Abstract
Background
Eosinophilic esophagitis is a chronic, immune-mediated inflammatory disorder that responds to dietary therapy; however, data evaluating the effectiveness of dietary therapeutic strategies is limited.
Objective
This study compared the effectiveness of three frequently prescribed dietary therapies [elemental, six-food elimination, and skin prick and atopy patch-directed elimination] and assessed the remission predictability of skin tests and their utility in directing dietary planning.
Methods
A retrospective cohort of proton-pump inhibitor-unresponsive, non-glucocorticoid-treated eosinophilic esophagitis patients who had two consecutive endoscopic biopsies associated with dietary intervention was identified. Biopsy histology and remissions (< 15 eosinophils/high-power field) following dietary therapy and food reintroductions were evaluated.
Results
Ninety-eight of 513 patients met eligibility criteria. Of these 98, 50% (49), 27% (26), and 23% (23) received elemental, six-food elimination, and directed diets, respectively. Remission occurred in 96%, 81%, and 65% of patients on elemental, six-food elimination, and directed diets, respectively. The odds of post-diet remission vs. non-remission were 5.6-fold higher (P=0.05) on elemental vs. six-food elimination, 12.5-fold higher (P=0.003) on elemental vs. directed, and were not significantly different (P=0.22) on six-food elimination vs. directed diets. Following 116 single-food reintroductions, the negative predictive value of skin testing for remission was 40%–67% (milk 40%, egg 56%, soy 64%, and wheat 67%).
Conclusion
All three dietary therapies are effective; however, an elemental diet is superior at inducing histologic remission compared with six-food elimination and skin test-directed diets. Notably, an empiric six-food elimination diet is as effective as a skin test-directed diet. The negative predictive values of foods most commonly reintroduced in single-food challenges are not sufficient to support the development of dietary advancement plans solely based on skin tests.
Keywords: Eosinophilic esophagitis, eosinophils, histologic remission, pediatric, dietary therapy, food allergy, negative predictive values, elemental diet, six-food elimination diet, skin test-directed elimination diet
INTRODUCTION
Eosinophilic esophagitis (EoE) is an emerging gastrointestinal disorder characterized by marked esophageal eosinophilia that is etiologically linked to immune hypersensitivity to dietary antigens (hereto referred to as foods)1 and is a chronic disorder than persists from childhood into adulthood2. The high response rate to food elimination diets, especially amino acid-based elemental diets3–6, and the frequent recurrence of disease with food reintroduction imply that the disease is mediated by allergic sensitization to foods7–9. Indeed, experimental EoE in mice can be induced by exposure to diet and/or aeroallergens via a variety of entry points including the skin, respiratory and gastrointestinal tracts10–12. Consistent with an allergic etiology rather than an acid-induced esophagitis4, swallowed glucocorticoids with limited systemic effects have also been shown to be effective for the treatment of EoE13–18 and elicit local changes in gene expression in the esophagus19. Furthermore, the esophagi of EoE patients express a unique transcription profile with an upregulation of genes involved in allergic inflammation20, 21.
Although the role of allergy testing in EoE remains controversial, evaluation by an allergist is recommended to be part of the diagnostic work-up, especially for the treatment of co-existing allergic disorders1. Current clinical practice for the allergic evaluation of patients with EoE mainly relies on skin prick tests (SPTs)1, 22. For IgE-mediated immediate hypersensitivity, SPT can provide a rapid means to detect sensitization when combined with a comprehensive history of food-induced symptoms23. In patients with allergic disease, negative SPT responses have a negative predictive value (NPV) of 95% to 100% for 4 common allergenic foods: egg, milk, peanut, and fish23, 24. However, the specificity of this test is approximately 50%, making positive results especially difficult to interpret25. Because EoE is thought to be primarily non-IgE-mediated, skin testing based on delayed hypersensitivity to foods via atopy patch tests (APTs) has been advocated7, 26, 27. Unfortunately, interpreting APT results is subjective, prone to significant inter-observer variation, hindered by the lack of standardized extracts, and has not been validated in an EoE population with the use of a control group25, 28, 29. Liacouras and Spergel demonstrated that 60–80% of patients with EoE respond to a directed elimination diet, which removes all of the patient’s SPT- and APT-positive foods, and that disease reoccurs upon food reintroduction5, 26.
Recently, an alternative approach to food elimination has been recommended based on empiric avoidance of the six most common allergenic foods in the U.S. (milk, egg, soy, wheat, peanuts/tree nuts, and fish/shellfish)6. Using a response cut-off of ≤ 10 eosinophils/high-power field (HPF), Kagalwalla et al. initially reported a histologic response of 74% for this empiric six-food elimination diet (SFED). Complete elimination of eosinophilic inflammation (≤ 1 eosinophil/HPF) was reported in 29% (10/35) patients in the SFED group and in 56% (14/25) of patients in the elemental diet group6. These findings are consistent with the original report by Kelly et al. that 50% of patients with EoE have complete histologic resolution and 100% have partial resolution following an elemental diet3. Uncovering whether dietary therapy based on skin testing (a directed diet) is better than empiric removal of foods (SFED) could have significant impact on clinical practice, which is currently based on the common paradigm of skin testing before food elimination.
Herein, we report comparison of remission rates among three dietary therapies frequently prescribed in pediatric EoE and implemented in a clinical setting: elemental diet, SFED, and a skin test-directed elimination diet. In addition, we evaluate the possibility of using skin tests for dietary planning and report the NPVs for single-food reintroductions.
METHODS
Study Design
A retrospective convenience cohort study consisted of patients seen from January 1999 to October 2011 at the Cincinnati Center for Eosinophilic Disorders (CCED) (http://www.cincinnatichildrens.org/service/c/eosinophilic-disorders/default/), Cincinnati Children’s Hospital Medical Center (CCHMC). Participants were recruited from the Eosinophilic Gastrointestinal Disease (EGID) database, an IRB-approved repository of disease-related information from patients who have eosinophilic disorders. Written, informed consent was obtained from parents, and written, informed assent was obtained from children 11 years and older.
Subjects
Subjects recruited for the EGID database were only those seen at the CCED by one of two gastroenterologists. Patient records were selected for review if they met the following eligibility criteria: 1) diagnosis of EoE1, 29; diagnostic criteria consisted of having ≥ 15 eosinophils/HPF in at least one esophageal biopsy specimen, having no response to a proton-pump inhibitor prescribed in varying doses (up to 2 mg/kg/day) for at least 6 weeks or having normal results on multichannel intraluminal impedance pH or pH probe, and the exclusion of other causes of esophageal eosinophilia; 2) having at least two consecutive esophagogastroduodenoscopies (EGDs) to monitor dietary therapy; 3) not having received oral or topical glucocorticoids for at least 2 months prior to and during the duration of the study; and 4) being ≤ 21 years of age throughout the study duration. Patients were excluded if they were diagnosed with other diseases or conditions associated with eosinophilia (e.g., celiac disease, Crohn’s disease, hypereosinophilic syndrome), were diagnosed with a mitochondrial disorder30, were enrolled in a concomitant drug trial, were not following any of the three dietary therapies under study, or were identified as non-compliant with dietary therapy (i.e., documentation by a physician that the diet was not followed as prescribed). Medications not known to affect esophageal eosinophilia, as well as asthma medications (including nasal and inhaled glucocorticoids), were permitted. A clinical history of allergic disease (i.e., asthma, allergic rhinitis or atopic dermatitis) was recorded. Evaluation of food reintroductions occurred only in patients who had both SPT and APT performed at the CCED. Patient demographic and disease characteristics were evaluated. Duration of follow-up at the CCED was defined as the number of years patients were followed at the CCED since their first CCED EGD. Patients were categorized as being local if their home zip code was included in CCHMC’s designated regional catchment area.
Dietary Therapy
Three commonly prescribed diet therapies, the elemental, the SFED, and the allergy test directed elimination diet were evaluated in this study. Patients were treated with one of three food elimination therapies as the sole intervention, except for acid-suppression therapy between 2 endoscopic assessments. The initial dietary therapy chosen for each patient was not randomly assigned but was negotiated between physicians and patient based on multiple factors after comprehensive medical history (including social history) and physical examination. Medical history factors included a patient’s response to any dietary therapies implemented prior to evaluation at our institution that may have precluded the use of one of the dietary therapy options under study and the assessment of the child and family’s ability and willingness to implement a dietary therapy.
The 3 dietary therapy interventions evaluated in this study were defined in the following manner: 1) An elemental diet was defined as eliminating all foods and providing complete nutrition by the exclusive use of formula that contained crystalline amino acids, such as Neocate or E028 Splash (Nutricia North America, Rockville, MD) or EleCare (Abbott Laboratories, Columbus, OH). 2) The SFED encompasses two variations: the classical SFED of which 42% (11/26) of patients empirically avoided the 6 most common allergenic foods (i.e., milk, soy, wheat, egg, peanuts/tree nuts, and fish/shellfish) regardless of allergy test results, and the modified SFED of which 58% (15/26) avoided foods that tested positive on SPT and APT in combination with the avoidance of the 6 most common allergenic foods. The number of foods eliminated in the SFED, the modified SFED, and the directed diets were 7 (6–11), 8 (7–9), and 5 (3–11) (median, interquartile range), respectively. No significant differences were detected between demographic or disease-related variables or remission (< 15 eosinophils/HPF) among the classical and modified SFED (data not shown); therefore, these data were combined and were referred to as the SFED. 3) A skin test-directed elimination (directed) diet was defined as the avoidance of only those foods that tested positive by SPT and/or APT, resulted in anaphylaxis, or were avoided due to known oral allergy syndrome.
The duration of dietary therapy was defined as the number of months that patients received initial dietary therapy intervention.
Esophageal Histology
Esophageal biopsies were fixed in formalin and embedded in paraffin; five-micron sections were stained with hematoxylin and eosin (H&E). The peak eosinophil count/HPF was defined as the highest eosinophil count in either the distal or proximal esophagus. The peak eosinophil count in biopsies was determined at 400X magnification (area 0.3 mm2) by CCHMC board-certified pathologists. For patients who had begun dietary therapy prior to their initial CCED visit, the peak eosinophil count from biopsy slides provided from the outside institutions was determined by a board-certified pathologist (author MHC) at CCHMC.
Remission Status
Due to the departure from symmetry, the median pre- and post-diet peak eosinophil counts were calculated. Remission status was determined using the post-diet peak eosinophil count and was initially defined using 4 categories: complete remission ≤ 1 eosinophil/HPF; partial remission 2–5 eosinophils/HPF; partial resolution 6–14 eosinophils/HPF; and non-remission or active disease ≥ 15 eosinophils/HPF. For greater simplicity, remission status following dietary therapy and food reintroduction was dichotomized with remission being defined as < 15 eosinophils/HPF and non-remission being defined as ≥ 15 eosinophils/HPF. Complete elimination of eosinophilic inflammation, previously defined as ≤ 1 eosinophil/HPF4, was referred to as complete remission and was compared among dietary therapies. The odds of post-diet remission vs. non-remission were calculated among dietary therapies.
Evaluation of Atopic Sensitization
Skin prick tests
For consistency of the allergen extract and the interpretation, only SPTs performed at the CCED were included. Foods tested were individually selected by the allergist based on clinical history and dietary intake. Patients had SPTs to as many as 62 foods and 11 environmental allergen extracts. The type, concentrations, and manufacturer of the allergen extracts and grading system used have been published previously31. Histamine (1 mg/mL) and albumin in saline were positive and negative controls, respectively. Tests were read after 15 minutes and interpreted as follows: 0 = negative control; 1+ = very small induration, erythema present; 2+ = 50.0% of histamine control; 3+ = histamine control; and 4+ ≥ histamine control of pseudopodia. Tests graded as 2+ or higher were considered positive, as were tests in which the largest wheal diameter measured ≥ 3 mm larger than the negative control. It is generally accepted that a mean wheal size ≥ 3 mm larger than the negative control is suggestive of food allergy32.
Atopy patch tests
Only patch tests performed at the CCED were included in the analysis. All available foods were tested by APT, except for foods to which the patient had a history of allergic reaction or a positive SPT. Details related to APT allergen manufacturers, preparation, and placements have been reported previously31. The patches were removed after 48 hours and scored at 72 hours as follows: 0, no visible findings; 1, erythema but no induration; 2, erythematous, generalized induration and/or a few scattered papules; 3, erythematous, marked induration/papules; and 4, erythematous papules and vesicular eruption. A score of 2 or above was considered positive.
Atopy
Patients were considered atopic if they had a history of asthma, allergic rhinitis, or atopic dermatitis and had a positive SPT.
Food Reintroduction
Investigation of single, multiple, and combined (both single and multiple) food reintroductions are reported only for patients who underwent an allergy evaluation and received both SPT and APT at the CCED. A multiple food reintroduction was defined as more than one food being reintroduced within one histologic evaluation interval. Food reintroductions were initiated only when the peak eosinophilic count post-diet therapy was <15 eosinophils/HPF. If symptoms occurred after reintroduction of a food, patients were instructed to discontinue that food, wait approximately 10 to 14 days, and then reintroduce another food. The frequency and type of food reintroductions performed were identified. Remissions occurring as a result of initial dietary therapy (food elimination) and esophageal eosinophilia or symptoms recurring after food reintroduction were documented. A food reintroduction was considered successful if no symptoms were reported and the post-peak eosinophil count/HPF was < 15 and considered unsuccessful if symptoms returned or if the post-peak eosinophil count/HPF was ≥ 15. Due to the paucity of dual SPT/APT-positive results (1/116), NPVs were calculated using only SPT results in order to assess its utility in dietary planning.
Statistical Analysis
Pre- and post-diet therapy peak eosinophil counts/HPF and percent of patients in remission (<15 eosinophils/HPF) were compared among each dietary therapy using the Kruskal-Wallis test and X2 test, respectively. If significant differences (P < 0.05) were detected, pairwise comparisons were performed. Adjusted p-values were reported for significant pairwise comparisons using the Dwass, Steel, Critchlow, Fligner correction for continuous variables and the Hochberg correction for discrete variables. This approach was repeated using each remission category: complete remission (≤ 1 eosinophil/HPF); partial remission (2–5 eosinophils/HPF); partial resolution (6–14 eosinophils/HPF); and non-remission (≥ 15 eosinophils/HPF). Comparisons of demographic and disease-related variables by dietary therapies were performed using the Kruskal-Wallis or Mann Whitney U tests for continuous variables and the X2 and Fisher’s exact test for discrete variables. The odds ratios and their confidence intervals for post-diet remission vs. non-remission among dietary therapies were calculated using logistic regression. The Wilcoxon paired signed rank test was used to determine statistical significance between pre- and post-diet therapy median peak eosinophil counts/HPF among each dietary therapy. Statistical analyses were performed using PASW Statistics 18.0 (SPSS Inc., Chicago, IL) and SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Enrollment
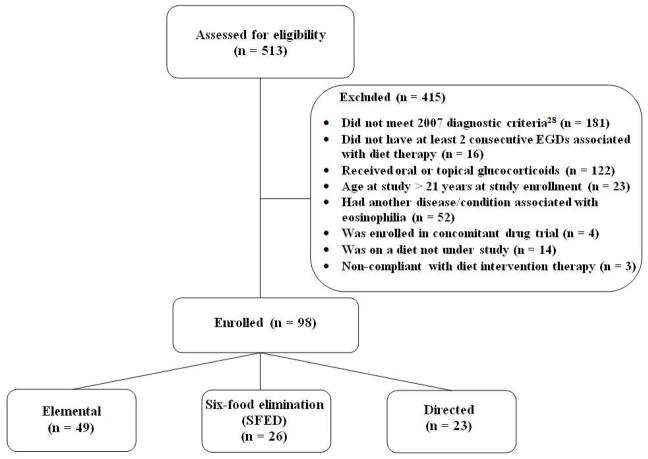
A flow diagram of participant recruitment is shown in Figure 1. A total of 513 patients were assessed for eligibility, of which 81% (415) were excluded and 19% (98) were enrolled in the study. Reasons for exclusion are as follows: not meeting the consensus disease criteria (n = 181); not having two consecutive EGDs separated by dietary intervention therapy (n = 16); having received oral or topical glucocorticoids (n = 122); patient age > 21 years (n = 23); the presence of another eosinophilia-associated condition or disorder (n = 52); enrollment in a concomitant drug trial (n = 4); on a diet that was not under study (n = 14); and obvious non-compliance with dietary intervention therapy (n = 3).
Figure 1.
Flow diagram of study participants.
Dietary Therapy
Of the 98 patients who met eligibility criteria, 50% (49), 27% (26), and 23% (23) received an elemental diet, SFED, and directed elimination diet at enrollment, respectively.
Subjects
Of the 98 enrolled patients, 50% were diagnosed with EoE prior to an age of 3.5 years. The mean age (± SD) at EoE diagnosis and upon the first patient visit at the CCED was 5.1 (± 4.2) and 5.9 (± 4.4) years, respectively; neither differed significantly among dietary therapies. Demographic and disease characteristics for each dietary therapy are outlined in Table 1. An approximate 3:1 male-to-female ratio was identified. On average, patients had 8.5 EGDs (± 6.3) (range, 1–24) performed at the CCED, with the number of EGDs being significantly greater among patients with the elemental diet compared to the SFED (P = 0.017) or to the directed diet (P = 0.039). Seventy-six percent (74) of patients had at least one EGD performed at an outside site prior to their first visit to the CCED. Seven percent (7) of patients were seen at the CCED only once, for an initial evaluation. The mean number of EGDs (± SD) performed per year in the 91 patients who returned to the CCED following their initial visit was 3.8 (± 1.8), and no statistical differences were detected among dietary therapies (P = 0.26). The duration of CCED follow-up, mean years (± SD) was 2.5 (± 2.2) years (range, 0–8.5), with there being a significantly longer interval for the elemental diet compared to the SFED (P = 0.01). A total of 68% of patients (67) were atopic; this percentage did not differ significantly among the examined dietary therapies. Approximately 67% (66) of patients lived outside CCHMC’s regional catchment area; this percentage did not differ significantly among the examined dietary therapies. Of the 49 patients on an elemental diet, 55% (27) ingested formula by mouth, and the remainder received tube feedings. Supplemental elemental formula for nutritional support was used in 27% (7) of the patients on the SFED and 35% (8) of the patients on the directed diet.
Table 1.
Patient demographic and disease-related characteristics among three EoE dietary therapies (n = 98).
| Elemental (n = 49) | SFED (n = 26) | Directed (n = 23) | P-value* | ||||
|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | ||
| Age at study, years | 5.6 | 0.9 – 19.7 | 6.6 | 2.2 – 20.8 | 5.2 | 0.9 – 15.0 | 0.32 |
| Age at first EGD¶, years | 4.3 | 0.7 – 16.2 | 5.5 | 0.6 – 20.3 | 4.6 | 0.1 – 13.6 | 0.25 |
| Duration between first EGD and EoE diagnosis, years | 0.5 | 0 – 4.3 | 0.4 | 0 – 2.9 | 0.2 | 0 – 1.6 | 0.73 |
| Dietary therapy duration**, months | 4.5 | 0.8 – 23.2 | 4.4 | 2.3 – 10.5 | 3.9 | 2.4 – 7.3 | 0.79 |
| Total number of EGDs | 11.1† | 2 – 26 | 6.2† | 3 – 12 | 8.4 | 3 – 22 | 0.005 |
| Number of EGDs at the CCED | 8.5‡,§ | 1.0 – 24.0 | 4.0‡ | 1.0 – 8.0 | 5.2§ | 1.0 – 19.0 | 0.006 |
| Duration of follow-up at the CCED††, years | 2.9|| | 0 – 8.5 | 1.1|| | 0 – 2.6 | 2.1 | 0 – 6.8 | 0.009 |
| Atopy‡‡, n (%) | |||||||
| Yes | 32 (65.3) | 20 (76.9) | 15 (65.2) | 0.55 | |||
| Gender, n (%) | |||||||
| Male | 36 (73.5) | 19 (73.1) | 19 (82.6) | 0.66 | |||
| Race n (%) | |||||||
| Caucasian | 44 (89.8) | 25 (96.2) | 22 (95.7) | ||||
| African American | 2 (4.1) | 1 (3.8) | 1 (4.3) | ||||
| Other | 3 (6.1) | 0 | 0 | 0.54 | |||
| Ethnicity, n (%) | |||||||
| Non-Hispanic | 48 (98.0) | 25 (96.2) | 22 (95.7) | 0.84 | |||
| Residence, n (%) | |||||||
| Non-local | 34 (69.4) | 16 (61.5) | 16 (69.6) | 0.76 | |||
Diet therapies were compared statistically using the Kruskal-Wallis test for continuous variables and the X2 test or the Fisher’s exact test for discrete variables. If significant differences were detected, pairwise comparisons were performed. Adjusted P-values using the Dwass, Steel, Critchlow, Fligner correction for multiple pairwise comparisons that were significantly different:
elemental vs. SFED, P = 0.004;
elemental vs. SFED, P = 0.017;
elemental vs. directed, P = 0.039; elemental vs. SFED, P = 0.01.
EGD, esophagogastroduodenoscopy.
Duration dietary therapy was defined as the number of months patients received initial dietary therapy intervention.
Duration of follow-up at the CCED was defined as the number of years patients were followed at the CCED since their first CCED EGD.
Atopy was defined as having a history of asthma, allergic rhinitis, or atopic dermatitis and had a positive skin prick test.
Post-diet Therapy Esophageal Histology
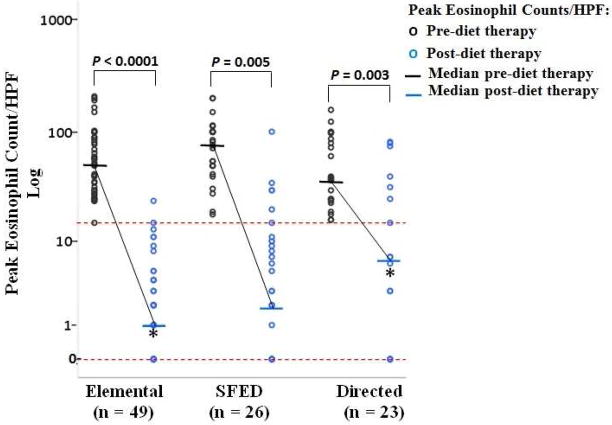
Comparison of the pre- and post-diet therapy peak eosinophil counts/HPF for each dietary therapy is shown in Figure 2. For each dietary therapy, the median pre-diet therapy eosinophil count/HPF was significantly higher than the median post-diet therapy eosinophil count/HPF (elemental diet P < 0.0001, SFED P = 0.005, and directed diet P = 0.003).
Figure 2.
Comparisons of pre- and post-diet therapy peak eosinophil counts/HPF for diet therapies using Kruskal-Wallis test. Data points between the dashed horizontal lines represent remission (0 to < 15 eosinophils/HPF), and data points above the upper dashed line represent non-remission (≥ 15 eosinophils/HPF). The Wilcoxon paired signed rank-test was used to compare pre- and post-diet therapy median peak eosinophil counts/HPF. The solid horizontal lines represent median values.
*Adjusted P-value using the Dwass, Steel, Critchlow, Fligner correction for pairwise comparison between the post-diet therapy peak eosinophil counts/HPF for the elemental and directed diet, P = 0.01.
Table 2 compares the median pre- and post-diet therapy peak eosinophil count/HPF among dietary therapies and presents the interquartile range for each diet therapy. Pairwise comparison revealed a significantly higher median pre-diet therapy peak eosinophil count/HPF in the SFED compared to the directed diet, P = 0.036. No significant differences in the pre-diet therapy peak eosinophil count/HPF were detected in the elemental diet compared to the SFED (P = 0.34) or compared to the directed diet (P = 0.41).
Table 2.
Comparison of pre- and post- diet therapy peak eosinophil counts/HPF and remission status among diet therapies (n = 98).
| Diet Therapy
|
|||
|---|---|---|---|
| Elemental (n = 49) | SFED (n = 26) | Directed (n = 23) | |
| Pre-diet Therapy: | |||
| Peak eosinophil count/HPF | |||
| Median | 51.0 | 76.5* | 38.0* |
| Interquartile range | 28.0 – 90.0 | 48.0 – 101.0 | 23.0 – 87.0 |
| Post-diet Therapy: | |||
| Peak eosinophil count/HPF | |||
| Median | 1.0† | 2.5 | 7.0† |
| Interquartile range | 0 – 3.5 | 0 – 10.3 | 0 – 25.0 |
| Remission Types||: | |||
| Remission, n (%) | 47 (96)‡ | 21 (81) | 15 (65)‡ |
| Complete remission, n (%) | 29 (59) § | 10 (39) | 7 (30) § |
| Partial remission, n (%) | 13 (27) | 6 (23) | 2 (9) |
| Partial resolution, n (%) | 5 (10) | 5 (19) | 6 (26) |
| Non-remission, n (%) | 2 (4)‡ | 5 (19) | 8 (35)‡ |
Remmission types were compared statistically using the Kruskal-Wallis test. If significant differences were detected, pairwise comparisons were performed. Adjusted P-values using Hochberg correction for multiple pairwise comparisons that were significantly different:
SFED vs. directed, P = 0.036;
elemental vs. directed, P = 0.01;
elemental vs. directed, P = 0.001;
elemental vs. directed, P = 0.04.
Remission types were defined as: remission < 15 eosinophils/HPF; complete remission ≤ 1 eosinophil/HPF; partial remission 2–5 eosinophils/HPF; partial resolution 6–14 eosinophils/HPF; and non-remission ≥ 15 eosinophils/HPF.
Pairwise comparison demonstrated a significantly lower median post-therapy peak eosinophil count/HPF in the elemental diet compared to the directed diet, P = 0.01. The post-diet therapy peak eosinophil count/HPF did not differ significantly between the elemental diet (P = 0.26) and the SFED or between the SFED and the directed diet (P = 0.35).
Remission Status
Table 2 compares the remission status among dietary therapies. Remission (< 15 eosinophils/HPF) was attained in 96% (47/49) of patients on the elemental diet, 81% (21/26) of patients on the SFED [82% (9/11) of patients on the classical SFED and 80% (12/15) of patients on the modified SFED], and 65% (15/23) of patients on the directed diet. Pairwise comparison revealed a significantly higher complete remission rate (≤ 1 eosinophil/HPF, P = 0.04) and significantly lower non-remission rate (≥ 15 eosinophils/HPF, P = 0.001) for the elemental diet compared to the directed diet. There were no significant differences in any type of remission between the elemental diet and the SFED or between the SFED and the directed diet.
The odds of post-diet remission (< 15 eosinophils/HPF) vs. non-remission (≥ 15 eosinophils/HPF) were 5.6-fold (95% CI, 1.0–31.2, P = 0.05) greater on the elemental diet compared with the SFED, 12.5-fold (95% CI, 2.3–65.6, P = 0.003) greater on the elemental diet compared with the directed diet, and not significantly different 2.2-fold (95% CI, 0.12–1.64, P = 0.22) greater on the SFED compared with the directed diet.
Food Reintroduction
Approximately 70% (69/98) of patients underwent both SPT and APT at the CCED. Table 3 shows the following variables by dietary therapy: the number of patients who underwent food reintroductions, the duration of the food reintroduction phase, and the total number of single-, multiple-, and combined-food reintroductions performed. Seventy-five percent of patients (51/69) underwent food reintroductions. No significant difference in the distribution of patients that underwent any type of food reintroduction (single, multiple, combined) was detected among the 3 dietary therapies, P = 0.10. Forty-eight percent (25/51) of food reintroductions were performed in patients on the elemental diet, 29% (15/51) in patients on the SFED, and 21% (11/51) in patients on the directed diet. No significant difference in the percentage of patients with atopy was detected among dietary therapies (P = 0.24). A total of 33 foods were reintroduced in 116 single-food challenges conducted in a total of 42 patients (21 patients who underwent single only reintroductions plus 21 patients who underwent combination reintroductions that included single-food challenges). Twelve percent (14/116) of single-food reintroductions tested positive on SPT; only one single-food reintroduction tested positive to both SPT and APT as APT was not performed for the other 13 foods that tested positive on SPT. Of the 42 patients (Table 3) that underwent single-food reintroductions, 5% (2/42) had positive findings to more than one food. A total of 285 foods were reintroduced in 99 multiple-food challenges in 30 patients. On average, 2.9 foods were reintroduced in each multiple-food challenge.
Table 3.
Food reintroduction* characteristics among dietary therapies.
| Diet Therapy
|
P- value† | |||
|---|---|---|---|---|
| Elemental (n = 49) | SFED (n = 26) | Directed (n = 23) | ||
| SPT‡ and APT§ performed at the CCED, n (%) | 28 (57.1) | 22 (84.6) | 19 (83.3) | n/a|| |
| Atopy, n (%) | 17/28 (60.7) | 18/22 (81.8) | 15/19 (78.9) | 0.24 |
| Food reintroduction(s)*, n (%) | 25/28 (89.3) | 15/22 (68.1) | 11/19 (57.9) | 0.78 |
| Number of patients who underwent food reintroduction(s), n (%) | ||||
| Single only | 6/25 (24.0) | 9/15 (60.0) | 6/11 (54.6) | |
| Multiple only | 5/25 (20.0) | 3/15 (20.0) | 1/11 (9.0) | |
| Combination (single and multiple) | 14/25 (56.0) | 3/15 (20.0) | 4/11 (36.4) | 0.10 |
| Duration of food reintroduction phase, years | ||||
| Mean (± SD) | 2.1 (±1.8) | 0.9 (±0.5) | 1.6 (±1.7) | 0.11 |
| Range | 0.2 – 6.4 | 0.2 – 2.0 | 0.2 – 5.9 | n/a|| |
Food reintroductions were conducted in 75% of the 69 patients who had both SPT and APT performed at the CCED.
Diet therapies were compared statistically using the X2 test or Fisher’s exact test for discrete variables and the Kruskal-Wallis test for continuous variables. No significant differences for pairwise comparisons were detected.
SPT, skin prick test;
APT, atopy patch test;
n/a = not applicable.
Of the 50% (58/116) of single-food reintroductions that failed, 12% (7/58) failed as a result of symptoms. The remaining 88% (51/58) failed single-food reintroductions based on histologic results. Thirty-seven percent (104/285) of the foods that were reintroduced during multiple-food challenges failed histologically. Table 4 outlines the single- and multiple-food reintroduction results. The percent of food reintroductions that passed histologic evaluation (peak eosinophil count < 15/HPF) ranged from 35% to 63%, and the NPVs for the foods most often reintroduced during single-food challenges ranged from 40% to 67%. These 4 foods (milk, egg, soy, and wheat; Table 4) represent 48% (56/116) of all single-food reintroductions. For the foods most often reintroduced during multiple-food challenges, the percent of food reintroductions that passed histologic evaluation appear in Table 4. The 15 foods listed under multiple reintroductions in Table 4 represent 53% (150/285) of all of the foods reintroduced in multiple-food challenges.
Table 4.
Single- and multiple-food reintroduction results.
| Type of Food Reintroduction | ||||
|---|---|---|---|---|
| Single (n = 116) | Multiple* (n = 99) | |||
| Food (n) | % Pass† | NPV‡ (%) | Food (n) | % Pass† |
| Wheat (16) | 63 | 67 | Cocoa (9) | 78 |
| Soy (13) | 62 | 64 | Pork (12) | 75 |
| Egg (10) | 60 | 56 | Grapes (11) | 73 |
| Milk (17) | 35 | 40 | Oats (10) | 70 |
| Sweet Potatoes (10) | 70 | |||
| Rice (11) | 64 | |||
| Apple (13) | 62 | |||
| Chicken (12) | 58 | |||
| Carrot (9) | 55 | |||
| Banana (10) | 50 | |||
| Broccoli (7) | 43 | |||
| Tomato (7) | 43 | |||
| Beef (12) | 42 | |||
| Corn (10) | 40 | |||
| Strawberry (7) | 29 | |||
A multiple-food reintroduction was defined as more than one food was being challenged within one histologic evaluation interval.
Reintroductions that passed were based on a post-peak eosinophil count < 15/HPF.
NPV, negative predictive value for SPT.
DISCUSSION
Herein, we report the first comparative study to determine the effectiveness of three different dietary therapies for EoE. We demonstrate that dietary therapy is highly effective at inducing disease remission in EoE and that the elemental diet is superior to restricted dietary therapies. These findings are consistent with a dose-response relationship between the number of immunologically reactive foods and the presence of active allergic inflammation in the esophagus. Furthermore, we demonstrate that the empiric removal of the most common allergens from the diet (SFED) is notably no less successful than the directed diet that is based on skin (prick and patch) testing alone. Consistent with the unreliability of skin testing at guiding dietary management for disease remission, the predictive value of SPT for maintenance of disease remission following food reintroduction remained low (≤ 67%). Taken together, our data substantiate an immune etiology for EoE and yet undermine the value of skin test-directed dietary management. The failure of skin testing to identify causative, specific food hypersensitivities may be explained by the local generation of immunoglobulins (including IgE) in the esophagus, suggesting that the skin is not a good surrogate for tissue-specific responses33. Our findings may also be reconciled by a disease mechanism that does not depend upon IgE-mediated responses, consistent with studies in mice that have elicited experimental EoE in B cell-deficient mice34 and a recent preliminary report that anti-IgE therapy is unsuccessful for EoE35.
The objectives of this study were to evaluate remission rates among three dietary therapies implemented in a focused population of children who have EoE and to assess the utility of skin testing in directing dietary planning. Access to our EGID data repository permitted comprehensive, longitudinal evaluation of histologic remission associated with dietary therapies and subsequent food reintroduction. These data reflect the evolution of dietary-based treatment options offered to patients with EoE over the past decade. To avoid the confounding influence of glucocorticoid treatment on remission, no patients received topical or systemic steroids for a minimum of 2 months prior to and during the duration of the study. This allowed comparison of remission response among dietary therapies but not other mainstays of treatment, such as topical steroids. Notably, there are not yet formal standards for defining histologic remission in response to therapy; thus, the standards employed vary widely among published studies.
Results from this retrospective observational study identified the superiority of inducing histologic remission with the elemental diet compared to the SFED and to the directed diet. No statistical difference in remission (overall, complete remission, partial remission, or partial resolution) was detected among the SFED and the directed diet in our study. While we suspect that differences in secondary clinical outcomes may exist among dietary therapies, such as more rapid individual diet optimization (symptomatic and histologic tolerance of desired foods) in patients on the SFED compared to patients on the elemental diet (who underwent more EGDs) or differences in quality of life and dietary adherence36, these secondary clinical outcomes and associated costs were not measured in this study. Multiple studies indicate that adherence is inversely related to the number of foods eliminated25, 37. Ensuring a nutritionally adequate dietary intake is more difficult for patients on a restrictive diet that eliminates the most common allergenic foods compared to the elemental diet38, but intensive education by a registered dietitian and interval monitoring of growth makes the diet manageable for most families. Interestingly, complete remission (≤ 1 eosinophil/HPF) occurred in only 30 to 60% of patients, with a higher percentage occurring in patients on an elemental diet and a lower percentage in patients on a directed elimination diet. These results differ from a previous study that demonstrated a significant improvement in esophageal eosinophilia (mean ± SD, 1.1 ± 0.6 eosinophils/HPF) in 97% (160/164) of patients on an elemental diet5. In this same study, 57% (75/132) of patients demonstrated significant improvement in esophageal eosinophilia (mean ± SD, 5.3 ± 2.7 eosinophils/HPF) in patients on a directed elimination diet5. In our study the odds of post-diet remission vs. non-remission were 5.6-fold greater on an elemental diet compared to the SFED, 12.5-fold greater on an elemental diet compared to the directed diet and not significantly different on the SFED compared to the directed diet.
A single-food reintroduction followed by histologic evaluation is the gold standard method for determining whether a food is tolerated1. In order to reduce variation when interpreting results, only food reintroductions conducted in patients who received both SPT and APT at the CCED were included. A total of 116 single-food reintroductions were evaluated. The use of combined SPT and APT results has been advocated when identifying foods as a causative agent in EoE7, 26, 27. It was our original intent to utilize combined SPT and APT results to calculate both negative and positive predictive values for foods reintroduced. However, due to the paucity of dual SPT/APT-positive results (1/116), the NPVs were calculated using only SPT results in order to assess its utility in dietary planning. Moreover, because we implemented a clinical practice-based retrospective study design that did not require reintroduction of skin test-positive foods, very few patients elected to reintroduce skin test-positive foods. As a result, the true and false positive rates for foods reintroduced are unknown; thus, only the NPV based on SPT alone could be reliably calculated. This differs from studies conducted by Spergel et al., which required reintroduction of skin test-positive foods followed by histologic evaluation26, 27. This permitted their assessment of the diagnostic accuracy of skin testing using sensitivity, specificity, and positive and negative predictive values based on single and combined SPT and APT results. Limitations of this study are linked to the use of a retrospective study design. Patients were not randomly assigned to dietary therapies. Therefore, bias related to dietary therapy selection is unknown and may not be equal among dietary therapies even though demographic and disease-related characteristics measured were not significantly different, except the number of EGDs obtained and the duration of follow-up at the CCED. The evaluation of additional secondary clinical outcomes that could identify potential selection bias, such as a measure of disease severity, were not available or have not been developed at this time. Inferences made related to remission outcomes are based on a mutual agreement made between patients and their CCED gastroenterologist regarding dietary therapy selection. In addition, because of the stringent inclusion/exclusion criteria applied, the external validity is limited to identifiable populations that meet this study’s eligibility criteria.
The 4 foods most often reintroduced in a single-food challenge were milk, wheat, soy and egg. The NPV for each of these foods was low, ranging from 40% (milk) to 67% (wheat). A low NPV indicates that using skin testing to predict the absence of adverse food reactions is not diagnostically adequate and risks recurrent esophagitis despite a negative SPT to a particular food. Therefore, it does not seem plausible to design a successful dietary plan that is only based on SPT. This finding is aligned with the 2010 NIAID-sponsored guidelines for managing food allergies, which states skin testing (either alone or in combination) are not diagnostic of food allergies39. Spergel et al.’s SPT-only NPVs were generally higher than the NPVs of our study, with the exception of wheat: milk, 58% vs. 40%; wheat, 65% vs. 67%; soy, 69% vs. 64%; and egg, 75% vs. 56%. Perhaps these differences are due in part to our adherence to more stringent cut-points for defining whether a food reintroduction passed (< 15 compared to 20 eosinophils/HPF) and our higher threshold for considering an APT as positive (a score of 2+ compared with Spergel’s 1+).
It is not uncommon for parents of children with EoE to prefer non-pharmacologic treatment to pharmacologic therapies; thus, comparative analysis of histologic remission rates in three frequently prescribed dietary therapies is clinically relevant. As evidenced by our data, the implementation of the elemental diet is highly effective compared to the SFED and to the directed elimination diet in attaining histologic remission. Further prospective study is warranted to elucidate the limitations and strengths of each dietary therapy, to optimize the initial diet in such a way as to obviate the need for food reintroductions, and to determine which patients require an elemental diet at presentation. Taken together, our study does not substantiate a reliable role for skin testing in dietary therapy for EoE.
KEY MESSAGES.
Skin test (prick and patch)-directed elimination diets show no superiority compared with empiric six-food elimination diets for the treatment of pediatric eosinophilic esophagitis.
Both of these diet plans are inferior at inducing remission compared with an elemental diet.
Skin testing has limited usefulness in directing dietary planning for remission and food reintroduction.
Acknowledgments
This publication was supported by the Campaign Urging Research for Eosinophilic Disease (CURED), by the Buckeye Foundation, and The International Group of Eosinophilic Researchers (TIGER), and by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 5UL1RR026314. “Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.”
The authors gratefully acknowledge Shawna KB Hottinger, Scientific Writer in the Division of Allergy and Immunology for her valuable and much appreciated editorial assistance.
ABBREVIATIONS
- APT
Atopy patch test
- CCED
Cincinnati Center for Eosinophilic Disorders
- CCHMC
Cincinnati Children’s Hospital Medical Center
- EoE
Eosinophilic esophagitis
- EGID
Eosinophilic Gastrointestinal Diseases
- EGD
Esophagogastroduodenoscopy
- HPF
High-power field
- NPV
Negative predictive value
- SFED
Six-food elimination diet
- SPT
Skin prick test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. e6. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Debrosse CW, Franciosi JP, King EC, Buckmeier Butz BK, Greenberg AB, Collins MH, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol. 2011;128(1):132–8. doi: 10.1016/j.jaci.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109(5):1503–12. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98(4):777–82. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 5.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3(12):1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 6.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109(2):363–8. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]
- 8.Wang FY, Gupta SK, Fitzgerald JF. Is there a seasonal variation in the incidence or intensity of allergic eosinophilic esophagitis in newly diagnosed children? J Clin Gastroenterol. 2007;41(5):451–3. doi: 10.1097/01.mcg.0000248019.16139.67. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen RG, Husby S. Eosinophilic oesophagitis: epidemiology, clinical aspects, and association to allergy. J Pediatr Gastroenterol Nutr. 2007;45(3):281–9. doi: 10.1097/MPG.0b013e31806210c8. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology. 2009;137(4):1238–49. doi: 10.1053/j.gastro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. J Clin Invest. 2001;107(1):83–90. doi: 10.1172/JCI10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology. 2005;129(3):985–94. doi: 10.1053/j.gastro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Teitelbaum JE, Fox VL, Twarog FJ, Nurko S, Antonioli D, Gleich G, et al. Eosinophilic esophagitis in children: immunopathological analysis and response to fluticasone propionate. Gastroenterology. 2002;122(5):1216–25. doi: 10.1053/gast.2002.32998. [DOI] [PubMed] [Google Scholar]
- 14.Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131(5):1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 15.Liacouras CA, Wenner WJ, Brown K, Ruchelli E. Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J Pediatr Gastroenterol Nutr. 1998;26(4):380–5. doi: 10.1097/00005176-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102(10):2271–80. doi: 10.1111/j.1572-0241.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer ET, Fitzgerald JF, Molleston JP, Croffie JM, Pfefferkorn MD, Corkins MR, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol. 2008;6(2):165–73. doi: 10.1016/j.cgh.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139(5):1526–37. 37 e1. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 19.Caldwell JM, Blanchard C, Collins MH, Putnam PE, Kaul A, Aceves SS, et al. Glucocorticoid-regulated genes in eosinophilic esophagitis: a role for FKBP51. J Allergy Clin Immunol. 2010;125(4):879–88. e8. doi: 10.1016/j.jaci.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest. 2006;116(2):536–47. doi: 10.1172/JCI26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard C, Stucke EM, Rodriguez-Jimenez B, Burwinkel K, Collins MH, Ahrens A, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127(1):208–17. 17 e1–7. doi: 10.1016/j.jaci.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heine RG, Nethercote M, Rosenbaum J, Allen KJ. Emerging management concepts for eosinophilic esophagitis in children. J Gastroenterol Hepatol. 2011;26(7):1106–13. doi: 10.1111/j.1440-1746.2011.06757.x. [DOI] [PubMed] [Google Scholar]
- 23.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–6. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 24.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100(4):444–51. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 25.Hong S. Food allergy and eosinophilic esophagitis: Learning what to avoid. Cleve Clin J Med. 2010;77(1):51–9. doi: 10.3949/ccjm.77a.09018. [DOI] [PubMed] [Google Scholar]
- 26.Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Ann Allergy Asthma Immunol. 2005;95(4):336–43. doi: 10.1016/S1081-1206(10)61151-9. [DOI] [PubMed] [Google Scholar]
- 27.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119(2):509–11. doi: 10.1016/j.jaci.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Heine RG, Verstege A, Mehl A, Staden U, Rolinck-Werninghaus C, Niggemann B. Proposal for a standardized interpretation of the atopy patch test in children with atopic dermatitis and suspected food allergy. Pediatr Allergy Immunol. 2006;17(3):213–7. doi: 10.1111/j.1399-3038.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 29.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Miles MV, Putnam PE, Miles L, Tang PH, DeGrauw AJ, Wong BL, et al. Acquired coenzyme Q10 deficiency in children with recurrent food intolerance and allergies. Mitochondrion. 2011;11(1):127–35. doi: 10.1016/j.mito.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Assa’ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119(3):731–8. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 32.Eigenmann PA, Sampson HA. Interpreting skin prick tests in the evaluation of food allergy in children. Pediatr Allergy Immunol. 1998;9(4):186–91. doi: 10.1111/j.1399-3038.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 33.Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59(1):12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol. 2007;81(4):916–24. doi: 10.1189/jlb.1106653. [DOI] [PubMed] [Google Scholar]
- 35.Rocha R, Vitor AB, Trindade E, Lima R, Tavares M, Lopes J, et al. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur J Pediatr. 2011;170(11):1471–4. doi: 10.1007/s00431-011-1540-4. [DOI] [PubMed] [Google Scholar]
- 36.Franciosi JP, Hommel KA, Debrosse CW, Greenberg AB, Greenler AJ, Abonia JP, et al. Quality of life in paediatric eosinophilic oesophagitis: what is important to patients? Child Care Health Dev. 2011 doi: 10.1111/j.1365-2214.2011.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hommel KA, Franciosi JP, Hente EA, Ahrens A, Rothenberg ME. Treatment Adherence in Pediatric Eosinophilic Gastrointestinal Disorders. J Pediatr Psychol. 2011 doi: 10.1093/jpepsy/jsr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuling MB, Noel RJ. Medical and nutrition management of eosinophilic esophagitis in children. Nutr Clin Pract. 2010;25(2):166–74. doi: 10.1177/0884533610361608. [DOI] [PubMed] [Google Scholar]
- 39.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010;126(6):1105–18. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]