Abstract
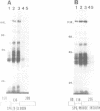
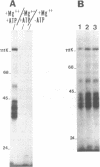
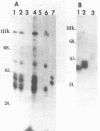
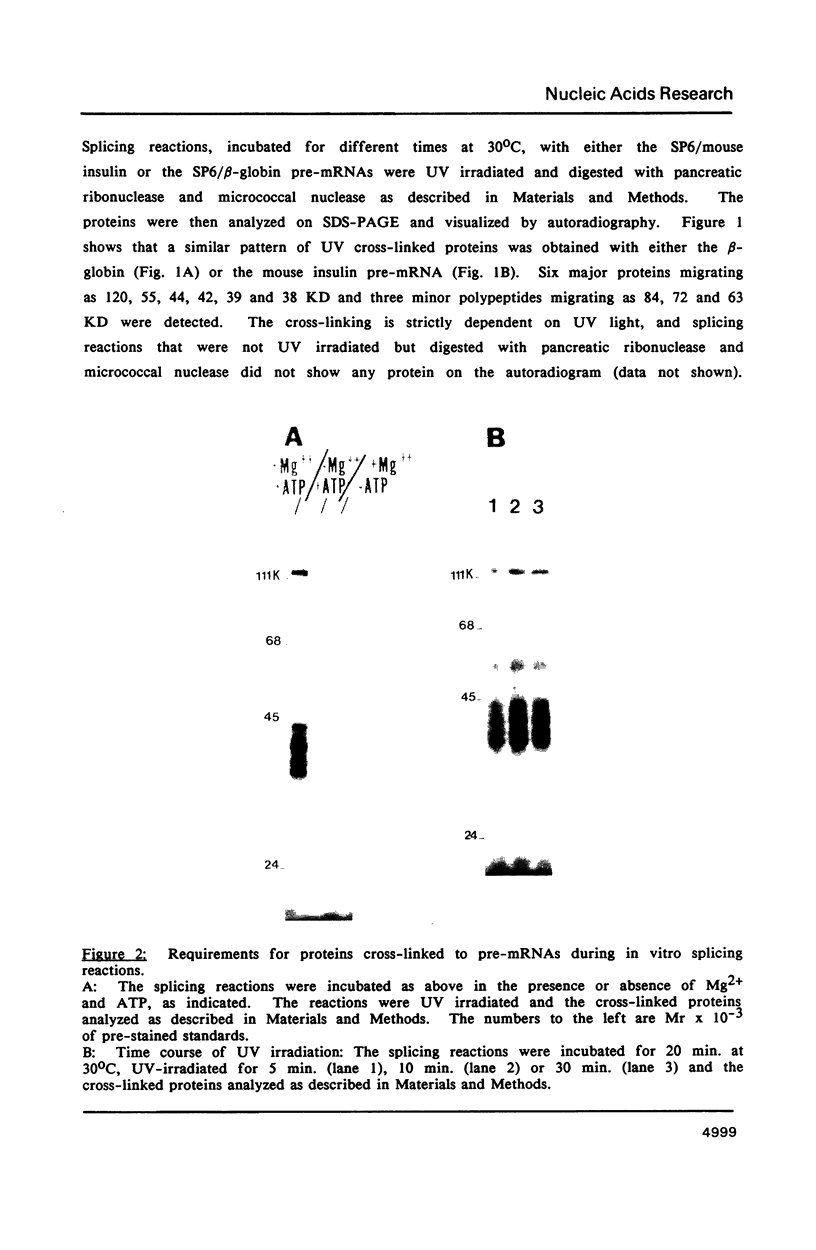
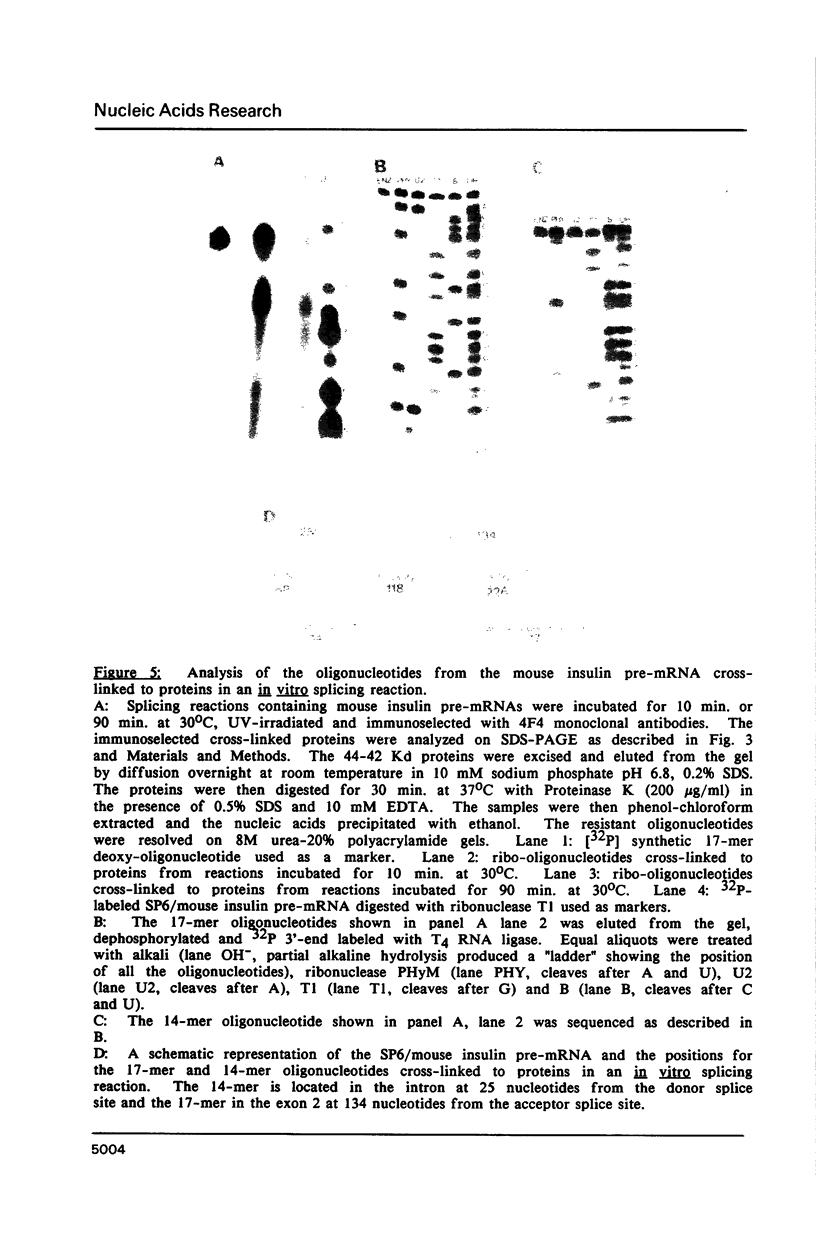
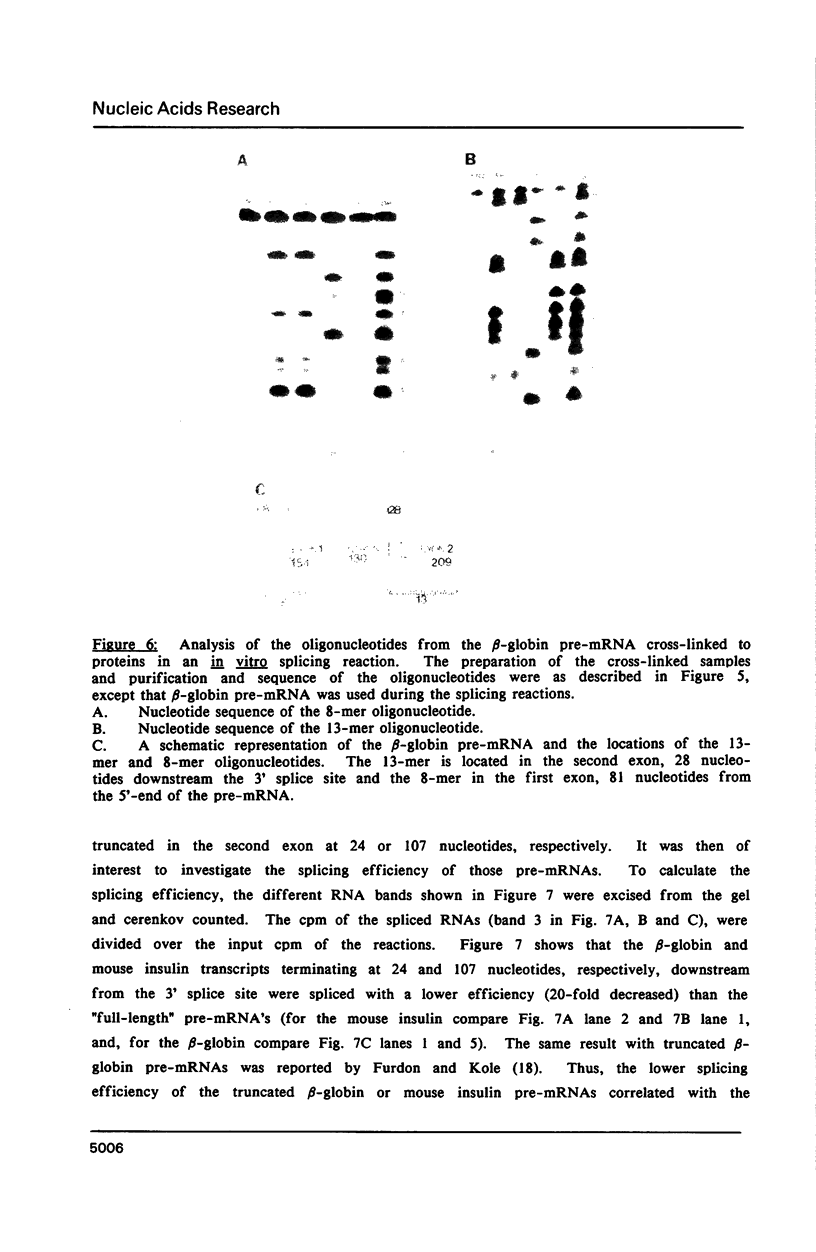
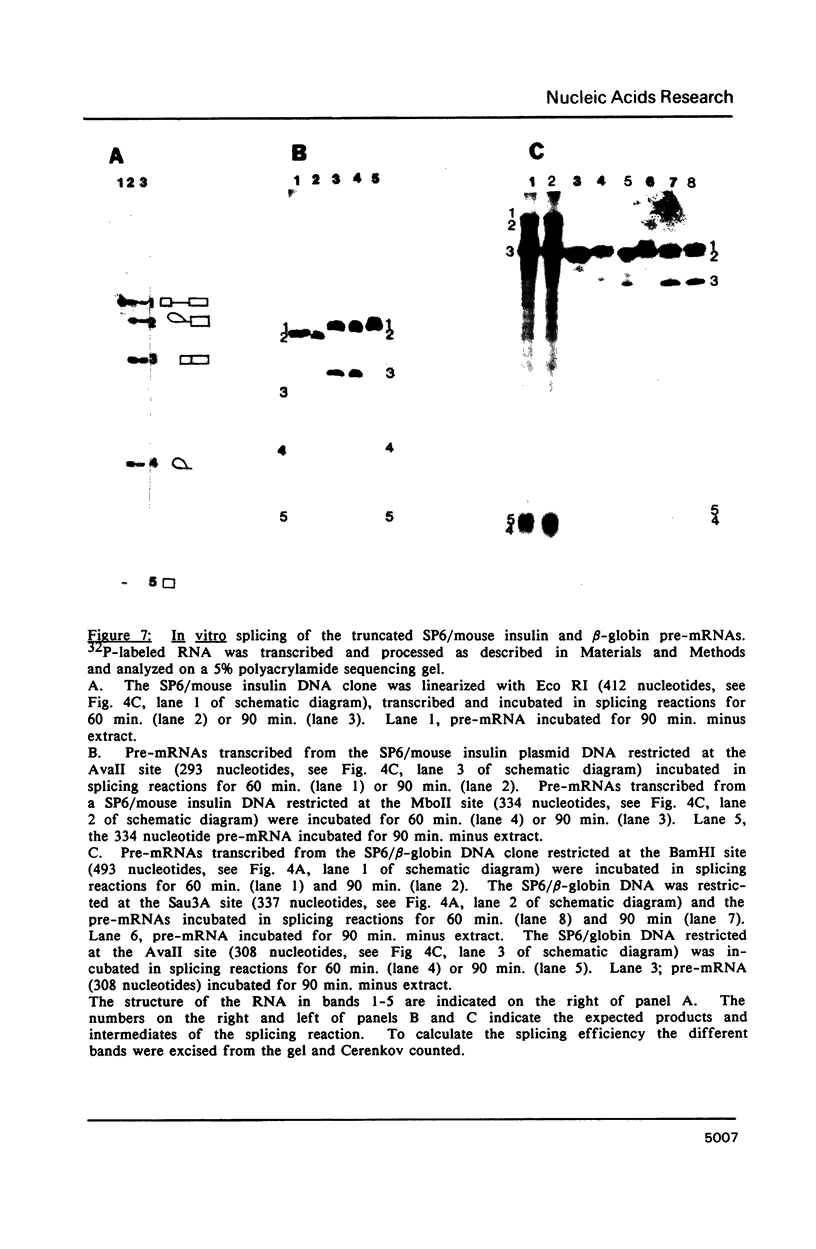
The proteins that are in direct contact with the pre-mRNA in an in vitro splicing reaction were analyzed by UV cross-linking experiments. Six major proteins (120, 55, 44, 42, 39 and 38 KD) and three minor polypeptides (84, 72 and 63 KD) were detected. The predominant proteins 44, 42 KD belong to the class of hnRNP C proteins since they were immunoprecipitated by monoclonal antibodies directed against hnRNP C proteins. The cross-linked proteins were not detected in the absence of Mg2+, ATP or when RNA lacking introns were used as substrates in the splicing reactions. The effect of exon sequences on the binding efficiency for the photocrosslinked proteins was investigated. Transcripts containing a second exon of 24 nucleotides for the beta-globin or 107 nucleotides for the mouse insulin, yielded a reduced amount of cross-linked proteins when compared with "full length" pre-mRNAs. Sequences within the first exon of the beta-globin pre-mRNA did not affect the binding efficiency of these proteins. The reduced binding efficiency of the cross-linked proteins for the truncated beta-globin or mouse insulin pre-mRNAs correlated with the lower efficiency for in vitro splicing. Substitutions with unrelated sequences in the beta-globin second exon restore the binding of the cross-linked proteins indicating that the length of the second exon and not specific sequences are relevant for the binding efficiency of these proteins. The SP6/mouse insulin oligonucleotides cross-linked to the hnRNP C proteins were isolated and sequenced. A 17-mer was located in the second exon (134 nucleotides downstream from the 3' splice site) and a 14-mer in the intron region (25 nucleotides downstream the 5' splice site). The beta-globin oligonucleotides cross-linked to the hnRNP C proteins were a 13-mer in the second exon (28 nucleotides downstream the 3' splice site) and an 8-mer in the first exon (81 nucleotides downstream the 5' end of the pre-mRNA). Our results indicate that the hnRNP C proteins interact with those oligonucleotides located in different regions of the pre-mRNA. The binding efficiency of those proteins, however, depends on the length of the second exon and the presence of intron sequences (secondary and/or tertiary pre-mRNA structure).
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Shih D. S., Zimmern D., Kaesberg P. Two-step binding of eukaryotic ribosomes to brome mosaic virus RNA3. Nature. 1979 Sep 27;281(5729):277–282. doi: 10.1038/281277a0. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Bouton A. H., Miller O. L., Jr Correlation of hnRNP structure and nascent transcript cleavage. Cell. 1981 Oct;26(2 Pt 2):155–165. doi: 10.1016/0092-8674(81)90299-3. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Christensen M. E., Walker B. W., LeStourgeon W. M. Identification and characterization of the packaging proteins of core 40S hnRNP particles. Cell. 1977 May;11(1):127–138. doi: 10.1016/0092-8674(77)90323-3. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Miller O. L., Jr, McKnight S. L. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980 May;20(1):75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Bindereif A., Green M. R. Ribonucleoprotein complex formation during pre-mRNA splicing in vitro. Mol Cell Biol. 1986 Jul;6(7):2582–2592. doi: 10.1128/mcb.6.7.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Black D. L., Steitz J. A. Pre-mRNA splicing in vitro requires intact U4/U6 small nuclear ribonucleoprotein. Cell. 1986 Aug 29;46(5):697–704. doi: 10.1016/0092-8674(86)90345-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chabot B., Black D. L., LeMaster D. M., Steitz J. A. The 3' splice site of pre-messenger RNA is recognized by a small nuclear ribonucleoprotein. Science. 1985 Dec 20;230(4732):1344–1349. doi: 10.1126/science.2933810. [DOI] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. Isolation of the heterogeneous nuclear RNA-ribonucleoprotein complex (hnRNP): a unique supramolecular assembly. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7471–7475. doi: 10.1073/pnas.81.23.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. D., Dreyfuss G. Monoclonal antibody characterization of the C proteins of heterogeneous nuclear ribonucleoprotein complexes in vertebrate cells. J Cell Biol. 1984 Dec;99(6):1997–1204. doi: 10.1083/jcb.99.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. D., Grabowski P. J., Sharp P. A., Dreyfuss G. Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science. 1986 Mar 28;231(4745):1534–1539. doi: 10.1126/science.3952495. [DOI] [PubMed] [Google Scholar]
- DiMaria P. R., Kaltwasser G., Goldenberg C. J. Partial purification and properties of a pre-mRNA splicing activity. J Biol Chem. 1985 Jan 25;260(2):1096–1102. [PubMed] [Google Scholar]
- Dreyfuss G., Choi Y. D., Adam S. A. Characterization of heterogeneous nuclear RNA-protein complexes in vivo with monoclonal antibodies. Mol Cell Biol. 1984 Jun;4(6):1104–1114. doi: 10.1128/mcb.4.6.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin A., Jove R., Hemenway C., Keiser H. D., Manley J. L., Prives C. Splicing pathways of SV40 mRNAs in X. laevis oocytes differ in their requirements for snRNPs. Cell. 1984 Jul;37(3):927–936. doi: 10.1016/0092-8674(84)90427-6. [DOI] [PubMed] [Google Scholar]
- Frendewey D., Keller W. Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell. 1985 Aug;42(1):355–367. doi: 10.1016/s0092-8674(85)80131-8. [DOI] [PubMed] [Google Scholar]
- Furdon P. J., Kole R. Inhibition of splicing but not cleavage at the 5' splice site by truncating human beta-globin pre-mRNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):927–931. doi: 10.1073/pnas.83.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V., Steitz J. A. A protein associated with small nuclear ribonucleoprotein particles recognizes the 3' splice site of premessenger RNA. Cell. 1986 Dec 26;47(6):973–984. doi: 10.1016/0092-8674(86)90812-3. [DOI] [PubMed] [Google Scholar]
- Grabowski P. J., Seiler S. R., Sharp P. A. A multicomponent complex is involved in the splicing of messenger RNA precursors. Cell. 1985 Aug;42(1):345–353. doi: 10.1016/s0092-8674(85)80130-6. [DOI] [PubMed] [Google Scholar]
- Kaltwasser G., Spitzer S. G., Goldenberg C. J. Assembly in an in vitro splicing reaction of a mouse insulin messenger RNA precursor into a 60-40S ribonucleoprotein complex. Nucleic Acids Res. 1986 May 12;14(9):3687–3701. doi: 10.1093/nar/14.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler J. T. An assessment of the evidence for the role of ribonucleoprotein particles in the maturation of eukaryote mRNA. Int Rev Cytol. 1983;84:103–153. doi: 10.1016/s0074-7696(08)61016-5. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T. Multiple factors including the small nuclear ribonucleoproteins U1 and U2 are necessary for pre-mRNA splicing in vitro. Cell. 1985 Oct;42(3):725–736. doi: 10.1016/0092-8674(85)90269-7. [DOI] [PubMed] [Google Scholar]
- Krupp G., Gross H. J. Rapid RNA sequencing: nucleases from Staphylococcus aureus and Neurospora crassa discriminate between uridine and cytidine. Nucleic Acids Res. 1979 Aug 10;6(11):3481–3490. doi: 10.1093/nar/6.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A., Keller W., Appel B., Lührmann R. The 5' terminus of the RNA moiety of U1 small nuclear ribonucleoprotein particles is required for the splicing of messenger RNA precursors. Cell. 1984 Aug;38(1):299–307. doi: 10.1016/0092-8674(84)90551-8. [DOI] [PubMed] [Google Scholar]
- Mayrand S. H., Pedersen N., Pederson T. Identification of proteins that bind tightly to pre-mRNA during in vitro splicing. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3718–3722. doi: 10.1073/pnas.83.11.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheim Y. N., Miller O. L., Jr, Beyer A. L. RNP particles at splice junction sequences on Drosophila chorion transcripts. Cell. 1985 Nov;43(1):143–151. doi: 10.1016/0092-8674(85)90019-4. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Mount S. M., Steitz J. A., Sharp P. A. Splicing of messenger RNA precursors is inhibited by antisera to small nuclear ribonucleoprotein. Cell. 1983 Nov;35(1):101–107. doi: 10.1016/0092-8674(83)90212-x. [DOI] [PubMed] [Google Scholar]
- Perkins K. K., Furneaux H. M., Hurwitz J. RNA splicing products formed with isolated fractions from HeLa cells are associated with fast-sedimenting complexes. Proc Natl Acad Sci U S A. 1986 Feb;83(4):887–891. doi: 10.1073/pnas.83.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierakowska H., Szer W., Furdon P. J., Kole R. Antibodies to hnRNP core proteins inhibit in vitro splicing of human beta-globin pre-mRNA. Nucleic Acids Res. 1986 Jul 11;14(13):5241–5254. doi: 10.1093/nar/14.13.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazi J., Alibert C., Temsamani J., Reveillaud I., Cathala G., Brunel C., Jeanteur P. A protein that specifically recognizes the 3' splice site of mammalian pre-mRNA introns is associated with a small nuclear ribonucleoprotein. Cell. 1986 Dec 5;47(5):755–766. doi: 10.1016/0092-8674(86)90518-0. [DOI] [PubMed] [Google Scholar]
- Wollenzien P. L., Goswami P., Teare J., Szeberenyi J., Goldenberg C. J. The secondary structure of a messenger RNA precursor probed with psoralen is melted in an in vitro splicing reaction. Nucleic Acids Res. 1987 Nov 25;15(22):9279–9298. doi: 10.1093/nar/15.22.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang V. W., Lerner M. R., Steitz J. A., Flint S. J. A small nuclear ribonucleoprotein is required for splicing of adenoviral early RNA sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1371–1375. doi: 10.1073/pnas.78.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]