Abstract
We conducted a systematic review investigating body fat distribution in older adults and its association with morbidity and mortality. Our search yielded 2,702 citations. Following three levels of screening, 25 studies were selected to evaluate the association between body fat distribution and comorbidity, and 17 studies were used in the mortality analysis. Most of the selected studies in our analyses used anthropometric measures, e.g., body mass index (BMI), waist circumference, and waist-hip ratio; relatively few studies used direct measures, such as body fat/lean mass, and percentage body fat. Studies reported inconsistent findings regarding the strongest predictor(s) of morbidity and mortality. However, the majority of studies suggested that BMI per se was not the most appropriate predictor of morbidity and mortality in the elderly because of its inability to discern or detect age-related body fat redistribution. In addition, studies using BMI found that the optimal BMI range for the lowest mortality in the elderly was overweight (25 kg/m2 ≤ BMI < 30 kg/m2) or mildly obese (30 kg/m2 ≤ BMI < 35 kg/m2). Our findings suggest that the current clinical guidelines, recommending that overweight and obesity are major risk factors for increased morbidity and mortality are not applicable to this population. Therefore, the central message of this review is to admonish the government to establish new guidelines specifically for this population, using a combination of body fat distribution measurements, and to certify that these guidelines will not be applied to inappropriate populations.
Keywords: body fat distribution, visceral fat, central obesity, morbidity, mortality, elderly
1. INTRODUCTION
While overweight and obesity are associated with increasing risk of morbidity and mortality in middle-aged adults, relatively few studies focus on older populations, among which, most found that overweight was protective. Based on these conflicting findings, it appears that the change of the role of overweight from being a risk factor for younger adults to being a protective factor for older adults is due to the influence of aging, which may be related to differences in fat distribution in the aging process.
As individuals age, bone mineral density and lean mass decrease, while body fat mass1 increases and is distributed specifically in the abdominal region [1]. A preferential increase in visceral fat (VF), combined with a decrease in lower body subcutaneous fat can occur independent of changes in body weight, total adiposity, or waist circumference (WC) [2]. Lifestyle factors that contribute to these age-associated changes in body fat distribution include dietary changes, with higher intakes of saturated fats and simple sugars, and reduced physical activities, with less skeletal muscle mass and reduced strength [3].
Increases in VF is strongly associated with many adverse health conditions, such as metabolic syndrome, inflammation, dyslipidemia , insulin resistance, type-2 diabetes, cardiovascular diseases, some cancers [4–9], and, ultimately, death [10]. Consequently, for an older population, body fat distribution seems to be a better indicator to predict morbidity and mortality.
Current clinical guidelines [11] were almost exclusively based on studies that predominantly included young and middle-aged populations and concluded that overweight and obesity2 were major risk factors for increased morbidity and mortality for adults aged 18 years and older without recommending age-specific cut points. Therefore, recommendations on optimal body shape and body weight specifically for the elderly are lacking and in need.
We performed a systematic review to determine the strength of evidence with regards to the existing findings for the relationship between body fat distribution and mortality in the elderly. The objective of this systematic review is to gain a better understanding of the complex relationship between mortality and morbidity risks associated with changes in body composition during the aging process. This knowledge will not only clarify the discordant evidence on BMI-based mortality risks for middle-aged adults, but also inform policy makers of the direction of prevention and control in current guidelines for this population.
2. MATERIALS AND METHODS
This systematic review was conducted and reported according to established guidelines [12]. A detailed research protocol was prepared a priori and was followed throughout.
2.1 Data sources and literature search
A search strategy was created by a MLIS qualified librarian. Databases, such as MEDLINE, EMBASE, CENTRAL, SCOPUS, COCHRANE, and CINAHL were searched to March 1st, 2012, using the following search terms: body fat (distribution), body composition, adiposity, waist circumference, waist-hip ratio, adipose tissue, fat tissue, subcutaneous fat, VF, or waist in conjunction with aged, old/older individuals/adults/people/persons/subjects/population, or elderly. We also reviewed references from retrieved articles when necessary.
2.2 Screening criteria
Search results were put through three levels of screening. The first screening level involved scanning abstracts for the following exclusion criteria: animal studies; languages other than English; duplicate studies; and studies not containing at least one type of relevant information (measurement of body fat distribution, morbidity, and mortality). After removing abstracts that fit the exclusion criteria during the first level of screening, full articles were obtained and studies were screened at the second level based on the following inclusion criteria: (i) studies needed to report at least one outcome of interest, including mortality and morbid conditions, focusing on insulin resistance, type-2 diabetes mellitus, cardiovascular disease, hypertension, and cancer; (ii) studies included subjects whose age is older than 55 years; (iii) studies used at least one clearly defined measurement of body fat distribution.
2.3 Study selection
In the third screening level, articles were selected to be included in both comorbidity and mortality analyses based on a second set of inclusion criteria: (i) the conclusion was based on stratified analysis and/or adjusted for appropriate covariates; (ii) studies included a minimum of 100 participants to boost statistical power; and (iii) for the mortality analysis, studies had to have 3 or more follow-up years, studies including BMI had to include at least one other measurement of body fat distribution, study subjects had to be non-institutionalized, and studies had to report the relationship between body fat distribution measurements and mortality.3 Three investigators independently reviewed the studies, abstracted outcomes, and resolved disagreements by consensus.
3. RESULTS
3.1 Data Retrieval
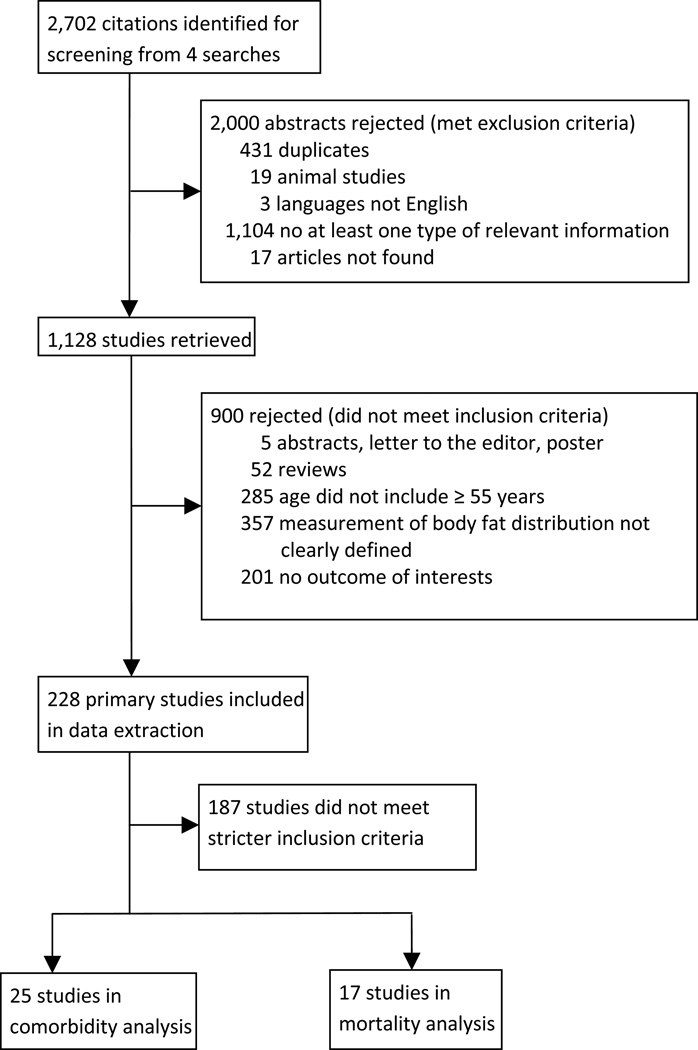
A flow diagram outlining the systematic review process is provided in Figure 1. The initial search resulted in 2,702 articles. After removing duplicate studies and reviewing all abstracts at the first level of screening, 1,128 abstracts remained. Full articles were retrieved, and after a second level of screening for exclusion and inclusion criteria, 228 articles were left for further selection. One hundred and eighty-seven studies did not meet the second set inclusion criteria. Among the selected studies, 25 were selected for comorbidity analysis and 17 were selected for mortality analysis.
Figure 1.
Study attrition diagram
3.2 Study and Sample Characteristics
Among the studies extracted for this article, 10 articles were published prior to the year 2002, and 31 articles were published during or after 2002 (Table 1). Eighteen studies initiated recruitment before the year 1995. The remaining 18 studies began recruitment during or after 1995. In 13 studies, the follow up period was equal to or greater than 5 years. Four studies had a follow up period of less than 5 years. The studies were carried out primarily in Europe (n= 17) and North America (n=13). Eight studies were conducted in Asian and only 1 was carried out in Brazil.
Table 1.
Study and sample characteristicsa
| Study characteristics | No. of studies | n |
|---|---|---|
| Publication year | ||
| <2002 | 10 | 87,856 |
| ≥2002 | 31 | 784,288 |
| Recruitment period | ||
| <1995 | 18 | 139,640 |
| ≥1995 | 18 | 730,253 |
| Follow-up yearsb | ||
| ≥ 5 years | 13 | 90,964 |
| < 5 years | 4 | 6,795 |
| Study location | ||
| North America | 13 | 501,151 |
| Europe | 17 | 358,825 |
| Asia | 8 | 14,473 |
| Others | 1 | 1,494 |
| Body fat distribution measurement | ||
| BMI | 37 | 864,420 |
| WC | 29 | 452,549 |
| WHR | 18 | 382,874 |
| Body composition | 9 | 32,483 |
| Comorbidities | ||
| Insulin resistance | 6 | 155,036 |
| Type 2 diabetes | 6 | 155,036 |
| Hypertension | 6 | 112,645 |
| Metabolic syndrome | 5 | 10,187 |
| Cardiovascular disease | 10 | 121,608 |
| Cancers | 5 | 590,976 |
| Mortality | ||
| All-cause | 17 | 66,057 |
| Circulatory | 3 | 47,335 |
| Cancer | 2 | 32,502 |
| Sample characteristics | n/Total (%) | |
| Sexc | ||
| Female | 455,820/868,910 (52.46) | |
| Male | 413,090/868,910 (47.54) | |
| Raced | ||
| Whites | 132,007/185,951 (70.99) | |
| Blacks | 35,730/185,951 (19.21) | |
| Asians | 18,214/185,951 (9.80) | |
n: number of participants
For articles that used the same study, we treated them as different samples.
Follow-up years only applied to articles included in mortality analysis.
Some studies did not provide information on numbers of men and women.
The numbers were computed from the studies that provided race information.
We included 868,910 individuals from the 41 extracted studies in our analysis. In studies reporting sex, 52% of participants were female and 48% were male. Among the articles that reported race, 71% of study participants were white, 19% were black, and about 10% were Asian. For the articles that reported comorbidities, 6 studies involving 155,036 participants discussed type-2 diabetes and insulin resistance, 6 targeted hypertension, 10 considered cardiovascular disease, and 5 studies investigated cancer.
3.3 Measurements of Body Fat Distribution
While the commonly used BMI provides a measure that allows comparison between individuals of different heights and weights, it cannot capture the differences in body composition and body fat distribution. Nonetheless, different mathematical formulae across studies were provided to relate BMI to body fat [13–15]. Therefore, BMI is often seen as a comparator among various measurements of body fat distribution in the literature. For the elderly population, BMI is known as an inappropriate measure of body fat and is limited in its predictive ability for mortality [16, 17] due to three reasons: (i) it does not differentiate between fat and lean body mass, and the latter is progressively lost with increasing age [18, 19]; (ii) height measurement is unreliable due to shrinkage and vertebral collapse [19]; (iii) height and weight information is often recalled and self-reported rather than measured, causing spurious and inaccurate BMI estimates in the elderly.
Anthropometric4 measurements include circumferences of various body parts, e.g., waist, hip, thigh, calf, and sagittal abdominal diameter, as well as skinfold thickness. Circumference measurements are often used alone or in combination with other measures, e.g., waist-hip ratio (WHR), waist-height ratio. Skinfold thickness is measured by calipers at standardized skin pinch points to determine the subcutaneous fat layer thickness.
Direct measures of body fat distribution include hydrodensitometry, air displacement plethysmography and bioelectrical impedance analysis (BIA) which provide information on the two-compartment model (fat mass and fat-free mass) of body composition [20]. Dual energy X-ray absorptiometry (DXA) adds a third compartment, bone mineral content, to the body composition model. Imaging methods, such as computed tomography (CT) and magnetic resonance imaging scanning can differentiate between subcutaneous and visceral adipose tissue and between subcutaneous and intermuscular adipose tissue [21]. These are suitable for smaller detailed research studies but not broader population health studies.
3.4 Body Fat Distribution and Risk of Morbidity in the Elderly
Adipose tissue is an endocrine organ that secretes inflammatory and immune mediators. Consequently, in evaluating body fat distribution, it is important to note that hepatic fat accumulation is more important than total body fat mass. Visceral adipose tissue (abdominal obesity) places individuals at a greater risk of metabolic syndrome disorders than adipose tissue located in the lower body or subcutaneously [22]. The accumulation of fat in the viscera is associated with a plethora of health conditions namely cardiovascular disease (CVD), insulin resistance and type-2 diabetes mellitus, metabolic syndrome (MS) and cancers. Its association with these diseases is due to its strong pro-inflammatory characteristics [23], and its close proximity to the portal vein, which carries blood from the intestinal area to the liver. Free fatty acids and other substances released by VF enter through the portal vein and travel to the liver, where they can influence the production of blood lipids [24].
VF also secretes several inflammatory hormones, cytokines, immune system chemicals, namely, tumor necrosis factor and interleukin-6 [24], which increases the risk of CVD by promoting insulin resistance, and low-level chronic inflammation. Moreover, evidence suggests that VF may have deleterious effects on cells’ sensitivity to insulin, blood pressure, and blood clotting [25]. Table 2 lists the selected studies reporting comorbidity outcomes of the elderly and associations with body fat distribution, using various methods of measurements described in Section 3.3. For studies that also included sample younger than 55 years, only the information and analysis concerning those 55 years and older were included. Twenty five studies [6, 26–47] were included in the comorbidity analysis, of which 5 focused on women only [31, 34, 41, 42, 45] and 4 focused on men [27, 32, 33, 43].
Table 2.
Selecteda studies reporting association between body fat distribution and comorbidites for the elderly
| Source, year/ Study/Geo- graphic location |
Recruitment period/ follow-up (years) |
No. of participants (Women; Men) |
Age range at baseline (Years) |
Measurement of body fat distribution |
Conclusion of comorbidity | Control for other risk factors |
|---|---|---|---|---|---|---|
| Koster et al., 2010/ The Health ABC Study/United States | 1997–1998/ -- | 729 (424 women; 305 men) | 70–79 | BMI, WC, fat compositionb,c | In women, per standard deviation higher in VF, the likelihood of MS significantly increased (OR: 2.16, 95% CI: 1.59–2.94). In contrast, the likelihood of MS decreased in both men (OR: 0.56, 95% CI: 0.39–0.80) and women (OR: 0.49, 95% CI: 0.34–0.69) with each standard deviation higher in thigh subcutaneous fat. | Age, race, site, education, physical activity, smoking, alcohol intake, height, total fat, IL-6, CRP, TNF-α, and PAI-1 |
| Levitan et al., 2009/Swedish Mammograph y Cohort/Sweden | 1998–2004/ -- | 80,360 (36,873 women; 43,487 men) | Women: 48–83; Men: 45–79 | BMI and WC | In women, HR for an interquartile range higher BMI were 1.39 (95% CI: 1.15–1.68) at age 60 and 1.13 (95% CI: 1.02–1.27) at 75. In men, HRs were 1.54 (95% CI: 1.37–1.73) at 60 and 1.25 (95% CI: 1.16–1.35) at 75. A 10-cm higher WC was associated with 15% (95% CI: 2%-31%) and 18% (95% CI: 4%-33%) higher HF rates among women with BMI 25 and 30 kg/m2, respectively; HRs for 1 kg/m2 higher BMI were 1.00 (95% CI: 0.96–1.04) and 1.01 (95% CI: 0.98–1.04) for WC 70 and 100 cm, respectively. In men, a 10-cm higher WC was associated with 16% and 18% higher rates for BMI 25 and 30 kg/m2, respectively; a 1 kg/m2 higher BMI was associated with 4% higher HF rates regardless of WC. | Age, education, smoking, alcohol consumption, total physical activity, post-menopausal hormone(W) therapy, living alone (W), marital status (M) and family history of myocardial infarction |
| Nakajima et al., 2009/ -- /Japan | 2006 | 2,675 (-- women; -- men) | 30–80 | BMI and WC | In elderly ≥ 55 Logarithmic C-reactive protein showed a sigmoid curve against BMI with a base at BMI ∈ [21.0, 22.9] kg/m2, but not against WC, indicating that the elderly with low body weight may have subtle low-grade inflammation irrespective of a favorable cardiovascular risk. | Age, sex, smoking status and weight change over the past 2 years |
| Wise et al., 2008/Black Women Health Study/United States | 1997–2003/4, 6, 8 | 33,403 women | ≥30 | BMI, WC, and WHR | The trend overall for a risk of polyps was associated with WHR in post-menopausal women P=0.02. | Age, physical activity, family history of colorectal cancer, smoking education, nonsteroidal antiinflammatory drug use, menopausal status postmenopausal hormone use, red meat intake, fiber intake and energy |
| Balkau et al., 2007/DECODE Study / Europe | --/10 | 2,790 men | 50–69 | WC | A large WC (> 102 cm) carried an OR of 2.24 (35% CI: 1.05–4.76) in low CVD risk men. | Age and study center |
| Carlsson et al., 2007/ -- /Sweden | 1997–1999/ -- | 4,228 (2,192 women; 2,036 men) | 60 | WC | WC ≥ 95 cm (quintiles 3–5) in men and ≥ 88.5 cm (quintiles 4–5) in women was associated with newly diagnosed high blood pressure. | Education, regular physical activity, alcohol consumption, and diabetes |
| Friedenrich et al., 2007/European Prospective Investigation into Cancer and Nutrition/Europe | 1992–2000/6.4 | 223,008 women | 35–70 | BMI, WC, HC, and WHR | Weight, BMI, WC, HC, and WHR were strongly associated with increased risk of endometrial cancer. RR for endometrial cancer WC of ≥ 88 cm vs. < 80 cm was 1.76, 95% CI: 1.42–2.19. | Total physical activity level, age at menarche, menopausal status, age at menopause, number of full term pregnancies, age of birth of last child, ever use oral contraceptives, ever use hormone replacement therapy, education, smoking status, hypertension, diabetes, fruit and vegetable intake, fiber intake, carbohydrate intake, and energy intake |
| Janssen et al, 2007/Cardiovascular Health Study/United States | 1989–1990/9 | 4,968 (2,752 women; 2,216 men) | ≥65 | BMI | Compared with the normal-weight group, the risks of myocardial infarction, stroke, sleep apnea, urinary incontinence, cancer, and osteoporosis were not different in the overweight group. The risks for arthritis and physical disability were modestly increased in the overweight group, whereas the risk for type-2 diabetes was increased by 78% in the overweight group. In the obese BMI group, the risk estimates were increased for type-2 diabetes, arthritis, sleep apnea, and physical disability and decreased by 26% for osteoporosis. | Age, sex, race, socioeconomic status, smoking, and physical activity, previous coronary heart disease events, previous stroke events, and history of cancer (at last 5 years prior to baseline examination) |
| Wright et al., 2007/NIH-AARP Diet and Health Study/United States | 1995–1996/5 | 287,760 Men | 50–71 | BMI | Higher baseline BMI was associated with significantly reduced total prostate cancer incidence. (RR: 0.67, 95% CI: 0.50–0.89) | Age, race, smoking status, education, personal history of diabetes, and family history of prostate cancer |
| Ramsay et al., 2006/The British Regional Heart Study/United Kingdom | 1998–2000/20 | 4,252 men | 60–79 | BMI, WC, FM, and fat-free massb | Adjusted ORs of CVD (1st vs. 5th) were 1.58 (95% CI: 1.23–2.03) for FMI, 1.45 (95% CI: 1.14–1.86) for BMI, and 1.27 (95% CI: 0.99–1.62) for WC. Body fatness, not fat-free mass, is associated with CVD in older men. BMI and WC are good indicators of the likelihood of morbidity in older men. | Age, social class, smoking, alcohol intake, and physical activity |
| Dekker et al., 2005/Hoorn Study/The Netherlands | 1989–1990/10 | 1,364 (749 women; 615 men) | 50–75 | BMI, WC, and WHR | In men, WC > 102 cm or BMI ≥ 30 kg/m2 was associated with fatal or/and non-fatal CVD; WHR was not associated with any CVD incidence. In women, WC > 80 or 88 cm was associated with non-fatal CVD; BMI ≥ 30 kg/m2 was not associated with any CVD incidence; WHR was not associated with any CVD incidence. | Age |
| Goodpaster et al., 2005/Health ABC Study/United States | 1997–1998/-- | 3,035 (1,562 women; 1,473 men) | 70–79 | Visceral adipose tissue | VF was associated with the MS in men with normal weight (OR: 2.1, 95% CI: 1.6–2.9), overweight (1.8, 1.5–2.1), and obese (1.2, 1.0–1.5), in women normal weight (3.3, 2.4–4.6), overweight (2.4, 2.0–3.0), and obese (1.7, 1.4–2.1). Subcutaneous abdominal adipose tissue was associated with the MS in normal-weight men (1.3, 1.1–1.7). Intermuscular adipose tissue was associated with the MS in normal-weight (2.3, 1.6–3.5) and overweight (1.2, 1.1–1.4) men. Subcutaneous thigh adipose tissue was inversely associated with MS in obese men (0.9, 0.8–1.0) and women (0.9, 0.9–1.0). | Race |
| Huang et al., 2005/Elderly Nutrition and Health Survey /Taiwan | 1999–200 | 2,432 (1,189 women; 1,243 men) | ≥ 65 | BMI, WC, and WHR | Odds of developing CVD increased with incremental increase in WC, WHR, and BMI | Age |
| Lawlor et al., 2005/The British Women’s Heart and Health Study/United Kingdom | 1999–2001/4 | 3,589 women | 60–79 | BMI, WC, and WHR | The age-adjusted HR (95% CI) was 1.03 (0.89, 1.19) for BMI, 1.15 (1.02, 1.31) for WHR and 1.12 (0..89, 1.27) for WC and coronary heart disease | Age |
| Passos et al., 2005/BamBui Health and Aging Study/Brazil | 1997/ -- | 1,276 (765 women; 511 men) | ≥ 60 | BMI and WHR | In elderly adults with family history of diabetes, diabetes was associated with BMI of 25–29 kg/m2, BMI ≥ 30 kg/m2, and increased WHR | Gender, age, family history of diabetes, alcohol use, HDL-C, Triglycerides |
| Patterson et al., 2004/Vitamins and Lifestyle Cohort Study/United States | 2000–2002/ -- | 73,003 (37,005 women; 35,998 men) | 50–76 | BMI | For women and men, respectively, the highest ORs comparing obese II/III to normal weight were diabetes (OR: 12.5 and 8.3) and hypertension (OR: 5.4 and 5.6). | Age, race/ethnicity, education and smoking status |
| Santos et al., 2003/ -- /Portugal | --/ -- | 1,470 (873 women; 563 men) | ≥ 18 | WC | Low prevalence of large WC in men and women with the metabolic syndrome (14.9% and 40.6% respectively) was found. | Age |
| Woo et al., 2002/ -- /China | --/3 | 2,032 (1,033 women and 990 men) | 80.1 (mean) | BMI and WC | BMI and WC were positively associated with diabetes in men. WC was positively associated with hypertension in men and women. WHR was not associated with any outcome measures. | Adjusted (not specified) |
| Wu et al., 2001/ -- / Taiwan | 1993–1995/ -- | 215 (101 women; 114 men) | ≥ 60 | BMI, WHR, % abdominal and femoral fatd | Centrality index (the ratio of abdominal fat divided by femoral fat) showed a better linear correlation with cardiovascular dysmetabolic than BMI, and centrality index was higher in men. | Age and sex |
| Folsom et al., 2000/The Iowa Women’s Health Study/United States | 1986/10 | 31,702 women | 55–69 | BMI, WC, and WHR | BMI and WC were associated with breast cancer and colon cancer. BMI was associated negatively with lung cancer and WC and WHR were associated with Ovarian cancer. BMI, WHR, and WC were strong predictors of incident self-reported diabetes. BMI, WHR, and WC were associated with self-reported blood pressure. | Age |
| Ghosh et al., 2000/ -- /India | 1998/ -- | 210 men | > 55 | BMI, WC, and HC | There were significant differences between normotensive (NT) and hypertensive (HT) subjects in the mean values for weight, BMI, WC, and HC, WHR, and fat free mass. Percentile distributions for all these variables and indices showed consistently higher values among the HT patients as compared with NT subjects. | Not-specified |
| Turcato et al., 2000/ -- / Italy | --/ -- | 229 (146 women; 83 men) | 67–68 | BMI, WHR, WC, and SAD | A significant association was found between triglycerides, basal glucose, 2 h glucose during oral glucose tolerance test, systolic blood pressure, diastolic blood pressure and anthropometric variables, which were associated with CVD risk factors in the elderly independently of BMI. Among them, WC and SAD were mostly closely related to CVD risks. | Age and BMI |
| Lempiainen et al., 1999/ -- /Finland | 1986–1988/7 | 1,069 (673 women; 396 men) | 65–74 | BMI and WHR | In men, the insulin resistance factor, which reflected primarily BMI, WHR, triglycerides, fasting plasma glucose, and insulin predicted CHD events (HR: 1.33, 95% CI: 1.08–1.65). | Age, previous MI, previous stroke, LVH |
| Woo et al., 1998/ -- / Hong Kong | 1991–1992/1.5 | 2,032 (999 women; 1,033 men) | ≥ 70 | BMI, WHR, total body fat, and fat-free masse | Among survivors, the only significant associations observed were a negative association BMI and the development of heart disease, and a positive association between systolic blood pressure and development of stroke. | Age and sex |
| Sellers et al., 1994/The Iowa Women’s Health Study / United States | 1986/3.5 | 41,837 women | 55–69 | BMI and WHR | High BMI and high WHR were associated with increased risk of self-reported diabetes. | Age |
BMI: body mass index; WHR: waist-hip ratio; WC: waist circumference; HC: hip circumference; SAD: sagittal abdominal diameter; VF: visceral fat; FM: fat mass; FMI: fat mass index (fat mass in kilogram/the square of height, kg/m2); LVH: left ventricular hypertrophy; MS: metabolic syndrome; HF: heart failure; DXA: Dual-energy x-ray absorptiometry; CT: computerized tomography; HR: hazard ratio; RR: relative risk; OR: odds ratio; CI: confidence interval; MI: myocardial Infarction; --: not available.
The selection was based on 3 levels of screening described in Section 2.2 and 2.3.
Body fat composition was measured by bioelectrical impedance analysis.
Body fat composition was measured by computed tomography.
Body fat composition was measured by dual-energy x-ray absorptiometry.
Total body fat was computed, using skin fold thickness, measured by Holtain calipers, which was then converted by the equation in Durnin and Womersley (1974).
3.4.1 Insulin resistance and type-2 diabetes
Body fat distribution is associated with numerous metabolic alterations in old age such as insulin resistance and type-2 diabetes. In the Iowa Women’s Health Study cohort, of 30,000 women aged 55–69 years BMI and WHR were significantly associated with incident diabetes and hypertension [48]. Passos et al. reported similar findings indicating central adiposity expressed in terms of WHR having greater predictive ability in diabetes development in the elderly and also possibly a better predictor than BMI [38]. Consistent with these reported findings, central obesity was predictive of other disorders important in the clinical development of insulin resistance and diabetes, such as lipid profile, blood pressure, and glycemic indices [37].
To the best of our knowledge, the literature on body fat distribution and insulin resistance/diabetes is sparse when looking at elderly ≥ 55 years. Nonetheless, surrogate anthropometric measures of abdominal fat appear to be better predictors of insulin resistance and type-2 diabetes than BMI [46]. It is possible that BMI is unable to detect increases in abdominal adiposity related to the aging process [49]. This may explain the reduced predictive ability of BMI to detect risk for insulin resistance and type-2 diabetes in the elderly. BMI does not appear to be as important a measure for predicting the development of these diseases.
3.4.2 Hypertension
Differences in adipose distribution tend to also influence hypertension. Gosh et al. investigated Bengalee Hindu elderly ≥ 55 years [27]. They reported that there were significant differences between normotensive and hypertensive individuals in the mean values for BMI, WC, hip circumference, and WHR. They found that the aforementioned indices had consistently higher values when compared with persons in the normotensive group. Moreover, in a study of 60-year-old men and women, a WC ≥ 95 cm in men and ≥ 88.5 cm in women was associated with incident high blood pressure. Newly diagnosed hypertensives in the study had a large WC, high BMI, high fasting glucose and insulin values. Similar findings of surrogate measures of abdominal fat being associated with blood pressure have being observed in other studies [37]. Again in older adults measures of central adiposity appear to be better predictors of disease risk.
3.4.3 Metabolic syndrome
Body fat is strongly correlated with MS. It has been postulated that it is a cardiovascular risk factor by acting through other risk factor states, such as dyslipidemia, hypertension, insulin resistance, inflammation and prothrombotic states. Adipocytes release non-esterfied fatty acids and inflammatory cytokines that can increase insulin resistance, promoting atherogenic dyslipidemia which is associated with prothrombotic and proinflammatory states [50, 51]. MS is also considered the primary risk factor for diabetes and cardiovascular disease [6]. It is defined as a cluster of 3 or more characteristics, namely WC > 102 cm in men > 88 cm in women, triglycerides levels 150 mg/dl, high density lipoprotein cholesterol < 40 mg/dl in men and < 50 mg/dl in women, blood pressure 130/85 mmHg and fasting serum glucose 110 mg/dL [29].
In studies investigating distribution of body fat and MS, one study did not observe a high prevalence of WC among persons with MS [29]. In contrast, in a cohort of 70–79 year old men and women, subcutaneous abdominal adipose tissue was associated with MS in normal weight men. Intermuscular adipose tissue was associated with MS in normal weight and overweight men. However, subcutaneous thigh adipose tissue was inversely associated with MS in obese men and women [6]. Similarly, another study reported the odds of having two or more MS clinical disorders increased with incremental increases in anthropometric measures [35]. In the Health Aging and Body Composition Study, individuals with MS had significantly more abdominal visceral fat and less thigh subcutaneous fat. Moreover, per standard deviation higher visceral fat, the odds of developing MS increased in women [40]. The present studies of older adults indicated that abdominal fat and intermuscular fat was associated with MS and that thigh subcutaneous fat was more protective.
3.4.4 Cardiovascular disease
Several studies reported that odds of developing CVD increased significantly with incremental increases in WC, WHR, and BMI [7, 52, 53]. WC was associated with CVD risk factors to a greater extent compared to BMI and WHR in Taiwanese [35]. Turcato et al. also reported similar findings in 229 men and women aged 67–78 years. Indicators of body fat distribution were associated with CVD risk factors independent of BMI [36]. In contrast one study noted a negative association between BMI and heart disease [54].
It is clear from the literature that certain specific body fat distribution indices may confer a greater risk for adverse health outcomes in the elderly. In particular, measures of central adiposity consistently appear to be stronger predictors of CVD risk compared to other measures [33]. These findings confirm that possibly VF more so than total fat is the main correlate of CVD.
3.4.5 Cancers
Studies of body fat distribution and cancer have produced inconsistent results. In the European Prospective Investigation into Cancer and Nutrition Study, anthropometric measurements were associated with endometrial cancer. Specific measures associated with cancer were BMI, WC, and WHR. The authors reported a two-fold increased risk in postmenopausal women with a BMI greater than 30 kg/m2 and three-fold increased risk for morbidly obese women, compared with women in other BMI categories [45]. In contrast to the previous results, higher BMI was associated with significantly reduced incidence of prostate cancer but associated with a risk of dying from prostate cancer [32]. Folsom et al. also reported both positive and negative associations between anthropometric measures and cancer [48]. They found that BMI and WC were moderately associated with incident breast cancer and colon cancer; BMI was negatively associated with lung cancer; BMI was not but WC and WHR were associated with incident ovarian cancer; and BMI and WC were strong predictors of incident uterine cancer.
Studies of body fat distribution and cancer have produced mixed results, indicating that specific measures of body fat may not confer a greater risk for some cancers, but higher anthropometric measures are still associated with other deleterious health outcomes [41].
3.5 Body Fat Distribution and Mortality in the Elderly
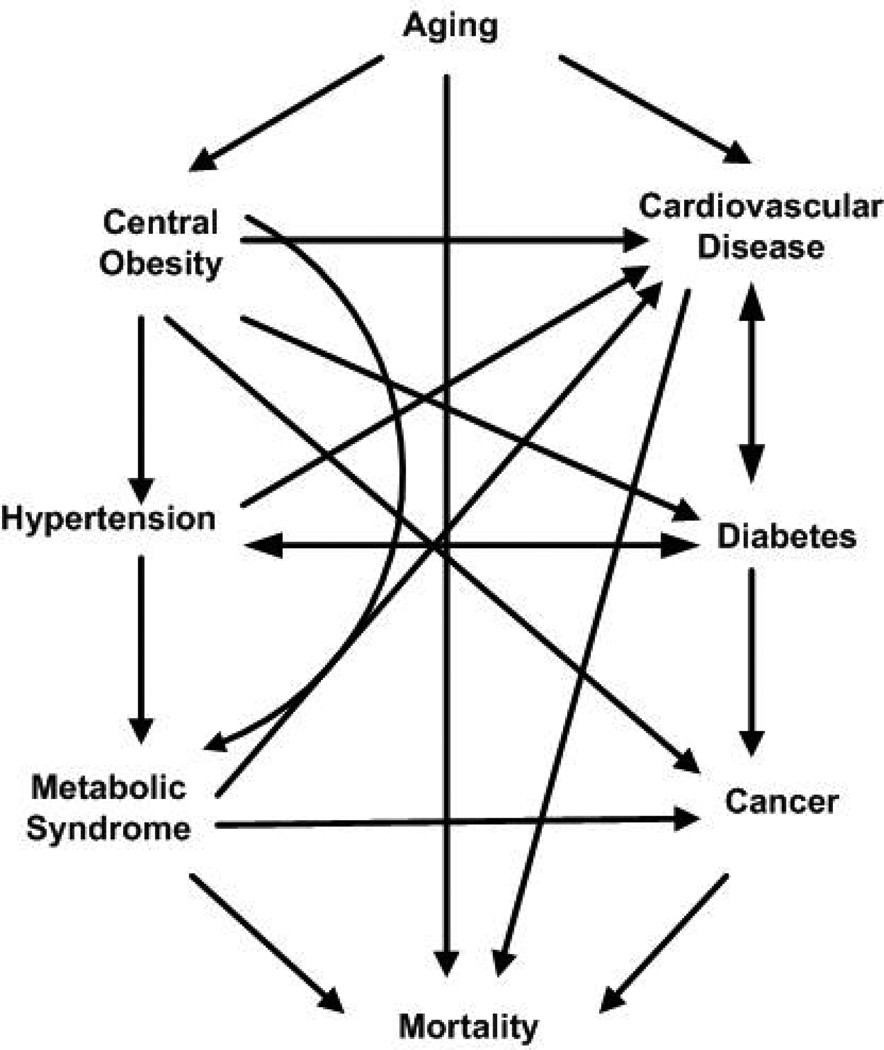
The associations between aging, central obesity, and chronic conditions, and mortality are depicted in Figure 2. The former association was explained in Section 3.4 and the latter will be described in this section. Table 3 lists the selected studies reporting mortality outcomes of the elderly and associations with body fat distribution. For studies that also included participants younger than 55 years, only the information and analysis concerning those 55 years and older were included. Seventeen studies [17, 19, 55–69] were selected for mortality analysis, of which, 3 studies [64, 68, 69] focused on men only and 2 studies focused on women only [55, 59]. In terms of measurements of body fat distribution, all of the studies used BMI; 13/8/2 studies used WC/WHR/ skinfold thickness; 5 studies [57–59, 62, 66] used direct measurements of body fat distribution (BIA, DXA, or CT) ; 2 studies [60, 64] used mathematical formulas transforming body weight or potassium to body fat.
Figure 2.
Association of aging and mortality via central obesity and associated comorbidities
Table 3.
Selecteda studies reporting association between body fat distribution and mortality for the elderly
| Source, year/ Study/Geo- graphic location |
Recruitment period/ follow-up (years) |
No. of participants (Women; Men) |
Age range at baseline (Years) |
Measurement of body fat distribution |
Conclusion of mortality | Control for other risk factors |
|---|---|---|---|---|---|---|
| Lisko et al., 2011/Vitality 90+ Study /Finland | 2000/4 | 257 (192 women; 65 men) | ≥ 90 | BMI, WC, and WHR | In men, normal weight indicated a three times higher mortality risk (HR: 3.09, 95% CI: 1.35–7.06) compared with overweight, and WC was inversely associated with mortality (HR: 0.96, 95% CI: 0.93–1.00). In women, the univariate WHR (HR: 1.43, 95% CI 1.06–1.92) and BMIadjusted WHR (HR: 1.45, 95% CI 1.07–1.97) were positively associated with mortality. Also, overweight women whose WC was < 86 cm had lower mortality than normal weight women with similar WC (HR: 0.34, 95% CI 0.12–0.97). Optimal values for lowest mortality: BMI∈[25, 30) for men and women; BMI∈[25, 30) and WC < 86 cm for women |
Chronic conditions (cardiovascular disease, cancer, diabetes, respiratory disease, infectious disease, and Mini-Mental State Examination score of 0–22), functional status, smoking, and alcohol intake |
| Han et al., 2010/Korean Longitudinal Study on Health and Aging /South Korea | 2005/3.5 | 877 (412 women/465 men) | 65–98 | BMI, WC, LMb, FMb, fat proportion, and LMI. | BMI, WC, and fat composition were not correlated with mortality, but higher LM and LMI were considered predictors of lower mortality. Higher LM and higher LMI are associated with better survival in the elderly Asian population. Optimal values for lowest mortality: LM: ≥ 44.7 kg; LMI: ≥ 16.8 kg/m2 |
Age, sex, hypertension, diabetes mellitus, preexisting history of coronary heart disease and cerebrovascular accident, ever drinking alcohol, smoking, hemoglobin, albumin, cholesterol, and regular exercise |
| Auyeung et al., 2010/ -- /Hong Kong | 2001–2003/ 5.3 (median) | 4,000 (2,000 women; 2,000 men) | ≥ 65 | BMI, WHR, BFIc, RTFc, and BMMIc | In men, HR decreased consistently by 0.85, 0.86, and 0.86 per every quintile increase in BMI, BFI, and BMMI, respectively. The minimum values of WHR and RTF were at the 3rd quintile and 4th quintile. In women, no corresponding relationship was observed except for WHR. Optimal values for lowest mortality: WHR: 0.92–0.94 for men, 0.90–0.94 for women |
Age |
| Cesari et al., 2009/ InCHIANTI study/Italy | 1998–2000/5.1 (mean) | 934 (513 women; 421 men) | 65–102 | BMI and a right leg pQCT scan was performed to evaluate calf muscle and fat areas. | Unadjusted analyses showed significant associations of mortality with muscle density (HR: 0.78, 95% CI: 0.69–0.88), muscle area (HR: 0.75, 95% CI 0.66–0.86), and fat area (HR: 0.82, 95% CI 0.73–0.92). After adjustment for potential confounders, no body composition parameter was significantly associated with mortality. Walking speed confirmed its well-established association with mortality risk (HR: 0.73, 95% CI: 0.60–0.88). Optimal values for lowest mortality: not available |
Height, weight, age, gender, site, education, Mini-Mental State Exam Score, Depression scale score, physical activity, congestive heart failure, coronary artery disease, hypertension, peripheral artery disease, respiratory disease, osteoarthritis, stroke, interleukin-6, C-reactive protein, and tumor necrosis factor |
| Guallar-Castillón et al., 2009/ --/Spain | 2000–2001/5.7 (mean) | 3,536 (-- women; -- men) | ≥ 60 | BMI and WC | Before adjusting for WC, mortality in the upper quartile of BMI was 15% lower than in the lower quartile (HR: 0.85, 95% CI: 0.66–1.08). After adjusting for WC, the association was even stronger, so that mortality in the upper quartile of BMI was 37% lower than in the lower quartile (HR: 0.63, 95% CI: 0.45–0.88). Before adjusting for BMI, no association was observed between WC and mortality. After adjusting for BMI, WC was positively associated with mortality (HR for upper vs. lower quartile of WC: 1.48, 95% CI: 1.07–2.05). BMI has an inverse, and WC has a direct, independent association with mortality in older adults, particularly in those with worse health status. Optimal values for lowest mortality: BMI ∈[28, 30.7] kg/m2 for men, BMI ∈[29, 32.4] kg/m2 for women |
Sex, age, education, smoking, alcohol consumption, physical activity, chronic obstructive lung disease, cancer, untreated cataracts, depression requiring treatment, dementia, and SF-36 mental summary component |
| Kuk et al., 2009/NHANES/United States | 1988–1994/7.1 (mean) | 9,603 (1,215 women; 1,101 men) | ≥ 65 | BMI, WC, WHR, HC, fatfree mass indexb, % fatfree massb, % body fatb, and sum of skinfolds | Optimal values for lowest mortality: In older adults aged 65–75, the lowest HR occurs at mildly obese group; at % body fat ∈[24.1, 29.4] and ≤ 24.3 and ∈[42.5, 46.4] for men and women, respectively; at sum of skinfolds ≥ 32.9 mm and ≥ 46.5 mm for men and women, respectively. | Income, ethnicity, smoking status, physical activity, high dietary fat, and alcohol consumption |
| Srikanthan et al., 2009/ MacArthur Successful Aging Study/United States | 1988–1989/ 5.9 (median) | 1,189 (659 women; 530 men) | 70–79 | BMI, WC, and WHR | There was no association between all-cause mortality and BMI or WC in either unadjusted or adjusted analyses. In contrast, all-cause mortality increased with WHR. There was an interaction with sex, so that there was a graded relationship between WHR and mortality in women (HR: 1.28 per 0.1 increase in WHR, 95% CI: 1.05–1.55) and a threshold relationship in men (HR: 1.75 for WHR > 1 compared to WHR ≤ 1, 95% CI: 1.06–2.91). Optimal values for lowest mortality: BMI∈(26, 28] kg/m2, WC∈(87, 92] cm, WHR ≤ 0.75 |
Age, sex, race, and smoking |
| Dolan et al., 2007/ -- / United States | 1986–1988/8 | 8,029 women | ≥ 65 | BMI, WC, fat and lean massb and % body fatb | Mortality was lowest among women in the middle of the distribution of each body size measure. For BMI, the lowest mortality rates were in the range 24.6 to 29.8 kg/m2. The U-shaped relations were seen throughout the age ranges included in this study and were not attributable to smoking or measures of preexisting illness. Body composition measures were not better predictors of mortality than BMI or waist girth. Optimal values for lowest mortality: for non-smokers, lean mass∈(38.34, 40.43] kg, fat mass∈(19.49, 23.63] kg, % body fat∈(40.91, 44.25] %, BMI∈(26.73, 29.82] kg/m2, WC ∈(80.0, 85.7] cm |
Age, smoking, self-reported health, grip strength, nonthiazide diuretic use, and femoral neck bone mineral density |
| Gale et al., 2007/ -- / United Kingdom | 1973–1974/24 | 800 (348 women; 452 men) | ≥ 65 | BMI, fat free mass, and % body fatd |
All-cause mortality: BMI was inversely associated with total mortality in both men and women; % body fat was inversely associated with mortality in men, but not in women; fat-free mass was not a predictor of mortality. Cardiovascular mortality: None of the measurements of body fat distribution was significant associated with cardiovascular mortality in either men or women, except for BMI in women. BMI was positively associated with cardiovascular mortality. Cancer mortality: Fat-free mass was not significantly associated with cancer mortality in both men and women, but % body fat and BMI were inversely associated with cancer mortality in men, but not in women. Optimal values for lowest mortality: not available |
Age, height, social class, smoking, reported change in weight, daily calorie intake, physical activity, grip strength, arm muscle area, and diagnosed disease at baseline |
| Wannamethee et al., 2007/The British Regional Heart Study / United Kingdom | 1978–1980/6 (mean) | 4,107 men | 60–79 | BMI, WC, WHR, and MAMC | After the exclusion of the underweight, increased adiposity (BMI, WC, and WHR) showed little relation with mortality. MAMC was significantly and inversely associated with mortality. After adjustment for MAMC, obesity markers, particularly high WC (>102 cm) and WHR (top quartile), were associated with increased mortality. A composite measure of MAMC and WC most effectively predicted mortality. Men with low WC (≤ 102 cm) and above-median MAMC showed the lowest mortality risk. Men with WC > 102 cm and above-median MAMC showed significantly increased mortality (RR: 1.36, 95% CI: 1.07, 1.74), and in those men with WC > 102 and low MAMC, the RR increased to 1.55, 95% CI: 1.01, 2.39. Optimal values for lowest mortality: MAMC > 26.43 cm and WC ≤ 102 cm |
Age, social class, physical activity, alcohol intake, and cigarette smoking |
| Hays et al., 2006/Established Population for Epidemiologic Studies of the Elderly/United States | 1986–1987/4 | 1,920 (1,278 women; 642 men) | ≥ 65 | BMI and WC | BMI did not confer significant mortality risk among any gender or race group in this sample. WC (measured either in dichotomous or continuous format) was not predictive of survival among men or women in controlled analyses. Optimal values for lowest mortality: not available |
Age, living with others, income, smoking and alcohol use, cognitive status, and overall self-rated health |
| Price et al., 2006/ -- / United Kingdom | 1990/ 5.9 (median) | 14,833 (13,667 women; 7,892 men) | ≥ 75 | BMI, WC, and WHR | In nonsmoking men and women, compared with the lowest quintile of BMI, HRs were < 1 for all other quintiles of BMI. Increasing WHR was associated with increasing HRs in men and women. BMI was not associated with circulatory mortality in men and was negatively associated in women. WHR was positively related to circulatory mortality in both men and women. WC was not associated with all-cause or circulatory mortality. Optimal values for lowest mortality: BMI∈(29, 40.4] kg/m2, WC ∈(99.5, 106.6] cm, WHR∈[0.74, 0.89] for non-smoking men; BMI∈(29.7, 45.2] kg/m2, WC ∈(88.4, 95.6] cm, WHR∈[0.67, 0.79] for non-smoking women |
Height, age, psychosocial factors, cognitive impairment, socioeconomic factors, former smoking, recent alcohol use, and unexplained recent weight loss |
| Janssen et al., 2005/Cardiovascular Health Study /United States | 1989–1990/9 | 5,200 (2,938 women; 2,262 men) | ≥ 65 | BMI and WC | When examined individually, BMI and WC were both negative predictors of mortality, but when BMI and WC were examined simultaneously, BMI was a negative predictor of mortality, whereas WC was a positive predictor of mortality. After controlling for WC, mortality risk decreased 21% for every standard deviation increase in BMI. After controlling for BMI, mortality risk increased 13% for every standard deviation increase in WC. Optimal values for lowest mortality: not available |
Age, sex, race, socioeconomic status, smoking, physical activity, and prevalent disease (diabetes, coronary heart disease, congestive heart failure, stroke, cancer, lung disease) |
| Visscher et al., 2001/The Rotterdam Study /The Netherlands | 1990–1993/5.4 (mean) | 6,296 (3,694 women; 2,602 men) | 55–102 | BMI, WC, and WHR | Only the highest category of BMI (BMI > 30 kg/m2) among never smoking men was related to increased mortality, compared to normal BMI (HR: 2.6, 95% CI: 1.3–5.3). WC ∈ [94, 102] cm and WC ≥ 102 cm were related to increased mortality, compared to normal WC (HR: 1.7, 95% CI: 1.1–2.8 and 1.6, 95% CI: 1.0–2.8, respectively). The proportion of mortality attributable to large WC among never smoking men was three-fold the proportion attributable to high BMI. Among never smoking women and ex- and current smokers, categories of large body fatness did not predict increased mortality. Optimal values for lowest mortality: for never-smoking men, BMI > 26.2 kg/m2, WC∈(86, 90] cm, WHR∈(0.96, 1]; for never-smoking women, BMI > 25.5 kg/m2, WC < 79 cm, WHR<0.8 |
Age |
| Folsom et al., 2000/The Iowa Women’s Health Study/ United States | 1986/10 | 31,702 women | 55–69 | BMI, WC, and WHR |
Total mortality: The WHR was the best anthropometric predictor of total mortality, with the multivariable-adjusted relative risk for quintile 5 vs 1 of 1.2 (95% CI: 1.1–1.4), compared with 0.91 (95% CI: 0.8–1.0) for BMI and 1.1 (95% CI: 1.0–1.3) for WC. Coronary heart disease mortality: A higher BMI and WHR were associated with greater coronary heart disease-related mortality. Other CVD mortality: The WHR was associated positively, BMI was associated negatively, and WC was not associated with other CVD-related deaths. Cancer mortality: BMI, WHR, and WC were not significantly associated with cancer mortality. Optimal values for lowest age-adjusted mortality: BMI ∈(27.06, 30.21] kg/m2, WC∈(80, 87.3] cm, WHR<0.762 |
Age, BMI, smoking, education level, estrogen use, age of first live birth, vitamin use, pack-years of cigarette smoking, energy, whole grain, fruit, vegetable, fish, Keys score, high blood pressure, and alcohol use |
| Heitmann et al., 2000/ -- /Swedan | 1972/22 | 735 men | 60 | BMI, WC, % body fate | % fat and fat-free mass are a positive and a negative linear function of mortality, respectively. The highest risk was observed for men in the highest quintile of % body fat, with RR: 1.5, 95% CI 1.11–2.00, compared with men belonging to the lowest quintile. There was a U-shaped association between BMI and mortality: the highest quintile of BMI has RR: 1.5, 95% CI: 1.06–1.96, compared to the middle quintile. Optimal values for lowest mortality: not available |
Smoking habit and physical activity |
| Kalmijn et al., 1999/The Honolulu Heart Porgram/ United States | 1991–1993/ 4.5 (mean) | 3,741 men | 71–93 | BMI, WHR, and skinfold thickness | BMI was a negative predictor of mortality (RR of highest vs lowest quintile: 0.5, 95% CI: 0.4–0.6). Results were independent of WHR. A higher WHR steadily increased the risk of dying (RR highest vs lowest quintile: 1.5, 95% CI: 1.1–2.0). Especially in subjects with a high BMI, there was a positive association between WHR and mortality. The results for skinfold thickness were similar to the results for BMI, but less strong. Optimal values for lowest mortality: BMI∈[24.2, 39.3] kg/m2, WHR∈[0.73, 0.90] |
Age, years of standard education, physical activity index, alcohol consumption, number of cigarettes/d, systolic blood pressure, diastolic blood pressure, serum total cholesterol level, fasting serum glucose and insulin concentrations |
BMI: body mass index; WHR: waist-hip ratio; WC: waist circumference; HC: hip circumference; LM: lean mass; FM: fat mass; LMI: lean mass index (lean mass in kilogram/the square of height, kg/m2); BFI: body fat index (total body fat in kilogram /the square of height in meter); RTF: relative trunk fat (trunk fat/total body fat); BMMI body muscle mass index (total body muscle mass/ the square of height in meter); MAMC: mid-arm muscle circumference; pQCT: peripheral quantitative computerized tomography; CVD: cardiovascular disease; HR: hazard ratio; RR: relative risk; CI: confidence interval; --: not available.
The selection was based on 3 levels of screening described in Section 2.2 and 2.3.
Body fat composition was measured by bioelectrical impedance analysis.
Body fat composition was measured by dual-energy x-ray absorptiometry.
% body fat was derived using the four average skinfold thickness measurements in the formulae devised by Durnin and Wormesley (1974).
Total body potassium was measured in a whole body counter, and lean body mass calculated using the assumption of a potassium content of 68.1 mEq/kg lean body mass. Fat mass was calculated by subtracting the lean body mass from body weight.
3.5.1 The Best predictor of all-cause mortality
Different studies used different sets of measurements of body fat distribution to look at the relationship between mortality and body fat distribution in different samples of old subjects. Therefore, the conclusions of the best predictor of mortality are inconsistent across studies. Most of the studies found association between body fat distribution and mortality. Folsom et al., analyzing finding from the Iowa Women’s Health Study, claimed that WHR was the best anthropometric predictor in women [55]. Han et al. in a study consisting of all Asian elderly (aged 65–98 years) argued that lean body mass should be the best predictor of mortality for Asians [62].
Some studies found that a combination of measurements most effectively predict mortality. In a study that only recruited men, Wannamethee et al. concluded that a composite measure of midarm muscle circumference and WC (>102 cm) was the best predictor of mortality for a male older population [68]. In the Cardiovascular Health Study, Janssen et al. found that after controlling for both WC and BMI, BMI was a negative predictor, whereas WC was a positive predictor [65].
Among those studies including body composition measures, Han et al. found lean body mass was the strongest predictor for Asian men and women, as previously mentioned. Looking also at the older Asian population, Auyeung et al. reported that in older men, mortality was related to body composition, whereas in women, no corresponding relationship could be revealed, except in WHR [57]. Kuk et al. found that low percentage body fat (≤ 24.3 % for women and ≤ 15 % for men) is strongly associated with a high mortality risk (4/2 folds higher than that for women with percentage body fat ∈ [24.4, 35.9] %/men with percentage body fat ∈ [15.1, 24.0] %) [66]. On the other hand, Dolan et al. contended that body composition measures were not better predictors than BMI or WC [59]. Cesari et al. also found that no body composition parameters were significantly associated with mortality [58].
In summary, the contradictory conclusions across different studies can be attributed to variations of the following: (i) study populations; (ii) statistical models; (iii) measurement of body fat distribution; and (iv) accuracy of the measurements.
3.5.2 Optimal BMI, WC, WHR, and body fat composition for the elderly
Thirteen out of 17 articles gave information about optimal ranges of body fat distribution for mortality for elderly populations.5 In terms of BMI, 8 studies provided optimal BMI range for the elderly. With the exception of one study using a sample aged 90+ suggested a range [25, 30) kg/m2, the rest of the studies all suggested higher ranges. Six studies had this information on WC. For men, Wannamethee et al., Hays et al., and Visscher et al. suggested WC should be < 102 cm, within (99.5, 106.6] cm, and within (86, 90] cm, respectively; for women, Dolan et al., Hays et al., and Visscher et al. had the optimal ranges of (80, 85.7] cm, (88.4, 95.6] cm, and <79 cm, respectively. Srikanthan et al. and Folsom et al. suggested 87–92 cm and 80–87.3 cm, respectively, for older populations in general. WHR, on the other hand, appears to be the smaller the better. Srikanthan et al. and Folsom et al. showed that older adults in their samples with WHR ≤ 0.75 and < 0.762, respectively, had the lowest mortality. Two studies provided optimal percentage body fat. Kuk et al. found that the lowest mortality occurred at [24.1, 29.4] % for men and [42.5, 46.4] % for women. Dolan also found that percentage body fat in the range of (40.91, 44.25] % is the most beneficial.
3.5.3 Other-cause mortality
Three studies discussed cause-specific mortality [19, 55, 60]. Gale et al. and Folsom et al. did not find associations between any of their measurements of body fat distribution and cancer mortality in elderly women, but an inverse association of percentage body fat as well as BMI and cancer mortality in elderly men was observed by Gale et al. For the cardiovascular mortality, WC has the worst predictive power in both men and women. Price et al. and Folsom et al. both showed that WHR was positively associated with circulatory- and cardiovascular-related death, respectively. Without including WHR as one of their measurements of body fat distribution, Gale et al. concluded that only BMI (neither fat free mass nor percentage body fat) showed positive association with cardiovascular mortality in women. However, Price et al. contradictorily found that BMI was negatively associated with circulatory mortality in women.
3.5.4 Obesity paradox
Although obesity is a risk factor for the aforementioned comorbidities, studies have shown that when overweight and obese people develop these diseases they have better survival outcomes than their normal weight counterparts. This has been termed the “obesity paradox” [70]. The obesity paradox was found in the elderly in most of the studies investigating BMI threshold as a risk factor in the older population [17, 19, 71–75]. However, the National Institutes of Health guidelines [11] on overweight and obesity define adults with a BMI ≥ 25 kg/m2 as being at risk, and recommend treatment for people with a BMI ≥ 30 kg/m2 or for those with a BMI ∈ [25, 30) kg/m2 and either ≥ 2 comorbidities or a high WC. The obesity paradox suggests that these recommendations are inappropriate for older adults [19, 74, 76].
3.6 Economic Burden
Death is only part of the picture of the burden of chronic diseases caused by body fat redistribution for the elderly. These conditions can cause tremendous economic burden on the health care system. The comorbidities associated with body fat redistribution in the process of aging will be a focus of medical care as the population continues to age and live longer than ever before. According to a report by Centers for Disease Control and Prevention [77], currently about 80% of Americans older than 65 years are living with at least one chronic condition. And the older population is growing due to longer life spans and aging baby boomers. However, the cost of providing health care for an older adult is three to five times greater than the cost for an adult younger than 65. As a result, by 2030, the U.S. health care spending is projected to increase by 25% due to the demographic shifts.6
Although premature death caused by those comorbidities associated with age-related body fat redistribution, ironically, generates a direct medical savings [78], the improved medical care and prevention efforts have prolonged life expectancy. To the extent that the age-associated fat deposition contributes to those comorbidities but does not hasten death, which translates to marked health care costs that greatly outperform the savings, the financial impact on the health care system is substantial. In a study that included 3,789 individuals aged ≥ 65 years, it was found that abdominal obesity was associated with 15% higher costs controlling for age, sex, race/ethnicity, and other factors [79].
4. DISCUSSION
We performed a comprehensive search and conducted a systematic review of the literature to examine the existing findings on the relationship between body fat distribution and mortality. We found that the majority of studies conducted body fat distribution and mortality in a general population context; relatively few studies focused on the elderly.7 Our review included 25 studies in the analysis of comorbidities associated with age-related body fat redistribution and 17 studies in the analysis of mortality.
Our review revealed several important findings. As fat tissue increases toward the center of the body in the process of aging, central adiposity becomes one of the major risk factors of chronic diseases, including type-2 diabetes, hypertension, cardiovascular diseases, and some cancers, and, ultimately, death.8 We found that the commonly used measures for body fat distribution in the literature were anthropometric measures, e.g., BMI, WC, and WHR, and direct measures, e.g., body fat/lean mass, and percentage body fat. Among these measures, studies have had inconsistent conclusions with regard to the strongest predictor(s) of incidence of the aforementioned comorbidities and mortality. But almost all of the studies alluded to the fact that BMI per se was not the most appropriate predictor for this population due to the age-related body fat redistribution. Furthermore, studies using BMI found that the optimal BMI range for the lowest mortality in the elderly was overweight and mildly obese. These suggest that the current National Institutes of Health guidelines [11] advising that overweight and obesity are major risk factors for increased morbidity and mortality are not applicable to this population.
There are at least four reasons that can explain the inconsistent findings regarding the best predictor of morbidity/mortality across different studies: (i) although the study samples belong to an older population, findings may be very different across different age subgroups, e.g., the youngest old and oldest old, even though age is controlled for; (ii) pre-baseline weight loss, the extent of which increases with age, can confound the estimation of risk due to adiposity at baseline in a cohort study [80]; (iii) the measurements of body fat distribution deviate from study to study, e.g., fat mass could be measured by DXA, BIA, and even from body weight; and (iv) the selections of covariates vary across studies, making back-to-back comparisons difficult. The heterogeneity in the studies and in the measurements of body fat distribution was one of the reasons that a systematic review is preferred over a meta-analysis in this paper.
Our study is restrained by the limitations of the quality of the selected studies for the following reasons. First, some of the studies used self-reported information, which might result in biased estimation and erroneous conclusion. Second, although we excluded those studies that only reported unadjusted estimates or raw correlations, bias still exist in the adjusted model due to important risk factors that were left out in the model. Third, quite a few studies reported insignificant individual tests results, which should be noted that the insignificance may not result from the weak correlation between the target covariates and the outcome; rather, the insignificance came from the strong correlation between covariates, which could be resolved by using a joint test.
Nevertheless, our review has several strengths. First, we performed a systematic review with a priori criteria to determine the strength of evidence in relevant studies and selected the studies of highest quality to synthesize their findings. Second, our conclusions help readers gain a better understanding of the complex relationship between mortality and the morbidity risks associated with age-related body fat composition. Third, our findings help clarify the misleading BMI-based mortality risks for middle-aged adults.
In conclusion, our findings have provided evidence supported by existing literature suggesting that the current clinical guidelines require updated recommendations using combinations of body fat distribution measurements, including height, weight, waist and hip circumferences, direct measurements, and so on, specifically for older adults. At the same time, the updated version should make itself clear that it will not be misapplied back to a general population or younger populations. As the demographic shifts toward an aging society, it is imperative to establish new guidelines for the older population in terms of optimal body fat distribution for chronic disease prevention and control.
Acknowledgements
We acknowledge Susan Fowler, a MLIS qualified librarian, for her contribution to help construct a search strategy and perform literature searches for this study.
Funding
Funding from the Foundation for Barnes-Jewish Hospital and U54 CA 155496 supported this research. G. Colditz is supported by an American Cancer Society Clinical Research Professorship.
Abbreviations
- BMI
body mass index
- WHR
waist-hip ratio
- WC
waist circumference
- VF
visceral fat
- SAD
sagittal abdominal diameter
- BIA
bioelectrical impedance analysis
- DXA
Dual energy X-ray absorptiometry
- BIA
bioelectrical impedance analysis
- CT
computed tomography
- CVD
cardiovascular disease
- MS
metabolic syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Fat mass consists of essential fat and storage fat, the former being the fat necessary to sustain normal physiological function and the latter comprising primarily adipose tissue. Lean mass includes muscle, water, bone, connective tissue and internal organs.
Overweight and obesity were defined as body mass index (BMI, weight in kilograms divided by the square of height in meters) ∈ (25, 30] kg/m2 and ≥ 30 kg/m2, respectively.
Less stringent criteria at level 3 were applied to the studies for comorbidity analysis to include more articles due to the limited number of studies for the elderly.
The term “anthropometric” refers to measurements made of various parameters of the human body.
If the studies used models with body fat distribution measurements in a continuous fashion, then the information on the optimal value is not available.
It is projected that there will be 71 million older adults by 2030, accounting for roughly 20% of the U.S. population.
Even though a lot of studies cover the range of our target age group, they did not stratify in terms of age, so the estimates that they reported were mixed and not applicable to our analyses.
We focused on these major diseases and did not cover other conditions attributable to age-related body fat redistribution, e.g., sarcopenia, left ventricular mass, pulmonary disease, and disability.
Contributors
S.-H. Chang: study design, data extraction, data synthesis and analysis, interpretation of results, and writing of the manuscript; T. Beason: study design, data extraction, data synthesis and analysis, interpretation of results, and writing of manuscript; J. Hunleth: data extraction, interpretation of results, and writing of manuscript; G. Colditz: study design, interpretation of results, and critical review of manuscript. All authors approved the final version.
Competing interest
None declared.
Contributor Information
Su-Hsin Chang, Email: changsh@wudosis.wustl.edu.
Tracey S. Beason, Email: beasont@wudosis.wustl.edu.
Jean M. Hunleth, Email: hunlethj@wudosis.wustl.edu.
Graham A. Colditz, Email: colditzg@wudosis.wustl.edu.
REFERENCES
- 1.St-Onge MP, Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26(2):152–155. doi: 10.1016/j.nut.2009.07.004. Epub 2009/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing research reviews. 2009;8(4):339–348. doi: 10.1016/j.arr.2009.06.001. Epub 2009/07/07. [DOI] [PubMed] [Google Scholar]
- 3.Gambert SRP S. Emerging Epidemic: Diabetes in Older Adults: Demography, Economic Impact, and Pathophysiology. Diabetes Spectrum. 2006;19(4):221–228. [Google Scholar]
- 4.Cefalu WT, Wang ZQ, Werbel S, Bell-Farrow A, Crouse JR, 3rd, Hinson WH, et al. Contribution of visceral fat mass to the insulin resistance of aging. Metabolism: clinical and experimental. 1995;44(7):954–959. doi: 10.1016/0026-0495(95)90251-1. Epub 1995/07/01. [DOI] [PubMed] [Google Scholar]
- 5.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. Epub 2006/12/15. [DOI] [PubMed] [Google Scholar]
- 6.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Archives of internal medicine. 2005;165(7):777–783. doi: 10.1001/archinte.165.7.777. Epub 2005/04/13. [DOI] [PubMed] [Google Scholar]
- 7.Huang KC, Lee MS, Lee SD, Chang YH, Lin YC, Tu SH, et al. Obesity in the elderly and its relationship with cardiovascular risk factors in Taiwan. Obesity research. 2005;13(1):170–178. doi: 10.1038/oby.2005.22. Epub 2005/03/12. [DOI] [PubMed] [Google Scholar]
- 8.MacInnis RJ, English DR, Hopper JL, Gertig DM, Haydon AM, Giles GG. Body size and composition and colon cancer risk in women. International journal of cancer Journal international du cancer. 2006;118(6):1496–1500. doi: 10.1002/ijc.21508. Epub 2005/09/28. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Lahmann PH, Boeing H, Tjonneland A, Halkjaer J, Overvad K, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC) International journal of cancer Journal international du cancer. 2006;118(3):728–738. doi: 10.1002/ijc.21398. Epub 2005/08/12. [DOI] [PubMed] [Google Scholar]
- 10.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14(2):336–341. doi: 10.1038/oby.2006.43. Epub 2006/03/31. [DOI] [PubMed] [Google Scholar]
- 11.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. The American journal of clinical nutrition. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. Epub 1998/10/15. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. Epub 2009/07/23. [DOI] [PubMed] [Google Scholar]
- 13.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. The British journal of nutrition. 1991;65(2):105–114. doi: 10.1079/bjn19910073. Epub 1991/03/01. [DOI] [PubMed] [Google Scholar]
- 14.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1998;22(12):1164–1171. doi: 10.1038/sj.ijo.0800741. Epub 1999/01/07. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? American journal of epidemiology. 1996;143(3):228–239. doi: 10.1093/oxfordjournals.aje.a008733. Epub 1996/02/01. [DOI] [PubMed] [Google Scholar]
- 16.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. The New England journal of medicine. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. Epub 1999/10/08. [DOI] [PubMed] [Google Scholar]
- 17.Visscher TL, Seidell JC, Molarius A, van der Kuip D, Hofman A, Witteman JC. A comparison of body mass index, waist-hip ratio and waist circumference as predictors of all-cause mortality among the elderly: the Rotterdam study. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(11):1730–1735. doi: 10.1038/sj.ijo.0801787. Epub 2001/12/26. [DOI] [PubMed] [Google Scholar]
- 18.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annual review of nutrition. 2002;22:309–323. doi: 10.1146/annurev.nutr.22.010402.102715. Epub 2002/06/11. [DOI] [PubMed] [Google Scholar]
- 19.Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. The American journal of clinical nutrition. 2006;84(2):449–460. doi: 10.1093/ajcn/84.1.449. Epub 2006/08/10. [DOI] [PubMed] [Google Scholar]
- 20.Heymsfield SB, Nunez C, Testolin C, Gallagher D. Anthropometry and methods of body composition measurement for research and field application in the elderly. European journal of clinical nutrition. 2000;54(Suppl 3):S26–S32. doi: 10.1038/sj.ejcn.1601022. Epub 2000/10/21. [DOI] [PubMed] [Google Scholar]
- 21.St-Onge MP. Relationship between body composition changes and changes in physical function and metabolic risk factors in aging. Current opinion in clinical nutrition and metabolic care. 2005;8(5):523–528. Epub 2005/08/05. [PubMed] [Google Scholar]
- 22.Fan JG, Farrell GC. VAT fat is bad for the liver, SAT fat is not! J Gastroenterol Hepatol. 2008;23(6):829–832. doi: 10.1111/j.1440-1746.2008.05474.x. Epub 2008/06/21. [DOI] [PubMed] [Google Scholar]
- 23.Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochimica et biophysica acta. 2009;1790(10):1117–1123. doi: 10.1016/j.bbagen.2009.01.008. Epub 2009/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human model. Obesity. 2006;14(Suppl 1):20S–24S. doi: 10.1038/oby.2006.278. Epub 2006/04/29. [DOI] [PubMed] [Google Scholar]
- 25.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. European heart journal. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. Epub 2008/09/09. [DOI] [PubMed] [Google Scholar]
- 26.Carlsson ACW PE, de Faire U, Hellenius ML. Risk factors associated with newly diagnosed high blood pressure in men and women. American journal of hypertension. 2008;21(7):771–777. doi: 10.1038/ajh.2008.167. Epub 2008/04/26. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh AB K, Das Chaudhuri AB. Comparison of anthropometric characteristics between normotensive and hypertensive individuals among a population of Bengalee Hindu elderly men in Calcutta, India. Journal of The Royal Society for the Promotion of Health. 2000;120(2):100–106. doi: 10.1177/146642400012000207. [DOI] [PubMed] [Google Scholar]
- 28.Lempiainen PM L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation. 1999;100(2):123–128. doi: 10.1161/01.cir.100.2.123. Epub 1999/07/14. [DOI] [PubMed] [Google Scholar]
- 29.Santos ACL C, Barros H. Prevalence of metabolic syndrome in the city of Porto. Revista portuguesa de cardiologia : orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology : an official journal of the Portuguese Society of Cardiology. 2004;23(1):45–52. Epub 2004/04/03. [PubMed] [Google Scholar]
- 30.Patterson REF LL, Kristal AR, White E. A comprehensive examination of health conditions associated with obesity in older adults. American Journal of Preventive Medicine. 2004;27(5):385–390. doi: 10.1016/j.amepre.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor DAS GD, Ebrahim S. Does the new International Diabetes Federation definition of the metabolic syndrome predict CHD any more strongly than older definitions? Findings from the British Women's Heart and Health Study. Diabetologia. 2006;49(1):41–48. doi: 10.1007/s00125-005-0040-3. Epub 2005/12/27. [DOI] [PubMed] [Google Scholar]
- 32.Wright MEC SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–684. doi: 10.1002/cncr.22443. Epub 2007/01/11. [DOI] [PubMed] [Google Scholar]
- 33.Ramsay SEW PH, Shaper AG, Wannamethee SG. The relations of body composition and adiposity measures to ill health and physical disability in elderly men. American journal of epidemiology. 2006;164(5):459–469. doi: 10.1093/aje/kwj217. Epub 2006/07/05. [DOI] [PubMed] [Google Scholar]
- 34.Folsom ARK LH, Anderson KE, Mink PJ, Olson JE, Hong C, Sellers TA, Lazovich D, Prineas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Archives of internal medicine. 2000;160(14):2117. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 35.Huang KCL MS, Lee SD, Chang YH, Lin YC, Tu SH, Pan WH. Obesity in the elderly and its relationship with cardiovascular risk factors in Taiwan. Obesity research. 2005;13(1):170–178. doi: 10.1038/oby.2005.22. Epub 2005/03/12. [DOI] [PubMed] [Google Scholar]
- 36.Turcato E, Bosello O, Di Francesco V, Harris TB, Zoico E, Bissoli L, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: their relation with cardiovascular risk factors. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000;24(8):1005–1010. doi: 10.1038/sj.ijo.0801352. Epub 2000/08/22. [DOI] [PubMed] [Google Scholar]
- 37.Wu CH, Yao WJ, Lu FH, Yang YC, Wu JS, Chang CJ. Sex differences of body fat distribution and cardiovascular dysmetabolic factors in old age. Age and ageing. 2001;30(4):331–336. doi: 10.1093/ageing/30.4.331. Epub 2001/08/18. [DOI] [PubMed] [Google Scholar]
- 38.Passos VMB SM, Diniz LM, Lima-Costa MF. Type 2 diabetes: prevalence and associated factors in a Brazilian community--the Bambui health and aging study. Sao Paulo medical journal = Revista paulista de medicina. 2005;123(2):66–71. doi: 10.1590/S1516-31802005000200007. Epub 2005/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dekker JMG C, Rhodes T, Nijpels G, Stehouwer CDA, Bouter LM, Heine RJ. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112(5):666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. [DOI] [PubMed] [Google Scholar]
- 40.Koster A, Stenholm S, Alley DE, Kim LJ, Simonsick EM, Kanaya AM, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring, Md) 2010;18(12):2354–2361. doi: 10.1038/oby.2010.86. Epub 2010/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wise LAR L, Palmer JR, Adams-Campbell LL. Anthropometric risk factors for colorectal polyps in African-American women. Obesity (Silver Spring, Md) 2008;16(4):859–868. doi: 10.1038/oby.2007.139. Epub 2008/02/02. [DOI] [PubMed] [Google Scholar]
- 42.Sellers TAS JM, Gapstur SM, Rich SS, Potter JD, Ross JA, McGovern PG, Nelson CL, Folsom AR. Does body fat distribution promote familial aggregation of adult onset diabetes mellitus and postmenopausal breast cancer? Epidemiology (Cambridge, Mass) 1994;5(1):102–108. doi: 10.1097/00001648-199401000-00015. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 43.Balkau B. Does diagnosis of the metabolic syndrome detect further men at high risk of cadiovascular death beyound those identified by conventional cadiovascular risk score? The DECODE Study: The Diabetes Epidemiology: Collaborative analysis of Diagnostic Criteria in Europe (DECODE) Study group. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14:192. [Google Scholar]
- 44.Levitan EBY AZ, Wolk A, Mittleman MA. Adiposity and incidence of heart failure hospitalization and mortality: a population-based prospective study. Circulation Heart failure. 2009;2(3):202–208. doi: 10.1161/CIRCHEARTFAILURE.108.794099. Epub 2009/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedenreich CC A, Lahmann PH, Steindorf K, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S, Linseisen J, Rohrmann S, Boeing H, Pischon T, Tjonneland A, Halkjaer J, Overvad K, Mendez M, Redondo ML, Garcia CM, Larranaga N, Tormo MJ, Gurrea AB, Bingham S, Khaw KT, Allen N, Key T, Trichopoulou A, Vasilopoulou E, Trichopoulos D, Pala V, Palli D, Tumino R, Mattiello A, Vineis P, Bueno-de-Mesquita HB, Peeters PHM, Berglund G, Manjer J, Lundin E, Lukanova A, Slimani N, Jenab M, Kaaks R, Riboli E. Anthropometric factors and risk of endometrial cancer: The European prospective investigation into cancer and nutrition. Cancer Causes and Control. 2007;18(4):399–413. doi: 10.1007/s10552-006-0113-8. [DOI] [PubMed] [Google Scholar]
- 46.Woo JH SC, Yu AL, Sham A. Is waist circumference a useful measure in predicting health outcomes in the elderly? International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26(10):1349–1355. doi: 10.1038/sj.ijo.0802080. Epub 2002/10/02. [DOI] [PubMed] [Google Scholar]
- 47.Woo JH SC, Yuen YK, Yu LM, Lau J. Cardiovascular risk factors and 18-month mortality and morbidity in an elderly Chinese population aged 70 years and over. Gerontology. 1998;44(1):51–55. doi: 10.1159/000021983. Epub 1998/01/22. [DOI] [PubMed] [Google Scholar]
- 48.Folsom ARK LH, Anderson KE, Mink PJ, Olson JE, Hong CP, Sellers TA, Lazovich D, Prineas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Archives of internal medicine. 2000;160(14):2117–2128. doi: 10.1001/archinte.160.14.2117. Epub 2000/07/25. [DOI] [PubMed] [Google Scholar]
- 49.Kuk JLS TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing research reviews. 2009;8(4):339–348. doi: 10.1016/j.arr.2009.06.001. Epub 2009/07/07. [DOI] [PubMed] [Google Scholar]
- 50.Calling SH B, Engstrom G, Berglund G, Janzon L. Effects of body fatness and physical activity on cardiovascular risk: risk prediction using the bioelectrical impedance method. Scandinavian journal of public health. 2006;34(6):568–575. doi: 10.1080/14034940600595621. Epub 2006/11/30. [DOI] [PubMed] [Google Scholar]
- 51.Nakajima KY H, Morita K, Ebata M, Eguchi S, Muneyuki T, Munakata H. Elderly people with low body weight may have subtle low-grade inflammation. Obesity (Silver Spring, Md) 2009;17(4):803–808. doi: 10.1038/oby.2008.596. Epub 2009/01/10. [DOI] [PubMed] [Google Scholar]
- 52.Dekker JM, Girman C, Rhodes T, Nijpels G, Stehouwer CD, Bouter LM, et al. Metabolic syndrome and 10-year cardiovascular disease risk in the Hoorn Study. Circulation. 2005;112(5):666–673. doi: 10.1161/CIRCULATIONAHA.104.516948. Epub 2005/08/03. [DOI] [PubMed] [Google Scholar]
- 53.Group DS. Does diagnosis of the metabolic syndrome detect further men at high risk of cardiovascular death beyond those identified by a conventional cardiovascular risk score? The DECODE Study. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2007;14(2):192–199. doi: 10.1097/01.hjr.0000230107.78524.da. Epub 2007/04/21. [DOI] [PubMed] [Google Scholar]
- 54.Woo J, Ho SC, Yuen YK, Yu LM, Lau J. Cardiovascular risk factors and 18-month mortality and morbidity in an elderly Chinese population aged 70 years and over. Gerontology. 1998;44(1):51–55. doi: 10.1159/000021983. Epub 1998/01/22. [DOI] [PubMed] [Google Scholar]
- 55.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Archives of internal medicine. 2000;160(14):2117–2128. doi: 10.1001/archinte.160.14.2117. Epub 2000/07/25. [DOI] [PubMed] [Google Scholar]
- 56.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Annals of epidemiology. 2009;19(10):724–731. doi: 10.1016/j.annepidem.2009.05.003. Epub 2009/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Auyeung TWL JS, Leung J, Kwok T, Leung PC, Woo J. Survival in older men may benefit from being slightly overweight and centrally obese--a 5-year follow-up study in 4,000 older adults using DXA. The journals of gerontology Series A, Biological sciences and medical sciences. 2010;65(1):99–104. doi: 10.1093/gerona/glp099. Epub 2009/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cesari MP M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, Guralnik JM, Ferrucci L. Skeletal muscle and mortality results from the InCHIANTI study. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2009;64(3):377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dolan CMK H, Browner W, Ensrud K, Kelsey JL. Associations between body composition, anthropometry, and mortality in women aged 65 years and older. American journal of public health. 2007;97(5):913–918. doi: 10.2105/AJPH.2005.084178. Epub 2007/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gale CRM CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. International journal of epidemiology. 2007;36(1):228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 61.Guallar-Castillon PB-C T, Lopez-Garcia E, Leon-Muoz LM, Gutierrez-Fisac JL, Banegas JR, Rodriguez-Artalejo F. BMI, waist circumference, and mortality according to health status in the older adult population of Spain. Obesity. 2009;17(12):2232–2238. doi: 10.1038/oby.2009.115. [DOI] [PubMed] [Google Scholar]
- 62.Han SSK KW, Kim KI, Na KY, Chae DW, Kim S, Chin HJ. Lean mass index: a better predictor of mortality than body mass index in elderly Asians. Journal of the American Geriatrics Society. 2010;58(2):312–317. doi: 10.1111/j.1532-5415.2009.02672.x. Epub 2010/01/15. [DOI] [PubMed] [Google Scholar]
- 63.Hays JCK HH, Østbye T. The effects of nutrition-related factors on four-year mortality among a biracial sample of community-dwelling elders in the North Carolina Piedmont. J Nutr Elder. 2006;25(2):41–67. doi: 10.1300/j052v25n02_04. [DOI] [PubMed] [Google Scholar]
- 64.Heitmann BLE H, Ellsinger BM, Mikkelsen KL, Larsson B. Mortality associated with body fat, fat-free mass and body mass index among 60-year-old Swedish men - A 22-year follow-up. The study of men born in 1913. Int J Obes. 2000;24(1):33–37. doi: 10.1038/sj.ijo.0801082. [DOI] [PubMed] [Google Scholar]
- 65.Janssen IK PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. Journal of the American Geriatrics Society. 2005;53(12):2112–2118. doi: 10.1111/j.1532-5415.2005.00505.x. Epub 2006/01/10. [DOI] [PubMed] [Google Scholar]
- 66.Kuk JLA CI. Influence of age on the association between various measures of obesity and all-cause mortality. Journal of the American Geriatrics Society. 2009;57(11):2077–2084. doi: 10.1111/j.1532-5415.2009.02486.x. Epub 2009/09/17. [DOI] [PubMed] [Google Scholar]
- 67.Lisko IT K, Stenholm S, Luukkaala T, Hervonen A, Jylhä M. Body mass index, waist circumference, and waist-to-hip ratio as predictors of mortality in nonagenarians: The vitality 90+ study. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2011;66 A(11):1244–1250. doi: 10.1093/gerona/glr147. [DOI] [PubMed] [Google Scholar]
- 68.Wannamethee SGS AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. The American journal of clinical nutrition. 2007;86(5):1339–1346. doi: 10.1093/ajcn/86.5.1339. Epub 2007/11/10. [DOI] [PubMed] [Google Scholar]
- 69.Kalmijn S, Curb JD, Rodriguez BL, Yano K, Abbott RD. The association of body weight and anthropometry with mortality in elderly men: the Honolulu Heart Program. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1999;23(4):395–402. doi: 10.1038/sj.ijo.0800832. Epub 1999/05/26. [DOI] [PubMed] [Google Scholar]
- 70.Chapman IM. Obesity paradox during aging. Interdisciplinary topics in gerontology. 2010;37:20–36. doi: 10.1159/000319992. Epub 2010/08/13. [DOI] [PubMed] [Google Scholar]
- 71.Dolan CM, Kraemer H, Browner W, Ensrud K, Kelsey JL. Associations between body composition, anthropometry, and mortality in women aged 65 years and older. American journal of public health. 2007;97(5):913–918. doi: 10.2105/AJPH.2005.084178. Epub 2007/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han SS, Kim KW, Kim KI, Na KY, Chae DW, Kim S, et al. Lean mass index: a better predictor of mortality than body mass index in elderly Asians. Journal of the American Geriatrics Society. 2010;58(2):312–317. doi: 10.1111/j.1532-5415.2009.02672.x. Epub 2010/01/15. [DOI] [PubMed] [Google Scholar]
- 73.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. Journal of the American Geriatrics Society. 2005;53(12):2112–2118. doi: 10.1111/j.1532-5415.2005.00505.x. Epub 2006/01/10. [DOI] [PubMed] [Google Scholar]
- 74.Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity. 2009;17(6):1232–1239. doi: 10.1038/oby.2008.664. Epub 2009/02/07. [DOI] [PubMed] [Google Scholar]
- 75.Testa G, Cacciatore F, Galizia G, Della-Morte D, Mazzella F, Langellotto A, et al. Waist circumference but not body mass index predicts long-term mortality in elderly subjects with chronic heart failure. Journal of the American Geriatrics Society. 2010;58(8):1433–1440. doi: 10.1111/j.1532-5415.2010.02979.x. Epub 2010/07/31. [DOI] [PubMed] [Google Scholar]
- 76.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Archives of internal medicine. 2001;161(9):1194–1203. doi: 10.1001/archinte.161.9.1194. Epub 2001/05/18. [DOI] [PubMed] [Google Scholar]
- 77.Merck Institute of Aging & Health., Gerontological Society of America., Merck Company Foundation. The state of aging and health in America. Washington, D.C.: Merck Institute of Aging & Health; 2002. p.v. [Google Scholar]
- 78.Finkelstein EA, DiBonaventura M, Burgess SM, Hale BC. The costs of obesity in the workplace. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2010;52(10):971–976. doi: 10.1097/JOM.0b013e3181f274d2. Epub 2010/10/01. [DOI] [PubMed] [Google Scholar]
- 79.Curtis LH, Hammill BG, Bethel MA, Anstrom KJ, Gottdiener JS, Schulman KA. Costs of the metabolic syndrome in elderly individuals: findings from the Cardiovascular Health Study. Diabetes care. 2007;30(10):2553–2558. doi: 10.2337/dc07-0460. Epub 2007/07/12. [DOI] [PubMed] [Google Scholar]
- 80.Kyulo NL, Knutsen SF, Fraser GE, Singh PN. Effect of weight loss in adults on estimation of risk due to adiposity in a cohort study. Obesity. 2012;20(1):206–213. doi: 10.1038/oby.2011.281. Epub 2011/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]