Abstract
The blood-brain barrier (BBB) provides significant protection against microbial invasion of the brain. However, the BBB is not impenetrable, and mechanisms by which viruses breach it are becoming clearer. In vivo and in vitro model systems are enabling identification of host and viral factors contributing to breakdown of the unique BBB tight junctions. Key mechanisms of tight junction damage from inside and outside cells are disruption of the actin cytoskeleton and matrix metalloproteinase activity, respectively. Viral proteins acting in BBB disruption are described for HIV-1, currently the most studied encephalitic virus; other viruses are also discussed.
Keywords: blood-brain barrier, viral encephalitis, tight junctions, matrix metalloproteinases
Viral entry to the brain
Viral encephalitis is a potentially deadly sequela of viral infection for which there are few treatment options. It is frequently associated with blood-brain barrier (BBB; see Glossary) disruption, enabling entry of virus, inflammatory cells, and deleterious molecules into the brain parenchyma. Members of at least 11 virus families, including DNA viruses, retroviruses, and RNA viruses, cause encephalitis with significant morbidity and mortality [1]. There are a variety of means by which viruses enter the brain, primarily via neuronal transport or by crossing of one of several barriers to the central nervous system (CNS), including the BBB or the blood-cerebrospinal fluid barrier (choroid plexus). Several recent reviews covered viral entry via axonal transport and the resulting neuronal damage [2–4]. This review will focus on CNS entry mechanisms used by viruses that breach the BBB. Cell culture and animal studies of viral encephalitis have recently progressed through approaches used in studies of pathogenic conditions of the CNS such as ischemic stroke and multiple sclerosis. We first present background information on the structure, function, and disruption of the BBB, followed by a discussion of specific mechanisms by which viruses breach the BBB.
Components of the BBB
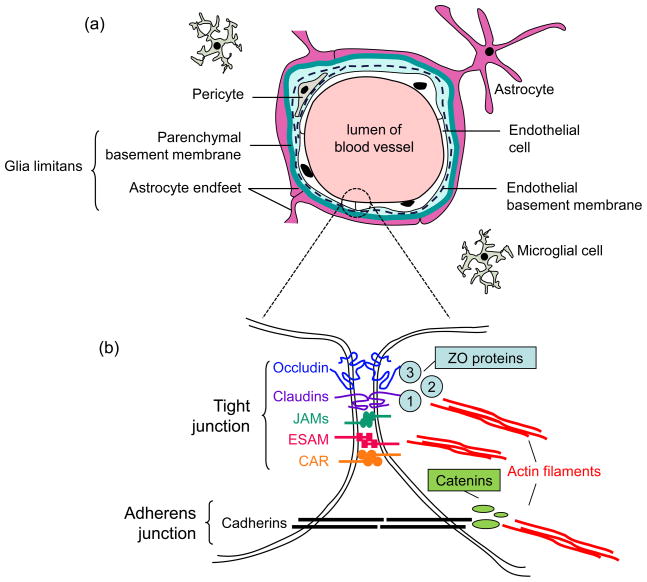
The BBB is a physical, metabolic, and transport barrier between the peripheral circulation and the CNS [5]. The function of the barrier is contributed by features specific to brain microvascular endothelial cells, which form the walls of brain capillaries, and the interactions of these cells with other components of the neurovascular unit (NVU), especially astrocyte endfeet and extracellular matrix [6]. The NVU also includes pericytes, microglia, and neurons (Figure 1). Brain endothelial cells form extremely tight cell-cell junctions that are distinct from tight junctions of endothelia and epithelia elsewhere in the body, due to brain endothelial cells’ morphology, biochemistry, and interactions with other cells of the NVU [5–7]. Brain endothelial cells lack fenestrations, have high numbers of mitochondria, are very thin, and have a low rate of pinocytosis, characteristics that relate to their specialized function. For example, high mitochondrial content in the NVU relative to other tissues is likely important for the energy required to maintain the structure and function of the BBB. Endothelial cells and associated pericytes are ensheathed by an endothelial cell basal lamina (vascular basement membrane). The composition of the endothelial basement membrane is distinct from that of a second basal lamina, the parenchymal basal lamina. This parenchymal basal lamina together with the astrocyte endfeet is termed the glia limitans. The perivascular space has been compared to a castle moat, between the vascular basement membrane (outer castle wall) and the glia limitans (inner wall) [8]. Leukocytes accumulate in this cerebrospinal fluid-filled perivascular space (moat), where immune surveillance occurs. When leukocytes are presented with their cognate antigens, they are activated and cross the glia limitans into the brain parenchyma. Brain endothelial cells have active transporters expressed on their apical and basal surfaces that exclude potentially detrimental molecules or enable passage of essential nutrients such as glucose and amino acids into the brain parenchyma. Intact brain endothelium in vivo is characterized by a very high transendothelial electrical resistance (TEER) due to its complex tight junctions, which result in both the effective block of passage of macromolecules and restricted diffusion of ions and polar solutes. The consequence of these features of brain endothelial cells is a restrictive barrier with controlled entry of plasma components.
Figure 1.
The neurovascular unit and junctions between endothelial cells. (a) cross-section of a brain microvessel, showing cells of the neurovascular unit. Neurons and their contacts with astrocytes are not shown. Cells in the lumen (bloodstream) include red blood cells, lymphocytes, monocytes, and neutrophils (not shown). Note the two basement membranes; the space between them is known as the perivascular space. (b) Enlargement of an endothelial cell-cell junction. Major molecules of the tight junction and adherens junction are shown; no order of the tight junction proteins within the tight junction is implied by the figure. Abbreviations: JAMs, junctional adhesion molecules; ESAM, endothelial cell-selective adhesion molecule; CAR, Coxsackie and adenovirus receptor; ZO, zona occludens. The figure is based on figures in [7,95].
Tight junctions in the brain
The complexes holding brain endothelial cells together are adherens junctions and tight junctions (Figure 1) [5]. The adherens junctions, composed of transmembrane cadherin proteins linked to the cell cytoskeleton by catenins, provide structural support and are important for the development of tight junctions. Tight junction complexes consist of both integral transmembrane proteins and peripheral membrane proteins. The integral transmembrane tight junction proteins include occludin, claudins, junctional adhesion molecules (JAMs), endothelial cell-selective adhesion molecule (ESAM), and the Coxsackie and adenovirus receptor (CAR) [5,9]. Occludin and claudins have external loops that mediate intercellular adhesion by interaction with occludin and claudins of neighboring cells [6]. The tight junction proteins that span the gap between cells can be altered in their localization or cleaved during BBB damage resulting from viral infections and other pathological conditions.
Cytosolic tails of the transmembrane tight junction proteins are associated with any of a large number of peripheral membrane (cytosolic) tight junction proteins, which can serve adaptor, scaffolding, signaling, or transcriptional activator functions [9]. In particular, claudins and occludin interact with zona occludens (ZO)-1, -2, and -3, which in turn link to the actin cytoskeleton. The endothelial cytoskeleton is critical for integrity of tight junctions: actin stress fibers and microtubules contribute to tension force and isometric cellular contraction required for barrier function [10]. Disruption of the endothelial cytoskeleton can contribute to or be a result of tight junction dysfunction. Disruption of occludin, claudin-5 and ZO-1 is an indicator of functional breakdown of the BBB [11]. The mechanisms that specific viruses use to alter tight junction proteins are discussed below.
BBB disruption by viruses
BBB disruption can be both a cause and effect of viral and non-viral CNS disease such as stroke, cancer, traumatic brain injury, and multiple sclerosis [6]. Viruses known to cause disruption of the BBB or endothelial junctions include HIV-1, human T-cell leukemia virus (HTLV-1), lymphocytic choriomeningitis virus (LCMV), West Nile virus (WNV), and mouse adenovirus type 1 (MAV-1) (Table 1) [1,12–14]. HIV-1, simian immunodeficiency virus (SIV-1), feline immunodeficiency virus, and WNV are thought to invade the brain parenchyma by a ‘Trojan horse’ mechanism, through diapedesis of infected immune cells that either cross the BBB paracellularly (between cells) or transcellularly (through cells) [15–17]. WNV cell-free viruses and virus-like particles (VLPs) may transit human endothelial cells via a transcellular pathway that does not affect the integrity of the BBB [16,18]. Important mechanisms of BBB disruption associated with paracellular entry of viruses that are discussed in this review include alterations in expression or phosphorylation of tight junction proteins, disruption of the basal lamina, and disruption of the actin cytoskeleton. Only in a few cases have viral gene products been directly implicated in BBB disruption (e.g. HIV-1, see below); in the absence of such mechanisms, indirect effects of viruses on the immune system are likely causes of barrier disruption.
Table 1.
Viruses and their effects on the BBB
| Virus | Effects on BBB | Refs |
|---|---|---|
| Herpes simplex virus | Increased MMP2 and MMP9 activity | [71] |
| HIV-1 | Alteration of tight junction protein expression, which is likely CCL2-dependent | [12, 33, 35, 36] |
| Increased MMP2 and MMP9 expression in in vitro culture supernatants | [33] | |
| Activation of Ras signaling | [41–44] | |
| Inhibition of tight junction protein expression with concomitant increase in MMP9 cleavage of tight junction proteins due to Tat | [45, 46] | |
| Higher vessel permeability due to presence of secreted gp120 | [47] | |
| Increased expression of MMP2 and MMP9 with decreased expression of claudin-5 and laminin due to oxidative stress | [49] | |
| HTLV-1 | Alteration of tight junction protein expression | [14] |
| Increased barrier permeability of cells and higher migration of infected lymphocytes in in vitro co-culture model | [14] | |
| Japanese encephalitis virus (JEV) | Increased MMP9 expression | [60] |
| Lymphocytic choriomeningitis virus (LCMV) | CD8+T cell-dependent BBB disruption | [68] |
| Mouse adenovirus type 1 (MAV-1) | Alteration of tight junction protein expression | [13] |
| Rabies virus | CD4+ T cell-dependent BBB disruption | [66, 67] |
| SIV | Alteration of tight junction protein expression associated with increased FAK expression | [39] |
| Theiler’s virus | Alteration of tight junction protein expression | [68, 69] |
| West Nile virus (WNV) | Increased tight junction protein expression | [16] |
| Increased MMP expression and activity | [59, 62] | |
| Increased TLR3-dependent inflammatory response leading to increased BBB disruption | [61] | |
| Various viruses | Increased ROS and RNS production | [27] |
There are many modulators of transcellular and paracellular BBB permeability, including vasogenic factors, growth factors, cytokines and chemokines, matrix metalloproteinases (MMPs), free radicals, and lipid mediators [10]. Accordingly, the mechanisms used by infectious agents to compromise the BBB vary. In some cases BBB disruption may be caused directly by microbial products, but in most cases, multiple factors are likely to play a role. For example, induction of cytokines and chemokines upon viral infection of brain cells and leukocytes homing to the brain could cumulatively contribute to BBB disruption.
Disruption of tight junctions and basal lamina by secreted MMPs
MMPs are key mediators of tight junction protein alterations leading to BBB dysfunction [19,20]. These zinc-dependent enzymes have proteolytic activity that acts on the extracellular matrix, such as basal laminae in the NVU. MMP activity induced in pathological conditions causes BBB disruption not only by basement membrane degradation, but also by cleavage of tight junction proteins occludin and claudin-5 [21–24]. MMPs can also cleave cytokines, modulating their activity and thus inflammation [11]. MMPs are synthesized as inactive enzymes (zymogens), and their activity is regulated at four levels: gene expression, activation of proenzyme, enzyme inactivation by association with endogenous inhibitors (tissue inhibitors of metalloproteinases, TIMPs), and cellular compartmentalization [9]. Activation of MMPs occurs by cleavage by other MMPs or proteases, or by direct or indirect exposure to oxidative stress.
Increased production of reactive oxygen species (ROS) correlates with increased MMP activity during brain injury, and markers of oxidative stress colocalize with active MMPs [25]. Non-viral inducers of ROS alter tight junction protein expression and phosphorylation, stimulate increased MMP activity, and increase permeability of the BBB [26]. Viral infections of the CNS can directly increase ROS and reactive nitrogen species (RNS) through stimulation of intracellular signaling by virion components or cytotoxic effects of viral nonstructural proteins [27]. The host inflammatory response to viral infection can also generate ROS and RNS, which in turn stimulate inflammatory cytokines and MMP secretion by cells of the NVU. Thus viral infections of the CNS have the potential to cause BBB disruption by inducing oxidative damage that alters MMP activity.
Some MMPs are membrane-bound (membrane-type MMPs, MT-MMPs), with extracellular catalytic domains [28]. However, most MMPs are secreted, although they may stay localized to the cell surface by association with MT-MMPs or other cell surface molecules. In the neuroinflammatory response, the primary secreted MMPs are MMP2, MMP3, and MMP9 [11]. MMP9 and MMP3 are inducible MMPs involved in inflammatory responses in the brain, whereas MMP2 is constitutively expressed by astrocytes, present in zymogen form throughout the brain, and activated upon host response to injury. MMP2, MMP3, and MMP9 all cleave tight junction proteins [21,23,24]. All cell types in the NVU are able to produce MMPs, and in pathological conditions (including viral infections), alterations in MMP mRNA and enzymatic activity have been found in endothelial cells, astrocytes, and microglia of the NVU, and in macrophages and neutrophils recruited from the circulation [28].
Disruption of tight junctions by alterations to the actin cytoskeleton and peripheral membrane tight junction protein complexes
Tight junctions can also be disrupted from within cells. Changes to the actin cytoskeleton are likely to occur upon alterations to the tight junction proteins, resulting in paracellular permeability changes [10,29]. ROS play a role in disrupting tight junctions from within cells, by induction of the small GTPase RhoA, PI3 kinase and protein kinase B (PKB/Akt) signaling pathways with a concomitant rearrangement of the actin cytoskeleton, altered localization of occludin and claudin-5, and altered BBB integrity [30]. Other evidence that BBB disruption can be initiated from within cells is that alteration of the actin cytoskeleton induced by hypoxic stress is correlated with changes in BBB permeability and ZO-1 localization [31]. Furthermore, the tight junction proteins occludin, ZO-1, ZO-2, and claudin-5 are phosphoproteins; changes in their phosphorylation result in changes in localization and/or interaction, affecting BBB permeability [10]. Expression of monocyte chemoattractant protein-1 (CCL2) alters the actin cytoskeleton and localization of tight junction proteins in brain endothelium, disrupting the BBB [32]. HIV-1 alteration of tight junction proteins is dependent on CCL2 [33]. Many virus infections alter the integrity of the cytoskeleton [34], but the role of this in viral disruption of the BBB has not been well studied.
Breaching of the BBB by retroviruses
HIV-1 is one of the most-studied viruses with respect to viral and host processes involved in encephalitis and disruption of the BBB. Accordingly, for HIV-1 encephalitis there are data for many of the mechanisms of barrier disruption described above, particularly alterations of tight junction protein expression [12]. Both transcellular and paracellular diapedesis of infected leukocytes are involved in HIV-1 transit across the BBB [15]. Retroviral-associated neural disease caused by HTLV-1 is also characterized by alteration of tight junction protein expression [14].
Alteration of BBB function by HIV-1 infection is seen both in patients and in vitro models. Post-mortem brain samples of HIV-1-positive patients with encephalitis or HIV-1-associated dementia show increased monocyte infiltration and fragmented or reduced expression of the tight junction proteins ZO-1, occludin, and claudin-5; such disruption is not seen in controls (HIV-1 patients without encephalitis, HIV-1 seronegative patients, or cases who die of non-HIV-1 causes) [35,36]. In vitro culture systems for HIV-1 infection studies use primary human brain-derived microvascular or umbilical vein-derived endothelial cells, often co-cultured across transwell inserts with astrocytes [33]. Compared with uninfected activated peripheral blood mononuclear cells, HIV-1-infected cells introduced into culture systems cross the endothelial cell monolayers, altering tight junction protein expression and increasing permeability and MMP2 and MMP9 expression.
An important recent development in the study of human encephalitic pathogens, particularly lentiviruses, has been the use of the immortalized, well-characterized brain endothelial cell line hCMEC/D3, obtained by expressing human telomerase reverse transcriptase (hTERT) and simian virus 40 (SV40) large T antigen [37]. This new cell line recapitulates important characteristics of primary human brain endothelial cells and has been successfully used in in vitro BBB studies with pathogenic microbes [14,17,38]. For example, hCMEC/D3 cells infected by co-culture with a HTLV-1-producing cell line have higher permeability than when co-cultured with a control line not producing viral particles; and migration of infected lymphocytes across hCMEC/D3 cells is higher than migration of control lymphocytes [14]. Another culture system is Rhesus macaque brain microvessels, which when incubated with SIV-infected leukocytes show a significant loss of ZO-1 expression associated with increased expression of focal adhesion kinase (FAK) [39].
In addition to host components, viral proteins contribute to changes in BBB function during HIV-1 infection [12]. Three HIV-1 proteins have been implicated in altering BBB integrity, Tat, gp120, and Nef. Tat is a multifunctional viral protein that acts as a transactivator, enhancing initiation and elongation of viral transcription. Tat is secreted from infected cells, and it can enter other cells and affect their function. There is evidence for several mechanisms by which Tat contributes to BBB disruption. A key target of Tat is vascular endothelium, where it activates inflammation and angiogenesis. Specifically, Tat acts on endothelial cells, inducing proliferation, expression of adhesion molecules, release of proteolytic enzymes, and adhesion to the extracellular matrix via focal adhesions [40]. Introduction of Tat into human brain microvascular endothelial cells results in altered expression of tight junction proteins and activation of Ras signaling [41–44]. Similarly, injection of Tat into mice results in altered expression of tight junction proteins occludin and ZO-1, accumulation of inflammatory cells, and activation of mitogen-activated protein kinase (MAPK) [12]. Tat interacts with MMP pathways, and may thus alter BBB function during HIV infection. For example, Tat was recently reported to inhibit endothelial cell occludin expression and promote its cleavage by MMP9, by a RhoA-dependent pathway [45]. MMP9 mRNA, protein, and enzymatic activity levels are increased when astrocytes are treated with Tat [46]. This upregulation of expression is dependent on MAPK, NF-κB (nuclear factor-kappa B) and Tat-induced tumor necrosis factor-alpha production. It should be noted that the physiological relevance of effects of Tat seen in vitro is controversial, because it is not clear whether sufficient levels of extracellular Tat in the circulation are achieved, even in microenvironments [12].
Another HIV-1 protein that affects BBB integrity is gp120, a virion envelope protein [12]. gp120 can be found in patient serum, and it crosses brain endothelial cells in culture by adsorptive endocytosis, giving it access to cells in the NVU. A transgenic mouse model in which gp120 is secreted was used to examine the role of circulating gp120 [47]. Compared to wild-type controls, transgenic mice had more brain blood vessels with permeability to albumin, indicating damage to the BBB. Additional experiments with endothelial cells from both transgenic or control mice, incubated with serum from transgenic mice, showed that the effect is gp120-dependent [48]. In another animal model, injection of gp120 alters the BBB in rats, increasing expression of MMP2 and MMP9 and decreasing expression of claudin-5 and laminin, a component of the BBB basal lamina; oxidative stress is implicated as a contributing mechanism [49]. A role for gp120 in BBB disruption is also supported by in vitro evidence that gp120 added to cultured human brain endothelial cells enhances monocyte migration, increases permeability, decreases TEER, and disrupts expression of tight junction proteins ZO-1, ZO-2, and occludin (but not claudins or actin) [50,51]. A proteasomal mechanism is apparently involved in the gp120-induced degradation of the ZO proteins, but how that is initiated and the mechanism for degradation of other tight junction proteins remain unknown [52]. Taken together, these in vivo and in vitro data suggest that circulating gp120 in HIV-infected humans may contribute to BBB disruption.
Nef is a third HIV-1 protein implicated in damage of the BBB. Nef is a pleiotropic accessory protein, whose best characterized activities are alteration of antigen presentation by major histocompatibility complex class I (MHC-I) and downregulation of CD4 [53]. Nef is expressed in astrocytes of AIDS patients, particularly those with moderate to severe dementia [54]. Nef expression activates cultured astrocytes, elevating expression of markers associated with brain inflammation [55]. Nef also increases sensitivity of cultured astrocytes to hydrogen peroxide, a ROS [56]. It is hypothesized that Nef may contribute to pathology in HIV-1-infected people through this mechanism, particularly in the absence of adequate functional glutathione peroxidase. HIV-1 infection of astrocyte cultures in an endothelial cell co-culture model increases permeability of the endothelium, causing endothelial cell apoptosis, altered astrocyte endfoot formation, and signaling between uninfected and infected cells in a gap junction-dependent mechanism [57]. This bystander effect of HIV-1-infected astrocytes on neighboring cells has in vivo support from experiments with astrocytes of SIV-infected macaques. HIV-1 infection of astrocytes also increases their production of pro-MMP2 and pro-MMP9 [46,58]. Due to the importance of astrocyte endfeet in BBB integrity, it will be interesting to learn whether Nef is responsible for these reported HIV-1 effects in astrocytes, thereby contributing to disruption of BBB integrity. Taken together, studies on Tat, gp120, and Nef indicate that BBB disruption during viral infection can be attributed to specific viral proteins. Similar analysis of viral protein involvement for other viruses will increase our understanding of virus-host interations in encephalitis.
Breaching of the BBB by RNA viruses
Flaviviridae are among the best-studied RNA viruses that compromise the BBB. As described above, WNV traffics across the BBB both by a Trojan horse mechanism and as a cell-free virus [16]. Remarkably, transit of cell-free virus or VLPs does not alter permeability of the BBB, and rather than a decrease in tight junction protein expression in cultured brain endothelial cells (as seen in other virus infections), there is an increase at the time of peak viral replication [16,18]. WNV infection of cultured brain cortical astrocytes, but not endothelial cells, increases MMP mRNA, protein, and activity, and increases expression of TIMPs [59]. Incubation of WNV-infected astrocyte supernatant with brain endothelial cells results in degradation of tight junction proteins, with a concomitant loss of TEER and barrier integrity. Infection of cultured rat astrocytes with another flavivirus, Japanese encephalitis virus (JEV), increases MMP9 expression in a NF-κB-, MAPK- and ROS-dependent manner [60].
In vivo, WNV infection of wild-type mice results in BBB permeability that is greater than in mice deficient for Toll-like receptor 3 (TLR3) [61]. The Tlr3−/− mice are more resistant to lethal WNV infection, indicating that the TLR3-mediated inflammatory response increases the ability of WNV to enter the brain. WNV infection increases activity and mRNA expression of MMP9 in mouse brains; and from experiments with MMP9−/− mice, MMP9 has been shown to disrupt the BBB and lead to virus entry into the brain [62]. This study also showed that MMP9 protein is elevated in cerebrospinal fluid of WNV-infected patients compared to uninfected controls. Interestingly, lethal WNV infection in some mouse strains or hamsters occurs without BBB breakdown [63]. The mechanism for this is not understood, but the authors note that lymphocytic cells may traffic to the CNS by routes other than the BBB. As with WNV, infection of mice with JEV results in deformation of tight junctions and increased permeability of Evans blue dye in the brain [64].
The dissociation of BBB permeability from lethality occurs in infection by another RNA virus, rabies virus. Rabies virus is a neurotropic negative-sense RNA virus that enters the brain via retrograde axonal transport rather than by disrupting the BBB [1]. Nonetheless, in rabies virus infections of mice, increased BBB permeability and inflammation occur differentially in various parts of the brain, accompanied by clearance of virus and a lack of neurological sequelae [65]. The BBB disruption is dependent on CD4+ T cells, and mice in which there is more extensive BBB permeability and CNS inflammation survive a lethal rabies virus infection better [66,67]. This indicates that in rabies virus infection, BBB disruption enables infiltration of immune effectors critical for survival.
Effects of two other RNA viruses on CNS vascular pathology have recently been reviewed. Some strains of Theiler’s murine encephalomyelitis virus, a positive-sense RNA virus, induce acute encephalitis with alterations in tight junction protein expression [68,69]. In meningitis caused by LCMV, a negative-sense RNA virus, virus-specific CD8+ T cells are required for induction of disease; they are recruited into the CNS, resulting in increased vascular permeability and BBB disruption [68]. However, uncal herniation due to ventricular leakage and edema, rather than the BBB damage, appear to be the cause of the fatal choriomeningitis [68,70]. Thus, similar to WNV and rabies, LCMV-induced BBB disruption itself is not lethal; instead ventricular failure is likely responsible for mortality.
Breaching of the BBB by DNA viruses
The mechanisms that DNA viruses use to enter the brain include both neural spread and breaching of the BBB. Herpes simplex virus-1 (HSV-1) causes rare but severe encephalitis, responsible for the majority of sporadic fatal viral encephalitis cases in the United States [71]. Similar to rabies virus, HSV-1 enters the brain via a neuronal route, and in HSV-1 encephalitis (HSE) BBB damage is seen. In a mouse model of HSE, MMP2 and MMP9 activity are increased, and in situ zymography indicates that MMP9 activity is centered around meninges and parenchymal blood vessels in the brain [71].
MAV-1 causes a fatal encephalomyelitis in susceptible strains of mice, its natural host [72,73]. The virus infects endothelial cells and causes significant histopathology in brain vasculature [72–74]. MAV-1 infection induces increased BBB permeability that is largely independent of inflammation [13]. Primary mouse brain endothelial cells infected with MAV-1 have decreased TEER, tight junction mRNA and protein levels compared to mock-infected cells. MAV-1 replication in the brain is limited to perivascular regions, leading to the hypothesis that the virus crosses the BBB by direct endothelial cell infection. However, entry into the CNS by a Trojan horse mechanism via monocytes, which are also infected by MAV-1, cannot be ruled out [74,75]. Although MAV-1 infection results in inflammatory cell infiltration in the brain, in infected mice in which inflammation is greatly reduced, BBB disruption is equivalent to that in control mice [13]. MAV-1 may thus stimulate an innate host response in infected endothelial cells that induces BBB disruption prior to and/or independent of cellular inflammation, possibly by increasing MMP activity of cells in the NVU and circulation.
Other virus-tight junction interactions: comparative lessons
Endothelia and epithelia share some features, such as tight and adherens junctions, although their permeabilities vary. For example, skin epithelium is a tighter barrier than intestinal epithelium [76]; and due to the unique properties of brain endothelial cells discussed above, the complex tight junctions of brain endothelium are nearly impenetrable compared to other less structurally organized endothelia. Some aspects of tight junction regulation are shared between endothelia and epithelia, including modulation by phosphorylation and oxidative stress [77]. The protein compositions of tight junctions of endothelia and epithelia are generally similar. However, BBB tight junctions have specific claudins (i.e., claudins 3, 5, and 12) that are key to maintenance of barrier function [5,6,78].
Viruses interact not only with tight junctions in the brain, but also tight junctions of airway and intestinal epithelial cells [79]. Some components of tight junctions or adherens junctions are viral attachment receptors or entry factors. For example, JAM-1 is a receptor for reovirus [80] and feline calicivirus [81]; occludin is required for Coxsackievirus B3 entry [82]; and claudin-1 and occludin are hepatitis C virus (HCV) entry factors [83,84]. Nectin-4, an adherens junction protein, was recently identified as an epithelial cell receptor for measles virus [85,86]. Some proteins were first identified as viral receptors and subsequently shown to be components or possible regulators of tight junctions, including CAR [87] and the receptor for human hepatitis A virus, respectively [88]. Why viruses use tight junction proteins as attachment or entry receptors is poorly understood [79]. However, in the setting of the BBB, targeting of tight junctions may enable viral access to the brain parenchyma. For example, recently HCV RNA has been demonstrated to be present in brain tissues of infected individuals, and in vitro brain endothelial cell infection by HCV appears to be claudin-1-dependent [17].
Despite use of tight junction components as receptors by a number of viruses few correlations have been made with junctional damage upon virus binding. However, there is ample evidence for disruption of tight junctions of non-neural vasculature or epithelia at mucosal surfaces as a direct or indirect result of viral infection. For example, HCV infection promotes expression of vascular endothelial growth factor, which alters tight junction integrity and polarity of hepatocytes [89]. Hemorrhagic hantavirus infection of renal epithelial and endothelial cells results in redistribution and reduction in tight junction protein ZO-1 and reduced transepithelial electrical resistance [90]. Dengue virus infection increases vascular permeability, particularly in severe dengue hemorrhagic fever and dengue shock syndrome. It does so at least in part by inducing macrophage migration inhibitory factor, which causes redistribution of the tight junction protein ZO-1 [91]. In cases of severe influenza, in which there is multi-organ failure with edema and high levels of cytokine production, there is increased vascular permeability that is associated with loss of ZO-1 [92]. Given the variety of viruses that utilize tight junction and adherens junction proteins as attachment receptors or entry factors, and the known disruption of tight junctions outside the CNS by viruses, a fruitful area of study will be to investigate junctional damage that results in the BBB as a result of specific virus-host protein interactions.
Concluding remarks
The interplay between viruses and their hosts at the BBB is complex. From HIV-1, we have learned that specific viral genes affect (i) signaling pathways that lead to oxidative stress, (ii) expression of enzymes such as MMPs that disrupt the structure of tight junctions, iii) cytokines that affect inflammation, and (iv) proteasomal degradation of tight junction proteins from within cells. The cumulative effects of these are BBB damage. Much less is known about other encephalitic viruses, but accumulating evidence demonstrates that they too use a variety of mechanisms to breach the BBB. Many viruses disrupt the actin cytoskeleton as part of their life cycle [34], and since cytoskeletal integrity is essential for BBB function, these effects on the cytoskeleton likely play an underappreciated role in BBB disruption.
Much knowledge about BBB disruption by viruses has been gained by extending studies of non-viral CNS disease pathogenesis, including ischemic stroke and multiple sclerosis. Comparing and contrasting viral infections that alter tight junctions in the brain with those that do so in the respiratory, gastrointestinal, or genital tracts will also be informative in future research investigating both viral genes and host response involved in tight junction disruption. Similarly, we can also learn from studies of parasites that breach or damage the BBB, such as Plasmodium spp., Toxoplasma gondii, and trypanosomes, which alter permeability by modifying expression of tight junction proteins and MMPs [38,68,93,94].
The development of good in vitro models, such as immortalized endothelial cells that retain key endothelial properties [37], holds promise for exciting new developments in the study of encephalitic viruses and how they disrupt the BBB (Box 1). Infections of small animals with viruses are important in vivo models that provide insight into virus-host interactions at the whole animal level. Moreover, expanded use of genetically altered mice, particularly knockouts in innate immune system components such as TLRs and inflammasome components, will enable studies of the impact of the host response to BBB disruption that are not possible in vitro. The use of sensitive in situ zymography to detect active MMPs [7], and live cell imaging, including intravital microscopy [4], will extend the use of in vivo and in vitro models. Currently we have a basic knowledge of how viruses disrupt the BBB. Advances in understanding virus-host interactions are likely to be forthcoming as researchers apply powerful genetic, immunological, biochemical, and cell biology approaches to this inquiry.
Box 1. Outstanding questions.
To what extent do virus-induced cytoskeletal changes in brain endothelial cells contribute to BBB disruption?
What is the sequence of events in BBB damage by encephalitic viruses, i.e. disruption from within the cell, or degradation of basal lamina or tight junction proteins from outside? How does this differ among viruses?
What viral gene products or host responses induce changes in tight junction protein expression in endothelial cells, leading to tight junction protein relocalization, altered phosphorylation, and/or degradation? What mechanisms are involved?
To what extent do viral interactions with non-endothelial cells of the NVU (e.g. astrocytes) contribute to BBB disruption?
What is the biological significance of viral CNS infection? What is the advantage to the virus of infecting this site?
Acknowledgments
We apologize to authors whose work we did not have space to cite. We thank Jason Weinberg for comments on the manuscript. K.R.S. has been supported by NIH grants AI023762, AI068645, and AI091721. T.-H. Hsu has been supported by NIH National Research Service Award T32 GM07544, a University of Michigan (U-M) Rackham Graduate School fellowship and Rackham research grants, a U-M Frances Wang Chin Fellowship, and the U-M Endowment for the Development of Graduate Education.
Glossary
- Adherens junctions
Protein complexes located at cell-cell contacts in endothelium and epithelium. Adherens junctions are essential for formation of tight junctions and are anchored on actin cytoskeletons
- Astrocytes
Cells with long processes (‘star’ shaped) that comprise of the majority of neuroglial cells in the brain and spinal cord. Astrocytes are important for development and/or maintenance of BBB characteristics
- Blood-brain barrier (BBB)
The interface between the brain and peripheral circulation. It is composed of specialized capillaries and adjoining cells that function to strictly regulate substances entering the brain from the peripheral circulation
- Central nervous system (CNS)
The part of the nervous system that contains the brain and the spinal cord. Responsible for the control and coordination of the entire body
- Claudins
A family of small transmembrane proteins important for tight junction formation
- Endothelial cells
Cells forming the main structural component of blood vessels
- Junctional adhesion molecules (JAMs)
Members of the immunoglobulin family involved in cell-cell adhesion
- Lymphocytes
A subset of white blood cells of the immune system that includes T cells, B cells and NK cells
- Neurovascular unit (NVU)
An association of endothelium, extracellular matrix, astrocytes, pericytes, microglia, and neurons that contributes structurally and functionally to permeability of the microvasculature
- Matrix metalloproteinases (MMPs)
Zinc-dependent endopeptidases that are involved in a variety of processes including tissue repair, angiogenesis, cell division, apoptosis and host immunity. These proteins can be soluble, matrix- bound or cell-associated
- Microglia
Resident macrophages of the brain and spinal cord. They are the initial and main host immune system responders in the CNS
- Occludin
Transmembrane tight junction protein
- Paracellular transport
Transport of substances between or around cells
- Reactive oxygen and nitrogen species (ROS
RNS), Highly reactive molecules or free radicals that contain oxygen or nitrogen, respectively. These chemicals can mediate cellular damage by attacking biological molecules
- Tight junctions
Protein complexes that include transmembrane and cytoplasmic proteins. Tight junctions are located in intercellular clefts and form close associations that restrict the passage of molecules
- Tissue inhibitors of MMP (TIMPs)
Natural regulators of MMPs
- Transcellular transport
Transport of substances through a cell
- Zona (or zonula) occludens (ZO) proteins
A family of intracellular scaffolding proteins important for the structural integrity of intercellular tight junctions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knipe DM, Howley PM, editors. Fields Virology. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Salinas S, et al. A hitchhiker’s guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol. 2010;8:645–655. doi: 10.1038/nrmicro2395. [DOI] [PubMed] [Google Scholar]
- 3.Griffin DE. Viral encephalomyelitis. PLoS Pathog. 2011;7:e1002004. doi: 10.1371/journal.ppat.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott NJ, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 7.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: Function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS. 2011;8:4. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehner C, et al. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid Redox Signal. 2011;15:1305–1323. doi: 10.1089/ars.2011.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamatovic SM, et al. Brain endothelial cell-cell junctions: How to “open” the blood brain barrier. Curr Neuropharmacol. 2008;6:179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candelario-Jalil E, et al. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strazza M, et al. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gralinski LE, et al. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J Virol. 2009;83:9398–9410. doi: 10.1128/JVI.00954-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afonso PV, et al. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog. 2008;4:e1000205. doi: 10.1371/journal.ppat.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivey NS, et al. Acquired immunodeficiency syndrome and the blood-brain barrier. J Neurovirol. 2009;15:111–122. doi: 10.1080/13550280902769764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma S, et al. West Nile virus infection modulates human brain microvascular endothelial cells tight junction proteins and cell adhesion molecules: Transmigration across the in vitro blood-brain barrier. Virology. 2009;385:425–433. doi: 10.1016/j.virol.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher NF, et al. The neuropathogenesis of feline immunodeficiency virus infection: barriers to overcome. Vet J. 2011;188:260–269. doi: 10.1016/j.tvjl.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasebe R, et al. Transcellular transport of West Nile virus-like particles across human endothelial cells depends on residues 156 and 159 of envelope protein. BMC Microbiol. 2010;10:165. doi: 10.1186/1471-2180-10-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng S, et al. Matrix metalloproteinase-2 and -9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS One. 2011;6:e20599. doi: 10.1371/journal.pone.0020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 21.Gurney KJ, et al. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Reijerkerk A, et al. Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J. 2006;20:2550–2552. doi: 10.1096/fj.06-6099fje. [DOI] [PubMed] [Google Scholar]
- 23.Bojarski C, et al. The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci. 2004;117 (Pt10):2097–2107. doi: 10.1242/jcs.01071. [DOI] [PubMed] [Google Scholar]
- 24.Giebel SJ, et al. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- 25.Wakisaka Y, et al. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. 2010;30:56–69. doi: 10.1038/jcbfm.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haorah J, et al. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 27.Valyi-Nagy T, Dermody TS. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol Histopathol. 2005;20:957–967. doi: 10.14670/HH-20.957. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, et al. Multiple roles of metalloproteinases in neurological disorders. Prog Mol Biol Transl Sci. 2011;99:241–263. doi: 10.1016/B978-0-12-385504-6.00006-3. [DOI] [PubMed] [Google Scholar]
- 29.Lai CH, et al. Critical role of actin in modulating BBB permeability. Brain Res Brain Res Rev. 2005;50:7–13. doi: 10.1016/j.brainresrev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Schreibelt G, et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:3666–3676. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- 31.Hicks K, et al. TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am J Physiol Cell Physiol. 2010;298:C1583–1593. doi: 10.1152/ajpcell.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatovic SM, et al. Potential role of MCP-1 in endothelial cell tight junction ‘opening’ signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–4628. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- 33.Eugenin EA, et al. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: A potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor MP, et al. Subversion of the actin cytoskeleton during viral infection. Nat Rev Microbiol. 2011;9:427–439. doi: 10.1038/nrmicro2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dallasta LM, et al. Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis. Am J Pathol. 1999;155:1915–1927. doi: 10.1016/S0002-9440(10)65511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boven LA, et al. Monocyte infiltration is highly associated with loss of the tight junction protein zonula occludens in HIV-1-associated dementia. Neuropathol Appl Neurobiol. 2000;26:356–360. doi: 10.1046/j.1365-2990.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- 37.Weksler BB, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 38.Zougbédé S, et al. Metabolic acidosis induced by Plasmodium falciparum intraerythrocytic stages alters blood-brain barrier integrity. J Cereb Blood Flow Metab. 2011;31:514–526. doi: 10.1038/jcbfm.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivey NS, et al. Association of FAK activation with lentivirus-induced disruption of blood-brain barrier tight junction-associated ZO-1 protein organization. J Neurovirol. 2009:1–12. doi: 10.1080/13550280902998413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avraham HK, et al. HIV-1 Tat-mediated effects on focal adhesion assembly and permeability in brain microvascular endothelial cells. J Immunol. 2004;173:6228–6233. doi: 10.4049/jimmunol.173.10.6228. [DOI] [PubMed] [Google Scholar]
- 41.Andras IE, et al. Signaling mechanisms of HIV-1 Tat-induced alterations of claudin-5 expression in brain endothelial cells. J Cereb Blood Flow Metab. 2005;25:1159–1170. doi: 10.1038/sj.jcbfm.9600115. [DOI] [PubMed] [Google Scholar]
- 42.Gandhi N, et al. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol. 2010;16:294–305. doi: 10.3109/13550284.2010.499891. [DOI] [PubMed] [Google Scholar]
- 43.Zhong Y, et al. Caveolin-1 regulates human immunodeficiency virus-1 Tat-induced alterations of tight junction protein expression via modulation of the Ras signaling. J Neurosci. 2008;28:7788–7796. doi: 10.1523/JNEUROSCI.0061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahajan SD, et al. Tight junction regulation by morphine and HIV-1 tat modulates blood-brain barrier permeability. J Clin Immunol. 2008;28:528–541. doi: 10.1007/s10875-008-9208-1. [DOI] [PubMed] [Google Scholar]
- 45.Xu R, et al. HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res. 2011;1436:13–19. doi: 10.1016/j.brainres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 46.Ju SM, et al. Extracellular HIV-1 Tat up-regulates expression of matrix metalloproteinase-9 via a MAPK-NF-kappaB dependent pathway in human astrocytes. Exp Mol Med. 2009;41:86–93. doi: 10.3858/emm.2009.41.2.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toneatto S, et al. Evidence of blood-brain barrier alteration and activation in HIV-1 gp120 transgenic mice. AIDS. 1999;13:2343–2348. doi: 10.1097/00002030-199912030-00005. [DOI] [PubMed] [Google Scholar]
- 48.Cioni C, Annunziata P. Circulating gp120 alters the blood-brain barrier permeability in HIV-1 gp120 transgenic mice. Neurosci Lett. 2002;330:299–301. doi: 10.1016/s0304-3940(02)00814-5. [DOI] [PubMed] [Google Scholar]
- 49.Louboutin JP, et al. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol. 2010;69:801–816. doi: 10.1097/NEN.0b013e3181e8c96f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanmogne GD, et al. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: Implications for the pathogenesis of HIV-associated dementia. J Neuropathol Exp Neurol. 2005;64:498–505. doi: 10.1093/jnen/64.6.498. [DOI] [PubMed] [Google Scholar]
- 51.Kanmogne GD, et al. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab. 2007;27:123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamuta S, et al. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol. 2008;14:186–195. doi: 10.1080/13550280801993630. [DOI] [PubMed] [Google Scholar]
- 53.Wonderlich ER, et al. HIV immune evasion disruption of antigen presentation by the HIV Nef protein. Adv Virus Res. 2011;80:103–127. doi: 10.1016/B978-0-12-385987-7.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ranki A, et al. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995;9:1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Kohleisen B, et al. Stable expression of HIV-1 Nef induces changes in growth properties and activation state of human astrocytes. AIDS. 1999;13:2331–2341. doi: 10.1097/00002030-199912030-00004. [DOI] [PubMed] [Google Scholar]
- 56.Masanetz S, Lehmann MH. HIV-1 Nef increases astrocyte sensitivity towards exogenous hydrogen peroxide. Virol J. 2011;8:35. doi: 10.1186/1743-422X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eugenin EA, et al. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci. 2011;31:9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lév que T, et al. Differential regulation of gelatinase A and B and TIMP-1 and -2 by TNFα and HIV virions in astrocytes. Microbes Infect. 2004;6:157–163. doi: 10.1016/j.micinf.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Verma S, et al. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology. 2010;397:130–138. doi: 10.1016/j.virol.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tung WH, et al. Japanese encephalitis virus induces matrix metalloproteinase-9 in rat brain astrocytes via NF-kappaB signalling dependent on MAPKs and reactive oxygen species. Br J Pharmacol. 2010;161:1566–1583. doi: 10.1111/j.1476-5381.2010.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang T, et al. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 62.Wang P, et al. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J Virol. 2008;82:8978–8985. doi: 10.1128/JVI.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrey JD, et al. Increased blood-brain barrier permeability is not a primary determinant for lethality of West Nile virus infection in rodents. J Gen Virol. 2008;89:467–473. doi: 10.1099/vir.0.83345-0. [DOI] [PubMed] [Google Scholar]
- 64.Liu TH, et al. The blood-brain barrier in the cerebrum is the initial site for the Japanese encephalitis virus entering the central nervous system. J Neurovirol. 2008;14:514–521. doi: 10.1080/13550280802339643. [DOI] [PubMed] [Google Scholar]
- 65.Phares TW, et al. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 66.Roy A, Hooper DC. Lethal silver-haired bat rabies virus infection can be prevented by opening the blood-brain barrier. J Virol. 2007;81:7993–7998. doi: 10.1128/JVI.00710-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phares TW, et al. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-alpha is neither necessary nor sufficient. J Immunol. 2007;178:7334–7343. doi: 10.4049/jimmunol.178.11.7334. [DOI] [PubMed] [Google Scholar]
- 68.Kang SS, McGavern DB. Microbial induction of vascular pathology in the CNS. J Neuroimmune Pharmacol. 2010;5:370–386. doi: 10.1007/s11481-010-9208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suidan GL, et al. Induction of blood brain barrier tight junction protein alterations by CD8 T cells. PLoS One. 2008;3:e3037. doi: 10.1371/journal.pone.0003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matullo CM, et al. Lymphocytic choriomeningitis virus-induced mortality in mice is triggered by edema and brain herniation. J Virol. 2010;84:312–320. doi: 10.1128/JVI.00727-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sellner J, et al. Herpes-simplex virus encephalitis is characterized by an early MMP-9 increase and collagen type IV degradation. Brain Res. 2006;1125:155–162. doi: 10.1016/j.brainres.2006.09.093. [DOI] [PubMed] [Google Scholar]
- 72.Guida JD, et al. Mouse adenovirus type 1 causes a fatal hemorrhagic encephalomyelitis in adult C57BL/6 but not BALB/c mice. J Virol. 1995;69:7674–7681. doi: 10.1128/jvi.69.12.7674-7681.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kring SC, et al. Susceptibility and signs associated with mouse adenovirus type 1 infection of adult outbred Swiss mice. J Virol. 1995;69:8084–8088. doi: 10.1128/jvi.69.12.8084-8088.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kajon AE, et al. Distribution of mouse adenovirus type 1 in intraperitoneally and intranasally infected adult outbred mice. J Virol. 1998;72:1219–1223. doi: 10.1128/jvi.72.2.1219-1223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ashley SL, et al. Mouse adenovirus type 1 infection of macrophages. Virology. 2009;390:307–314. doi: 10.1016/j.virol.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moens E, Veldhoen M. Epithelial barrier biology: good fences make good neighbours. Immunology. 2012;135:1–8. doi: 10.1111/j.1365-2567.2011.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Z, et al. Molecular regulation of the intestinal epithelial barrier: implication in human diseases. Front Biosci. 2012;17:2903–2909. doi: 10.2741/3888. [DOI] [PubMed] [Google Scholar]
- 78.Nitta T, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergelson JM. Intercellular junctional proteins as receptors and barriers to virus infection and spread. Cell Host Microbe. 2009;5:517–521. doi: 10.1016/j.chom.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 80.Barton ES, et al. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–451. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 81.Makino A, et al. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J Virol. 2006;80:4482–4490. doi: 10.1128/JVI.80.9.4482-4490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coyne CB, et al. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–192. doi: 10.1016/j.chom.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans MJ, et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- 84.Ploss A, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mühlebach MD, et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–583. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noyce RS, et al. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7:e1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen CJ, et al. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin TA, et al. HAVcR-1 reduces the integrity of human endothelial tight junctions. Anticancer Res. 2011;31:467–473. [PubMed] [Google Scholar]
- 89.Mee CJ, et al. Hepatitis C virus infection reduces hepatocellular polarity in a vascular endothelial growth factor-dependent manner. Gastroenterology. 2010;138:1134–1142. doi: 10.1053/j.gastro.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krautkrämer E, et al. Pathogenic old world hantaviruses infect renal glomerular and tubular cells and induce disassembling of cell-to-cell contacts. J Virol. 2011;85:9811–9823. doi: 10.1128/JVI.00568-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chuang YC, et al. Macrophage migration inhibitory factor induced by dengue virus infection increases vascular permeability. Cytokine. 2011;54:222–231. doi: 10.1016/j.cyto.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 92.Wang S, et al. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J Infect Dis. 2010;202:991–1001. doi: 10.1086/656044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Prato M, et al. Natural haemozoin modulates matrix metalloproteinases and induces morphological changes in human microvascular endothelium. Cell Microbiol. 2011;13:1275–1285. doi: 10.1111/j.1462-5822.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 94.Lacerda-Queiroz N, et al. Plasmodium berghei NK65 induces cerebral leukocyte recruitment in vivo: an intravital microscopic study. Acta Trop. 2011;120:31–39. doi: 10.1016/j.actatropica.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 95.Francis K, et al. Innate immunity and brain inflammation: The key role of complement. Expert Rev Mol Med. 2003;5:1–19. doi: 10.1017/S1462399403006252. [DOI] [PubMed] [Google Scholar]