 years old). Potential mechanisms may include epigenetic (e.g., methylation) events and morphological changes in utero. There is mounting evidence, for example, that prenatal exposure to cigarette smoke results in DNA methylation (which plays a role in gene expression) that has been measured at birth,18 childhood,19, 20 and adulthood,21 suggesting that in utero exposure can lead to modifications in DNA that persist long after the exposure has occurred. In utero exposure to tobacco smoke is also associated with aberrant development of the fetal lung22, 23 and decreased lung function.5, 24, 25 Given that the majority of lung development occurs during the first trimester, and the observation that nicotine accelerates lung branching morphogenesis during this period,23 in utero smoking may potentially lead to poor asthma control via dysynaptic lung development (i.e., disproportionate growth) and subsequent obstructive lung disease. This potential mechanism is consistent with our finding that the association between poor asthma control and in utero smoking is stronger for children who were exposed during the first trimester (Table II), though the reliability of trimester-specific estimates may be compromised by the low number of children exposed within a given trimester of pregnancy. The lack of a robust association between poor asthma control and current SHS exposure in our study is consistent with reports in the literature26–29 that the contribution of SHS is more prominent among younger children. Mannino and colleagues,26 for example, report that asthma prevalence in the Third National Health and Nutrition Examination Survey was associated with biomarker-determined tobacco smoke exposure among 4- to 6-year-olds but not among older children. A review of longitudinal studies of asthma incidence has also reported a stronger association with SHS among younger children than for older children.28
Given the strong association between in utero smoking and poor asthma control, the low smoking cessation rates during pregnancy among Puerto Ricans and Blacks present public health opportunities for targeted interventions. In our study sample, 9% of subjects were exposed to in utero smoking. Assuming minimal bias and confounding in our effect estimates, 10% of children would have had better asthma control had they not been exposed to in utero smoking (see Methods in the Online Repository). This observation lends additional urgency to tobacco control efforts and supports the practice of inquiring about cigarette use and counseling smoking cessation at all clinical encounters.30
Our results should be interpreted with an understanding of this study’s strengths and limitations. Since asthma is known to be more prevalent among males in young children and among females in older children, gender and hormonal differences in our study population may have affected our results. We therefore re-examined the association between tobacco smoke exposure and asthma control for males and females, doing separate analyses for subjects aged 8–11 and 12–17 (Table E5). While the odds ratios for older and younger males were fairly similar, the association between in utero smoking and poor asthma control seemed stronger among younger females (OR = 1.9, 95% CI: 0.9–4.2) than among older females (1.3, 95% CI: 0.7–2.4) (Table E5). However, we did not see evidence of a gender*age interaction between in utero smoking and poor asthma control (interaction P-value = 0.94). The absence of an interaction may be due to a lack of heterogeneity of the association or a lack of statistical power after stratifying the sample into age/gender strata. To maintain statistical power, we therefore reported the unstratified associations, using regression models that include variables to represent gender and age category (under 12 years versus 12 and older). Subjects were recruited as part of a clinic-based case-control study. Therefore, our estimates of SHS should not be interpreted as prevalence or extrapolated to the general population. Because the current environment for some of our older participants may be very different from those in which they were raised, we included postnatal SHS exposure during participants’ first 2 years of life. Our method, however, may still not have adequately accounted for a participant’s history of exposure to tobacco smoke. Our assessment of current smoking was not confirmed by biomarker (e.g., cotinine) measurement and the retrospective design may have affected exposure estimates. Subjects, for example, may have underreported in utero smoking to provide socially desirable answers (which could have underestimated the effect). Alternatively, our estimates could have been inflated if recall of in utero smoking was over-reported, though this type of upward bias appears to be uncommon when assessing smoking during pregnancy.31–33 However, the proportion of in utero smoking in our study is consistent with national estimates, and the concordance between our observations with published data for similar measures suggests that our estimate of in utero smoking is accurate.34, 35 Puerto Rican children in our study population were recruited from Puerto Rico as well as from the mainland United States. We therefore re-examined the association between asthma control and tobacco smoke exposure after classifying Puerto Rican subjects based on their recruitment site but we did not observe a distinguishable change our measures of association. However, we acknowledge that our approach may not have adequately controlled for important differences between island- and mainland-recruited Puerto Rican children, posing potential challenges to our interpretation of results for this subgroup. Selection bias may have affected our study: because we used a case-control study design, we were unable to confirm the temporality between exposure to SHS and worsening of asthma control. Parents of children with poorly controlled asthma may be more likely not to smoke so as not to exacerbate their child’s asthma. Thus, we may have underestimated the strength of the association between poor asthma control and current SHS exposure, and a longitudinal study comparing the effects between in utero and current SHS on asthma control (e.g., Table IV) may produce different estimates. Self-report may have influenced our outcome measures but the bias is unlikely to be severe. The associations we found are robust and remained significant after controlling for SHS exposure at other time points and from other sources. Additionally, our estimates for poor asthma control by race/ethnicity are consistent with national vital statistics data, lending convergent validity to the observation that asthma mortality is lowest in Mexicans and highest in Puerto Ricans and Blacks.36
To our knowledge, we have conducted the largest investigation of the association between in utero smoking and current SHS exposure with asthma control among minority children. Our estimates are unlikely to be highly confounded given our use of a detailed questionnaire, which allowed us to control for potential confounding between tobacco smoke exposure and asthma control. Our study’s large size helped produce more precise estimates. We also measured tobacco smoke exposure from multiple time points and sources to reduce the influence of residual confounding. Our study population was composed of young Latinos and Blacks of predominantly low SES. Consequently, our results generalize toward populations disproportionately affected by asthma. Furthermore, our focus on Latinos and Blacks has significant public health implications given the discrepancy in asthma burden relative to the lack of minority representation in clinical and basic asthma research.
Conclusions
We demonstrate that tobacco smoke exposure while in utero (a relatively short duration) is associated with poor asthma control, suggesting that prenatal exposures have lingering effects at least 8 years post-exposure. Asthma is a lifelong disease negatively affected by cigarette smoke. Preventing cigarette smoke exposure during pregnancy will have important implications for improving asthma control and reducing health disparities.
Clinical Implications.
In utero smoke exposure is associated with poor asthma control and early onset asthma in subjects assessed at 8–17 years. These findings underscore the importance of smoking prevention and cessation.
Capsule Summary.
In utero smoke exposure is independently associated with poor asthma control and early onset asthma in subjects assessed at 8–17 years, underscoring the importance of tobacco prevention and cessation efforts in women of childbearing age.
Acknowledgments
Declaration of all sources of funding: This research was supported in part by National Institutes of Health (ES015794, AI077439, HL088133, HL078885, CA113710, AI079139, AI061774, HL079055, DK064695, and M01-RR00188); Flight Attendant Medical Research Institute (FAMRI); RWJF Amos Medical Faculty Development Award (to EGB); the Sandler Foundation; the American Asthma Foundation (to EGB and LKW); Ernest S. Bazley Grant (to PCA); the Fund for Henry Ford Hospital (to LKW). SSO and EGB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors acknowledge the families and patients for their participation and thank the numerous health care providers and community clinics for their support and participation in GALA II and SAGE II. In particular, the authors thank study coordinator Sandra Salazar; the recruiters who obtained the data: Duanny Alva, MD, Gaby Ayala-Rodriguez, Lisa Caine, Elizabeth Castellanos, Jaime Colon, Denise DeJesus, Blanca Lopez, Brenda Lopez, MD, Louis Martos, Vivian Medina, Juana Olivo, Mario Peralta, Esther Pomares, MD, Jihan Quraishi, Johanna Rodriguez, Shahdad Saeedi, Dean Soto, Ana Taveras; and Celeste Eng, who processes and manages the data.
Abbreviations used
- SHS
Secondhand Smoke
- NHLBI
National Heart, Lung, and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U. S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: A report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Vital signs: Nonsmokers’ exposure to secondhand smoke --- United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2010;59:1141–1146. [PubMed] [Google Scholar]
- 3.Farber HJ, Knowles SB, Brown NL, Caine L, Luna V, Qian Y, et al. Secondhand tobacco smoke in children with asthma: Sources of and parental perceptions about exposure in children and parental readiness to change. Chest. 2008;133:1367–1374. doi: 10.1378/chest.07-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaakkola JJ, Jaakkola MS. Effects of environmental tobacco smoke on the respiratory health of children. Scand J Work Environ Health. 2002;28 (Suppl 2):71–83. [PubMed] [Google Scholar]
- 5.Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348:1060–1064. doi: 10.1016/s0140-6736(96)04446-7. [DOI] [PubMed] [Google Scholar]
- 6.Best D. From the American Academy of Pediatrics: Technical report--secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009;124:e1017–1044. doi: 10.1542/peds.2009-2120. [DOI] [PubMed] [Google Scholar]
- 7.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 8.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163:429–436. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 9.Leonardi-Bee J, Britton J, Venn A. Secondhand smoke and adverse fetal outcomes in nonsmoking pregnant women: A meta-analysis. Pediatrics. 2011;127:734–741. doi: 10.1542/peds.2010-3041. [DOI] [PubMed] [Google Scholar]
- 10.Hu FB, Persky V, Flay BR, Zelli A, Cooksey J, Richardson J. Prevalence of asthma and wheezing in public schoolchildren: Association with maternal smoking during pregnancy. Ann Allergy Asthma Immunol. 1997;79:80–84. doi: 10.1016/S1081-1206(10)63090-6. [DOI] [PubMed] [Google Scholar]
- 11.Stein RT, Holberg CJ, Sherrill D, Wright AL, Morgan WJ, Taussig L, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: The Tucson children’s respiratory study. Am J Epidemiol. 1999;149:1030–1037. doi: 10.1093/oxfordjournals.aje.a009748. [DOI] [PubMed] [Google Scholar]
- 12.National Asthma Education and Prevention Program (National Heart Lung and Blood Institute) Guidelines for the diagnosis and management of asthma: Full report 2007. Bethesda, MD: U.S Dept. of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; 2010. Third Expert Panel on the Management of Asthma. [Google Scholar]
- 13.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 14.American Lung Association Epidemiology and Statistics Unit. Trends in asthma morbidity and mortality. [cited 2011 Jul 12]. Available from: http://www.lungusa.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf.
- 15.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: Data from the third National Health and Nutrition Examination Survey. Chest. 2002;122:409–415. doi: 10.1378/chest.122.2.409. [DOI] [PubMed] [Google Scholar]
- 16.California Environmental Protection Agency. Health effects of exposure to environmental tobacco smoke: The report of the California Environmental Protection Agency. Sacramento, CA: California Environmental Protection Agency, Office of Environmental Health Hazard Assessment; 1997. [Google Scholar]
- 17.Akuete K, Oh SS, Thyne S, Rodriguez-Santana JR, Chapela R, Meade K, et al. Ethnic variability in persistent asthma after in utero tobacco exposure. Pediatrics. 2011;128:e623–e630. doi: 10.1542/peds.2011-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerrero-Preston R, Goldman LR, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter FR, et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics. 2010;5:539–546. doi: 10.4161/epi.5.6.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breton CV, Salam MT, Gilliland FD. Heritability and role for the environment in DNA methylation in axl receptor tyrosine kinase. Epigenetics. 2011;6:895–898. doi: 10.4161/epi.6.7.15768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry MB, Ferris JS, Pilsner R, Flom JD, Tehranifar P, Santella RM, et al. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:2306–2310. doi: 10.1158/1055-9965.EPI-08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu ZX, Hunter DD, Kish VL, Benders KM, Batchelor TP, Dey RD. Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environ Health Perspect. 2009;117:1434–1440. doi: 10.1289/ehp.0800511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wongtrakool C, Roser-Page S, Rivera HN, Roman J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L611–618. doi: 10.1152/ajplung.00038.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167:917–924. doi: 10.1164/rccm.200206-616OC. [DOI] [PubMed] [Google Scholar]
- 25.Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55:271–276. doi: 10.1136/thorax.55.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155:36–41. doi: 10.1001/archpedi.155.1.36. [DOI] [PubMed] [Google Scholar]
- 27.Stein RT, Holberg CJ, Sherrill D, Wright AL, Morgan WJ, Taussig L, Martinez FD. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children’s Respiratory Study. Am J Epidemiol. 1999;149:1030–7. doi: 10.1093/oxfordjournals.aje.a009748. [DOI] [PubMed] [Google Scholar]
- 28.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53:204–12. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Wypij D, Gold DR, Speizer FE, Ware JH, Ferris BG, Jr, Dockery DW. A longitudinal study of the effects of parental smoking on pulmonary function in children 6–18 years. Am J Respir Crit Care Med. 1994;149:1420–5. doi: 10.1164/ajrccm.149.6.8004293. [DOI] [PubMed] [Google Scholar]
- 30.From the American Academy of Pediatrics: Policy statement--Tobacco use: a pediatric disease. Pediatrics. 2009;124:1474–1487. doi: 10.1542/peds.2009-2114. [DOI] [PubMed] [Google Scholar]
- 31.Dietz PM, Homa D, England LJ, Burley K, Tong VT, Dube SR, Bernert JT. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173:355–9. doi: 10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]
- 32.Orr ST, Newton E, Tarwater PM, Weismiller D. Factors associated with prenatal smoking among black women in eastern North Carolina. Matern Child Health J. 2005;9:245–52. doi: 10.1007/s10995-005-0010-x. [DOI] [PubMed] [Google Scholar]
- 33.Wright TE, Milam KA, Rougee L, Tanaka MD, Collier AC. Agreement of umbilical cord drug and cotinine levels with maternal self-report of drug use and smoking during pregnancy. J Perinatol. 2011;31:324–9. doi: 10.1038/jp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Center for Health Statistics. Health, United States, 2010: With special feature on death and dying. Hyattsville, Maryland: 2011. [PubMed] [Google Scholar]
- 35.Perreira KM, Cortes KE. Race/ethnicity and nativity differences in alcohol and tobacco use during pregnancy. Am J Public Health. 2006;96:1629–1636. doi: 10.2105/AJPH.2004.056598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homa DM, Mannino DM, Lara M. Asthma mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban heritage, 1990–1995. Am J Respir Crit Care Med. 2000;161:504–509. doi: 10.1164/ajrccm.161.2.9906025. [DOI] [PubMed] [Google Scholar]