Abstract
Rats emit ultrasonic vocalizations (USVs) at ~22 kHz and ~50 kHz, respectively, during negative and positive affective states. Among rats raised in a naturalistic social context, 22-kHz USVs serve as "alarm cries" that can elicit freezing behavior. By contrast, several studies show that naive laboratory rats do not freeze in response to alarm cries. An obvious and consistent interpretation of these facts is that USV-elicited freezing depends on a type of social learning that ordinarily does not occur in the laboratory. However, the present study explored an alternative and explicitly non-social learning mechanism. Animals in the experimental group received multiple footshocks that elicited 22-kHz USVs. Animals in the control group were exposed to the same chamber but did not receive footshocks and, therefore, did not vocalize. When subsequently tested in a novel context, experimental animals froze in response to a novel 22-kHz USV but were unresponsive to a novel 50-kHz USV. Vocalizing during the aversive experience was predictive of subsequent freezing to the 22-kHz USV. As expected from previous studies, control animals failed to freeze to either USV. We propose that the experimental animals learned to associate their own 22-kHz USVs with an internal fear state and selectively generalized this "autoconditioning" to a novel 22-kHz USV. This non-social form of learning seems sufficiently rapid, reliable, and stimulus-specific to be ethologically adaptive.
Rat ultrasonic vocalizations (USVs) are conspecific social signals. USVs in the 22-kHz range are emitted during negative situations such as predatory encounters, defeat, or fear conditioning [1, 2]. Sometimes called “alarm cries” [3], 22-kHz USVs are produced in conjunction with freezing behavior [1, 4, 5], a conventional index of fear in rats. By contrast, 50-kHz USVs are generated during positive situations, including mating, play, and reward anticipation [1, 2]. Dubbed “rat laughter” by some [6], 50-kHz USVs are usually accompanied by increased locomotor activity [7]. There is general agreement that 22-kHz USVs and 50-kHz USVs are reliable markers, respectively, of negative and positive affective states [1, 8].
Whereas the capacity to emit USVs seems innate, reactivity to these social signals evidently requires experience. Naïve Sprague-Dawley rats do not freeze in response to pre-recorded 22-kHz USVs [8–10]. An excellent review of the somewhat conflicting literature concluded that 22-kHz USVs are not innately recognized as alarm cries [2], prompting the question, what learning experience causes rats to fear alarm cries? One interpretation of the preceding facts is that reactivity to alarm cries requires a type of social learning that does not ordinarily occur in the laboratory.
The present study explored an alternative mechanism that does not entail social learning. The central tenet is that the learning results from "autoconditioning" [11], a hypothetical mechanism in which a rat hears its own 22-kHz USVs, which become associated with a concomitant state of fear caused by aversive stimulation. To be ethologically-viable, a theory of autoconditioning minimally requires three additional assumptions.
First, the conditioning must generalize to 22-kHz USVs produced by other conspecifics. Although the spectrotemporal structures of these USVs vary considerably [3, 10, 12], all of them are discontinuous and have a root frequency near 22 kHz [1]. Second, this stimulus generalization must not extend to 50-kHz USVs. Such an extension would presumably be maladaptive.
Third, the essential conditioning must occur through gross temporal contiguity, analogous to contextual fear conditioning. The required contiguity is between vocalization and an internal fear state, as measured by freezing. Unlike typical cued fear conditioning, where the conditional stimulus (CS) predicts the time of occurrence of the unconditional stimulus, autoconditioning does not require a precisely-predictive temporal relationship. Autoconditioning can occur if vocalization and fear co-occur or co-vary across some time interval.
Subjects were adult male Sprague-Dawley rats (N = 48; 250–350 g; Charles-River). They were individually housed on a 12 h light/dark cycle, had ad libitum access to food and water, and were handled for 5 d before experimentation. All procedures were in strict compliance with Yale University’s Institutional Animal Care and Use Committee guidelines.
Two chambers were used for conditioning and testing (dimensions of 25.4 cm wide × 29.4 cm deep × 32.0 cm high; Coulbourn Instruments). Both chambers were housed within sound-attenuating enclosures located in separate rooms. Chamber A, which had a stainless-steel grid floor, was used for administering footshocks and testing context conditioning. Footshocks were delivered to the grid by a shock-generator and grid scrambler (MED Associates). During the experiment, both Chamber A and the experimental room were illuminated. Before each session, the underlying tray was sprayed with a 30% vinegar/water solution. Chamber B, which had a linoleum floor, served as a context shift for presenting ultrasonic stimuli. Before each session, the underlying tray was sprayed with Febreeze® and the chamber and experimental room were darkened.
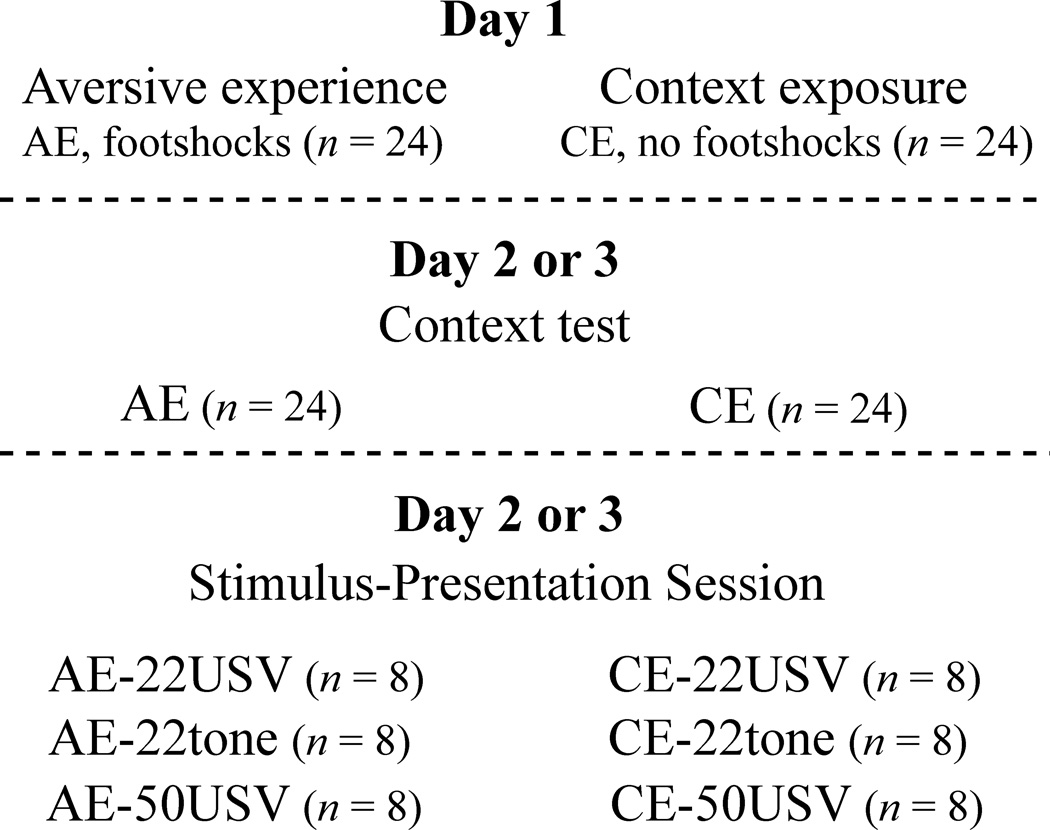
Behavioral procedures took place over three days and involved six groups of animals (Fig. 1). On day 1, the “aversive experience” group (AE; n = 24) was exposed to a 180 s baseline period followed by 5 unsignaled footshocks (1 mA, 0.5 s) with an inter-trial interval of 180±21 s. The “context exposure” group (CE; n = 24) was exposed to Chamber A for the same amount of time under identical conditions except that they did not receive footshocks. Over the next 2 days, both groups were re-exposed (in counter-balanced order) to Chamber A and presented with an ultrasonic stimulus in Chamber B. The context test consisted of exposure to Chamber A for 8 min. The stimulus-presentation session in Chamber B consisted of a 120 s baseline period followed by presentation of a 22-kHz USV, a 50-kHz USV, or a 22-kHz tone for 6 min. Thus, the AE group was subdivided into 3 groups (abbreviated as AE-22USV, AE-22tone, and AE-50USV) and the CE group was subdivided into 3 groups (abbreviated as CE-22USV, CE-22tone, and CE-50USV).
Figure 1. Experimental design.
On day 1, subjects either underwent an “aversive experience” (5 unsignaled footshocks) or “context exposure” (no footshocks). On days 2 and 3, subjects were tested for context conditioning and reactivity to ultrasonic stimuli in counterbalanced order. The context test consisted of re-exposing subjects to the conditioning chamber. During the stimulus-presentation session, subjects were presented one of three stimuli in a novel chamber. The stimuli were a 22-kHz USV, a 22-kHz tone, or a 50-kHz USV. Freezing and vocalizations were measured throughout all stages.
The 22-kHz USV was recorded from a Sprague-Dawley rat that was given multiple footshocks (1–1.2 mA, 1 s). The USV had a 19 kHz root frequency and 7.92 s bout duration. The 22-kHz tone was matched to the 22-kHz USV in terms of root frequency and duration. The 50-kHz USV, recorded during rough-and-tumble play, had a 53 kHz root frequency and 6.72 s bout duration. All stimuli were matched on loudness (65 dB SPL; Ultraprobe 9000; UE Systems). An Enhanced Real-Time Processor and an Electrostatic Speaker Driver (Tucker Davis Technologies) were used to present the stimuli free-field. Frequency spectrograms and amplitude plots of these stimuli are published [8, 10, 12, 13].
Freezing and emission of 22-kHz USVs were concurrently measured throughout the experiment. Freezing was monitored via an infrared-CCD camera (CB-21; Circuit Specialists) mounted to the chamber ceilings. The USVs were monitored with a Mini-3 bat detector (NHBS) set to the 20 kHz frequency. The audio-visual data were recorded for offline analysis (WinTV; Hauppauge Computer Works).
Freezing behavior, defined as immobilization lasting ≥3 s, was measured using video-analysis software [13]. The result was converted to a percentage of time spent freezing, termed "percent freezing". The amount of vocalization was measured with a stopwatch by a researcher who was blind to the experimental conditions. The time spent vocalizing was measured in 1-min bins and converted to a percentage of time spent vocalizing, termed "percent vocalization". Significant differences in freezing and vocalization were evaluated using t-tests or analysis of variance (ANOVA, F-tests). Significant F-tests were followed by Fisher’s Least-Significant-Difference post hoc tests (SPSS 19.0). Effect sizes were calculated using Cohen’s d [14].
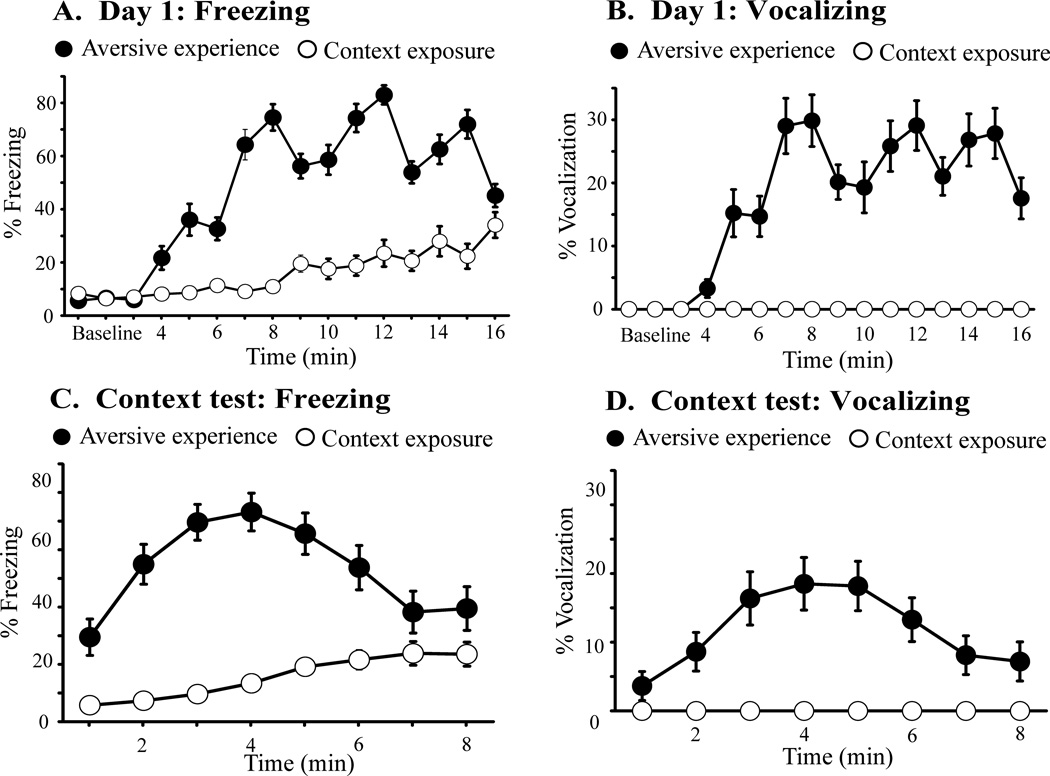
During day 1, the mean percent freezing was low (<10%) and comparable in the AE and CE groups during the baseline period (Fig. 2A). Following the first footshock at 4 min, freezing increased in the AE group and plateaued at ~75% (Fig. 2A, filled circles). Freezing remained low in the CE group, but increased slightly after min 8, possibly due to habituation (Fig. 2A, open circles). ANOVA revealed a significant effect of group (F1,46 = 380, p < 0.0001; d = 2.85) and time (F15 = 46.5, p < 0.0001) and a significant time × group interaction (F15 = 23.1, p < 0.0001).
Figure 2. Freezing and vocalization during day 1 and during the context test.
(A and B) Freezing and vocalization over time on day 1. The aversive-experience (AE) group froze (part A) and vocalized (part B) more than the context-exposure (CE) group. The first three minutes served as the baseline period. The AE group then received 5 shocks over the next 13 min. The CE group remained in the chamber for the same duration but did not receive shocks. (C and D) Freezing and vocalization over time during the context test. The AE group froze (part C) and vocalized (part D) more than the CE group.
None of the 24 CE animals vocalized during day 1 (Fig. 2B, open circles). By contrast, 22 of the 24 AE animals vocalized, including all eight AE22-USV animals. The mean percent vocalization in the AE group increased at 4 min and plateaued at ~30% (Fig. 2B, filled circles). The time course of vocalization (Fig. 2B) paralleled the time course of freezing (Fig. 2A). ANOVA revealed a significant effect of effect of group (F1,42 = 59, p < 0.0001; d = 2.37) and time on vocalization (F15 = 17.6, p < 0.0001) and a significant time × group interaction (F15 = 19.2, p < 0.0001).
An independent samples t-test determined whether there were significant differences in freezing during the context test or stimulus-presentation session based on the order (day 2 or 3) of these tests (Fig. 1). There was no order effect during either the context test (t46 = 0.78, p > 0.05) or the stimulus-presentation session (t46 = −0.65, p > 0.05).
During context testing, freezing in the AE group peaked at ~4 min (~75%) and gradually declined over 4 minutes (Fig. 2C, filled circles). Freezing in the CE group gradually increased but remained low (Fig. 2A, open circles). ANOVA revealed a significant effect of group (F1,46 = 161, p < 0.0001; d = 2.00) and time on freezing (F7 = 8.53, p < 0.0001) and a significant time × group interaction (F7 = 10.4, p < 0.0001). None of the CE subjects vocalized during the context text (Fig. 2D, open circles). Vocalization in the AE group peaked at ~4 min (~20%) and declined over the last 4 min (Fig. 2D, filled circles). ANOVA revealed a significant effect of group (F1,42 = 23.1, p < 0.0001; d = 1.49) and time (F7 = 5.29, p < 0.0001) and a significant time × group interaction (F7 = 5.29, p < 0.0001). The overall time-course of vocalization (Fig. 2D) closely paralleled the time-course of freezing (Fig. 2C), but the onset of freezing always preceded the onset of vocalization in each animal.
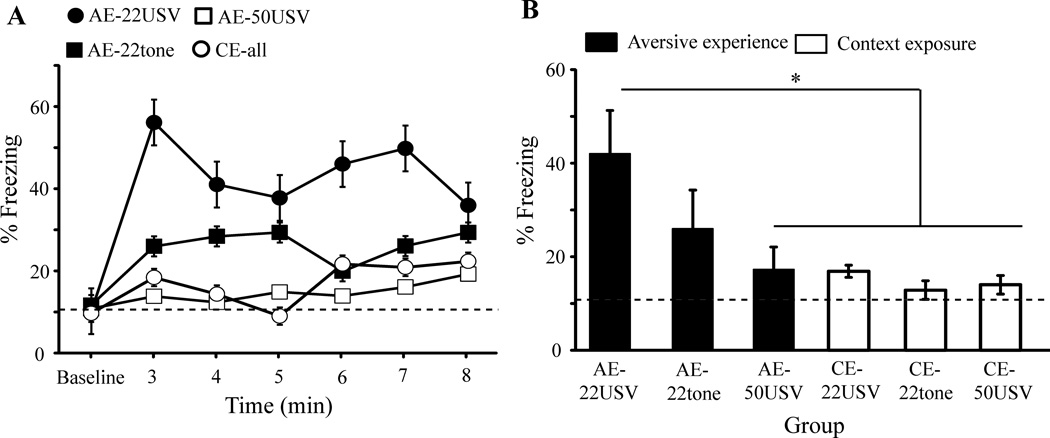
During the baseline period of the stimulus-presentation session, overall freezing was low (10.2±0.4%; Fig. 3A, dashed horizontal line) and there were no significant group differences (F5,42 = 1.94, p > 0.05). During the stimulus presentation, ANOVA revealed a significant effect of group (F5,42 = 3.95, p < 0.005) and time (F8 = 7.32, p < 0.0001) and a significant time × group interaction (F40 = 1.46, p < 0.05). The auditory stimulus elicited a significant increase (paired t-test) in the mean percent freezing, relative to the baseline level, in the AE-22USV group (t14 = 3.46, p < 0.01) and the AE-22tone group (t14 = 2.18, p < 0.05), but not the AE-50USV group (t14 = 1.47, p > 0.05). There was no significant increase in freezing in the CE-22USV (t14= 1.9, p > 0.05), CE-22tone (t14 = 0.55, p > 0.05), or CE-50USV (t14 = 0.96, p > 0.05) group. For graphical purposes only, the three CE groups are pooled in Figure 3A (CE-all).
Figure 3. Stimulus-elicited freezing in a novel context.
(A) Mean percent freezing as a function of time during the stimulus-presentation session. The three aversive experience (AE) groups are plotted separately, whereas the three context-exposure (CE) groups are combined. (B) Mean percent freezing during the stimulus presentation for the three AE and three CE groups. The AE-22USV group froze significantly more than the AE-50USV group, but not the AE-22tone group. The three CE groups failed to freeze to any stimuli. (A and B) The mean level of baseline freezing is indicated by the horizontal dashed lines. *, p < 0.005
ANOVA revealed significant group differences in freezing during the stimulus presentation (F5,42 = 3.95, p < 0.005). Post hoc tests showed that group AE-22USV froze significantly more than the AE-50USV, CE-22USV, CE-22tone, and CE-50USV groups (p < 0.005 and d ≥ 1.0 for all comparisons; Fig. 3B). The mean percent freezing in group AE-22tone during the stimulus presentation was intermediate between group AE-22USV and all other groups. Freezing in group AE-22tone did not differ significantly from any other group (p > 0.05). Averaging across all CE animals, freezing was generally low (~15%). There was no significant difference in freezing between the AE-50USV and CE-50USV groups (p > 0.05).
Animals in the CE-22USV, CE-22tone, CE-50USV, and AE-50USV groups did not vocalize during the stimulus-presentation session. The only animals that vocalized were from the AE-22USV (3 of 8) and AE-22tone (2 of 8) groups. In analyzing these two groups, ANOVA showed no significant effect of group (F1,3 = 0.83, p > 0.05) and time (F5 = 0.03, p > 0.05) and no significant time × group interaction (F5 = 1.36, p > 0.05).
The three AE groups did not differ in terms of percent freezing or vocalization on day 1. In terms of freezing, there was no significant effect of group (F2,21 = 1.97, p > 0.05) and no significant time × group interaction (F30 = 1.07, p > 0.05). In terms of vocalization, there was also no effect of group (F2,21 = 1.15, p > 0.05) and no significant time × group interaction (F30 = 0.87, p > 0.05).
Correlations between freezing and vocalizing [4, 5], in each stage of the experiment, were as follows: during day 1, r48 = 0.80; during the context test, r48 = 0.80; and after the baseline period during the stimulus-presentation session, r48 = 0.70 (all ps < 0.0001). Notably, the amount of vocalization on day 1 was strongly correlated with the level of freezing during the stimulus-presentation session (r48 = 0.55, p < 0.0001). This correlation was slightly larger when restricted to the AE-22USV and AE-22tone groups (r16 = 0.59, p < 0.05).
The present study was designed to elucidate how rats learn to freeze to 22-kHz USVs. In agreement with previous reports [8–10], naïve rats did not freeze to a pre-recorded 22-kHz USV (Figs. 3A–B). However, a prior aversive experience caused the animals to freeze robustly in response to the 22-kHz USV (Figs. 3A–B). The aversive experience did not cause freezing to a 50-kHz USV, which would be maladaptive. Since the experimental design eliminated social learning, or any social influences, autoconditioning emerges as the obvious learning mechanism.
After completing this study, another one [11] also found that a prior aversive experience is sufficient to cause freezing to 22-kHz USVs. In pair-housed rats, “sender” rats received 10 tone-shock pairings while “receiver” rats were given either three unsignaled footshocks or remained experimentally-naïve. When the pair was presented the tone in a novel chamber, the sender rats froze and emitted 22-kHz USVs. These conditional responses induced freezing in receiver rats that had undergone an aversive event, but did not influence experimentally-naïve rats. The conditional responses of the sender rats also failed to influence receiver rats that had undergone an aversive event immediately following reversible inactivation of the auditory thalamus. The inactivation presumably prevented animals from perceiving their own vocalizations.
Although the present and previous results can be explained by autoconditioning, it is worth considering in greater detail the one remaining possibility: that the effect of an aversive experience on USV responsiveness reflects sensitization, a non-associative process. The obvious problem with this hypothesis is that sensitization is, by definition, not stimulus-specific. By contrast, the present study demonstrated that an aversive experience causes rats to freeze in response to a 22-kHz USV, but not a 50-kHz USV (Fig. 3B).
However, a more complex version of the sensitization hypothesis deserves further consideration. First, suppose that rats are, indeed, genetically predisposed to fear 22-kHz USVs. Second, imagine that this predisposition cannot be detected by freezing behavior [8–10] because it is a relatively high-threshold behavior. Finally, assume that the aversive experience simply sensitizes preexisting stimulus-response tendencies. Arguably, these three assumptions might account for the present results.
One general reaction is that freezing is the most common and best-understood fear response in rats [15]. More to the point, analysis of other behaviors, such as avoidance, have also failed to detect an innate predisposition to fear alarm cries [2, 16]. Furthermore, this hypothesis predicts that 22-kHz USVs should be especially "salient" as CSs in cued fear-conditioning. In fact, however, USVs are no more salient than tones [10]. Finally, the sensitization hypothesis cannot explain the above-mentioned effects [11] of auditory thalamus inactivation.
Autoconditioning offers a new interpretation of “asymmetrical stimulus generalization” [10], a phenomenon discovered using a differential fear-conditioning paradigm in which 22-kHz and 50-kHz USVs served as CSs. One stimulus (the CS+) predicted the US and the other stimulus (the CS−) predicted no US. When functioning as a CS+, 22-kHz and 50-kHz USVs were equally effective in supporting fear conditioning. When a 22-kHz USV served as the CS+ and a 50-kHz USV served as the CS−, there was little generalization of freezing to the CS−. However, in the reverse case, rats froze substantially to the CS−. Theoretically, this asymmetry could reflect a biological predisposition in generalization [10]. Alternatively, because the subjects likely emitted 22-kHz USVs during fear conditioning, they may have undergone autoconditioning.
We suggest that autoconditioning is sufficiently rapid, reliable, and stimulus-specific to serve an adaptive defensive function in rats. Analogous learning might similarly enable playful pups to associate 50-kHz USVs with a positive affective state, thereby facilitating future sexual interactions [2, 17]. The results invite further investigations into the quantitative principles and neurobiological mechanisms that govern autoconditioning. One novel prediction is that autoconditioning may depend on perirhinal cortical function, since the latter is known to be critical for fear conditioning to USVs [12, 13, 18].
Highlights.
An aversive experience caused rats to freeze upon hearing a conspecific alarm cry.
By contrast, experimentally-naive rats did not freeze in response to this stimulus.
This effect can be explained by "autoconditioning", a non-social form of learning.
Autoconditioning is suitably stimulus-specific to serve an adaptive function.
Acknowledgments
Role of the funding source:
This work was supported by the National Institute of Health (NIH) research grant MH058405 and Yale University. These sponsors did not contribute to the experimental design, the data collection, analysis or interpretation, the writing of the report, or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brudzynski SM. Ultrasonic calls of rats as indicator variables of negative or positive states: acetylcholine-dopamine interaction and acoustic coding. Behav Brain Res. 2007;182:261–273. doi: 10.1016/j.bbr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Wohr M, Schwarting RKW. Activation of limbic system structures by replay of ultrasonic vocalization in rats. In: Brudzynski SM, editor. Handbook of mammalian vocalization. Oxford: Academic Press; 2010. pp. 113–124. [Google Scholar]
- 3.Litvin Y, Blanchard DC, Blanchard RJ. Rat 22kHz ultrasonic vocalizations as alarm cries. Behav Brain Res. 2007;182:166–172. doi: 10.1016/j.bbr.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 4.Lee HJ, Choi JS, Brown TH, Kim JJ. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci. 2001;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi JS, Brown TH. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. J Neurosci. 2003;23:8713–8721. doi: 10.1523/JNEUROSCI.23-25-08713.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res. 2000;115:25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- 7.Fu XW, Brudzynski SM. High-frequency ultrasonic vocalization induced by intracerebral glutamate in rats. Pharmacol Biochem Behav. 1994;49:835–841. doi: 10.1016/0091-3057(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 8.Parsana AJ, Li N, Brown TH. Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behav Brain Res. 2012;226:77–86. doi: 10.1016/j.bbr.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endres T, Widmann K, Fendt M. Are rats predisposed to learn 22 kHz calls as danger-predicting signals? Behav Brain Res. 2007;185:69–75. doi: 10.1016/j.bbr.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Bang S, Allen TA, Jones LK, Boguszewski P, Brown TH. Asymmetrical stimulus generalization following differential fear conditioning. Neurobiol Learn Mem. 2008;90:200–216. doi: 10.1016/j.nlm.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang S, Brown TH. Perirhinal cortex supports acquired fear of auditory objects. Neurobiol Learn Mem. 2009;92:53–62. doi: 10.1016/j.nlm.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kholodar-Smith DB, Allen TA, Brown TH. Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behav Neurosci. 2008;122:1178–1185. doi: 10.1037/a0012902. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. Statistical power analysis for the behavioral sciences. 1988 [Google Scholar]
- 15.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 16.Sadananda M, Wohr M, Schwarting RKW. Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett. 2008;435:17–23. doi: 10.1016/j.neulet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 18.Lindquist DH, Jarrard LE, Brown TH. Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals. J Neurosci. 2004;24:3610–3617. doi: 10.1523/JNEUROSCI.4839-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]