Abstract
Spinal GABAA receptor modulation with agonists and allosteric modulators evokes analgesia and antinociception. Changes in KCC2 expression or function that occur after peripheral nerve injury can result in an impairment in the Cl− extrusion capacity of spinal dorsal horn neurons. This, in turn, alters Cl− mediated hyperpolarization via GABAA receptor activation contributing to allodynia or hypersensitivity associated with nerve injury or inflammation. A gap in knowledge exists concerning how this loss of spinal KCC2 activity differentially impacts the analgesic efficacy or potency of GABAA agonists and allosteric modulators. We utilized intrathecal drug administration in the tail flick assay to measure the analgesic effects of general GABAA agonists muscimol and ZAPA, the ∂-subunit preferring agonist THIP and allosteric modulators of the benzodiazepine (midazolam) and neurosteroid (ganaxolone) class, alone, or in the presence of KCC blockade. Intrathecal muscimol, ZAPA, THIP midazolam and ganaxolone all evoked significant analgesia in the tail flick test. Co-administration of either agonists or allosteric modulators with DIOA (a drug that blocks KCC2) had no effect on agonist or allosteric modulator potency. On the other hand, the analgesic efficacy of muscimol and ZAPA and the allosteric modulator ganaxolone were markedly reduced while THIP and midazolam were unaffected. Finally, In the spared nerve injury (SNI) model, midazolam significantly reversed tactile hypersensitivity whilst ganaxolone had no effect. These results indicate that the KCC2-dependent Cl− extrusion capacity differentially regulates the analgesic efficacy of agonists and allosteric modulators at the GABAA receptor complex.
Perspective
Our work suggests that drug discovery efforts for the treatment of chronic pain disorders should target benzodiazepine or ∂-subunit-containing sites at the GABAA complex.
Keywords: KCC2, GABA, THIP, ganaxolone, benzodiazepine, tail flick, neuropathic pain
Introduction
Targeting spinal ionotropic gamma-aminobutyric acid (GABAA) receptors elicits analgesia in rodents 43 and humans 26, 58. The GABAA agonist muscimol possesses antinociceptive activity against acute nociception 1, 23, 37 and in the formalin model 17. The δ subunit preferring agonist THIP similarly induces analgesia and antinociception in rats and mice 25, 34, 35. In addition to agonists, positive allosteric modulators, such as benzodiazepines, are effective in producing spinally-mediated analgesia 6, 22, 63. GABAA -mediated inhibitory neurotransmission in both individual neurons and neuronal networks is modulated by cation-chloride cotransporter functional expression 30, 49, 52, 54. These cotransporters regulate neuronal Cl− homeostasis. The K+-Cl− cotransporter isoform 2 (KCC2) is largely responsible for Cl− extrusion in mature CNS neurons 7, 49. Dysregulation in Cl− homeostasis resulting from changes in functional KCC2 expression occurs in many CNS pathologies including epilepsy 27, 45, 57 neuronal trauma 46, 61 and chronic pain 13, 51. Because KCC2 maintains a low intracellular Cl− concentration in CNS neurons, a prerequisite for the generation of Cl−-dependent, hyperpolarizing GABAA-mediated responses 30, 49, 56, a disruption in functional KCC2 expression alters Cl− homeostasis and can consequently lead to a reduction in efficacy of GABAergic inhibition.
In the spinal dorsal horn, KCC2 plays a key role in regulating nociceptive circuits. Hypomorphic KCC2 mice show altered sensitivity to tactile and noxious thermal stimuli 60 and a reduction in nociceptive thresholds in rats is observed when KCC2 expression is knocked down or pharmacologically inhibited 13. Notably, reduced KCC2 expression or function has been implicated in the pathogenesis of neuropathic pain. Here, decreased functional KCC2 expression causes a depolarizing shift in GABAA Cl− reversal potential in lamina I/II neurons that leads to a reduction in GABAergic inhibitory efficacy in a subset of lamina I/II neurons 13, 15, 51. Despite these findings, there is strong evidence that modulation of spinal GABAA receptors in the setting of peripheral nerve injury or inflammation 4, 5, 32, 33 is still capable of producing antinociceptive or analgesic effects. Despite increasing knowledge of the analgesic and antinociceptive properties of subtype specific GABAA receptor agonists and allosteric modulators, the influence of reduced KCC2 activity on the spinal analgesic efficacy and/or potency of these agonists and allosteric modulators remains unknown. However, this information is likely important for the development of novel analgesics that target the GABAA receptor complex.
To directly address this question, we used the tail flick assay to measure the analgesic effects of GABAA receptor agonists muscimol, ZAPA and THIP, and allosteric GABAA modulators midazolam and ganaxolone. We utilized DIOA 21 and furosemide 48 as inhibitors of KCC2. Muscimol and ZAPA 3 are derived from the endogenous GABAA receptor agonist GABA and target all GABAA receptors without impacting GABAB receptors. THIP preferentially targets δ-subunit containing GABAA receptors 2 and activates a tonic current in spinal dorsal horn neurons 8. Midazolam and ganaxolone 11 belong to two major classes of GABAA allosteric modulators, benzodiazepines and neurosteroids, respectively. Benzodiazepines positively modulate both tonic and phasic currents in spinal dorsal horn neurons 38 while the effects of neurosteroids on these currents are unknown. Our results demonstrate that muscimol, ZAPA and ganaxolone lose analgesic efficacy, but not potency with KCC2 blockade while potency and efficacy of THIP and midazolam are unchanged. Moreover, midazolam significantly reverses tactile allodynia following peripheral nerve injury while ganaxolone is without effect. These findings indicate that impaired Cl− extrusion capacity resulting from decreased KCC2 activity reduces the analgesic efficacy of certain GABAA receptor agonists and allosteric modulators.
Methods
Drugs
Muscimol, ZAPA, THIP, ganaxolone, and clonidine hydrochloride were purchased from Tocris (Ellisville, MO). DIOA and furosemide were purchased from Sigma-Aldrich (St. Louis, MO). Midazolam was purchased from USP. Stock solutions of muscimol, ZAPA, clonidine and midazolam were made were made in distilled H2O. DIOA, furosemide, THIP and ganaxolone were made in 100% DMSO. DIOA was diluted in Ringer’s solution containing 10% DMSO. All other drugs were diluted to final doses in Ringer’s solution for injection. Final concentrations of DMSO in solutions used for intrathecal injection in the tail flick test were 10%. This concentration of DMSO had no effect on tail flick latency at any time point (baseline = 3.55 ± 0.33 s, 10 min post injection = 3.56 ± 0.40 s, 20 min post injection = 3.18 ± 0.25 s, 30 min post injection = 3.16 ± 0.13 sec, n = 7). Final concentrations of DMSO in intrathecal injections in neuropathic animals did not exceed 1%.
Animals
Male ICR mice (Harlan, 18–22g) were used for all studies. Animals were housed in a climate-controlled room on a 12-h light/dark cycle where food and water were available ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Arizona and were performed in accordance with the handling and use of laboratory animal guidelines of the International Association for the Study of Pain and National Institutes of Health.
Spared Nerve Injury (SNI)
Prior to surgery, all animals were assessed for mechanical withdrawal thresholds. SNI was performed by a lesion of two of the three terminal branches of the sciatic nerve (tibial and common peroneal nerves) leaving the remaining sural nerve intact as described previously 9. Under isoflurane anesthesia (induction = 5%, maintenance = 1.7–2% isoflurane in room air), an incision was made on the lateral surface of the thigh and a section was made directly through the biceps femoris muscle exposing the sciatic nerve and its three terminal branches: the sural, common peroneal and tibial nerves. The tibial and common peroneal nerves were tightly ligated with 5.0 silk and cut distal to the ligation, removing ~ 2mm of the distal nerve stump. The sural nerve was left intact. The skin incision was closed with staples. Mice were monitored daily following surgery and were tested for development of neuropathic allodynia 7 and 10 days following surgery. Testing with administration of intrathecal drugs was done on day 10–14 after surgery. The neuropathic pain state in these mice lasts for at least 4 weeks and is isolated to the ipsilateral paw 9.
Intrathecal injection
The intrathecal injection was done as previously described by Hylden and Wilcox 28 using a 25-μl Hamilton syringe with a 30 1/2-gauge needle. The injection was performed under brief (less than 3 min) isoflurane anesthesia (induction = 5%, maintenance = 1.7–2% isoflurane in room air) in a volume of 5μl. With the pelvic girdle held to keep the spinal cord in place, the needle was inserted into the intervertebral space between the L5 and L6 spinal cord level. A reflexive flick of the tail was used as an indicator of accurate needle placement and also as a confirmation of drug injection.
Tail Flick Latency
Analgesia was measured using the tail immersion method 29. The mice were restrained with the tail extending out. The distal portion of the tail (2–3cm) was immersed in a water bath thermostatically controlled at 52°C ± 0.5. The tail withdrawal reaction time (in seconds) was initially recorded as the tail flick latency before drug administration and then recorded at 10, 20, 30, 45 and 60 min after the administration of the test drug. A cut off latency of 10 seconds was maintained to prevent tissue damage. All the drugs were administered intrathecally. Percent maximum possible effect was calculated as:
Mechanical thresholds
Animals were placed in acrylic boxes with wire mesh floors and allowed to habituate for 1 hr followed by predrug mechanical thresholds recording. Using the up-down method 12, calibrated von Frey filaments (Stoelting, Wood Dale, IL) were used for mechanical stimulation of the plantar surface of the left hindpaw and withdrawal thresholds were calculated.
Statistics
All data are presented as Mean ± S.E.M unless otherwise stated. Graph plotting and statistical analysis were performed using Graphpad Prism Version 5.03 (Graph Pad Software, Inc. San Diego, CA, USA). Variable slope (4 parameters) nonlinear regression was used for dose response curves analysis. Measures of dose by time were done by two-way ANOVA with Bonferroni post-hoc test in tail flick tests. For analysis of mechanical thresholds in neuropathic animals, mechanical thresholds are expressed as log of gram force and analyzed by one-way ANOVA with Dunnett post-test (as described previously 40) where comparisons were made to baseline mechanical thresholds at 10–14 days after SNI. A priori level of significance at 95% confidence level was considered at p < 0.05.
Results
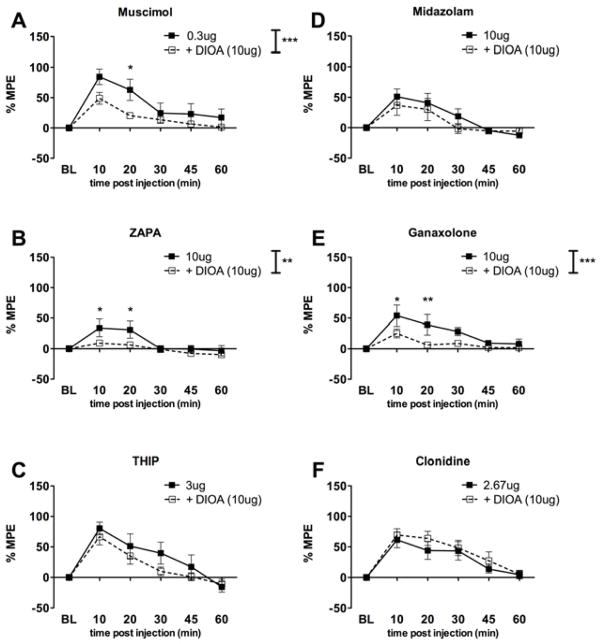
We first assessed the ability of GABAA receptor agonists and allosteric modulators to evoke analgesia in the tail flick test following intrathecal injection. The GABAA agonists muscimol (Fig 1A), THIP (Fig 1B) and ZAPA (Fig 1C) each caused significant analgesia in the tail flick test peaking at 0.3, 10 and 3 μg doses, respectively. Tail flick latencies returned to baseline levels within 60 min. Peak effects were observed at 10 or 20 min post injection. We then retested each of these agonists with co-intrathecal administration of the KCC2 inhibitor DIOA (10 μg). In the presence of DIOA, peak analgesic effects were also observed at 10 or 20 min post injection. The GABAA allosteric modulators midazolam (Fig 1D) and ganaxolone (Fig 1E) and the α2 adrenergic receptor agonist, clonidine (Fig 1F), also each caused significant analgesia in the tail flick test peaking at 10, 10 and 2.67 μg doses, respectively. Tail flick latencies returned to baseline levels within 60 min for each compound. We then retested each of these agonists with co-intrathecal administration of the KCC2 inhibitor DIOA (10 μg). Again, peak effects were observed at 10 or 20 min post injection in the presence and absence of DIOA. We reasoned that blockade of KCC2 with DIOA might alter either efficacy or potency (or both) for GABAA agonists or allosteric modulators in the tail flick test. We further anticipated that analgesic efficacy and potency of clonidine would be unaffected by KCC2 perturbation because this drug does not act on the GABAA receptor complex. In order to establish the analgesic efficacy and potency of the different agonists and allosteric modulators alone and in the presence of DIOA, dose response curves were constructed and evaluated. The dose response curves were graphed using the sum of the percent maximal effect (%MPE) at 10 and 20 min time points where peak analgesia was noted for all GABAA agonists and allosteric modulators used. Due to the steep and clear inverted U-shaped nature (in agreement with our previous observations and modeling studies 5, 18) of the dose response curves obtained, the curves for all compounds used were fitted with a fixed Emax (a measure of efficacy) based on the maximally effective doses listed above. Thus Emax values for the different agonists and allosteric modulators could not be found and evaluated through this method. Dose response curves were therefore evaluated using the ED50s (a measure of potency) and confidence intervals derived from these curves. A comparison of the ED50s and of the different agonists and allosteric modulators alone and in the presence of DIOA (Table 1) showed an overlap in confidence intervals for the different agonists and allosteric modulators alone and with DIOA present indicating that potency was unchanged by DIOA treatment. This result also illustrates that DIOA is not acting as a competitive antagonist at GABAA receptors in this assay.
Figure 1.
Dose response curves for intrathecal GABAA agonists and allosteric modulators with and without DIOA treatment in the tail flick test.
Log doses of drug in grams are shown on x-axis while sum of %MPE for 10 and 20 min time points are shown on y-axis. Dose response curves are shown with agonist or allosteric modulators ± DOIA. ED50s calculated from these curve fits (log(agonist) vs. response – Variable slope (four parameters)) are shown in Table 1.
Table 1.
ED50 of intrathecal GABAA agonists and allosteric modulators with and without DIOA (10 μg) treatment in the mouse tail flick test. No significant changes in ED50 were observed with DIOA co-treatment.
| Drug | ED50 | 95% CI | ED50 | 95% CI |
|---|---|---|---|---|
| Drug Alone | + DIOA (10μg)3 | |||
| Muscimol | 83.6 ng (0.42 nmoles) | 48.6 ng to 144 ng | 4.70 ng (0.024 nmoles) | 0.23 ng to 94.4 ng |
| THIP | 1.05 μg (5.9 nmoles) | 671 ng to 1.63 μg | 776 ng (4.4 nmoles) | 484 ng to 1.24 μg |
| ZAPA | 3.04 μg (12.4 nmoles) | 1.25 μg to 7.40 μg | 560 ng (2.3 nmoles) | 144 ng to 2.17 μg |
| Midazolam | 695 ng (2.1 nmoles) | 501 ng to 963 ng | 1.33 μg (4.1 nmoles) | 508 ng to 3.50 μg |
| Ganaxolone | 3.22 μg (9.7 nmoles) | 2.04 μg to 5.08 μg | 3.74 μg (11.2 nmoles) | 1.64 μg to 8.50 μg |
| Clonidine | 203 ng (0.76 nmoles) | 45.9 ng to 894 ng | 798 ng (3.0 nmoles) | 560 ng to 1.14 μg |
From the plotted %MPE data from figure 1, it was evident that the major effect of DIOA was a reduction in analgesic efficacy (e.g. reduced Emax) for some agonists or allosteric modulators. Thus, we compared the peak effect responses for each compound ± DIOA. For the general GABAA agonists muscimol (Fig 2A) and ZAPA (Fig 2B), DIOA co-treatment significantly reduced analgesic efficacy at either 10 or 20 min post injection indicating a reduction in analgesic efficacy in the presence of KCC2 blockade. In contrast, the δ-subunit preferring agonist THIP failed to lose analgesic efficacy with KCC2 blockade (Fig 2C). The benzodiazepine GABAA allosteric modulator, midazolam, showed no loss of analgesic efficacy with KCC2 blockade (Fig 2D) whereas the neurosteroid site ligand, ganaxolone did (Fig 2E). As expected, there was no change in analgesic efficacy in the presence of DIOA for clonidine (Fig 2F). To further support the reduced analgesic efficacy observed with DIOA treatment, we asked if similar effects would be observed with the structurally dissimilar KCC2 inhibitor furosemide 47. Furosemide (30 μg) did not influence the effects of THIP (Fig 3A) but reduced analgesic efficacy for ganaxolone (Fig 3B) consistent with findings with DIOA. Importantly, neither DIOA at 10 μg (%MPE at 10 min = 0.83 ± 3.28% and 20 min = 0.63 ± 4.21%) nor furosemide at 30 μg (%MPE at 10 min = 6.66 ± 8.94% and 20 min = 1.75 ± 4.28%) alone had an effect in the tail flick test.
Figure 2.
Peak-effect responses of intrathecal GABAA agonists and allosteric modulators with and without DIOA treatment in the tail flick test.
Maximal effective doses for each agonist or allosteric modulator are shown ± DIOA expressed as %MPE over a 60 min time course. DIOA reduced analgesic efficacy for agonists muscimol (A) and ZAPA (B) but did not influence THIP (C). DIOA did not influence midazolam (D) efficacy but did reduce ganaxolone efficacy (E). Clonidine (F) was unaffected by DIOA. Two-way ANOVA with Bonferroni post-test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Figure 3.
Peak-effect responses for intrathecal THIP and ganaxolone with and without furosemide treatment.
Furosemide had no effect on THIP (A) analgesic efficacy but significantly reduced ganaxolone analgesia (B). Two way ANOVA with Bonferroni post-test. ** p < 0.01, *** p < 0.001.
Reduced chloride extrusion capacity is thought to underlie neuropathic allodynia following peripheral nerve injury 15, 52, 54. This dysregulation in chloride extrusion capacity has been shown to occur via changes in functional KCC2 expression 13, 31. Because we observed differential effects of GABAA allosteric modulator analgesic efficacy with KCC2 blockade, we hypothesized that midazolam would retain anti-allodynic effects in the spared nerve injury model while ganaxolone would lack anti-allodynic effects. Using doses of midazolam and ganaxolone that were efficacious in the tail flick test under unperturbed KCC2 activity, midazolam significantly reversed neuropathic allodynia observed in the spared nerve injury model (Fig 4A) whereas ganaxolone had no effect (Fig 4B). This result confirms our hypothesis and suggests that the analgesic efficacy of allosteric modulators that differentially target the GABAA receptor complex are under control of KCC2-mediated Cl− extrusion capacity.
Figure 4.
Effect of intrathecal midazolam and ganaxolone on spared nerve injury-induced neuropathic allodynia.
SNI surgery was performed and mice were tested for mechanical allodynia on the ipsilateral hindpaw 10–14 days post surgery. (A) intrathecal injection of midazolam significantly reversed mechanical allodynia in the mouse SNI model whereas ganaxolone (B) was without effect. One way ANOVA with Dunnet post-test comparing to baseline. * p < 0.05, *** p < 0.001.
Discussion
Although there is ample evidence for the analgesic and antinociceptive properties of GABAA receptor agonists and allosteric modulators at the spinal level, the effect of loss of Cl− extrusion capacity on their analgesic and antinociceptive properties has hitherto been unexplored. Hence, the primary findings of the current work are that loss of Cl− extrusion capacity does not affect the potency (ED50) of either agonists or allosteric modulators of the GABAA receptor complex, but, rather, modulates the efficacy (Emax) of non-selective GABAA agonists and neurosteroid-derived allosteric modulators. Specifically, the agonists muscimol and ZAPA and the allosteric modulator ganaxolone lose analgesic efficacy whereas the δ-subunit preferring agonist THIP and the allosteric modulator midazolam maintain efficacy when Cl− extrusion capacity is inhibited. Importantly, our findings illustrate that spinal midazolam attenuates tactile hypersensitivity following peripheral nerve injury, which reduces spinal KCC2 expression 13, whilst ganaxolone is without effect. Thus, in clinical situations where spinal functional Cl− extrusion capacity is thought to be diminished, targeting spinal GABAA receptors containing the δ-subunit and/or sensitive to benzodiazepines is likely to represent a relevant therapeutic approach.
In spinal dorsal horn neurons, GABAA receptor agonists have been demonstrated to attenuate glutamate-induced and/or spontaneous excitability 14, 65 and GABAA agonists and/or allosteric modulators applied intrathecally increase nociceptive thresholds in rodents 1, 17, 23. Consistent with these results, our present findings indicate that the GABAA agonists muscimol, THIP and ZAPA and allosteric modulators midazolam and ganaxolone produce analgesia in the mouse tail flick test. An important question in terms of GABAA-based therapeutics for the treatment of pain in humans arises from our current understanding of the role of KCC2 in modulating GABAA-mediated currents in preclinical models. KCC2 expression is decreased in the spinal dorsal horn following peripheral nerve injury and after peripheral inflammation or injury 13, 39, 41, 44, 64. This change in KCC2 expression is thought to decrease GABAA-dependent inhibition in a subset of dorsal horn neurons and has been proposed as a primary mechanism for the generation of allodynia in preclinical neuropathic pain models 13, 15, 52, 54. However, it is also clear that benzodiazepine class allosteric modulators maintain efficacy for reducing neuropathic allodynia and/or inflammation-induced hyperalgesia 4, 5, 32, 33. These findings suggest that altered functional KCC2 expression may differentially modulate the analgesic efficacy of GABAA agonists or allosteric modulators 54. Our findings strongly support this conclusion and point to specific therapeutic avenues for the generation of effective spinal analgesics that target the GABAA receptor complex.
Heteropentameric GABAA receptors composed of multiple subunits (α1–6, β1–3, γ1–3, δ, ε, θ and ρ) mediate both phasic (synaptic) and tonic (extrasynaptic) inhibition 19, 20. The transient activation of phasic GABAA receptors composed of two α subunits combined with two β and a γ subunit produces phasic inhibition mediating fast GABAergic inhibitory transmission. Tonic inhibition is predominately generated by the persistent action of extrasynaptic receptors (composed of two α subunits combined with two β and a δ subunit or γ2 subunit) by ambient GABA. These GABAA receptors are based on high affinity isoforms (e.g. α6β×δ, α4β×δ and α5β×γ2) with slow desensitization kinetics 19, 20. Muscimol and ZAPA do not pharmacologically distinguish GABAA receptors of the tonic and phasic types 20; however, THIP preferentially acts at receptors of the tonic type 42. Electrophysiological studies have shown that THIP exhibits superagonist activity at α4β3δ containing receptors 2, 10 attributed to its efficacy at tonic receptors (Mortensen et al, 2010). Our current work shows that THIP, unlike muscimol and ZAPA, produces analgesia in the tail flick test which is unaffected by KCC2 inhibition. This suggests that reduced Cl− extrusion capacity does not modulate THIP’s analgesic activity. In agreement with our results, others have demonstrated the antinociceptive effects of THIP in preclinical models 8 where KCC2 expression is at least transiently decreased 44. We therefore propose that selective activation of tonic GABAA receptors in the setting of decreased Cl− extrusion capacity may represent a viable approach for pharmacological intervention at the spinal level in chronic pain states. Further electrophysiological work will be needed to test this hypothesis in more detail.
Ganaxolone is a high affinity synthetic neurosteriod with positive allosteric modulatory effects at the GABAA receptor complex. It has been shown to have anticonvulsive effects in preclinical models of seizure 55, 59. Ganaxolone potentiates GABAA-evoked Cl− currents with similar efficacy and potency as allopregnanolone at recombinant human α1β2γ2 and α2β2γ2 GABAA receptors 11. Classical benzodiazepines, such as midazolam, target GABAA receptors composed of α1, α2, α3 or α5 subunits together with β and γ2 subunits. Benzodiazepines potentiate GABAA channel opening by increasing agonist affinity 36. Benzodiazepines are known to elicit spinal analgesia and this effect is maintained in neuropathic and inflammatory pain models. It has recently been shown that midazolam enhances a tonic GABAA current in substantia gelatinosa neurons 38 indicating that spinal, tonic GABAA receptors are benzodiazepine sensitive. Here we show that midazolam maintains analgesic efficacy in the face of pharmacological perturbation of Cl− extrusion capacity whereas ganaxolone efficacy is strongly reduced. Moreover, in the SNI model, midazolam effectively reverses mechanical allodynia while ganaxolone is without effect at doses that are efficacious in the tail flick test without Cl− extrusion capacity manipulation. These findings, combined with data from GABAA agonists, suggest that ganaxolone (and other neurosteroids) may lack effects on presynaptic and/or tonic GABAA receptors in the spinal dorsal horn.
A major unresolved question from these studies is whether the primary site of action of GABAergic agonists and allosteric modulators employed here, in the presence and absence of Cl− extrusion capacity blockade, is pre- or post-synaptic. THIP and midazolam maintained analgesic efficacy even in the presence of Cl− extrusion capacity inhibition and midazolam reversed neuropathic allodynia in the SNI model. Recent findings concerning the nature of spinal analgesia induced by benzodiazepines suggest that this action is mediated primarily by α2-containing GABAA subunits 32 and that these receptors reside on presynaptic terminals of primary afferents in the spinal dorsal horn 62. Interestingly, THIP also has a strong effect on primary afferents and stimulates primary afferent depolarization 16, 50. Because primary afferents are devoid of KCC2 expression 53, it is tempting to speculate that the insensitivity of benzodiazepine- and THIP-induced analgesia to Cl− extrusion capacity inhibition is due to an action of these compounds on GABAA receptors expressed by primary afferents. If this is the case, analgesia mediated by these compounds is unlikely to be influenced by NKCC1 blockade because furosemide 47, which also inhibits NKCC1, also did not influence THIP-induced analgesia. As discussed above, THIP and benzodiazepines also have effects on postsynaptic tonic receptors in the spinal dorsal horn, hence, electrophysiological studies will be required to clearly define pre- vs. post-synaptic activities of these compounds in the presence of Cl− extrusion capacity blockade.
There are several limitations to the present study that we wish to acknowledge. First, we have utilized DIOA and furosemide to influence Cl− extrusion capacity in the spinal cord. While these compounds inhibit KCC2, they also have effects on other co-transporters: DIOA inhibits all KCCs and furosemide inhibits NKCC1 and 2 at least as potently as it influences KCCs 47. Second, we have previously observed dose-limiting effects of spinal benzodiazepines in preclinical neuropathic pain models as demonstrated by inverted U-shaped anti-allodynic dose response effects 5. Similar results were obtained in the present studies at high doses suggesting that Cl− extrusion capacity is insufficient to maintain GABAA mediated analgesia under conditions of intense receptor activation, a suggestion supported by recent modeling studies 18. Although doses of compounds higher than those shown in this study caused motor impairment, again, inverted U-shaped effects in the tail-flick test were observed at doses shown here strongly suggesting that analgesic effects were not due to motor impairment. Finally, although drugs were given intrathecally, we cannot completely rule out an effect on higher brain centers; however, GABAA agonist injection into the brainstem causes a decrease, not increase, in tail flick latency suggesting that the observed effects are largely due to a spinal action 24.
In conclusion, the present findings provide direct evidence for differential modulation of spinal GABAergic analgesia when Cl− extrusion capacity is reduced via KCC2 blockade. Our findings indicate that while potency remains unchanged, muscimol, ZAPA and ganaxolone demonstrate marked declines in analgesic efficacy. On the other hand, THIP and midazolam retain full efficacy when KCC2 is blocked. Because KCC2 expression is decreased in the spinal dorsal horn in preclinical neuropathic pain models 13, 39, 52, 54, these results have potential implications for the generation and clinical testing of GABAA agonists and allosteric modulators for the treatment of chronic pain disorders.
Acknowledgments
This work was supported by funds from The Rita Allen Foundation (TJP), NIH grant NS065926 (TJP), The Academy of Finland (KK), The Sigrid Jusélius Foundation (KK) and The Scan | Design Foundation BY INGER & JENNS BRUUN (TJP and KK). TJP is a Rita Allen Foundation Scholar in Pain.
Footnotes
Disclosures:
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aanonsen LM, Wilcox GL. Muscimol, gamma-aminobutyric acidA receptors and excitatory amino acids in the mouse spinal cord. J Pharmacol Exp Ther. 1989;248:1034–8. [PubMed] [Google Scholar]
- 2.Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–9. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- 3.Allan RD, Dickenson HW, Hiern BP, Johnston GA, Kazlauskas R. Isothiouronium compounds as gamma-aminobutyric acid agonists. Br J Pharmacol. 1986;88:379–87. doi: 10.1111/j.1476-5381.1986.tb10214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anseloni VC, Gold MS. Inflammation-induced shift in the valence of spinal GABA-A receptor-mediated modulation of nociception in the adult rat. J Pain. 2008;9:732–8. doi: 10.1016/j.jpain.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asiedu M, Ossipov MH, Kaila K, Price TJ. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain. 2010;148:302–8. doi: 10.1016/j.pain.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahar M, Cohen ML, Grinshpon Y, Chanimov M. Spinal anaesthesia with midazolam in the rat. Can J Anaesth. 1997;44:208–15. doi: 10.1007/BF03013011. [DOI] [PubMed] [Google Scholar]
- 7.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–38. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Bonin RP, Labrakakis C, Eng DG, Whissell PD, Koninck YD, Orser BA. Pharmacological enhancement of delta-subunit-containing GABA(A) receptors that generate a tonic inhibitory conductance in spinal neurons attenuates acute nociception in mice. Pain. 2011;152:1317–26. doi: 10.1016/j.pain.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Bourquin AF, Suveges M, Pertin M, Gilliard N, Sardy S, Davison AC, Spahn DR, Decosterd I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122(14):e1. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–74. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3alpha-hydroxy-3beta-methyl-5alpha-pregnan-20-one), a selective, high-affinity, steroid modulator of the gamma-aminobutyric acid(A) receptor. J Pharmacol Exp Ther. 1997;280:1284–95. [PubMed] [Google Scholar]
- 12.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 13.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–42. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 14.Curtis DR, Lodge D, McLennan H. The excitation and depression of spinal neurones by ibotenic acid. J Physiol. 1979;291:19–28. doi: 10.1113/jphysiol.1979.sp012796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharmacol. 2007;7:93–9. doi: 10.1016/j.coph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Desarmenien M, Feltz P, Occhipinti G, Santangelo F, Schlichter R. Coexistence of GABAA and GABAB receptors on A delta and C primary afferents. British journal of pharmacology. 1984;81:327–33. doi: 10.1111/j.1476-5381.1984.tb10082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirig DM, Yaksh TL. Intrathecal baclofen and muscimol, but not midazolam, are antinociceptive using the rat-formalin model. J Pharmacol Exp Ther. 1995;275:219–27. [PubMed] [Google Scholar]
- 18.Doyon N, Prescott SA, Castonguay A, Godin AG, Kroger H, De Koninck Y. Efficacy of synaptic inhibition depends on multiple, dynamically interacting mechanisms implicated in chloride homeostasis. PLoS Comput Biol. 2011;7:e1002149. doi: 10.1371/journal.pcbi.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 20.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 21.Garay RP, Nazaret C, Hannaert PA, Cragoe EJ., Jr Demonstration of a [K+,Cl−]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl−]-cotransport system. Mol Pharmacol. 1988;33:696–701. [PubMed] [Google Scholar]
- 22.Goodchild CS, Serrao JM. Intrathecal midazolam in the rat: evidence for spinally-mediated analgesia. Br J Anaesth. 1987;59:1563–70. doi: 10.1093/bja/59.12.1563. [DOI] [PubMed] [Google Scholar]
- 23.Hammond DL, Drower EJ. Effects of intrathecally administered THIP, baclofen and muscimol on nociceptive threshold. Eur J Pharmacol. 1984;103:121–5. doi: 10.1016/0014-2999(84)90197-3. [DOI] [PubMed] [Google Scholar]
- 24.Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: role in nociceptive modulation in the lightly anesthetized rat. Pain. 1991;47:105–13. doi: 10.1016/0304-3959(91)90017-R. [DOI] [PubMed] [Google Scholar]
- 25.Hill RC, Maurer R, Buescher HH, Roemer D. Analgesic properties of the GABA-mimetic THIP. Eur J Pharmacol. 1981;69:221–4. doi: 10.1016/0014-2999(81)90419-2. [DOI] [PubMed] [Google Scholar]
- 26.Ho KM, Ismail H. Use of intrathecal midazolam to improve perioperative analgesia: a meta-analysis. Anaesth Intensive Care. 2008;36:365–73. doi: 10.1177/0310057X0803600307. [DOI] [PubMed] [Google Scholar]
- 27.Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–73. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 29.Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. 1963;13:502–7. [PubMed] [Google Scholar]
- 30.Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 31.Keller AF, Beggs S, Salter MW, De Koninck Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol Pain. 2007;3:27. doi: 10.1186/1744-8069-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Mohler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–4. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- 33.Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–8. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Krogsgaard-Larsen P, Schultz B, Mikkelsen H, Aaes-Jorgensen T, Bogeso KP. THIP, isoguvacine, isoguvacine oxide, and related GABA agonists. Adv Biochem Psychopharmacol. 1981;29:69–76. [PubMed] [Google Scholar]
- 35.Krogsgaard-Larsen P, Frolund B, Liljefors T, Ebert B. GABA(A) agonists and partial agonists: THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem Pharmacol. 2004;68:1573–80. doi: 10.1016/j.bcp.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie AM, Twyman RE. Direct evidence for diazepam modulation of GABAA receptor microscopic affinity. Neuropharmacology. 1996;35:1383–92. doi: 10.1016/s0028-3908(96)00077-9. [DOI] [PubMed] [Google Scholar]
- 37.Liebman JM, Pastor G. Antinociceptive effects of baclofen and muscimol upon intraventricular administration. Eur J Pharmacol. 1980;61:225–30. doi: 10.1016/0014-2999(80)90124-7. [DOI] [PubMed] [Google Scholar]
- 38.Maeda A, Katafuchi T, Oba Y, Shiokawa H, Yoshimura M. Enhancement of GABAergic tonic currents by midazolam and noradrenaline in rat substantia gelatinosa neurons in vitro. Anesthesiology. 2010;113:429–37. doi: 10.1097/ALN.0b013e3181e19bd4. [DOI] [PubMed] [Google Scholar]
- 39.Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137:532–9. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain research. 2000;861:105–16. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- 41.Morales-Aza BM, Chillingworth NL, Payne JA, Donaldson LF. Inflammation alters cation chloride cotransporter expression in sensory neurons. Neurobiol Dis. 2004;17:62–9. doi: 10.1016/j.nbd.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol. 588:1251–68. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadeson R, Guo Z, Porter V, Gent JP, Goodchild CS. gamma-Aminobutyric acidA receptors and spinally mediated antinociception in rats. J Pharmacol Exp Ther. 1996;278:620–6. [PubMed] [Google Scholar]
- 44.Nomura H, Sakai A, Nagano M, Umino M, Suzuki H. Expression changes of cation chloride cotransporters in the rat spinal cord following intraplantar formalin. Neurosci Res. 2006;56:435–40. doi: 10.1016/j.neures.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Palma E, Amici M, Sobrero F, Spinelli G, Di Angelantonio S, Ragozzino D, Mascia A, Scoppetta C, Esposito V, Miledi R, Eusebi F. Anomalous levels of Cl− transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc Natl Acad Sci U S A. 2006;103:8465–8. doi: 10.1073/pnas.0602979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papp E, Rivera C, Kaila K, Freund TF. Relationship between neuronal vulnerability and potassium-chloride cotransporter 2 immunoreactivity in hippocampus following transient forebrain ischemia. Neuroscience. 2008;154:677–89. doi: 10.1016/j.neuroscience.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 47.Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. The American journal of physiology. 1997;273:C1516–25. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 48.Payne JA. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol. 1997;273:C1516–25. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 49.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 50.Polc P. Effects of GABA-mimetic agents on the cat spinal cord. Prog Neuropsychopharmacol. 1979;3:345–52. doi: 10.1016/0364-7722(79)90045-6. [DOI] [PubMed] [Google Scholar]
- 51.Prescott SA, Sejnowski TJ, De Koninck Y. Reduction of anion reversal potential subverts the inhibitory control of firing rate in spinal lamina I neurons: towards a biophysical basis for neuropathic pain. Mol Pain. 2006;2:32. doi: 10.1186/1744-8069-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price TJ, Cervero F, de Koninck Y. Role of cation-chloride-cotransporters (CCC) in pain and hyperalgesia. Curr Top Med Chem. 2005;5:547–55. doi: 10.2174/1568026054367629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price TJ, Hargreaves KM, Cervero F. Protein expression and mRNA cellular distribution of the NKCC1 cotransporter in the dorsal root and trigeminal ganglia of the rat. Brain research. 2006;1112:146–58. doi: 10.1016/j.brainres.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60:149–70. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 2010;89:254–60. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–5. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 57.Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol. 2002;159:747–52. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serrao JM, Marks RL, Morley SJ, Goodchild CS. Intrathecal midazolam for the treatment of chronic mechanical low back pain: a controlled comparison with epidural steroid in a pilot study. Pain. 1992;48:5–12. doi: 10.1016/0304-3959(92)90125-U. [DOI] [PubMed] [Google Scholar]
- 59.Snead OC., 3rd Ganaxolone, a selective, high-affinity steroid modulator of the gamma-aminobutyric acid-A receptor, exacerbates seizures in animal models of absence. Ann Neurol. 1998;44:688–91. doi: 10.1002/ana.410440417. [DOI] [PubMed] [Google Scholar]
- 60.Tornberg J, Voikar V, Savilahti H, Rauvala H, Airaksinen MS. Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur J Neurosci. 2005;21:1327–37. doi: 10.1111/j.1460-9568.2005.03959.x. [DOI] [PubMed] [Google Scholar]
- 61.Toth Z, Hollrigel GS, Gorcs T, Soltesz I. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci. 1997;17:8106–17. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Witschi R, Punnakkal P, Paul J, Walczak JS, Cervero F, Fritschy JM, Kuner R, Keist R, Rudolph U, Zeilhofer HU. Presynaptic alpha2-GABAA receptors in primary afferent depolarization and spinal pain control. J Neurosci. 2011;31:8134–42. doi: 10.1523/JNEUROSCI.6328-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanez A, Sabbe MB, Stevens CW, Yaksh TL. Interaction of midazolam and morphine in the spinal cord of the rat. Neuropharmacology. 1990;29:359–64. doi: 10.1016/0028-3908(90)90094-8. [DOI] [PubMed] [Google Scholar]
- 64.Zhang W, Liu LY, Xu TL. Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience. 2008;152:502–10. doi: 10.1016/j.neuroscience.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 65.Zieglgansberger W, Sutor B. Responses of substantia gelatinosa neurons to putative neurotransmitters in an in vitro preparation of the adult rat spinal cord. Brain Res. 1983;279:316–20. doi: 10.1016/0006-8993(83)90201-9. [DOI] [PubMed] [Google Scholar]