Abstract
Visual transduction in the Drosophila compound eye functions through a pathway that couples rhodopsin to phospholipase C (PLC) and the opening of Transient Receptor Potential (TRP) channels. This cascade differs from phototransduction in mammalian rods and cones, but is remarkably similar to signaling in mammalian intrinsically photosensitive retinal ganglion cells (ipRGCs). This review focuses on recent advances in the fly visual system, including the discovery of a visual cycle and insights into the machinery involved in generating a light response in photoreceptor cells. These latest findings suggest that the mechanism of light detection in flies and mammals have important parallels. Thus, a better understanding of phototransduction in the fly has the potential to contribute to our understanding of light detection in mammalian ipRGCs.
Keywords: TRP channels, rhodopsin, phototransduction, phospholipase C
Introduction
Drosophila phototransduction has been scrutinized for more than 40 years, and provides a genetic paradigm for signaling cascades that employ phosphoinositides and TRP channels [1–4]. During the last decade it has become clear that there exists a new class of photoreceptor cells in mammals, referred to as ipRGCs [5], which function through a signaling cascade akin to fly visual transduction [6–9]. The ipRGCs contribute primarily to photoentrainment of circadian rhythm, pupillary constriction and sleep [10–15]. The current review focuses on recent advances in understanding the fly visual system. Such findings include the discovery of a visual cycle, insights into the mechanism activating the TRP channels, the demonstration of dynamic interactions with the INAD (Inactivation but No Afterpotential D) signaling complex, and plastic changes in rhodopsin expression. Furthemore, the finding that light-dependent shuttling of a signaling protein depends on a second type of cascade that is coupled to rhodopsin will be discussed.
Anatomy of compound eye
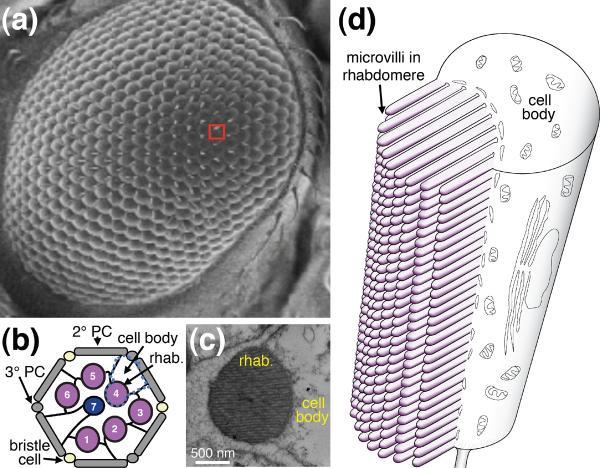
The Drosophila compound eye is comprised of ~750–800 reiterating hexagon-shaped ommatidia (Figure 1a), each of which contains 20 cells, including eight photoreceptors. Six photoreceptor cells (R1–6) extend the full depth of the retina, while the R7 and R8 cells are situated in the top (distal) and bottom (proximal) halves of the retina, respectively. Thus, only seven of photoreceptor cells lie in any plane of section (Figure 1b). Photoreception and signal transduction take place in the rhabdomeres, which are comprised of stacks of microvilli. ~50,000 are present in the larger R1–6 cells (Figure 1c and d). These ~1.2–1.5 μm long microvilli are only ~50 nm in diameter and contain an F-actin filament, but are devoid of internal organelles. The secondary retinal pigment cells (RPCs) are the main cells surrounding the photoreceptor cells (2° PC; Figure 1b). Tertiary RPCs and mechanosensory bristle cells occupy alternating vertices of the ommatidia (3° PC and bristle cell; Figure 1b).
Figure 1.
Anatomy of Drosophila compound eye and photoreceptor cells. (a) Scanning electron microscope (EM) image of an adult compound eye. The eye contains ~750 – 800 ommatidia. The red box indicates one ommatidium. (b) Cartoon illustrating the various cell types in a cross-sectional view of an ommatidium (distal region). Seven photoreceptor cells, each containing a rhabdomere, are shown: bristle cell, mechanosensory bristle cell; cell body, photoreceptor cell body; rhab., rhabdomere; 2° PC, secondary retinal pigment cells; 3° PC, tertiary retinal pigment cells. The dashed blue line indicates a single photoreceptor cell. (c) Transmission EM cross-section through one photoreceptor cell. (d) Cartoon showing a longitudinal view of one photoreceptor cell. ~50,000 microvilli are present in the rhabdomeres of each R1-6 cell. The microvilli are not drawn to scale. Normally there are 30–35 (50 nm wide) microvilli per cross-section.
In addition to the compound eye, adult flies have three smaller and simpler eyes (ocelli) located on the top of the head. Ocelli seem to be more important for detecting changes in light intensities during flight, than in image formation [16].
Fly phototransduction and its relationship to the cascades in rods and cones
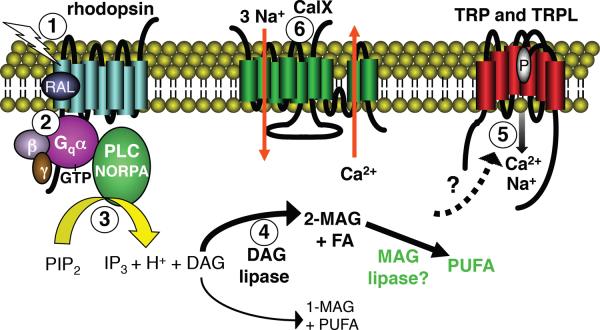
Phototransduction cascades serve to amplify single photon responses, and to allow the cells to adapt to light with intensities that differ over many orders of magnitude. The initiation of these signaling pathways depend on light sensors that are comprised of a seven transmembrane-containing protein (opsin) linked to a chromophore (3-hydroxy 11-cis-retinal in Drosophila, and 11-cis-retinal in rods and cones). Light promotes isomerization of the chromophore to the all-trans configuration, thereby inducing a conformation change in the protein subunit. The photoactivated visual pigment stimulates GDP/GTP exchange in a heterotrimeric G-protein. In the fly eye, the G-protein (Gq) [17] activates the PLC encoded by norpA [18], which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2), leading to opening of the TRP channel and the related TRP-like (TRPL) cation channel in photoreceptor cells (Figure 2) [19–23]. NORPA also accelerates the intrinsic GTPase activity of the Gqα [24–26]. Thus, NORPA promotes activation and negative feedback regulation, and does so through distinct domains [24–26]. The rise in Ca2+ is counterbalanced by extrusion through the Na+/Ca2+ exchanger, CalX [27].
Figure 2.
Model of the Drosophila phototransduction cascade. Events in phototransduction: 1) Light-activates rhodopsin. 2) Coupling of light-activated rhodopsin with the heterotrimer Gq protein. The activated Gqα subunit associates with GTP. 3) Stimulation of PLC leads to hydrolysis of PIP2 and production of IP3, DAG and H+. 4) A DAG lipase encoded by inaE hydrolyzes DAG to produce 2-MAG and FA. Minor products are 1-MAF and PUFA. The 2-MAG might be metabolized into PUFA by an unknown MAG lipase. 5) TRP and TRPL are activated following PLC stimulation, although the mechanism remains controversial. 6) Following activation of the channels, a Na+/Ca2+ exchanger (CalX) extrudes Ca2+ out of the photoreceptor cell. Abbreviations: DAG, diacylglycerol; FA, saturated fatty acid; IP3, inositol 1,4,5-trisphosphate; MAG, monoacylglycerol; PIP2, phosphatidylinositol 4,5-bisphosphate;; P, pore loop indicated in TRP; PUFA, polyunsaturated fatty acid; RAL, the chromophore (3-OH-11-cis-retinal).
Several of the key aspects of Drosophila phototransduction are distinct from the cascades in mammalian rods and cones. In flies, rhodopsin is bistable, i.e.—the chromophore usually stays bound to the opsin following exposure to light [28]. A second photon of light is necessary to convert the 3-OH-all-trans retinal back to the 3-OH-11-cis-retinal. If the flies are exposed to blue (480 nm) light, the major rhodopsin (Rh1) remains active in the dark. In rods and cones the light activated chromophore, all-trans-retinal, is released from the opsin and must be recycled through an enzymatic pathway [29, 30]. The heterotrimeric G-protein that is activated by the mammalian visual pigments (transducin; Gt) stimulates a phosphosphodiesterase, causing a decline in cGMP levels and subsequent closure of the cGMP-gated channels [31]. Thus, light induces opposite affects on the state of the cation channels in the rhabdomeres, and in the outer segments of rods and cones.
Mammals sensing light like flies
Direct light activation of the ipRGCs is mediated by the visual pigment melanopsin, which bears greater sequence homology to fly rhodopsin than to the mammalian rod and cone visual pigments [5, 8, 10]. Similar to the major Drosophila rhodopsin (Rh1), melanopsin is maximally activated by blue light, and appears to be a bistable photopigment [7, 32].
A variety of pharmacological, electrophysiological and expression studies in heterologous systems and in native cells indicate that melanopsin engages a Gq/PLC/TRPC signaling cascade [6, 7, 33, 34]. The requirement for PLC signaling has been solidified in a recent study demonstrating that loss of the mouse homolog of Drosophila NORPA (i.e. PLCβ4) dramatically reduces the light response of the M1-ipRGCs, which are the most light responsive ipRGCs [9]. Phototransduction in these cells depends on the TRP cation (TRPC) channels TRPC6 and TRPC7, since a double mutant eliminated photosensitivity [9]. Given that the Drosophila TRP and TRPL channels are the classical TRPC channels, these findings further underscore the common features of phototransduction in ipRGCs and fly photoreceptor cells.
The sphincter muscle cells in the iris of certain nocturnal and crepuscular (i.e. most active at dawn and dusk) mammals are also photosensitive and contribute to a local pupillary light response. It is proposed that the iris from nocturnal/crepuscular animals is directly photosensitive because their eyes are comprised mostly of rods, and under bright light the pupil may be constricted to such an extent that insufficient light reaches retinal photoreceptor cells to direct a pupillary response [9]. The phototransduction cascade in these photosensitive muscle cells is dependent on melanopsin and PLCβ4, as is the case for the ipRGCs [9]. However, their light response is not dependent on a TRPC channel, or on the TRP Vanilloid (TRPV) channel, TRPV4, which functions in smooth muscle cells [9]. Nevertheless, the phototransduction cascade in the iris may be dependent on another TRP channel. If so, light signaling in these sphincter muscle cells would represent another Drosophila-like phototransduction cascade.
Drosophila rhodopsins and plasticity in the adult eye
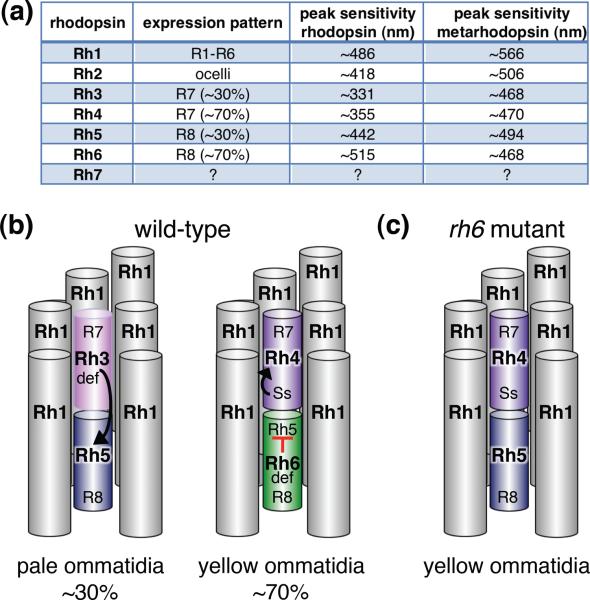
Drosophila encodes seven opsin genes (Rh1–Rh7), six of which (Rh1–6) fully account for the photoresponses of the different classes of photoreceptor cells in the adult compound eyes and ocelli (Figure 3a). The major rhodopsin, Rh1, is expressed in the six outer photoreceptor cells (R1–6 cells), which respond to dim light (Figure 3a and b). The R7/R8 photoreceptor cells each express one of four different rhodopsins, and are involved in color vision, including the detection of ultraviolet light by the R7 cells (Figure 3a and b) [35]. A random set of ~30% of the ommatidia coordinately express Rh3 in the R7 cells and Rh5 in the R8 cells (pale ommatidia; Rh3/Rh5; Figure 3b). Most of the remaining ~70% of ommatidia express Rh4 and Rh6 in the R7/R8 photoreceptor cell pairs (yellow ommatidia; Rh4/Rh6). In addition, there are two minor classes of ommatidia located near the dorsal rim that deviate from this pattern.
Figure 3.
Expression of Drosophila rhodopsins. (a) Five of the six characterized rhodopsins (Rh1 and Rh3 – Rh6) are expressed in photoreceptor cells in the compound eye. Rh2 is expressed in the ocelli. The peak light sensitivities of the non-activated (rhodopsin) and light activated pigments (metarhodopsin) are indicated. (b) Shown are the spatial distribution of Rh1 and Rh3 – Rh6 in the eight photoreceptor cells within an ommatidium (wild-type fly reared under a normal light/dark cycle). Rh1 is expressed in the R1-6 cells of all ommatidia. Rh3 – Rh6 are expressed in non-overlapping subsets of R7 and R8 cells as indicated. Yellow ommatidia normally express Rh4 and Rh6 in the R7 and R8 cells, respectively. Pale ommatidia express Rh3 and Rh5 in the R7 and R8 cells, respectively. Expression of Rh4 is induced by the Spineless (Ss) transcription factor [36]. If Ss is not expressed in a given Rh7 cell, then Rh3 is turned on as the default (def) state. Rh5 is induced in R8 cells that are below Rh3 expressed R7 cells. As a default, Rh6 is expressed in an R8 cell if it is not below an Rh7 cell that expresses Rh3. Expression of Rh6 in R8 cells inhibits expression of Rh5 in these cells. (c) Rh5 is turned on in nearly all yellow ommatidia of rh6 mutant flies [37]. If wild-type flies are maintained constantly in the dark, some yellow ommatidia express low levels of Rh5 in addition to Rh6 [37].
The control of one opsin to one R7 or R8 neuron in the pale and yellow ommatidia begins with stochastic expression of the Spineless (Ss) transcription factor in 70% of R7 cells, and induction of Rh4 expression in these cells [36]. As a default, R7 cells that do not express Ss turn on Rh3 (Figure 3b). The Rh7 cells that express Rh3 then induce expression of Rh5 in the underlying R8 cells in pale ommatidia (Figure 3b). Expression of Rh6 in the R8 cells is the default if Rh3 is not expressed (Figure 3a).
The continued exclusion of Rh5 from R8 cells in yellow ommatidia occurs through a mechanism involving a negative feedback signal induced by Rh6, which inhibits transcription of rh5 [37]. The signal requires activity of Rh6 and the Gqα, but not NORPA or the TRP and TRPL channels. In rh6 mutant flies, expression of Rh5 gradually expands with age to include nearly all R8 cells, including those in yellow ommatidia (Figure 3c) [37]. If wild-type flies are maintained in the dark, a low level of Rh5 expression is turned on in some yellow R8 cells that also express Rh6 [37]. The mechanism that links Rh6 activity with transcriptional repression of rh5 remains to be determined.
The finding that Rh6 prevents expression of Rh5 illustrates a role for a rhodopsin that is distinct from its classical function in vision. The major rhodopsin, Rh1, has two light-independent roles. These include a requirement for this rhodopsin for rhabdomere morphogenesis [38]. In addition, Rh1 initiates a thermosensory signaling cascade, which permits larvae to select their preferred temperature in the comfortable range [39]. Whether Drosophila rhodopsins have other light-independent or non-visual roles have not been reported, but are intriguing possibilities.
Bistable photopigments and an enzymatic visual cycle
The Drosophila visual pigments are bistable pigments, and therefore require absorption of one photon to activate rhodopsin, and a second photon to convert the light-activated metarhodopsin back to the non-active rhodopsin. Because the regeneration of the chromophore is normally a light-driven process, it has been assumed that Drosophila rhodopsins do not rely on an enzymatic cycle to regenerate the chromophore. However, recent findings demonstrate that flies use a visual cycle after all.
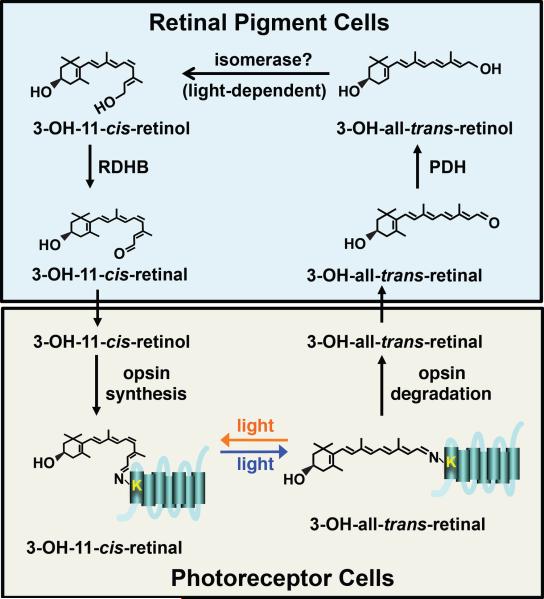
During light stimulation, a fraction of the rhodopsin pool is internalized and the opsin is degraded, thereby releasing the chromophore. The free 3-OH-all trans retinal is then recycled through an enzymatic pathway that includes the retinal dehydrogenases, PDH (Pigment Cell Dehydrogenase) [40] and RDHB (Retinal Dehydrogenase B) [41], and an isomerase that remains to be identified (Figure 4). These enzymatic steps take place in the RPCs that surround the photoreceptor cells. The essential role for the RPCs is reminiscent of the requirements for cells in the retinal pigment epithelium and Müller cells for regeneration of the chromophore used in rods and cones, respectively [29, 30].
Figure 4.
Model of the visual cycle. Following light stimulation of rhodopsin, a proportion of the rhodopsin pool is internalized and degraded, thereby liberating the 3-OH-all-trans-retinal. The 3-OH-all-trans-retinal is then transported to primary and secondary retinal pigment cells where it is converted into 3-OH-11-cis-retinal through several enzymatic steps that depend on at least two retinal dehydrogenases, PDH (Pigment Cell Dehydrogenase) [40] and RDHB (Retinal Dehydrogenase B) [41], and a putative isomerase that has not been identified. The 3-OH-11-cis-retinal is then transported into the photoreceptor cells.
In Drosophila the visual cycle allows the flies to maintain chromophore levels and a normal visual response under conditions in which dietary limitations prevent the flies from synthesizing new chromophore [40]. Melanopsin also appears to be a bistable pigment [7, 32], although it is not known if it depends on a visual cycle. If so, the Müller glia are candidate cells for participating in this process since they are situated near the ipRGCs and function in the visual cycle necessary for the cone pigments [15].
Mechanism of activation of TRP
TRP is the classical member of the TRP superfamily [19, 20, 42], and activation of this Ca2+-permeable cation channel is strictly dependent on stimulation of the PLCβ encoded by norpA [18]. However, the mechanism linking PLC with the opening of TRP, and the related TRPL channels, remains controversial. Stimulation of PLC results in hydrolysis of PIP2 and production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG; Figure 2). Although largely ignored, a single H+ is also generated [43] (Figure 2). Thus, TRP and TRPL could be activated either by a reduction in inhibitory PIP2, or an increase in H+, IP3, DAG, or by metabolites that are produced by these products, such as polyunsaturated fatty acids (PUFAs). IP3 does not appear to contribute to channel gating since release of caged IP3 does not mimic the light response, and mutation of the sole IP3 receptor encoded in the fly genome does not impair activation [44–46].
There are currently two prevailing models. The first is that PUFAs activate the channels. This concept is supported by whole-cell and single-channel recordings of isolated ommatidia indicating that TRPL is activated by PUFAs [47, 48]. DAG lipases metabolize DAG, and mutation of a gene (inaE) encoding a putative DAG lipase (Figure 2), severely impairs the light response [49]. A second model, supported by a recent study, is that a decrease in inhibitory PIP2, in combination with a local acidification, activates TRP and TRPL [43]. This work also provides in vivo evidence that light stimulation leads to PLC-dependent acidification. However, acidification with the protonophore 2,4-dinitrophenol is most effective at activating the channels, and this chemical is a mitochondrial uncoupler. Since metabolic stress can also activate the channels [50], it cannot be excluded that this is the basis for activation by 2,4-dinitrophenol. Another possibility is that full activation of the channel involves a decline in PIP2 in combination with a rise in DAG [51], PUFA, H+, and possibly other metabolites.
Light dependent movements of signaling proteins
Multiple signaling proteins in mammalian and fly photoreceptor cells undergo light dependent changes in spatial distribution [52]. In Drosophila photoreceptor cells, TRPL, Gq, and Arrestin1 and Arrestin2 (Arr1 and Arr2) shuttle in and out of the rhabdomeres dynamically [53–57]. These latter two proteins bind to rhodopsin, and contribute to termination of signaling by blocking the rhodopsin/Gq interaction.
The light-induced movements of some of these proteins are opposite in direction, and correlate with their roles during phototransduction. TRPL and Gq function in activation and are concentrated in the rhabdomeres of dark-adapted flies [56–59]. Upon light stimulation, these two proteins translocate to the cell bodies over the course of minutes. In contrast, Arr1 and Arr2 participate in termination of phototransduction, and shuttle from the cell bodies into the rhabdomeres in response to light [54, 55], and do so with a time-constant of under 10 seconds [60]. The opposite vector of these movements makes sense, since the light-dependent translocations participate in light adaptation [55, 56]. A decline in TRPL and Gq in the rhabdomeres, or an increase in Arr1 and Arr2, all serve to attenuate signaling.
Translocation of TRPL, Gq and Arr1/2 between the two major compartments of the photoreceptor cells depends on rhodopsin, which is expected since the trafficking is light dependent [59, 61, 62]. However, there are some surprising findings concerning the requirements for other phototransduction proteins. Two studies indicate that the phototransduction cascade comprised of PLC, TRP and TRPL are not required for movement of Gqα [57, 58]. Similarly, Arr2 can translocate into the rhabdomeres in the absence of any of classical proteins that function downstream of Rh1, such as PLC and TRP [61]. However, the kinetics of the movements is reduced at least 10-fold in the absence of these proteins due to a contribution of Ca2+ influx for the translocation [60]. Nevertheless, there is a Gq/PLC/TRP independent mechanism that contributes to light-induced movement of Arr2. This second pathway includes the small GTPase, Rac2, which associates either directly or with Rh1 in vivo [61]. Thus, in addition to the classical phototransduction cascade, there is a second Rac2-dependent cascade in Drosophila photoreceptor cells.
Several studies focusing on vertebrate rhodopsin indicate that a similar non-classical phototransduction pathway exists [63]. Mammalian Rac1, which is the homolog of fly Rac2, associates with mammalian rhodopsin and is activated by light. These findings raise the possibility that such as a cascade may also participate in Arr2 translocation in rod photoreceptor cells.
The effector proteins that couple with Rac2 in fly photoreceptor cells are not known. However, one candidate is phospholipase D (PLD), an enzyme that can be controlled by small G proteins [64]. G-protein coupled receptors of the rhodopsin family can activate PLD. Furthermore in bovine retinas the small GTPase RhoA has been reported to regulate PLD activity in a light-dependent manner [65], and the Drosophila genome encodes a PLD that appears to contribute to phototransduction [66].
The light-dependent movement of TRPL out of the rhabdomeres occurs in a two-step process. During the first stage, which occurs over the course of a few minutes, TRPL translocates to the apical region of the plasma membrane just outside the rhabdomeres (stalk membrane) [59]. The second stage proceeds for several hours and results in a redistribution of TRPL over the basolateral membrane of the photoreceptor cells. NORPA is required for the first stage, while the entire phototransduction cascade, including activation of the TRP channel participates in the second stage [59]. In addition, two small GTPases, Rab5 and RabX4 [67], as well as both the N- and C-terminal regions of TRPL appear to contribute to internalization of TRPL [68]. However, the proteins that bind to these domains and promote the translocation of TRPL are not known.
A controversial issue is whether the NINAC (Neither Inactivation Nor Afterpotential C) myosin III participates in dynamic movements of signaling proteins. Three independent studies have found that NINAC participates in the translocation of Gq, TRPL and Arr2 [58, 62, 69]. However, another study has challenged this conclusion, at least with respect to the contribution of NINAC to shuttling of Arr2 [53]. NINAC has been reported to promote the light-dependent translocation of TRPL from the rhabdomeres to the cell bodies [62]. In the case of Gqα (Gα49B) [58] and Arr2 [69], NINAC may function in movements from the cell bodies to the rhabdomeres, although this occurs under opposite light conditions—dark and light, respectively. The interaction between NINAC and Arr2 is indirect, and may be mediated by interactions with the same vesicles, since both proteins bind phosphoinositides [55, 69].
If NINAC participates in the movements of signaling proteins, the question arises as to whether this activity is mediated by the motor activity of this myosin III. Human myosin III is a plus ended motor [70]. However, NINAC motor activity has not been demonstrated, despite considerable effort (e.g. Porter, Sellers and Montell, unpublished observations). The plus end of F-actin orients towards the distal tips of the rhabdomeres [71], which is consistent with a function for NINAC in the translocation of Gq and Arr2 into the rhabdomeres. However, an activity as a plus-ended motor is inconsistent with a role in the shuttling of TRPL from the rhabdomeres to the cell bodies. Thus, NINAC may not retain motor activity. Rather, it is possible that the cell body and rhabdomere-specific isoforms of NINAC (i.e. p132 and p174, respectively) [72, 73] might function in dynamic spatial redistributions of signaling proteins by passive association, and therefore act as a sink in one compartment or the other. If so, this would support the recent contention that the shuttling of signaling proteins is driven by diffusion, rather than by an active motor [60].
The INAD signalplex and redox modulation of signaling
Many of the proteins that function in Drosophila phototransduction are grouped in the rhabdomeres into a large macromolecular assembly referred to as the signalplex [74–78]. The central scaffold in the signalplex is INAD, a protein consisting of five tandem ~90 amino protein interaction modules referred to as PDZ domains. The core complex includes INAD and three target proteins—TRP, PLC (NORPA) and a protein kinase C (PKC) encoded by the inaC gene [79]. Loss of INAD results in instability of these three proteins and disrupts their localization in the rhabdomeres [74, 80]. The stability and spatial distribution of INAD is reciprocally dependent on TRP [79, 81]. Thus, TRP is required both as a cation channel and as a molecular anchor. The concentration of INAD also declines if it not bound to the NINAC myosin III, or if there are mutations in a Membrane Occupation and Recognition Nexus (MORN)-domain containing protein, Retinophilin, which is required for stability of NINAC [82].
INAD also binds to itself and forms an homooligomer through a PDZ/PDZ interaction interface distinct from the surface groove that functions in target binding [77]. Thus, INAD may couple an extensive array of TRP channels with the other proteins required for phototransduction. Several other proteins that might bind to INAD include calmodulin, TRPL and a fraction of the rhodopsin (Rh1) pool [74, 77]. These signaling proteins are not dependent on this interaction for stability or localization, and their interactions with INAD might be dynamic. However, in contrast to the members of the core complex, there is not a consensus that these latter proteins bind INAD [83].
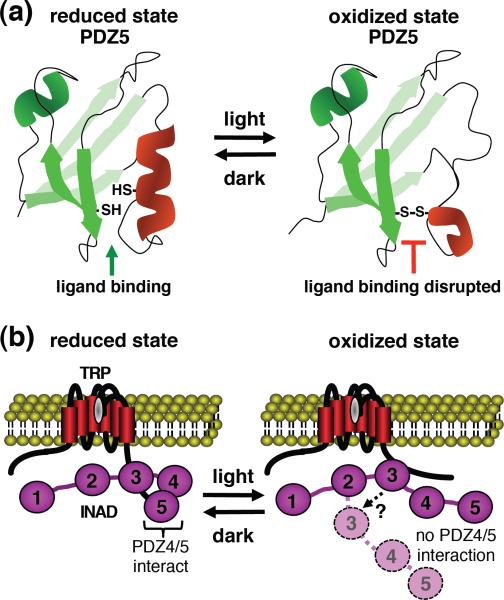
Two studies demonstrate that interactions of at least some of the signaling proteins that bind to INAD are dynamic, and are regulated by light-dependent conformational changes in PDZ5 [84, 85]. PDZ domains are β-barrel structures that contain a surface groove into which target proteins form hydrogen bonds [86]. PDZ5 in INAD is unusual in that two cysteines juxtaposed on either side of the surface groove form disulfide bonds, but only upon light stimulation (Figure 5a) [84]. Once generated, this precludes binding of target proteins. Under dark conditions, the disulfide bond is reduced, thereby creating a typical binding pocket [84].
Figure 5.
Dynamic binding of TRP with INAD. (a) The two cysteines lining the surface groove in INAD PDZ5 are reduced in the dark [84, 98]. As a consequence, PDZ5 can bind to binding proteins, including the C-terminus of TRP [84]. Light results in oxidation of the two key cysteines in PDZ5, which precludes target binding. (b) In the dark, PDZ4 and PDZ5 interact, thereby promoting the reduced state in PDZ5 [85]. Under these conditions TRP binds to PDZ5 through the C-terminus, and to PDZ3 via a separate binding site near the C-terminus. Following light stimulation, the PDZ4/PDZ5 interaction is disrupted, leading to oxidation of PDZ5 [85]. This prevents binding of the TRP C-terminus to PDZ5. Since the affinity of the internal binding site in TRP to PDZ3 is weak, binding to PDZ3 may dissociate as a secondary consequence of the oxidation of PDZ5. However, the light-induced impairment of the TRP/PDZ3 interaction is speculative, and is therefore indicated by a question mark.
The dynamic oxidation and reduction of the disulfide bonds in PDZ5 depend on interactions with the adjacent PDZ4 domain [85]. In the dark, the two PDZ domains interact stably (Figure 5b), thereby promoting the reduced state by increasing the redox potential of the disulfide bond by 330 mV. Upon exposure to light, PDZ4/PDZ5 coupling is disrupted, resulting in disulfide formation. It is proposed that light dissociates PDZ4 from PDZ5 due to the acidification generated by hydrolysis of PIP2 [85].
The light-induced oxidation of PDZ has physiological consequences, since it prevents target binding. One such INAD binding protein is TRP, which interacts with both PDZ3 and PDZ5 through distinct sites in the channel. TRP binds to PDZ5 through a classical C-terminal PDZ binding motif, and to PDZ3 via a site near the C-terminus [79, 85]. It is proposed that in the dark, when TRP is bound to both PDZ domains, TRP is maximally sensitive to activation [85]. Upon light stimulation, the TRP/PDZ5 interaction is disrupted, which is proposed to lead to full dissociation of the TRP/INAD interaction since TRP binding to PDZ3 is too weak to maintain the association between the two proteins (Figure 5b). As a consequence, the sensitivity of TRP to activation declines. Thus, production of H+ by PIP2 might contribute to both activation and negative feedback regulation.
Conclusions and future perspectives
The discovery that light-dependent changes in redox potential affects dynamic interactions of TRP with PDZ5 raises a number of questions. Is there a disulfide isomerase that promotes the conformational switch? A protein with this predicted activity is enriched in the fly eye [87]. Since the kinetics of the oxidation of PDZ5 appears to be too slow to account for the rapid kinetics of signaling, the mechanisms that work in concert with this reaction to promote the speed of phototransduction remain to be determined. If light causes full dissociation of TRP from INAD, how is TRP maintained in the rhabdomeres during light stimulation? This is an issue since TRP depends on its interaction with INAD for localization in the rhabdomeres [74], and TRP remains in the rhabdomeres during prolonged light stimulation [56]. Furthermore, mutation of the C-terminal PDZ5 binding site in TRP causes mislocalization of TRP [79]. Perhaps wild-type TRP remains bound to PDZ3 after dissociation from PDZ5, or the C-terminus of TRP binds to an alternative PDZ domain in INAD after its interaction with PDZ5 is occluded by the light-induced oxidation of PDZ5.
The nexus between PIP2 hydrolysis and activation of TRP and TRPL remains controversial. While it is an intriguing concept that a decline in inhibitory PIP2 combined with a rise in H+ might be the mechanism [43], a recent study argues that PUFAs gate TRPL [88], as proposed previously [47]. While there are many reports describing the biophysical properties of TRPL in heterologous expression system [89–94], until now there have been only three groups that have reported functional expression of TRP in vitro [90, 95, 96]. Apparently, this is due to difficulties in obtaining surface expression of TRP in expression systems. However, this obstacle may be reduced by the recent demonstration that a protein called XPORT augments the transport of TRP from the endoplasmic reticulum to the plasma membrane in tissue culture cells [97].
The recent insights concerning the machinery involved in the light response in Drosophila photoreceptor cells have potential implications for the ipRGCs, and for the light sensitive cells in the iris of nocturnal and crepuscular mammals [9]. Are mammalian TRPC6/TRPC7 activated by PUFAs or a combination of a decline in PIP2 and a rise in H+? The existence of a Drosophila visual cycle [40] despite the bistability of the fly visual pigments suggests the possibility that the melanopsin in ipRGCs also depends on an enzymatic pathway for regenerating the chromophore. The melanopsin and PLCβ-dependent light response in the iris sphincter muscle cells would appear to function through a TRP channel, although TRPC channels and TRPV4 have been excluded [9]. Nevertheless, the discovery of melanopsin-initiated signaling in mammalian eyes indicates that the mechanism of light detection in flies and mammals have a common and ancient origin.
Acknowledgments
I thank the National Eye Institute for supporting the research in my laboratory on Drosophila phototransduction, Wendy Novak for assistance with preparation of the Figures and Jinfei Ni for providing the EM image shown in Figure 1c.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pak WL. Why Drosophila to study phototransduction? J. Neurogenet. 2010;24:55–66. doi: 10.3109/01677061003797302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflügers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- 3.Pak WL, et al. Nonphototactic mutants in a study of vision of Drosophila. Nature. 1969;222:351–354. doi: 10.1038/222351a0. [DOI] [PubMed] [Google Scholar]
- 4.Hotta Y, Benzer S. Abnormal electroretinograms in visual mutants of Drosophila. Nature. 1969;222:354–356. doi: 10.1038/222354a0. [DOI] [PubMed] [Google Scholar]
- 5.Berson DM, et al. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 6.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 7.Panda S, et al. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 8.Provencio I, et al. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue T, et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hattar S, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panda S, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 12.Ruby NF, et al. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 13.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hankins MW, et al. Melanopsin: an exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt TM, et al. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmons PJ. The function of insect ocelli. Trends Neurosci. 1982;5:182–183. [Google Scholar]
- 17.Scott K, et al. Gqα protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 18.Bloomquist BT, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 19.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 20.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 21.Niemeyer BA, et al. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 22.Phillips AM, et al. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- 23.Montell C, et al. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- 24.Cook B, et al. Phospholipase C and termination of G-protein mediated signalling in vivo. Nat. Cell Biol. 2000;2:296–301. doi: 10.1038/35010571. [DOI] [PubMed] [Google Scholar]
- 25.Waldo GL, et al. Kinetic scaffolding mediated by a phospholipase C-β and Gq signaling complex. Science. 2010;330:974–980. doi: 10.1126/science.1193438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang T, et al. The SOCS box protein STOPS Is required for phototransduction through Its effects on phospholipase C. Neuron. 2008;57:56–68. doi: 10.1016/j.neuron.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T, et al. Light activation, adaptation, and cell survival functions of the Na+/Ca2+ exchanger CalX. Neuron. 2005;45:367–378. doi: 10.1016/j.neuron.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 28.Hillman P, et al. Transduction in invertebrate photoreceptors: the role of pigment bistability. Physiol.Rev. 1983;63:668–772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- 29.Travis GH, et al. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker RO, Crouch RK. Retinol dehydrogenases (RDHs) in the visual cycle. Exp. Eye Res. 2010;91:788–792. doi: 10.1016/j.exer.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu Y, Yau KW. Phototransduction in mouse rods and cones. Pflugers Arch. 2007;454:805–819. doi: 10.1007/s00424-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melyan Z, et al. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 33.Isoldi MC, et al. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc. Natl. Acad. Sci. USA. 2005;102:1217–1221. doi: 10.1073/pnas.0409252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham DM, et al. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J. Neurophysiol. 2008;99:2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- 35.Jukam D, et al. Rhodopsins in Drosophila Color Vision. In: Tombran-Tink J, Barnstable CJ, editors. Visual Transduction and Non-Visual Light Perception. Humana Press; 2008. pp. 252–266. [Google Scholar]
- 36.Wernet MF, et al. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasiliauskas D, et al. Feedback from rhodopsin controls rhodopsin exclusion in Drosophila photoreceptors. Nature. 2011;479:108–112. doi: 10.1038/nature10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leonard DS, et al. Degeneration of photoreceptors in rhodopsin mutants of Drosophila. J. Neurobiol. 1992;23:605–626. doi: 10.1002/neu.480230602. [DOI] [PubMed] [Google Scholar]
- 39.Shen WL, et al. Function of rhodopsin in temperature discrimination in Drosophila. Science. 2011;331:1333–1336. doi: 10.1126/science.1198904. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, et al. Requirement for an enzymatic visual cycle in Drosophila. Curr. Biol. 2010;20:93–102. doi: 10.1016/j.cub.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, et al. The Drosophila visual cycle and de novo chromophore synthesis depends on rdhB. J. Neurosci. 2012;32:3485–3491. doi: 10.1523/JNEUROSCI.5350-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montell C. The history of TRP channels, a commentary and reflection. Pflugers Arch. 2011;461:499–506. doi: 10.1007/s00424-010-0920-3. [DOI] [PubMed] [Google Scholar]
- 43.Huang J, et al. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr. Biol. 2010;20:189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Acharya JK, et al. InsP3 receptor essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18:881–887. doi: 10.1016/s0896-6273(00)80328-1. [DOI] [PubMed] [Google Scholar]
- 45.Raghu P, et al. Normal phototransduction in Drosophila photoreceptors lacking an InsP3 receptor gene. Mol. Cell. Neurosci. 2000;15:429–445. doi: 10.1006/mcne.2000.0846. [DOI] [PubMed] [Google Scholar]
- 46.Hardie RC, Raghu P. Activation of heterologously expressed Drosophila TRPL channels: Ca2+ is not required and InsP3 is not sufficient. Cell Calcium. 1998;24:153–163. doi: 10.1016/s0143-4160(98)90125-7. [DOI] [PubMed] [Google Scholar]
- 47.Chyb S, et al. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- 48.Delgado R, Bacigalupo J. Unitary recordings of TRP and TRPL channels from isolated Drosophila retinal photoreceptor rhabdomeres: activation by light and lipids. J. Neurophysiol. 2009;101:2372–2379. doi: 10.1152/jn.90578.2008. [DOI] [PubMed] [Google Scholar]
- 49.Leung HT, et al. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron. 2008;58:884–896. doi: 10.1016/j.neuron.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agam K, et al. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J. Neurosci. 2000;20:5748–5755. doi: 10.1523/JNEUROSCI.20-15-05748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estacion M, et al. Regulation of Drosophila transient receptor potential-like (TrpL) channels by phospholipase C-dependent mechanisms. J. Physiol. 2001;530:1–19. doi: 10.1111/j.1469-7793.2001.0001m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arshavsky VY. Protein translocation in photoreceptor light adaptation: a common theme in vertebrate and invertebrate vision. Sci. STKE 2003. 2003:PE43. doi: 10.1126/stke.2003.204.pe43. [DOI] [PubMed] [Google Scholar]
- 53.Satoh AK, Ready DF. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr. Biol. 2005;15:1722–1733. doi: 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 54.Kiselev A, et al. A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron. 2000;28:139–152. doi: 10.1016/s0896-6273(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, et al. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39:121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 56.Bähner M, et al. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- 57.Kosloff M, et al. Regulation of light-dependent Gqα translocation and morphological changes in fly photoreceptors. EMBO J. 2003;22:459–468. doi: 10.1093/emboj/cdg054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cronin MA, et al. Light-dependent subcellular translocation of Gq〈 in Drosophila photoreceptors is facilitated by the photoreceptor-specific myosin III NINAC. J. Cell Sci. 2004;117:4797–4806. doi: 10.1242/jcs.01371. [DOI] [PubMed] [Google Scholar]
- 59.Cronin MA, et al. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J. Cell Sci. 2006;119:2935–2944. doi: 10.1242/jcs.03049. [DOI] [PubMed] [Google Scholar]
- 60.Satoh AK, et al. Arrestin translocation is stoichiometric to rhodopsin isomerization and accelerated by phototransduction in Drosophila photoreceptors. Neuron. 2010;67:997–1008. doi: 10.1016/j.neuron.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elsaesser R, et al. Light-induced translocation of Drosophila visual Arrestin2 depends on Rac2. Proc Natl Acad Sci USA. 2010;107:4740–4745. doi: 10.1073/pnas.0906386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meyer NE, et al. Subcellular translocation of the eGFP-tagged TRPL channel in Drosophila photoreceptors requires activation of the phototransduction cascade. J. Cell Sci. 2006;119:2592–2603. doi: 10.1242/jcs.02986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balasubramanian N, Slepak VZ. Light-mediated activation of Rac-1 in photoreceptor outer segments. Curr. Biol. 2003;13:1306–1310. doi: 10.1016/s0960-9822(03)00511-6. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell R, et al. Rhodopsin-family receptors associate with small G proteins to activate phospholipase D. Nature. 1998;392:411–414. doi: 10.1038/32937. [DOI] [PubMed] [Google Scholar]
- 65.Salvador GA, Giusto NM. Phospholipase D from photoreceptor rod outer segments is a downstream effector of RhoA: evidence of a light-dependent mechanism. Exp. Eye Res. 2006;83:202–211. doi: 10.1016/j.exer.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 66.LaLonde M, et al. A role for Phospholipase D in Drosophila embryonic cellularization. BMC Dev. Biol. 2006;6:60. doi: 10.1186/1471-213X-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oberegelsbacher C, et al. The Drosophila TRPL ion channel shares a Rab-dependent translocation pathway with rhodopsin. Eur. J. Cell Biol. 2011;90:620–630. doi: 10.1016/j.ejcb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Richter D, et al. Translocation of the Drosophila transient receptor potential-like (TRPL) channel requires both the N- and C-terminal regions together with sustained Ca2+ entry. J, Biol. Chem. 2011;286:34234–34243. doi: 10.1074/jbc.M111.278564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee SJ, Montell C. Light-dependent translocation of visual arrestin regulated by the NINAC myosin III. Neuron. 2004;43:95–103. doi: 10.1016/j.neuron.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 70.Kambara T, et al. Human myosin III is a motor having an extremely high affinity for actin. J. Biol. Chem. 2006;281:37291–37301. doi: 10.1074/jbc.M603823200. [DOI] [PubMed] [Google Scholar]
- 71.Arikawa K, et al. Identification of actin filaments in the rhabdomeral microvilli of Drosophila photoreceptors. J. Cell Biol. 1990;110:1993–1998. doi: 10.1083/jcb.110.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Montell C, Rubin GM. The Drosophila ninaC locus encodes two photoreceptor cell specific proteins with domains homologous to protein kinases and the myosin heavy chain head. Cell. 1988;52:757–772. doi: 10.1016/0092-8674(88)90413-8. [DOI] [PubMed] [Google Scholar]
- 73.Porter JA, et al. Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J. Cell Biol. 1992;116:683–693. doi: 10.1083/jcb.116.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chevesich J, et al. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- 75.Shieh B-H, Zhu M-Y. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 1996;16:991–998. doi: 10.1016/s0896-6273(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 76.Wes PD, et al. Termination of phototransduction requires binding of the NINAC myosin III and the PDZ protein INAD. Nat. Neurosci. 1999;2:447–453. doi: 10.1038/8116. [DOI] [PubMed] [Google Scholar]
- 77.Xu XZ, et al. Coordination of an array of signaling proteins through homo-and heteromeric interactions between PDZ domains and target proteins. J. Cell Biol. 1998;142:545–555. doi: 10.1083/jcb.142.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huber A, et al. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 1996;15:7036–7045. [PMC free article] [PubMed] [Google Scholar]
- 79.Li HS, Montell C. TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J. Cell Biol. 2000;150:1411–1422. doi: 10.1083/jcb.150.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsunoda S, et al. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- 81.Tsunoda S, et al. Independent anchoring and assembly mechanisms of INAD signaling complexes in Drosophila photoreceptors. J. Neurosci. 2001;21:150–158. doi: 10.1523/JNEUROSCI.21-01-00150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Venkatachalam K, et al. Dependence on a retinophilin/myosin complex for stability of PKC and INAD and termination of phototransduction. J. Neurosci. 2010;30:11337–11345. doi: 10.1523/JNEUROSCI.2709-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huber A. Scaffolding proteins organize multimolecular protein complexes for sensory signal transduction. Eur. J. Neurosci. 2001;14:769–776. doi: 10.1046/j.0953-816x.2001.01704.x. [DOI] [PubMed] [Google Scholar]
- 84.Mishra P, et al. Dynamic scaffolding in a G protein-coupled signaling system. Cell. 2007;131:80–92. doi: 10.1016/j.cell.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 85.Liu W, et al. The INAD scaffold is a dynamic, redox-regulated modulator of signaling in the Drosophila eye. Cell. 2011;145:1088–1101. doi: 10.1016/j.cell.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 86.Lee HJ, Zheng JJ. PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun. Signal. 2010;8:8. doi: 10.1186/1478-811X-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu H, et al. A lysosomal tetraspanin associated with retinal degeneration identified via a genome-wide screen. EMBO J. 2004;23:811–822. doi: 10.1038/sj.emboj.7600112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lev S, et al. Signal dependent hydrolysis of PI(4,5)P2 without activation of phospholipase C: Implications on the gating of the Drosophila TRPL channel. J. Biol. Chem. 2011;287:1436–1447. doi: 10.1074/jbc.M111.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu Y, Schilling WP. Receptor-mediated activation of recombinant TRPL expressed in Sf9 cells. Biochem. J. 1995;305:605–611. doi: 10.1042/bj3050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu XZ, et al. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 91.Hardie RC, et al. Functional equivalence of native light-sensitive channels in the Drosophila trp301 mutant and TRPL cation channels expressed in a stably transfected Drosophila cell line. Cell Calcium. 1997;21:431–440. doi: 10.1016/s0143-4160(97)90054-3. [DOI] [PubMed] [Google Scholar]
- 92.Parnas M, et al. Open channel block by Ca2+ underlies the voltage dependence of drosophila TRPL channel. J. Gen. Physiol. 2007;129:17–28. doi: 10.1085/jgp.200609659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kunze DL, et al. Properties of single Drosophila Trpl channels expressed in Sf9 cells. Am. J. Physiol. 1997;272:C27–C34. doi: 10.1152/ajpcell.1997.272.1.C27. [DOI] [PubMed] [Google Scholar]
- 94.Zhang L, et al. Expression of Drosophila Ca2+ permeable transient receptor potential-like channel protein in a prostate cancer cell line decreases cell survival. Cancer Gene Ther. 2003;10:611–625. doi: 10.1038/sj.cgt.7700608. [DOI] [PubMed] [Google Scholar]
- 95.Vaca L, et al. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am. J. Physiol. 1994;266:C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- 96.Harteneck C, et al. The PDZ scaffold protein INAD abolishes apparent store-dependent regulation of the light-activated cation channel TRP. FASEB J. 2002;16:1668–1670. doi: 10.1096/fj.02-0192fje. [DOI] [PubMed] [Google Scholar]
- 97.Rosenbaum EE, et al. XPORT-dependent transport of TRP and rhodopsin. Neuron. 2011;72:602–615. doi: 10.1016/j.neuron.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montell C. Dynamic regulation of the INAD signaling scaffold becomes crystal clear. Cell. 2007;131:19–21. doi: 10.1016/j.cell.2007.09.022. [DOI] [PubMed] [Google Scholar]