Abstract
Objective
To determine the association between glaucomatous visual field (VF) loss and the amount of physical activity and walking in normal life.
Design
Prospective observational study.
Participants
Glaucoma suspects without significant VF or acuity loss (controls) and glaucoma subjects with bilateral VF loss between age 60 and 80.
Methods
Participants wore an accelerometer over 7 days of normal activity.
Main Outcome Measures
Daily minutes of moderate or vigorous physical activity (MVPA) was the primary measure. Steps/day was a secondary measure.
Results
Fifty-eight controls and 83 glaucoma subjects provided sufficient study days for analysis. Control and glaucoma subjects were similar in age, race, gender, employment, cognitive ability and comorbid illness (p>0.1 for all). Better-eye VF mean deviation (MD) averaged 0.0 dB in controls and −11.1 dB in glaucoma subjects.
The median control subject engaged in 16.1 minutes of MVPA daily and walked 5,891 steps/day, as compared to 12.9 minutes of MVPA/day (p=0.25) and 5,004 steps/day (p=0.05) for the median glaucoma subject. In multivariable models, glaucoma was associated with 21% less MVPA (95% CI = -53 - +32%; p=0.37) and 12% fewer steps/day (95% Confidence interval [CI] = -22 to +9%; p=0.21) than controls, though differences were not statistically significant. There was a significant dose-response relating VF loss to decreased activity with each 5 dB decrement in the better-eye VF associated with 17% less MVPA (95% CI = -30 to -2%; p=0.03) and 10% fewer steps/day (95% CI = -16 to -5%; p=0.001) . Glaucoma subjects in the most severe tertile of VF damage (better-eye VF MD worse than -13.5 dB) engaged in 66% less MVPA than controls (95% CI = -82 to -37%, p=0.001) and took 31% fewer steps/day (95% CI = -44 to -15%, p=0.001). Other significant predictors of decreased physical activity included older age, comorbid illness, depressive symptoms, and higher body-mass index.
Conclusions
Overall, no significant difference in physical activity was found between individuals with and without glaucoma, though substantial reductions in physical activity and walking were noted with greater levels of VF loss. Further study is needed to better characterize the relationship between glaucoma and physical activity.
INTRODUCTION
Glaucoma affects 2-4% of adults over the age of 40, and will become increasingly prevalent as the population ages.1 Individuals who acquire glaucoma later in life are unlikely to go blind during their lifetimes,2 but may still suffer substantial disability due to their disease.3 Understanding when and how glaucoma leads to visual disability in daily life is crucial for deciding when, and how aggressively, treatment should be administered. Better understanding of the specific functional deficits attributable to glaucoma would also help in the design of interventions to improve quality of life in individuals with existing visual field (VF) loss.
Mobility is the issue of greatest importance to glaucoma patients,4 and many lines of evidence suggest that glaucoma affects mobility.4,5 Research in controlled environments has indicated that individuals with glaucoma walk slower and with more difficulty and are more likely to bump into objects than age-matched controls.6,7 Glaucoma also affects balance5,8 and increases the risk of falls.9,10 However, whether individuals with glaucoma restrict their physical activity because of these functional limitations is unknown.
The ability to move freely and safely is not only an important determinant of quality of life, but higher levels of activity are associated with better overall health outcomes.11 There is strong scientific evidence linking lower physical activity levels with increased risk of chronic diseases of several types, depression, and all-cause mortality.12-15 Identifying individuals with lower physical activity levels could be an important first step in improving health outcomes.11
Accelerometers provide an important tool for directly measuring physical activity in the normal environment and routine.16 Several studies have demonstrated that questionnaires about physical activity correlate poorly direct measurement of physical activity. Therefore, significant error or bias may be present in the results reported by studies that evaluated physical activity through self-report.17
While there is a growing body of research on directly-measured physical activity levels, the relationship between visual field loss and directly-measured activity has not been studied in glaucoma. Substantial decreases in walking and physical activity were noted with acuity loss not due to refractive error in the National Health and Nutrition Evaluation Survey (NHANES),18 but the impact of VF loss of physical activity was not evaluated. In this report, we use accelerometers to provide the first objective assessment of how VF loss from glaucoma impacts daily physical activity in the normal daily living environment.
METHODS
All study procedures were approved by the Institutional Review Board of Johns Hopkins Medicine. Study participants gave written informed consent and completed the study procedures between July 2009 and June 2011.
Study Subjects
Subjects were recruited from a convenience sample of patients between the ages of 60 and 80 followed at the Glaucoma Clinic of the Wilmer Eye Institute at Johns Hopkins Hospital. Inclusion criteria included the ability to communicate in English and a willingness to wear an accelerometer for one week. Individuals were ineligible for the study if they had any laser procedure in the previous week, any hospitalization or non-ophthalmic surgery in the previous 2 weeks, or any ocular surgery in the previous 2 months. All subjects had VF testing performed within the last year (HFA2, Carl Zeiss Meditec, Inc., Dublin CA).
Two study groups were recruited, glaucoma suspects who served as controls, and those with bilateral VF loss from glaucoma. Controls had a chart diagnosis of glaucoma suspect or ocular hypertension, and were also required to have a presenting Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity of 20/40 or better in both eyes, and right and left eye VFs meeting the following criteria: (1) mean deviation (MD) better than -5 decibels (dB) in both eyes on a SITA standard 24-2 test (including at least one eye with a MD better than -3 dB); and (2) glaucoma hemifield test (GHT) result of “Within Normal Limits”, “Borderline”, or “General Reduction of Sensitivity”. Other GHT results were permitted if the VF test was noted to have “Low Test Reliability” or “Excessively High False Positives”.
Individuals were eligible for recruitment into the glaucoma group if they had a diagnosis of primary open angle glaucoma, primary angle closure glaucoma, pseudoexfoliation glaucoma, or pigment dispersion glaucoma. Recruited glaucoma subjects were also required to have VFs in both eyes with a MD worse than -3 dB and a GHT result of “Outside Normal Limits”, “Generalized Reduction of Sensitivity” or “Borderline”. Most testing was performed over the central 24 degrees using a size III stimulus and the standard Swedish interactive thresholding algorithm (SITA). All remaining VFs were performed using a 10-2 algorithm. For patients with recent 24-2 VFs in both eyes, the better-eye MD was taken from the VF with the higher (less negative) MD. For patients with a recent 24-2 VF in only one eye, and either no recent VF or a 10-2 VF in the second eye, the better-eye MD was taken from the recent 24-2 VF. In patients with only recent 10-2 VFs, the last 24-2 VFs completed for each eye were identified, and the better-eye MD was taken from the 24-2 VF with the higher MD.
Evaluation of Physical Activity
Physical activity was evaluated over 1 week19 of normal activities using an omnidirectional accelerometer (Actical, Respironics Inc). Subjects were instructed to clip the accelerometer to their waistband in front of their hip during all waking hours aside from time spent swimming or bathing. Motion detected by the accelerometer is used to record steps, and is also summarized as counts, which reflect a transformation of the acceleration detected into an arbitrary unit. The physical activity level occurring over each study minute was then classified as sedentary, light, moderate, or vigorous using count values and the cut points defined by Colley et al. based on previous work which compared Actical count values to Metabolic Equivalent of Task (METs) derived from treadmill testing.20 A value of 1,535 counts/minute or more was classified as moderate/vigorous physical activity (MVPA).
Morning phone calls were placed to subjects on each of the 7 study days to maximize compliance with wearing the device. Compliance was also assessed by estimated accelerometer wear time, defined as the time interval between the first and last minutes showing accelerometer counts for the given day. Days with less than 8 hours of estimated wear time were excluded, as it was assumed this indicated that the individual had not adequately worn the accelerometer.21 We also queried subjects directly about their wearing the device during the phone calls, and excluded days for which they admitted not wearing it. Individuals with fewer than 2 valid days were excluded from all analyses except those employing generalized estimating equations. Daily steps and time spent in MVPA were averaged over all valid days.
Measurement of Vision and Covariates
Visual acuity was measured with patients’ habitual distance correction using ETDRS chart. For statistical analysis, acuity was summarized as the negative logarithm of the minimum angle of resolution (logMAR) in the better-seeing eye.22 Patients unable to read letters on the ETDRS chart at 1 meter were assigned logMAR acuities of 1.8 for count fingers and 2.3 for hand motions or worse.23 Contrast sensitivity was measured as the number of letters read correctly on the Pelli-Robson chart under binocular conditions.
Lenticular changes were graded after pupillary dilation of both eyes. Significant lenticular changes were classified as present if nuclear sclerosis was graded as > 2 on the Wilmer Cataract Grading System,24 cortical changes were present in ≥ 4/16th of the retroilluminated pupil,25 or if any posterior subcapsular change was present in the central 3 mm of the posterior capsule. In pseudophakic eyes, lenticular change was classified as present if posterior capsular opacification (PCO) was greater than the mild PCO image shown by Findl et al.26 Significant lenticular changes were defined as present when findings were present in either eye.
Socio-demographic variables including age, race/ethnicity, education, and employment were gathered using standardized forms. Comorbid illness was quantified as the number of medical conditions (out of a list of 15 diseases) subjects reported having being diagnosed with by a physician. Conditions qualifying as a comorbid illness included arthritis, previous hip fracture, back problems, previous heart attack, angina/chest pain, congestive heart failure, peripheral vascular disease, hypertension, diabetes, emphysema, asthma, stroke, Parkinson’s, non-skin cancer, or vertigo/Meniere’s. The presence of depressive symptoms was assessed using the Geriatric Depression Scale, with scores of greater than 6 classified as having depressive symptoms.27 Cognitive ability was evaluated using a truncated version of the Mini Mental State Exam (MMSE) designed to remove vision-dependent items.28
As physical activity might be affected by weather conditions, weather information was obtained from the North East Regional Climate Center at Cornell University, NY. Data was taken for the home location using data from the nearest weather station with full weather and rainfall data. For each subject, weather was summarized as the proportion of rainy study days (0.2 inches of rainfall or greater) and the proportion of cold-weather study days (average daily temperature less than 45°F).
Sample Size Determination
A sample size of 60 subjects in both the glaucoma and control groups was chosen to detect a 25% difference in physical activity across groups, allowing for invalid/insufficient data in up to 10% of subjects. At an interim analysis, a decision was made to oversample the glaucoma group to increase the precision of activity estimates across the spectrum of glaucoma severity. Specifically, a total sample size of 84 was targeted in order to generate an interquartile range of physical activity equal to one-fourth the standard deviation for each tertile of subjects. Secondary analyses were performed using steps as an outcome measure.
Statistical Methods and Programming
Group differences in demographic, health, vision and study period features were analyzed using the Student’s t test for normally-distributed continuous variables, Wilcoxon rank-sum testing for non-normally distributed continuous variables, and chi-squared testing for categorical variables. Predictors of physical activity were also evaluated using univariable and multivariable negative binomial regression models. Negative binomial regression models were chosen as physical activity measures were in the form of over-dispersed count data (steps or minutes of MVPA) that were not normally distributed. Additionally, negative binomial models have been advocated as the best method for analyzing accelerometer data.29 Coefficients from negative binomial models are expressed as rate ratios (RR), reflecting the relative rate of events (i.e. steps or minutes of MVPA) associated with specific model elements. Variables were included in multivariable models if they demonstrated a significant impact (p<0.1) in univariable regression, or if they had been shown to impact physical activity measures in previous research.30 To exclude bias resulting from the exclusion of subjects with insufficient valid study days, confirmatory analyses were run using negative binomial models which assessed each person-day as a separate data point, and which used generalized estimating equations to account for correlation of physical activity measures for the same individual across different study days.
RESULTS
Sixty control subjects and 85 glaucoma subjects were enrolled in the study. Two control and 2 glaucoma subjects had fewer than 2 valid study days and were excluded from all analyses except for those employing generalized estimating equations. Included glaucoma subjects had an average of 6.69 valid days of accelerometer data, as compared to 6.75 valid days for included control subjects (p=0.78). Estimated accelerometer wear time over valid days was 15.8 and 15.6 hours for control and glaucoma subjects respectively (p=0.54).
Individuals with and without glaucoma were similar with regards to age, race, gender, education level and employment status (Table 1). Subjects with and without glaucoma were also similar with regards to measures of health, including number of comorbid illnesses, depressive symptoms, body mass index (BMI) and cognitive ability. In comparison to controls, individuals with glaucoma had greater VF loss (mean-deviation of -11.1 vs. 0.0 dB; p<0.001), lower contrast sensitivity (29.3 vs. 37.7 letters read; p<0.001), and average worse better-eye visual acuity (logMAR acuity of 0.27 vs. 0.08; p<0.001). Lesser group differences were noticed in median visual acuity (0.08 for controls vs. 0.16 for glaucoma, p<0.001). Glaucoma subjects were more likely than controls to have a significant level of cataract or PCO in at least 1 eye (p=0.05).
Table 1.
Characteristicsof subjects by glaucoma status.
| No glaucoma (n=58) | Glaucoma (n=83) | p value | |
|---|---|---|---|
| Vision | |||
| Visual field MD (better eye) | 0.0 | -11.1 | <0.001 |
| Better-eye Acuity, logMAR | 0.08 | 0.27 | <0.001 |
| Contrast sensitivity, letters* | 37.7 | 29.3 | <0.001 |
| Significant cataract or PCO** (%) | 22.2 | 38.6 | 0.05 |
| Demographics | |||
| Age (Years) | 69.3 | 70.3 | 0.86 |
| African-American Race (%) | 20.7 | 33.7 | 0.09 |
| Female Gender (%) | 62.1 | 54.2 | 0.35 |
| Education (years) | 15.3 | 14.7 | 0.17 |
| Employed (%) | 39.7 | 41.0 | 0.88 |
| Health | |||
| Comorbid illnesses (#) | 2.5 | 2.2 | 0.19 |
| Depressive symptoms (%) | 5.2 | 6.0 | 0.83 |
| BMI | 30.3 | 29.4 | 0.07 |
| MMSE-VI score | 20.7 | 20.6 | 0.69 |
logMAR = logarithm of the minimum angle of resolution; MD = mean deviation, PCO = Posterior Capsular Opacification; BMI = body-mass index; MMSE-VI = Mini-mental status examination for the visually impaired
Contrast sensitivity measured binocularly
In one or both eyes.
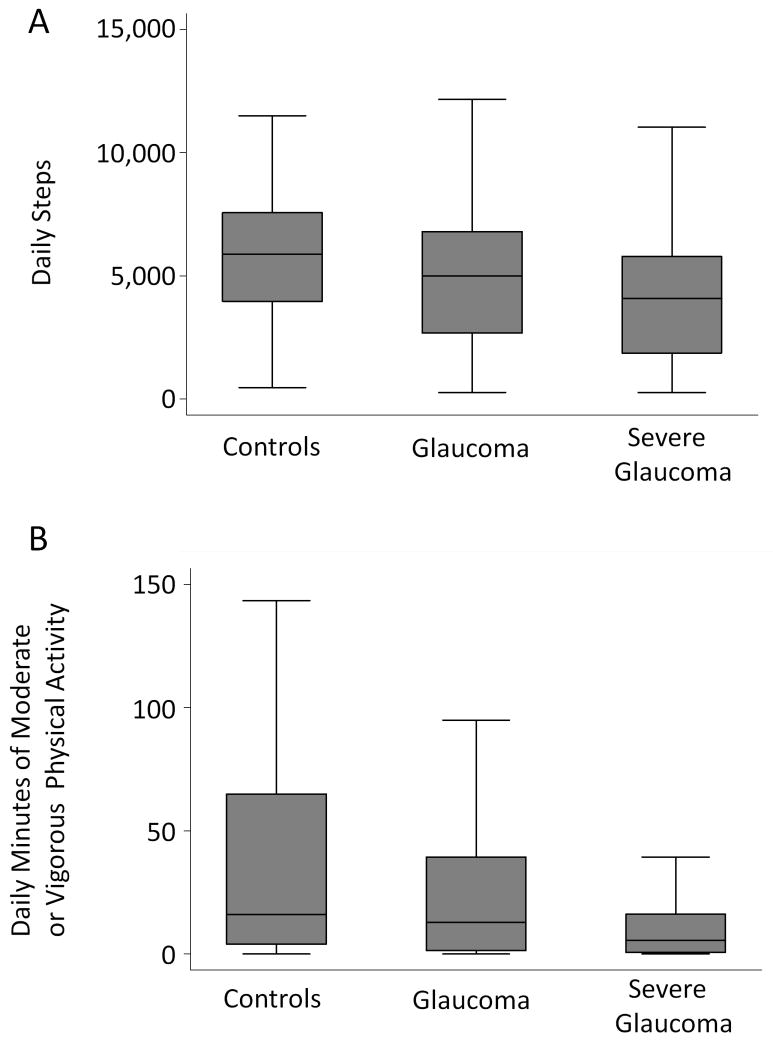
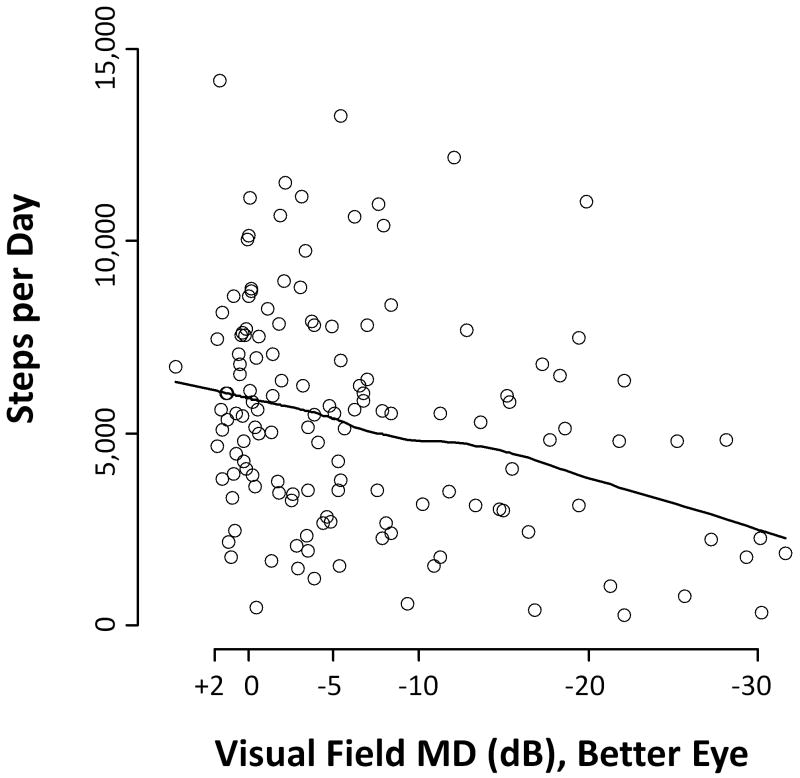
Median time spent in moderate or vigorous physical activity (MVPA) was 16.1 minutes (Interquartile range [IQR] = 4.0 to 64.9 minutes) for control subjects, as compared to 12.9 minutes for all glaucoma subjects (IQR = 1.3 to 39.4 minutes; p=0.25) and 5.6 minutes for subjects with only the most severe glaucoma (IQR = 0.5 to 16.3 minutes/day; p=0.01) (Figure 1). No significant differences were observed in the daily time spent in MVPA in control subjects as compared to subjects in the tertile of least VF damage (better eye MD better than −5.5 dB; median minutes of MVPA = 19.7 minutes; IQR=5.8 to 41.6 minutes; p=0.86) or subjects in the middle tertile of VF damage (better eye MD between −13.5 and −5.5 db; median minutes of MVPA = 23.4 minutes; IQR= 1.1 to 75 minutes; p=0.74). The median control subject walked 5,891 steps/day (Interquartile range [IQR] = 3,956 to 7,560), as compared to 5,004 steps/day (IQR = 2,673 to 6,800; p=0.05) for all glaucoma subjects, and 4,074 steps/day (IQR = 1,881 to 5,797; p=0.001) for subjects with severe glaucoma (defined as those with the lowest one-third of better eye MD scores, corresponding to a better-eye MD worse than -13.5 db) (Figure 1). Measures of physical activity were also observed to decrease with increasing VF damage (Figure 2).
Figure 1.
Daily walking and physical activity by glaucoma status
Severe glaucoma groups includes those with better eye visual field scores in the lowest tertile, corresponding to a better eye visual field mean deviation worse than -13.5 decibels.
Figure 2.
Lowess plot of Daily Step Counts by Extent of Better-Eye Visual Field Loss
MD = Mean deviation; dB = decibels
In univariate negative binomial regression analyses, glaucoma was associated with a non-statistically significant 21% decrease in daily time spent in MVPA (95% CI = -53 to +32%, p=0.37) and a 12% decrement in daily steps (95% CI = -22 to +9%; p=0.21) (Table 2). Amongst all subjects, a 5 dB increment of worsening in the MD of the better-eye VF was associated with a 17% decrease in MVPA (95% CI = -30 to -2%; p=0.03) and a 10% decrease in daily steps (95% CI = -16 to -5%; p=0.001), , while a 5 letter decrement in contrast sensitivity was associated with a 15% decrease in MVPA (95% CI = -27 to 0%, p=0.05 and a 10% decrease in daily steps (95% CI = -15 to -4%, p=0.002). Larger effects on both MVPA (64% fewer minutes/day; 95% CI = -82% to -30%; p=0.003) and walking (34% fewer steps/day; 95% CI = -50 to -13%; p=0.003) were observed in individuals with severe glaucoma. Both physical activity and steps were also observed with decreases in contrast sensitivity (p≤0.05 for both), but were not observed for changes in visual acuity (p>0.1 for both).
Table 2.
Factors Influencing Walking and Physical Activity, Univariate Analysis
| Daily Steps
|
Minutes Mod/Vig Activity
|
||||
|---|---|---|---|---|---|
| Variable | Interval | RR* | p value | RR | p value |
| Vision | |||||
| Glaucoma | Present | 0.88 | 0.21 | 0.79 | 0.37 |
| VF loss MD , bettereye | 5 dB worse | 0.90 | 0.001 | 0.83 | 0.03 |
| VA, better eye | 0.1 logMAR worse | 0.98 | 0.13 | 0.98 | 0.59 |
| Contrast Sensitivity | 5 letters worse | 0.90 | 0.002 | 0.85 | 0.05 |
| Demographics | |||||
| Age | 10 years older | 0.77 | 0.009 | 0.49 | 0.009 |
| Race | African-American | 0.64 | <0.001 | 0.36 | <0.001 |
| Gender | Female vs. Male | 0.96 | 0.48 | 0.90 | 0.42 |
| Education | 4 years more | 1.42 | <0.001 | 2.16 | <0.001 |
| Employed | Yes | 1.09 | 0.41 | 1.16 | 0.58 |
| Health | |||||
| Comorbidities | 1 more illness | 0.85 | <0.001 | 0.69 | <0.001 |
| Depressive symptoms | Present | 0.25 | <0.001 | 0.06 | <0.001 |
| BMI | 1 unit higher | 0.96 | <0.001 | 0.90 | <0.001 |
| MMSE-VI score | 5 points higher | 0.59 | 0.002 | 0.60 | 0.31 |
| Weather & Day of Week | |||||
| Daily Temperature | % days under 45°F | 1.02 | 0.14 | 1.06 | 0.12 |
| Rainfall | % days with > 0.2 inches | 0.99 | 0.67 | 0.98 | 0.84 |
| Weekend | % weekend days | 0.99 | 0.88 | 0.97 | 0.83 |
Rate ratio – Ratio of steps or minutes of moderate/vigorous activity for the given variable. An RR less than 1 Indicates less walking associated with the variable of interest.
Mod = moderate; Vig = vigorous; VF – Visual field; MD = Mean deviation; dB – decibels; VA – visual acuity; logMAR – Logarithm of the minimum angle or resolution; BMI – Body mass index; MMSE-VI – Mini-mental state exam for the visual impaired
Other univariate predictors of decreased walking and less MVPA included older age, African-American race, less education, more comorbid illnesses, depressive symptoms, and a higher BMI (p<0.01 for all) (Table 2). Cognitive function, inferred from the visually impaired version of the MMSE test, was significantly associated with fewer daily steps (p=0.002), but not with less time spent in MVPA. No significant association with walking or physical activity was observed with gender, employment status, proportion of weekend/holiday study days, cold weather, or rain (p>0.1 for all).
In multivariable models, glaucoma was associated with non-statistically significant decrement in MVPA (22% fewer minutes/day; 95% CI = -49 to 31%; p=0.41) and steps (13% fewer steps/day; 95% CI = -26 to 2%; p=0.09) (Table 3). Severe glaucoma was associated with 66% less MVPA (95% CI = -82 - -37%; p=0.001) and 31% fewer steps (95% CI = -44 to -15%; p=0.001) as compared to control subjects. In separate multivariable models incorporating the severity of VF or contrast sensitivity loss but not glaucoma status, each 5 dB decrement in the better-eye VF was associated with a 22% decrement in time spent in MVPA (95% CI = -32 to -9%; p=0.001) and a 10% decrement in daily steps (95% CI = -14 to -5%; p<0.001), while each 5 letter decrement in contrast sensitivity was associated with 17% less MVPA (95% CI = -30 to -1%, p=0.04 and 7% fewer steps (95% CI = -12 to -2%, p=0.005)). When both the extent of VF loss, visual acuity, and contrast sensitivity were included in the same multivariable models, only VF loss remained predictive of either MVPA or steps (p<0.01 for both). Increasing age, comorbid illness, depressive symptoms, and higher BMI were all associated with less time spent in MVPA and less walking (p<0.05 for all). African-American race, less education, and lower cognitive function were associated with less walking, but not less time spent in MVPA.
Table 3.
Variables influencing walking and physical activity, multivariable analysis
| Daily Steps
|
Minutes Mod/Vig Activity
|
||||
|---|---|---|---|---|---|
| Variable | Interval | RR* | p value | RR* | p value |
| Glaucoma | Present | 0.87 | 0.09 | 0.82 | 0.41 |
| Severe glaucoma† | Present | 0.69 | 0.001 | 0.34 | 0.001 |
| VF MD, bettereye | 5 dB worse | 0.90 | <0.001 | 0.78 | 0.001 |
| Age | 10 years older | 0.84 | 0.03 | 0.59 | 0.03 |
| Race | African-American | 0.83 | 0.05 | 0.66 | 0.19 |
| Gender | Female vs. Male | 0.99 | 0.81 | 0.99 | 0.95 |
| Education | 4 years more | 1.16 | 0.03 | 1.36 | 0.20 |
| Comorbidities | 1 more illness | 0.94 | 0.03 | 0.78 | 0.004 |
| Depressive symptoms | Present | 0.40 | <0.001 | 0.11 | <0.001 |
| BMI | 1 unit higher | 0.97 | <0.001 | 0.93 | 0.003 |
| MMSE-VI score | 5 points higher | 0.75 | 0.04 | 0.70 | 0.42 |
Rate ratio – Ratio of steps or minutes of moderate/vigorous activity for the given variable. An RR less than 1 Indicates less walking associated with the variable of interest.
Includes subjects in tertile of most severe glaucoma (better eye VF MD worse than -13.5 dB).
VF – visual field; MD – mean deviation; dB – decibels; BMI – Body mass index; MMSE-VI – Mini-mental state exam for the visually impair
Confirmatory analyses were performed using multivariable negative binomial models in which each person-day was analyzed as a separate observation and generalized estimating equations were used to account for correlation of physical activity measures across different study days of the same subject. Glaucoma was associated with a non-significant 24% decrease in MVPA (p=0.26) as compared to controls, while severe glaucoma was associated with a 58% decrease in MVPA (p<0.001), and each 5 dB decrement in the better-eye VF MD was associated with 17% less time in MVPA. Confirmatory findings were also obtained for step data.
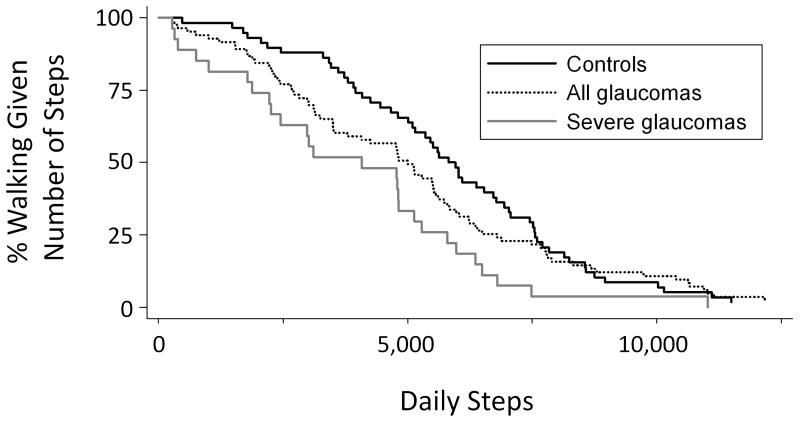
The portion of subjects meeting recommended activity levels of 10,000 steps/day was low in both the control (6.9%) and glaucoma (8.2%) groups, with both the full group of glaucoma subjects and subjects with the greatest tertile of VF damage more likely to not meet step thresholds within the limited (2,500 to 4,999 steps/day) and low-active (5,000 to 7,499 steps/day) range (Figure 3).31Multivariable logistic regression models were used to assess the odds of being in the lowest study quartile for steps taken (< 3,000 steps/day). When compared to controls, both glaucoma subjects (OR=3.1; 95% CI=1.0-9.4; p=0.04) and severe glaucoma subjects (OR=6.9; 95% CI=1.1-43.4; p=0.04) were more likely to not meet this activity threshold.
Figure 3.
Likelihood of meeting various step targets by glaucoma status
Severe glaucoma group includes subjects in the tertile of most severe glaucoma (better eye visual field mean deviation worse than -13.5 decibels).
DISCUSSION
This report is the first to quantify the impact of glaucomatous VF loss on walking and physical activity performed during the normal lives of patients. The study was designed to investigate if glaucoma subjects with bilateral VF loss engage in less physical activity than a control group consisting of glaucoma suspects with normal visual acuity and VFs. No group differences in MVPA were noted, possibly because glaucoma patients with mild bilateral VF loss had physical activity levels that are similar to, or only slightly less than controls. Indeed, physical activity levels were lower with greater levels of VF loss, with every 5 dB loss of MD associated with a 17% decrease in the amount of MVPA performed and a 10% decrease in steps taken, suggesting that VF loss from glaucoma did indeed impact physical activity. Additionally, individuals with the most severe glaucoma had severe reductions in both time spent in MVPA (66% less than controls) and steps per day (34% less than controls).These reductions exceeded the effects associated with older age, black race/ethnicity, co-morbid diseases, and higher BMI as measured in this study, and also the effects of other major conditions including arthritis, chronic obstructive pulmonary disorder, stroke, congestive heart failure, or a better-eye acuity of worse than 20/50 as determined in the National Health and Nutrition Evaluation Survey (NHANES).18
A decrease in physical activity in glaucoma might be expected given previous research suggesting that glaucoma significantly impairs mobility. Glaucoma subjects have been observed to walk more slowly, and be more likely to bump into objects in a mobility course as compared to subjects without glaucoma.6 Prior work has also suggested that individuals with glaucoma may experience falls at a rate as much as four-fold higher than individuals with normal sight.10,32 Impaired balance has been observed in individuals with glaucoma,5,8 and may explain the higher frequency of falls and bumps. Balance impairment and difficulty way finding may also produce fear of falling, which could lead to slower walking speed as well as the greater restriction of walking observed here.
While our data suggest that early VF loss may produce little or no activity restriction, the large effects of severe VF loss on measures physical activity were similar to those noted for subjects with visual impairment (better-eye visual acuity of 20/50 or worse) in the National Health and Nutrition Evaluation Survey (NHANES).18 Indeed, the effect of severe glaucoma (66% decrease in moderate/vigorous physical activity and 31% decrease in steps) exceeded the impact of visual impairment observed in NHANES (48% decrease in time spent in MVPA and 26% decrease in steps). These findings suggest that significant bilateral VF loss, like loss of central acuity, can strongly alter how much physical activity is performed. In the present study, neither visual acuity nor contrast sensitivity predicted decreased activity in models including VF loss severity, suggesting that neither is a significant driver of activity restriction in glaucoma patients. The lack of association between better-eye acuity and measures of physical activity also suggests that the higher prevalence of cataract noted in patients with glaucoma in this study is unlikely to explain the observed differences in physical activity.
The association of greater levels of VF loss with restriction of physical activity has significant implications for the health and well being of glaucoma patients. Daily walking in the population studied was in line with calculated averages for older adults,30 but few subjects in either study group met recommended activity guidelines for older adults, which include taking at least 7,000 steps/day and spending at least 150 minutes/week in MVPA. Individuals with glaucoma, particularly those more advanced VF loss, were less likely to meet activity thresholds. Previous research has associated lower levels of physical activity with a greater risk of mortality, as well as a greater risk of several specific diseases, including diabetes, heart disease, and osteoporosis.12-14 Additionally, lower levels of physical activity have been associated with decreased quality of life, decreased muscle mass, and other health consequences.11,33 While glaucoma subjects were not observed to have greater comorbid illnesses in the current study, it remains possible that these associations would only be seen in individuals suffering from greater levels of VF loss over prolonged durations. However, higher physical activity levels have been noted to produce noticeable changes in muscle mass and bone density even over shorter periods of time.33,34 Additionally, walking increases during travel outside the home,35 and is likely impacted by worsening VF loss.
The impact of severe VF loss on physical activity and walking highlights the need for better methods to identify and improve mobility outcomes in individuals with more advanced glaucoma. Mobility, particularly outdoor mobility, is a major stated concern for glaucoma patients.4 Referral rates of individuals with advanced glaucoma to low vision services and/or orientation and mobility training are unknown. However, less than one in three low vision rehabilitation entities/hospitals offers orientation and mobility training, while only 1 in 5 have an orientation and mobility specialist, suggesting that mobility training is likely rare even for glaucoma patients referred to low vision services.36 Additionally, low vision rehabilitation focuses more on safety and functional mobility (i.e. getting where one needs to go), and not necessarily on increasing overall step counts or physical activity levels. However, given the significant impact of greater VF loss on the amount of walking and physical activity performed, and the demonstrated health impacts of lower physical activity, encouraging greater physical activity should be an important goal of vision rehabilitation as long as greater physical activity does not translate into more injuries or falls.
Several factors limit the generalizability of our findings. The study was performed with patients in a tertiary glaucoma referral center and it required an additional visit to the clinic. Therefore, it is possible that individuals with severe mobility restriction may not have participated; however, this would most likely have resulted in an underestimate of disease impact. Patterns of walking and physical activity may also differ considerably in regions with different weather, different views about activity and exercise, and different built environments (which may be more or less conducive towards walking). Furthermore, only individuals between the ages of 60 and 80 were studied, and our findings may not generalize to younger or older age ranges.
Glaucoma suspects, and not individuals with no possible sign of eye disease were chosen as a control group. While it is possible that some of these individuals may have had the earliest stages of glaucoma, it is highly unlikely that any had meaningful mobility restriction resulting from their vision given a mean better-eye VF MD of 0.0 dB. Indeed, it has been shown previously that monocular glaucoma damage has little impact on mobility.6 We therefore restricted our analyses to individuals with bilateral VF loss from glaucoma and cannot make conclusions about the impact of unilateral VF loss on walking and physical activity. Finally, while it is clear individuals with severe glaucoma engage in less walking and physical activity, the cross-sectional study design prevents us from assessing when mobility declines are experienced.
Several limitations are inherent when inferring physical activity from accelerometers. First, one must assume that accelerometers measure steps and physical activity levels equally in individuals with and without glaucoma. Glaucoma does result in slower walking, which may lead to a differential detection in steps across groups. However, piezoelectric accelerometers have been shown to be accurate across a range of walking speeds,37 and walking speed should not affect classification of activity levels. Second, one must assume equal compliance with devices in individuals with and without glaucoma. Significant effort (i.e. daily morning phone calls) was expended to ensure compliance, and days of valid wear and daily wear time standards suggested equal compliance in subjects with and without glaucoma. It is possible that phone calls may have encouraged physical activity, though the very low levels of observed physical activity suggest no significant Hawthorne effect. Accelerometers also fail to capture upper-extremity exercise, swimming-related activities, or activity taking place when the device was not worn, which if more prevalent in glaucoma subjects, could bias our results towards a positive finding.
In summary, this study demonstrated no overall decrease in physical activity in a group of glaucoma subjects, though secondary analyses demonstrated that even a modest 5 dB decrement of better-eye VF is associated with a significant decrement in physical activity. These findings suggest a need for more study to better character when, and to what extent, physical activity is impacted by glaucomatous VF loss. Individuals with advanced bilateral VF loss demonstrate severe restrictions of walking and higher levels of physical activity and may be at greater risk for the numerous negative outcomes associated with lower levels of physical activity. Further research is needed to develop and validate interventions to encourage greater physical activity in individuals with severe glaucoma.
Acknowledgments
Financial Support: This work was supported in part by the Dennis W. Jahnigen Memorial Award, NIH Grant EY018595, the Research to Prevent Blindness Robert and Helen Schaub Special Scholar Award, and the Intramural Research Program of the NIH, National Institute of Aging. All funding organizations had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: No conflict of interest for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broman AT, Quigley HA, West SK, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49:66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramulu P. Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol. 2009;20:92–8. doi: 10.1097/ICU.0b013e32832401a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aspinall PA, Johnson ZK, Azuara-Blanco A, et al. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1907–15. doi: 10.1167/iovs.07-0559. [DOI] [PubMed] [Google Scholar]
- 5.Popescu ML, Boisjoly H, Schmaltz H, et al. Age-related eye disease and mobility limitations in older adults. Invest Ophthalmol Vis Sci. 2011;52:7168–74. doi: 10.1167/iovs.11-7564. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DS, Freeman E, Munoz B, et al. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology. 2007;114:2232–7. doi: 10.1016/j.ophtha.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Turano KA, Rubin GS, Quigley HA. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2803–9. [PubMed] [Google Scholar]
- 8.Black AA, Wood JM, Lovie-Kitchin JE, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008;85:489–97. doi: 10.1097/OPX.0b013e31817882db. [DOI] [PubMed] [Google Scholar]
- 9.Black AA, Wood JM, Lovie-Kitchin JE. Inferior field loss increases rate of falls in older adults with glaucoma. Optom Vis Sci. 2011;88:1275–82. doi: 10.1097/OPX.0b013e31822f4d6a. [DOI] [PubMed] [Google Scholar]
- 10.Haymes SA, Leblanc RP, Nicolela MT, et al. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1149–55. doi: 10.1167/iovs.06-0886. [DOI] [PubMed] [Google Scholar]
- 11.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, D.C.: U.S Department of Health and Human Services; 2008. [December 16, 2011]. pp. E4–E43. Available at: http://www.health.gov/paguidelines/report/pdf/CommitteeReport.pdf. [Google Scholar]
- 12.Leon AS, Connett J, Jacobs DR, Jr, Rauramaa R. Leisure-time physical activity levels and risk of coronary heart disease and death: the Multiple Risk Factor Intervention Trial. JAMA. 1987;258:2388–95. [PubMed] [Google Scholar]
- 13.Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS., Jr Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325:147–52. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 14.Cummings SR, Kelsey JL, Nevitt MC, O’Dowd KJ. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985;7:178–208. doi: 10.1093/oxfordjournals.epirev.a036281. [DOI] [PubMed] [Google Scholar]
- 15.Grand A, Grosclaude P, Bocquet H, et al. Disability, psychosocial factors and mortality among the elderly in a rural French population. J Clin Epidemiol. 1990;43:773–82. doi: 10.1016/0895-4356(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 16.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 17.Atienza AA, Moser RP, Perna F, et al. Self-reported and objectively measured activity related to biomarkers using NHANES. Med Sci Sports Exerc. 2011;43:815–21. doi: 10.1249/MSS.0b013e3181fdfc32. [DOI] [PubMed] [Google Scholar]
- 18.Willis JR, Vitale SE, Jefferys JL, Ramulu PY. Visual impairment, uncorrected refractive error, and accelerometer-defined physical activity in the United States. Arch Ophthalmol. doi: 10.1001/archopthalmol.2011.1773. In press. [DOI] [PubMed] [Google Scholar]
- 19.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? [December 16, 2011];Int J Behav Nutr Phys Act. 2011 8:62. doi: 10.1186/1479-5868-8-62. [serial online] Available at: http://www.ijbnpa.org/content/8/1/62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colley RC, Tremblay MS. Moderate and vigorous physical activity intensity cut-points for the Actical accelerometer. J Sports Sci. 2011;29:783–9. doi: 10.1080/02640414.2011.557744. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz JR, Ortega FB, Martinez-Gomez D, et al. HELENA Study Group. Objectively measured physical activity and sedentary time in European adolescents: the HELENA Study. Am J Epidemiol. 2011;174:173–84. doi: 10.1093/aje/kwr068. [DOI] [PubMed] [Google Scholar]
- 22.Bailey IL, Bullimore MA, Raasch TW, Taylor HR. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci. 1991;32:422–32. [PubMed] [Google Scholar]
- 23.Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–40. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 24.West SK, Munoz B, Schein OD, et al. Racial differences in lens opacities: The Salisbury Eye Evaluation (SEE) Project. Am J Epidemiol. 1998;148:1033–9. doi: 10.1093/oxfordjournals.aje.a009579. [DOI] [PubMed] [Google Scholar]
- 25.West SK, Munoz B, Wang F, Taylor H. Measuring progression of lens opacities for longitudinal studies. Curr Eye Res. 1993;12:123–32. doi: 10.3109/02713689308999480. [DOI] [PubMed] [Google Scholar]
- 26.Findl O, Buehl W, Menapace R, et al. Comparison of 4 methods for quantifying posterior capsule opacification. J Cataract Refract Surg. 2003;29:106–11. doi: 10.1016/s0886-3350(02)01509-2. [DOI] [PubMed] [Google Scholar]
- 27.Montorio I, Izal M. The Geriatric Depression Scale: a review of its development and utility. Int Psychogeriatr. 1996;8:103–12. doi: 10.1017/s1041610296002505. [DOI] [PubMed] [Google Scholar]
- 28.Busse A, Sonntag A, Bischkopf J, et al. Adaptation of dementia screening for vision-impaired older persons: Administration of the Mini-Mental State Examination (MMSE) J Clin Epidemiol. 2002;55:909–15. doi: 10.1016/s0895-4356(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 29.Oliver M, Schluter PJ, Schofield G. A new approach for the analysis of accelerometer data measured on preschool children. J Phys Act Health. 2011;8:296–304. doi: 10.1123/jpah.8.2.296. [DOI] [PubMed] [Google Scholar]
- 30.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. [December 16, 2011];Int J Behav Nutr Phys Act. 2011 8:80. doi: 10.1186/1479-5868-8-80. [serial online] Available at: http://www.ijbnpa.org/content/8/1/80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 32.Lamoureux EL, Chong E, Wang JJ, et al. Visual impairment, causes of vision loss, and falls: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49:528–33. doi: 10.1167/iovs.07-1036. [DOI] [PubMed] [Google Scholar]
- 33.Park H, Park S, Shephard RJ, Aoyagi Y. Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol. 2010;109:953–61. doi: 10.1007/s00421-010-1424-8. [DOI] [PubMed] [Google Scholar]
- 34.Niu K, Ahola R, Guo H, et al. Effect of office-based brief high-impact exercise on bone mineral density in healthy premenopausal women: the Sendai Bone Health Concept Study. J Bone Miner Metab. 2010;28:568–77. doi: 10.1007/s00774-010-0163-6. [DOI] [PubMed] [Google Scholar]
- 35.Ramulu PY, Chan ES, Loyd TL, et al. Comparison of home and away-from-home physical activity using accelerometers and cellular network-based tracking devices. J Phys Act Health. doi: 10.1123/jpah.9.6.809. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owsley C, McGwin G, Jr, Lee PP, et al. Characteristics of low-vision rehabilitation services in the United States. Arch Ophthalmol. 2009;127:681–9. doi: 10.1001/archophthalmol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maddocks M, Petrou A, Skipper L, Wilcock A. Validity of three accelerometers during treadmill walking and motor vehicle travel. Br J Sports Med. 2010;44:606–8. doi: 10.1136/bjsm.2008.051128. [DOI] [PubMed] [Google Scholar]