Abstract
Cancer stem cells (CSCs) play critical roles in cancer initiation, progression, and therapeutic refractoriness. Although many studies have focused on the genes and pathways involved in stemness, characterization of the factors in the tumor microenvironment that regulate CSCs is lacking. In this study, we investigated the effects of stromal fibroblasts on breast cancer (BC) stem cells. We found that compared to normal fibroblasts, primary cancer-associated fibroblasts (CAFs) and fibroblasts activated by co-cultured BC cells produce higher levels of chemokine (C-C motif) ligand 2 (CCL2), which stimulates the stem cell-specific, sphere-forming phenotype in BC cells and CSC self-renewal. Increased CCL2 expression in activated fibroblasts required STAT3 activation by diverse BC-secreted cytokines, and in turn, induced NOTCH1 expression and the CSC features in BC cells, constituting a “cancer-stroma-cancer” signaling circuit. In a xenograft model of paired fibroblasts and BC tumor cells, loss of CCL2 significantly inhibited tumorigenesis and NOTCH1 expression. In addition, upregulation of both NOTCH1 and CCL2 was associated with poor differentiation in primary BCs, further supporting the observation that NOTCH1 is regulated by CCL2. Our findings therefore suggest that CCL2 represents a potential therapeutic target that can block the cancer-host communication that prompts CSC-mediated disease progression.
Keywords: breast cancer, cancer stem cells, chemokine (C-C motif) ligand 2, tumor microenvironment, stromal fibroblasts
Introduction
Recent studies indicate that a subset of cancer cells possessing stem cell properties, referred to as cancer-initiating or cancer stem cells (CSCs), play crucial roles in tumor initiation, progression and therapeutic refractoriness (1–2). Similar to embryonic and somatic stem cells, the self-renewal and differentiation of CSCs are simultaneously regulated by intrinsic (cancer cell-endowed) and extrinsic (microenvironmental) factors. Despite the increasing number of studies on genes and pathways involved in cancer “stemness”, factors in the tumor microenvironment that regulate CSCs, and how cancer cells, in turn, modify the niche by influencing their neighboring cells remain largely uncharacterized. In this study, we focus on the regulation of CSCs by stromal fibroblasts, an important cellular component of the tumor-hosting niche in many human cancers, especially breast cancer (BC). Fibroblasts release a variety of growth factors, chemokines, and components of the extracellular matrix into the microenvironment and influence the differentiation and homeostasis of adjacent epithelia (3). Reconstitution of human-specific mammary glands in cleared mouse mammary fat pads using stem-cell-enriched human mammary epithelial cell organoids requires co-injection of human mammary fibroblasts (4), suggesting a critical role for fibroblasts in regulating stem cell functions. Fibroblasts interplay with cancer cells at all stages of cancer progression through complex paracrine mechanisms. Cancer-associated fibroblasts (CAFs) can promote cancer progression by modulating multiple components in the cancer niche to build a permissive and supportive microenvironment for tumor growth and invasion.
In the current study, we observed robust induction of the stem-cell-like mammosphere-forming phenotype in BC cells co-cultured with CAFs. The CSC-stimulating effect was attributed to the increased secretion of CCL2 (monocyte chemotactic protein-1; MCP-1) by CAFs when compared to normal cell-associated fibroblasts (NAFs). CCL2 signals through the G protein-coupled receptor chemokine (C-C motif) receptors CCR2 and CCR4, and is a potent chemoattractant for monocytes and other immune cells to areas of inflammation (5). Both monocytes and non-monocytic cells, such as fibroblasts and endothelial cells, secrete CCL2 in response to cytokine stimulation (6–7). Cancer cells also frequently overexpress CCL2 in order to modify their local environment. Here, we further found that CCL2 induced the self-renewal of CSCs by inducing NOTCH1 expression at both RNA and protein levels. NOTCH signaling has been recognized as a key regulator in normal and malignant stem cells from various tissues, including the breast (8–9). Upon ligand binding, NOTCH receptors are activated by sequential cleavages involving members of the ADAM (a disintegrin and metalloprotease domain) protease family (α-secretases) and γ-secretase. These cleavage events result in translocation of the NOTCH intracellular domain (NICD) to the nucleus, where it acts on downstream targets (10). Our identification of NOTCH-mediated activation by tumor environmental factor CCL2 therefore presents a unique mode of niche-conferred regulation of CSCs. These findings further emphasize the importance of simultaneously targeting cancer cells and events within the tumor microenvironment, such as production of CCL2 by stromal cells, in future anti-cancer therapies that also incorporate the latest stem cell research.
Materials and Methods
Tissue sources and cell purification
Human BC tissues were obtained from consented patients at City of Hope Medical Center (Duarte, CA) under approved institutional review board protocols. Tissues were mechanically and enzymatically dissociated, and epithelial tumor cells and fibroblasts (CAFs) were isolated as previously described (11). Briefly, BC tissue was mechanically minced into small pieces, placed in digestion solution containing collagenase III (Worthington Biochemical Corporation; Lakewood, NJ) and DNase I (AppliChem; St. Louis, MO), and incubated at 37°C for 2–3 h with occasional pipetting. The cells were separated by differential centrifugation at 90 ×g for 2 min. The epithelial (tumor) cells in the pellet were cultured in Iscove’s Modified Dulbecco’s Media (Invitrogen; Grand Island, NY) containing 0.7 mM L-glutamine (Mediatech/Cellgro; Manassas, VA), 5 μg/ml insulin (Lonza; Allendale, NJ), 5 μg/ml transferrin (Lonza), 5 ng/ml selenium (Lonza), and 20% fetal bovine serum (FBS; PAA Laboratories; Dartmouth, MA). The supernatant containing fibroblasts were centrifuged at 800 ×g for 10 min, resuspended and cultured in Dulbecco’s Modified Eagle Medium (Mediatech/Cellgro) containing 10% FBS on a non-treated dish. Purity of primary tumor cells and CAFs were confirmed by expression of Epithelial Specific Antigen (ESA) and Vimentin, respectively, in flow cytometry and immunofluorescence assays (Fig. S1). CAF265922 (primary CAFs) and XP265922 (primary tumor cells) were isolated from a primary triple-negative BC that was resistant to the chemotherapy regimen containing cisplatin, 5-fluorouracil, and docetaxel. CAF3 were isolated from a primary HER2-positive BC for which the primary tumor cells were not available. Normal human mammary fibroblasts (NAF2) were purchased from ScienCell (Carlsbad, CA). For immunohistochemistry in primary breast tumors, pretreatment core biopsies or surgical specimens were obtained from patients with HER2-positive (31 cases) or triple-negative (ER−/PR−/HER2−; 20 cases) BC. Specimens were collected and processed for formalin fixation and paraffin embedding in a time frame that would preserve the integrity of protein epitopes.
Cell lines, plasmids and viruses
Human BC cell lines BT474, MDA-MB-361 (MDA361) and MCF7, and the non-cancerous mammary epithelial cell line MCF10A were obtained from American Type Culture Collection (Manassas, VA) and cultured in the recommended media in a humidified 5% CO2 incubator at 37°C. Recombinant human CCL2 was purchased from R&D Systems (Minneapolis, MN). The STAT3 inhibitor Stattic, p38 MAPK inhibitor SB202190, and γ-secretase inhibitor DAPT were purchased from Sigma-Aldrich (St. Louis, MO). The α-secretase inhibitor INCB3619 was provided by Incyte Corporation (Wilmington, DE). For conditional knockdown of CCL2, the shRNA targeting the CCL2 mRNA (TRCN0000006283) was constructed into the pTIG (pHIV7-TetR-IRES-GFP) lentiviral vector (12) (kindly provided by Dr. Rossi) downstream of a Dox inducible U6 promoter, as described elsewhere (13). GFP-labeled CAF265922 were generated using pBABE-GFP retroviral vector. Production of viruses, as well as infection and selection of CAFs were carried out as previously described (13).
Mammosphere formation assay
Please see Supplemental Materials for procedures.
RNA extraction, reverse transcription (RT) and real-time quantitative PCR (qPCR)
Please see Supplemental Materials for procedures.
Cytokine antibody array and Western blot analyses
Please see Supplemental Materials for procedures.
Cell transfection, reporter assays, and RNAi studies
Please see Supplemental Materials for procedures.
Flow cytometry and cell sorting
Single-cell suspensions prepared from tumors or cell culture were stained with APC-conjugated human ESA antibody (Catalog #347200; BD Biosciences, Franklin Lakes, NJ) or analyzed by ALDEFLUOR assay kit (Catalog #01700; Stemcell Technologies, Vancouver, BC, Canada) following the manufacturer’s protocol. Flow cytometry assays were performed using a CyAn ADP cytometer (Dako; Carpinteria, CA) and analyzed with FlowJo software (TreeStar; Ashland, OR). Electronic cell sorting based on intensity of GFP, APC, PKH67 or ALDEFLUOR was done on a FACSAriaIII cell sorter (BD Biosciences).
Xenografts
All animal experiments were approved by the institutional animal care and use committee at City of Hope. Please see Supplemental Materials for procedures.
Immunohistochemistry (IHC)
IHC staining of formaldehyde-fixed, paraffin-embedded primary or xenograft tumor tissues was performed as previously reported (14) using the following antibodies and dilutions: human CCL2 (Catalog #ab9669; Abcam; Cambridge, MA), 1:75 dilution; NOTCH1 (for primary BC: Catalog #1935-1; Epitomics; Burlingame, CA; for xenograft tumors: Catalog #3608; Cell Signaling; Danvers, MA), 1:40 dilution; and SMA (Catalog #ab5694; Abcam), 1:100 dilution. For CCL2 and SMA, cytoplasmic staining was evaluated and for NOTCH1 nuclear staining was evaluated. Stained slides were scored according to intensity of staining (-: 0; +: 1; ++: 2; and +++: 3) and percentage of tumor cells staining positive for each antigen (0%: 0; 1~30%: 1; 31~70%: 2; and >70%: 3). The score for the intensity of immunostaining was multiplied by the score for the percentage of cells staining positive to obtain a final score, which was used in the statistical correlation analysis of CCL2 and NOTCH1.
Statistical analyses
All quantitative data are presented as mean ± standard deviation (SD). All results were confirmed in at least three independent experiments, and data from one representative experiment was shown. The statistical analysis was performed using a SPSS 13.0 software package. Student t test or ANOVA test was used for comparison of quantitative data. Values of P < 0.05 were considered significant. The differential expression of CCL2, CCR2 and NOTCH family of receptors and ligands between Grade 3 and Grade 1 BCs were analyzed using a logistic regression model after which an odds ratio (OR) was reported. For example, an OR of 13.72 (CCL2) means that tumors with each unit increase in CCL2 expression are 13.72 times more likely to be Grade 3, rather than Grade 1 (95% CI = 2.66–70.89). The linear dependence between CCL2 and NOTCH1 expression was evaluated by Pearson (for the microarray dataset) or Spearman correlation coefficient (for the IHC dataset). The microarray gene expression data and associated clinical data were extracted from a published 295 BC dataset (15–16).
Results
CAF-derived CCL2 induces the stem-cell-like mammosphere-forming phenotype in BC cells
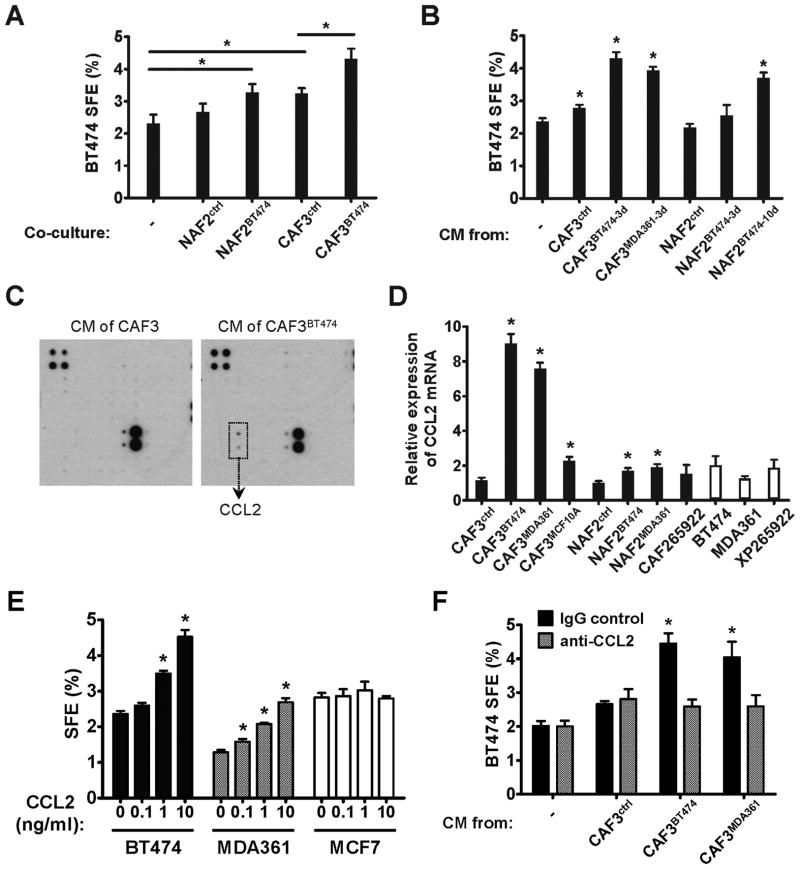
The mammosphere assay has been used to functionally characterize and enrich normal and malignant stem cells from the breast, relying on the unique feature of stem cells to escape anoikis and grow into spheres in anchorage-independent conditions (17–18). Using this approach, we examined the sphere-forming efficiency (SFE) in BT474 BC cells in the presence or absence of co-cultured primary human mammary NAFs or CAFs. Compared to the BC cells that were cultured alone, co-culture with CAFs, but not NAFs, significantly increased mammosphere formation in BC cells (Fig. 1A). When NAFs and CAFs were first “activated” in vitro by co-culturing with BT474 cells before being transferred to the mammosphere co-culture, both activated NAFs and the CAFs that were continuously activated by BC cells in vitro were able to induce BT474 mammosphere formation to a greater extent than their pre-activation counterparts (Fig. 1A). The mammosphere-inducing effect was also observed with the conditioned medium (CM) harvested from CAFs, but not NAFs. CM from CAFs that were further activated by BT474 or MDA361 BC cells exhibited an enhanced capacity for inducing mammosphere formation. In vitro activation of NAFs also resulted in mammosphere-inducing activity in the CM, which was more significant in NAFs that had been co-cultured with BT474 cells for a prolonged 10-day period (Fig. 1B). These results indicate that the continuous activation of stromal fibroblasts by BC cells leads to secretion of fibroblast-derived soluble factors that can induce the CSC-like phenotype.
Fig. 1. CCL2 secreted by cancer-activated fibroblasts induces mammosphere formation in BC cells.
(A) Mammosphere formation assay of BT474 cells co-cultured with various fibroblasts. NAFs and CAFs were first co-cultured with BT474 cells growing in transwell inserts for 3 days (NAF2BT474 and CAF3BT474), or cultured alone (NAF2ctrl and CAF3ctrl), before being transferred to transwell inserts and co-cultured with freshly plated BT474 cells for mammosphere assays. Spheres were counted on day 10, and sphere forming efficiency (SFE) was calculated. * p<0.01. (B) Mammosphere formation assay of BT474 cells exposed to the conditioned media (CM) of differentially treated fibroblasts. Fibroblasts were first treated with BT474- or MDA361-derived CM for 3 or 10 days as indicated by “3d” or “10d” in the superscript, or with regular medium (NAF2ctrl and CAF3ctrl). Treated fibroblasts were then cultured in sphere-forming media for 24 h to prepare CM subsequently used in BT474 sphere formation assays. * p<0.01 compared to the control (the first column). (C) CM was collected from CAF3 that had been previously treated for 3 days with BT474 CM (CAF3BT474), and from untreated CAF3 as a control. Concentrated CM was subjected to a cytokine antibody array. Induction of CCL2 protein was observed in the CM of CAF3BT474. (D) Relative expression of CCL2 mRNA in various cell types was determined by RT-qPCR. CAF265922 (primary CAFs) and XP265922 (primary tumor cells) were isolated from the same BC specimen. The superscripts denote CM treatments as in (B). The time length of CM treatment was 3 days in CAF3 and 10 days in NAF2. * p<0.01 compared to the untreated control CAF3 or NAF2. (E) BT474, MDA361, and MCF7 BC cells were assayed for sphere formation in the presence of CCL2 at the indicated concentrations. * p<0.01 compared to the control (no CCL2 treatment) in each cell line. (F) Effect of CAF CM (as indicated in B) on BT474 sphere formation in the presence or absence of a CCL2 neutralizing antibody. * p<0.01 compared to the control (the first column). Each bar represents the mean ± S.D. of 3 wells.
To identify these soluble factors, we performed a cytokine array assay using the CM of untreated and BT474-treated CAFs. Significant induction of CCL2 was observed following in vitro activation of CAFs (Fig. 1C). At the RNA level, expression of CCL2 was induced in CAFs 7 to 9-fold in response to activation by various BC cells, with BC-activated CAFs producing the highest amounts of CCL2 among various cell types, including NAFs and BC cells. Treatment of CAFs with the non-cancerous MCF10A human mammary epithelial cells, as well as short-term (3-day) treatments of NAFs with BC cells only modestly induced CCL2 expression (Fig. 1D). To examine the direct effect of CCL2 on CSCs, we added increasing amounts of recombinant CCL2 to various BC cells, and observed dose-dependent formation of mammospheres in BT474 and MDA361, but not MCF7 cells (Fig. 1E). Using a neutralizing antibody for CCL2, we further showed that depletion of functional CCL2 from the CM of activated CAFs abolished the CSC-inducing activity (Fig. 1F). Thus, we concluded that BC-activated CAFs regulate CSCs through expression and secretion of higher levels of CCL2.
CCL2 induces the self-renewing expansion of CSCs
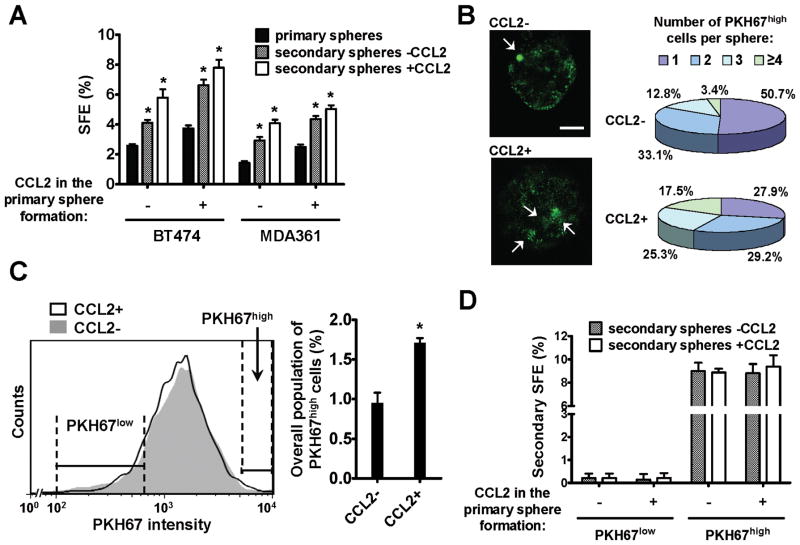
The sphere-initiating CSCs are maintained in the primary mammospheres through self-renewal, and are able to give rise to secondary mammospheres when cells from the primary spheres are dissociated and allowed to grow in anchorage-independent conditions (17). We therefore examined the effect of CCL2 on CSC self-renewal by secondary mammosphere culture. Interestingly, compared to the control spheres that had not been treated with CCL2, the first-passage spheres that had been treated with CCL2 contained higher numbers of CSCs capable of initiating secondary spheres, even in the absence of continuous CCL2 treatment (Fig. 2A). Based on this result, we hypothesized that CCL2 either promoted the self-renewal (to cause a self-renewing expansion) of existing CSCs, or, alternatively, promoted the conversion of non-CSCs to CSCs. These possibilities were examined using a reported approach involving the labeling of an initial cell population with PKH fluorescent dye and tracking the serial dilution of fluorescence in single-cell-formed spheres during the cell division events in mammosphere formation (19). Because the PKH dye binds to cell membranes and segregates in daughter cells after each cell division, the PKH fluorescence intensity of each cell in a sphere reflects its proliferation history. The PKHhigh cells, therefore, represent the stem cell population that has undergone a limited number of divisions during sphere formation, in contrast to the non-stem, PKHlow cells that are highly proliferative (19). Using PKH67-labeled BT474 cells, we generated primary mammospheres containing cells with various PKH67 intensities. In the absence of CCL2 treatment, about 50% of mammospheres carried 1 PKH67high cell per sphere, and about 33% and 13% carried 2 and 3 PKH67high cells, respectively. This distribution was altered in CCL2-treated spheres, where the percentage of spheres carrying a single PKH67high cell decreased to 28%, and of those carrying 3 or more PKH67high cells significantly increased (Fig. 2B). In spheres carrying multiple PKH67high cells, the PKH67high cells also exhibited slightly dimmer fluorescence comparing to those in spheres carrying single PKH67high cells (Fig. 2B and S2), likely indicating one or two more rounds of division (and PKH67 dilution) of the PKH67high cells before entering the stem-cell-like, slow-proliferating or quiescent state in the multi-PKH67high-cell spheres. The overall population of PKH67high cells in dissociated sphere cells was also ~2-fold higher in CCL2-treated spheres than untreated spheres, as determined by flow cytometric analysis (Fig. 2C). When the PKH67high and PKH67low sphere cells were purified by fluorescence-activated cell sorting, the PKH67high primary sphere cells exhibited the highest SFE in the secondary mammosphere formation assay when compared to the PKH67low cells (Fig. 2D), consistent with their CSC property. CCL2 treatment did not alter the SFE of PKH67low and PKH67high cells in the secondary sphere formation (Fig. 2D), indicating that CCL2 does not cause conversion of non-CSCs (PKH67low) to CSCs (sphere-initiating). Therefore, we conclude that CCL2 regulates CSCs by inducing their self-renewing expansion. Interestingly, a similar effect has been previously described for NOTCH activation on stem cells (8). The role of NOTCH pathway in mediating CSC regulation by CCL2 was further investigated in Fig. 4.
Fig. 2. CCL2 induces the self-renewing expansion of CSCs.
(A) Primary and secondary sphere formation in the presence or absence of CCL2 (10 ng/ml). * p<0.01 compared to the control (the first column in each cell line). (B) Left: Confocal images of representative spheres formed by PKH67-labeled BT474 cells in the presence or absence of CCL2. PKH67high cells are indicated by arrows. Scale bar equals 20 μm. Right: Summary of the numbers of PKH67high cells per sphere by counting 150 spheres formed in the presence or absence of CCL2. (C) A representative histogram indicating the flow cytometric profile of dissociated primary sphere cells and gating of PKH67low and PKH67high cells. Percentage of PKH67high cells was summarized in the bar graph. Each bar represents the mean ± S.D. of 3 independently treated sphere cultures. * p<0.01. (D) Secondary mammosphere formation of sorted PKH67high and PKH67low primary sphere cells. Each bar represents the mean ± S.D. of 3 wells.
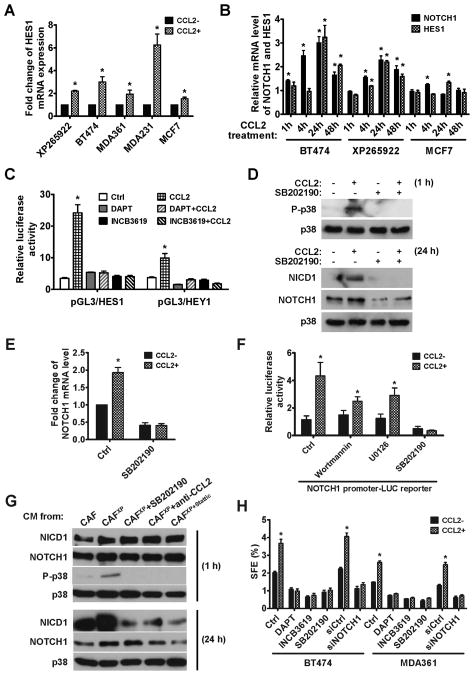
Fig. 4. CCL2 regulates CSC phenotype in BC cells by activating NOTCH signaling.
(A) Total RNA isolated from various BC cell lines treated with CCL2 or vehicle for 24 h were analyzed for the expression of HES1, a target gene activated by NOTCH signaling. Data of RT-qPCR were normalized to 18S in each sample. Each bar represents the mean ± S.D. of 3 wells. * p<0.001 compared to the control (the first column). (B) Expression of NOTCH1 and HES1 mRNAs upon CCL2 treatment in the indicated time course. The mRNA level at each time point was compared to that in untreated cells, which was set as 1. Each bar represents the mean ± S.D. of 3 wells. * p<0.01 compared to untreated cells. (C) Luciferase reporters of HES1 and HEY1 were transfected into XP265922 primary BC cells. Luciferase activity was analyzed at 24 h post CCL2 treatment +/− inhibitors of γ-secretase (DAPT; 10 μM) or α-secretase (INCB3619; 5 μM). Each bar represents the mean ± S.D. of 3 independently transfected wells. * p<0.001 compared to the control (the first column). (D) Western blot analysis of p38 and NOTCH1 at indicated time points following treatment conditions in XP265922 cells. (E) Expression of NOTCH1 mRNA in XP265922 cells treated with CCL2 +/− SB202190 (5 μM) for 24 h. * p<0.001 compared to the control (the first column). (F) A luciferase reporter containing a 6-kb NOTCH1 promoter was transfected into XP265922 cells to analyze NOTCH1 promoter regulation by CCL2 and various inhibitors (Wortmannin: 1 μM; U0126: 10 μM; SB202190: 5 μM). Each bar represents the mean ± S.D. of 3 independently transfected wells. * p<0.001 compared to the control (the first column). (G) XP265922 cells were treated with CM from indicated sources and analyzed for NOTCH1 and p38 expression by Western blot. (H) Mammosphere formation assay of BC cells treated with various inhibitors in the presence or absence of CCL2. Each bar represents the mean ± S.D. of 3 wells. * p<0.01 compared to the control (the first column in each cell line).
Paracrine signaling of cancer-secreted cytokines induces CCL2 production in fibroblasts via STAT3 activation
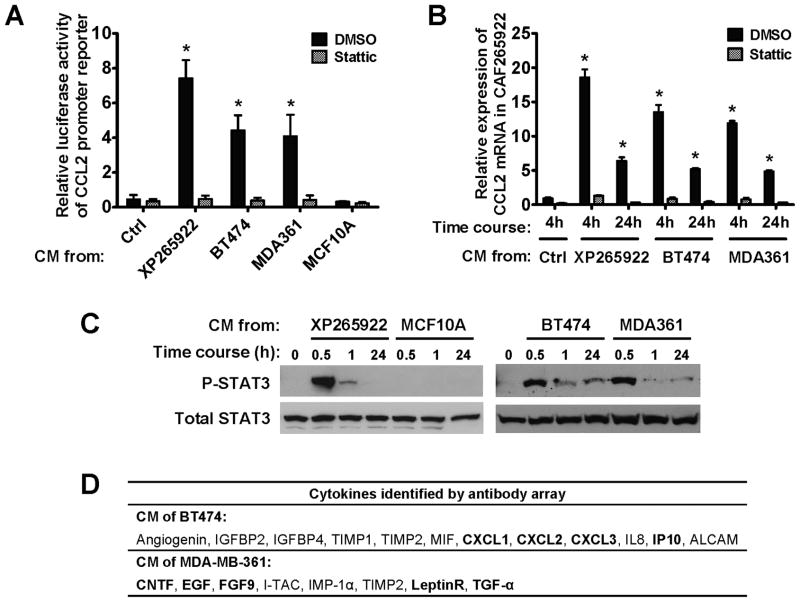
We then set out to identify the mechanism underlying increased CCL2 production in BC-activated CAFs. STAT3 has been recently reported to bind to and activate the promoter of CCL2 (20). To determine if BC cells induce CCL2 expression in fibroblasts through STAT3 activation, we first examined the effects of BC-derived CM and a STAT3 inhibitor on a previously reported CCL2 promoter reporter (21) and on CCL2 expression in CAFs freshly isolated from a triple-negative (ER−/PR−/HER2−) primary BC (CAF265922). CM from BC cells, including BT474, MDA361 and the primary BC cells isolated from the same BC specimen (XP265922), but not from MCF10A cells, markedly induced luciferase expression driven by the CCL2 promoter (Fig. 3A), as well as endogenous expression of CCL2 (Fig. 3B). These effects were abrogated by addition of the STAT3 inhibitor (Fig. 3A and 3B), indicating STAT3 involvement in mediating the induction of fibroblast-derived CCL2 expression. Similar results were also obtained using CAFs from a different primary BC (Fig. S3). Indeed, high levels of STAT3 phosphorylation were observed in CAFs as early as 30 min following treatment with BC-derived, but not MCF10A-derived, CM (Fig. 3C). The induction of CCL2 expression was sustained over a time course of 5 days in CAFs co-cultured with BC cells, and was abolished by inhibition of STAT3 (Fig. S4). Although CM from BT474, MDA361 and primary BC cells all induced STAT3 phosphorylation and CCL2 expression in CAFs, different cytokines were detected in the CM of BT474 and MDA361 (Fig. 3D). In either case, multiple cytokines with reported STAT3-activating effect were detected, suggesting that the STAT3 activation observed in BC-treated CAFs was likely a combined effect of various BC-secreted cytokines. Thus, BC cells derived from different origins and secreting different cytokines activate the STAT3 core pathway in fibroblasts of the tumor microenvironment, leading to STAT3-mediated promoter activation of CCL2.
Fig. 3. Paracrine signaling of cancer-secreted cytokines induces CCL2 production in fibroblasts via STAT3 activation.
(A) A luciferase reporter containing a 2.8-kb CCL2 promoter was transfected into CAF265922 cells. Luciferase activity was analyzed at 4 h post CM exposure in the presence of DMSO or Stattic (a STAT3 inhibitor; 5 μM). Each bar represents the mean ± S.D. of 3 independently transfected wells. * p<0.01 compared to the control (the first column). (B) Total RNA isolated from CAF265922 that had been treated with CM from indicated BC cells for 4 or 24 h was analyzed for CCL2 mRNA level by RT-qPCR. Data were normalized to 18S in each sample. Each bar represents the mean ± S.D. of 3 wells. * p<0.01 compared to the control (the first column). (C) CAF265922 cells were treated with CM from indicated cells and analyzed by Western blot. (D) Summary of the cytokine array data identifying cytokines constitutively secreted by BT474 and MDA361 BC cells. Cytokines that are known to activate STAT3 are in bold.
CCL2 induces CSCs by activating NOTCH signaling
To explore the molecular basis for CCL2-mediated regulation of CSCs, we surveyed the CCL2 responsiveness of genes involved in NOTCH, Wnt/β-catenin and Hedgehog pathways, which are known to regulate stem cells (8, 22–23). We found that expression of NOTCH1 and a target gene of NOTCH signaling, HES1, were induced by CCL2 in various BC cells, and that HES1 expression occurred subsequent to NOTCH1 expression (Fig. 4A and 4B). Interestingly, the NOTCH-activating effect of CCL2 was only subtle in MCF7 cells, which also failed to respond to CCL2-induced sphere formation (Fig. 1E). This may be related to the lower expression level of CCR2 in MCF7 compared to other cells (Fig. S5), making MCF7 less sensitive to CCL2-triggered effects. Transfected HES1 and HEY1 reporters were significantly activated by CCL2 in primary BC cells and BT474 cells, and these effects were abolished by inhibitors of the NOTCH-activating α- and γ-secretases (Fig. 4C and S6). Activation of NOTCH signaling by CCL2 was thus attributed to increased NOTCH1 expression, as CCL2 showed no effect on the activities of α- and γ-secretases as assessed by in vitro substrate cleavage assays (Fig. S7). Upon CCL2 treatment, a rapid and dramatic induction of p38 MAPK phosphorylation was observed, followed by the induction of NOTCH1 and NICD1 (cleaved NOTCH1) proteins at a later time point (Fig. 4D). Both p38 phosphorylation and NOTCH1/NICD1 induction were abolished by an inhibitor of p38 MAPK (Fig. 4D and 4E). Using a 6-kb NOTCH1 promoter reporter, we observed a ~4-fold induction of NOTCH1 promoter activity by CCL2 in primary BC cells, and this effect was also completely suppressed by the p38 MAPK inhibitor, but only partially suppressed by inhibitors of PI3K and MEK1/2 MAPK (Fig. 4F). This suggests that p38 MAPK plays an essential role in activating NOTCH1 promoter, whereas PI3K and MEK1/2 MAPK may play an accessory role. Induction of p38 MAPK phosphorylation and NOTCH1 proteins (full-length and cleaved) was also observed in XP265922 BC cells treated with CM from CAF265922 previously activated by co-culturing with XP265922. This effect was abolished by treatment with a p38 MAPK inhibitor or CCL2 neutralizing antibody, and also by using CM from CAF265922 previously co-cultured with XP265922 cells but in the presence of a STAT3 inhibitor (Fig. 4G). We therefore concluded that in BC cells, CCL2 induces NOTCH1 expression and its downstream signaling mainly through p38-dependent activation of the NOTCH1 promoter. In BT474 and MDA361 BC cells, CCL2-induced mammosphere formation was efficiently blocked by inhibitors of the α- and γ-secretases and p38 MAPK, and by RNA interference (RNAi) of NOTCH1 (Fig. 4H), indicating that activation of NOTCH1 mediates the effect of CCL2 on CSCs.
Fibroblast-specific knockdown of CCL2 or CCL2 depletion by a neutralizing antibody inhibits in vivo tumorigenesis
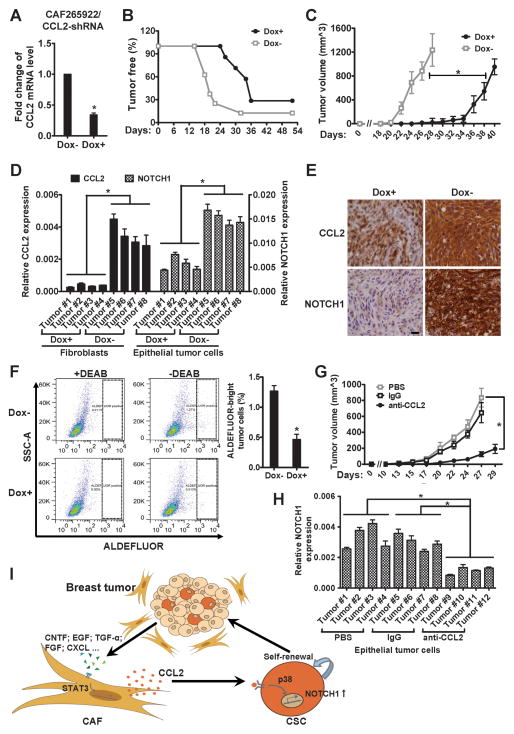
To obtain in vivo evidence of the CSC-regulating function of fibroblast-derived CCL2, we established an orthotopic xenograft model of co-transplanted primary CAFs and BC cells. CAF265922 were stably transduced to express doxycycline (Dox)-inducible short hairpin RNA (shRNA) of CCL2, which suppressed CCL2 expression in vitro by ~60% in the presence of Dox (Fig. 5A). The modified CAFs were then co-transplanted with BC cells from the same primary tumor (XP265922) into the mammary fat pads of female NOD/SCID/IL2Rγ-null (NSG) mice. Mammary tumor formation was monitored in transplanted mice with or without Dox treatment. Fibroblast-specific knockdown of CCL2 in the Dox+ group resulted in significantly delayed tumor formation and reduced tumor volume, compared to the Dox− control group (Fig. 5B and 5C). Xenograft tumors from both groups were harvested, and the fibroblast (labeled by lentiviral-encoded GFP) and tumor cell (positive for human ESA) components were separated for gene expression analyses. In Dox+ tumors, both fibroblast-derived CCL2 expression and tumor cell-derived NOTCH1 expression were significantly lower than their counterparts in Dox− tumors (Fig. 5D). Immunohistochemistry (IHC) staining also indicated lower levels of CCL2 and NOTCH1 proteins in the Dox+ tumors, compared to the Dox− tumors (Fig. 5E). We further analyzed the CSC population within all tumor cells in each xenograft tumor using ALDEFLUOR flow cytometry analysis. The ALDEFLUORbright CSC populations in Dox+ tumors were significantly smaller than those in Dox− tumors (Fig. 5F; ~0.45% vs. ~1.3%), indicating a decrease in the number of CSCs. Thus, fibroblast-derived CCL2 secretion appears to play an important role in tumorigenesis and in regulating NOTCH1 expression and the CSC population in BC cells. Consistent with these results, the CCL2 neutralizing antibody, but not a control IgG or PBS, significantly suppressed tumorigenesis and decreased NOTCH1 expression in tumor cells when XP265922 and GFP-labeled CAF265922 were co-transplanted into NSG mice (Fig. 5G, 5H and S8). Thus, based on the studies that have been described to this point, we propose a model of CSC generation that incorporates a crosstalk circuit involving STAT3, CCL2 and NOTCH pathways (Figure 5I).
Fig. 5. Fibroblast-specific CCL2 knockdown or CCL2 depletion by neutralizing antibody inhibits in vivo tumorigenesis.
(A) CAF265922 cells stably expressing Dox-inducible CCL2 shRNA were examined for CCL2 mRNA expression by RT-qPCR upon 48 h treatment of Dox (1 μg/ml) or vehicle. * p<0.001. (B) In vivo tumor formation was examined by co-transplanting unmodified XP265922 primary BC cells and the CAF265922 cells tested in (A) into the mammary fat pads of NOD/SCID/IL2Rγ-null mice, as described in Materials and Methods. The time-course of xenograft tumor formation in Dox-treated mice (Dox+) and control mice (Dox−) was compared. p<0.001 between the two groups. (C) Tumor volume determined in Dox+ and Dox− mice. * p<0.001 at each available time point starting from day 22. (D) Total RNA was isolated from fibroblasts and epithelial tumor cells purified from the Dox+ and Dox− xenograft tumors, and subjected to RT-qPCR for CCL2 and NOTCH1 expression, respectively. * p<0.001. (E) Representative immunohistochemistry images of Dox+ and Dox− xenograft tumor sections stained with antibodies against CCL2 and NOTCH1 (40×; bar=20 μm). (F) Representative flow cytometry dot plots indicating the ALDEFLUOR-bright tumor cells from Dox+ and Dox−xenograft tumors. Diethylaminobenzaldehyde (DEAB), an inhibitor of ALDH, was added in the left two panels. Bar graph: Averaged percentages of the ALDEFLUOR-bright population from bulk tumor cells in 4 Dox+ and 4 Dox− xenograft tumors. * p<0.001. (G) Tumor volume determined in mice treated with PBS, IgG, or CCL2 neutralizing antibody. * p<0.001. (H) Total RNA was isolated from epithelial tumor cells purified from the three groups of xenograft tumors, and subjected to RT-qPCR for NOTCH1 expression. * p<0.001. (I) A model of the CSC-stimulating crosstalk circuit that involves STAT3, CCL2 and NOTCH pathways. In the tumor microenvironment, paracrine signaling initiated by BC cells induces CCL2 production by stromal fibroblasts through STAT3 activation, and the fibroblast-derived CCL2, in turn, promotes cancer progression by regulating CSCs through activation of the NOTCH pathway.
Expression of CCL2 and NOTCH1 are correlated in primary BCs and associated with poor differentiation of tumor cells
To extend our findings to a larger number of primary BCs, odds ratio (OR) and 95% confidence internal (CI) were calculated using unconditional logistic regression to determine if the microarray-determined expression levels of CCL2, CCR2 and NOTCH family of receptors and ligands in a previously reported BC dataset (15–16) were associated with tumor grade (Table 1). Patients with Grade 3 (poorly-differentiated) and Grade 1 (well-differentiated) tumors were included in the analysis. We didn’t include the Grade 2 tumors because they fell between Grade 1 and Grade 3 and exhibited highly diverse levels of differentiation, compared to Grades 1 and 3. Patients with poor differentiation (Grade 3) were significantly associated with higher levels of CCL2 (OR = 13.72, 95% CI = 2.66–70.89), NOTCH1 (OR = 9.56, 95% CI = 1.54–59.27) and delta-like 3 (OR = 20.93, 95% CI = 1.53–289.94), as well as lower level of jagged 1 (OR = 0.14, 95% CI = 0.03–0.57).
Table 1.
Relative odds of having BC Grade 3 (N = 119) vs. Grade 1 (N = 75) associated with CCL2 and NOTCH related genes.
| Gene Symbol | Gene Name | OR* | CI** | p |
|---|---|---|---|---|
| CCL2 | chemokine (C-C motif) ligand | 13.72 | 2.66 – 70.89 | < 0.01 |
| CCR2 | chemokine (C-C motif) receptor 2 | 6.32 | 0.87 – 45.82 | 0.07 |
| NOTCH1 | Notch homolog 1 | 9.56 | 1.54 – 59.27 | 0.02 |
| NOTCH2 | Notch homolog 2 | 0.76 | 0.15 – 3.90 | 0.74 |
| NOTCH3 | Notch homolog 3 | 2.51 | 0.36 – 17.29 | 0.35 |
| NOTCH4 | Notch homolog 4 | 0.39 | 0.03 – 4.33 | 0.44 |
| DLL1 | delta-like 1 | 0.48 | 0.10 – 2.30 | 0.36 |
| DLL3 | delta-like 3 | 20.93 | 1.53 – 286.94 | 0.02 |
| DLL4 | delta-like 4 | 1.16 | 0.03 – 49.36 | 0.94 |
| JAG1 | jagged 1 | 0.14 | 0.03 – 0.57 | < 0.01 |
| JAG2 | jagged 2 | 0.31 | 0.08 – 1.19 | 0.09 |
Odds ratio of Grade 3 vs. Grade 1;
95% Confidence interval.
In addition, correlation coefficients were calculated between microarray-determined CCL2 and NOTCH1 expression and stratified by tumor grade and stage (Table 2, top part). A significant linear correlation was observed between CCL2 and NOTCH1 expression among all BCs (R = 0.18, p < 0.01). This association was especially pronounced in Grade 3 tumors (R = 0.19, p = 0.03), and in Stage 1 (R = 0.33, p < 0.01) and Stage 2a disease (R = 0.19, p = 0.02). We further evaluated the expression of CCL2 and NOTCH1 at the protein level by IHC in 51 HER2+ or triple-negative primary BCs. The correlation between two proteins was approaching statistical significance (R = 0.26, p = 0.06) among all tumors, and was indeed significant within HER2+ tumors (R = 0.40, p = 0.03), but not triple-negative BCs (Table 2, bottom part). Although the previously reported association of CCL2 with poor clinical outcome (24) was not detected in the current cohort, our results nevertheless support a relationship between CCL2 and NOTCH1 with effects on CSCs in primary BCs.
Table 2.
Correlation between CCL2 and NOTCH1 in primary human BC.
| Microarray-based expression
| |||
|---|---|---|---|
| Group | N (%) | R* | p |
| All | 295 (100) | 0.18 | < 0.01 |
| Grade | |||
| 1 | 75 (25.4) | 0.21 | 0.07 |
| 2 | 101 (34.2) | 0.02 | 0.81 |
| 3 | 119 (40.3) | 0.19 | 0.03 |
| Stage | |||
| 1 | 82 (27.8) | 0.33 | < 0.01 |
| 2a | 142 (48.1) | 0.19 | 0.02 |
| 2b | 41 (13.9) | 0.05 | 0.76 |
| 3–4 | 30 (10.2) | 0.13 | 0.49 |
| IHC-based expression
| |||
|---|---|---|---|
| Group | N (%) | R** | p |
| All | 51 (100) | 0.26 | 0.06 |
| Subtype | |||
| triple-negative | 20 (39.2) | 0.04 | 0.86 |
| HER2+ | 31 (60.8) | 0.40 | 0.03 |
Pearson correlation coefficient.
Spearman correlation coefficient.
Discussion
Fibroblasts are altered by cancer cells through non-genetic modifications, and in turn, effect direct changes on cancer cells or indirect changes on the tumor microenvironment to facilitate cancer growth and invasion. The resulting co-evolution of cancer and the hosting niche critically influences disease progression. Our attention to the influence of fibroblasts on CSCs, the “seeds” of cancer, showed that, upon cancer-mediated activation, human mammary fibroblasts secreted CCL2 to induce CSC generation through activation of NOTCH signaling. In the cancer niche, CCL2 may be produced and secreted into the extracellular environment by almost all cell types, including cancer cells, stromal fibroblasts, tumor-infiltrated monocytes, and endothelial cells. Nevertheless, our study indicated that: 1) CCL2 expression was 4 to 9-fold higher in activated CAFs than in BC cells (Fig. 1D); 2) fibroblast-specific knockdown of CCL2 significantly suppressed tumorigenesis and the CSC population in xenograft tumors (Fig. 5A-F); and 3) CCL2 is detected by IHC in both tumor cells and stromal fibroblasts in primary BC (Fig. S9). These data strongly suggest that CAFs are an important source of CCL2 in the cancer niche and a major environmental regulator of CSCs.
The CAF populations in tumor-associated stroma are known to include both fibroblasts and myofibroblasts. Myofibroblasts are endowed with the ability to promote tumor growth and associated with higher-grade malignancy and poor prognosis in cancer patients (25–26). These cells express α-smooth muscle actin (SMA) to be distinguished from fibroblasts. Kojima et al. recently show that through self-sustaining autocrine signaling of transforming growth factor β (TGF-β) and stromal cell-derived factor-1 (SDF-1), fibroblasts can transdifferentiate into myofibroblasts during tumor progression (26). The CAFs prepared and examined in our study indeed contained a subpopulation of SMA-expressing myofibroblasts, as indicated by immunofluorescent assay and immunohistochemistry (Fig. S1B and S8). However, the SMA+ myofibroblasts and SMA− fibroblasts produced CCL2 at comparable levels in the cancer niche (Fig. S8), suggesting that the increased CCL2 production and enhanced CSC-promoting capacity of the herein examined CAFs are unlikely related to the myofibroblast phenotype, but rather a general alteration in fibroblasts in response to cancer-derived stimulation. Our findings also support further studies on the regulation of CSCs and their non-cancerous counterparts by other physiological and therapeutic conditions that locally elevate CCL2 levels. Such conditions, including wound healing, inflammation, and chemotherapy (7, 27), are becoming increasingly appreciated for their relevance to the biology of normal and cancerous stem cells.
Our data indicate that the CCL2-producing and CSC-promoting ability of CAFs is conferred by BC-secreted soluble factors present in the CM, such as the cytokines listed in Fig. 3D. Although different BC cells appeared to secrete distinct sets of cytokines to induce CCL2 production in CAFs, these cytokines ultimately functioned through STAT3, and inhibition of STAT3 completely abrogated the induction of CCL2 by BC paracrine signaling (Fig. 3). Therefore, compared to the diverse signals released by cancer cells, the common effector STAT3 serves as a superior target to therapeutically block cancer-induced activation of stromal fibroblasts. STAT3 has been identified as an important effector and target in cancer cells and tumor-infiltrated immune cells (28). Our study now identifies STAT3-mediated fibroblast activation as a potential therapeutic target, further supporting the idea that anti-STAT3 therapies may exert dual effects on both cancer and host cells, halting their dynamic and mutual activation during cancer progression.
CCL2 has been implicated in breast cancer progression and metastasis (29). In primary breast tumors, CCL2 expression is correlated with the accumulation of tumor-associated macrophages, and is a significant indicator of early relapse (24, 30). Overexpression of CCL2 in BC cells promotes metastasis formation in lungs and bone through increasing macrophage infiltration and osteoclast differentiation, respectively (31). A recent report demonstrates that CCL2 produced by both tumor and stromal cells recruits the CCR2-expressing inflammatory monocytes to the pulmonary metastases of mammary tumors, where monocyte-derived factors promote endothelial permeability and extravasation of tumor cells (32). CCL2 expression is interactively regulated in the crosstalk between tumor and niche cells. Increased expression of CCL2 is detected in the bone marrow mesenchymal stem cells (MSCs) following stimulation by leukemia cells, resulting in enhanced cancer-promoting capacity of MSCs (33). Co-culture with MSCs, in turn, induces CCL2 expression in cancer cells (34). These previous studies have therefore established an important role of CCL2 in cancer-host crosstalk through the regulation of tumor cell homing and metastasis, angiogenesis and the immune system.
Here we show that CCL2 induces CSCs both in vitro and in vivo through activation of NOTCH (Fig. 4–5). NOTCH activation has been shown to promote the self-renewal of mammary stem cells (8). An important role for NOTCH signaling in human cancers has been long established (35), and several γ-secretase inhibitors (GSIs) are currently in early clinical development as potential NOTCH-targeting therapeutics. It is proposed that single-agent GSI therapy may be effective in triple-negative BCs, which are known to harbor CSC-like characteristics (36). Here, our data further support the use of NOTCH-targeting agents in efficiently blocking the stimulatory effect of stromal fibroblasts on CSCs. Our data also indicate that activation of p38 MAPK is required for CCL2-induced NOTCH1 expression (Fig. 4). The E2A-encoded transcription factors E12 and E47 have been shown to activate NOTCH1 expression through binding to multiple E-box sites in the 6-kb NOTCH1 promoter region (37). Phosphorylation of E47 by p38 MAPK and by MAPK-activated protein kinase 2 (MAPKAPK2), a kinase activated by p38, has been reported (38–39). The function of p38-mediated E47 phosphorylation in regulating NOTCH1 promoter activity is still unclear, and may underlie the induction of NOTCH1 by CCL2, which induces potent p38 activation in primary BC cells (Fig. 4D). In addition, the NOTCH-activating effect of CCL2 was only subtle in the ER+/PR+/HER2−MCF7 cells (Fig. 4A and 4B), which also failed to respond to CCL2-induced sphere formation (Fig. 1E). Whether the lower CCR2 level in MCF7 (Fig. S5) causes their low sensitivity to CCL2 effect, and whether levels of CCL2 receptors are associated with BC subtypes need to be further investigated. Nevertheless, IHC staining of primary BCs indicated a significant correlation between CCL2 and NOTCH1 in HER2+ tumors (Table 2, bottom part), suggesting that at least in these tumors, as observed in the HER2+ BT474 and MDA361 BC cells, the regulation of NOTCH signaling by CCL2 may indeed occur in vivo.
In summary, our study provides a model in which paracrine signaling initiated by BC cells induces CCL2 production by stromal fibroblasts through STAT3 activation. The fibroblast-derived CCL2, in turn, promotes cancer progression by regulating CSCs through NOTCH activation (Fig. 5I). The results described herein provide novel insights into understanding how CSCs are influenced by the tumor microenvironment during the co-evolution of cancer and the hosting niche, and identify CCL2, STAT3 and NOTCH1 as future therapeutic targets to efficiently block the CSC-stimulating cancer–host crosstalk to overcome CSC-mediated disease progression and treatment resistance.
Supplementary Material
Acknowledgments
Financial support: NCI R00 CA125892 (SEW) and P30 CA033572; National Natural Science Foundation of China Grant Number 30872986 (XR) and 81171983 (HL)
We acknowledge Drs. Leonid S. Metelitsa and Warren S. Pear for kindly providing the plasmid constructs of CCL2 and NOTCH1 promoter reporters. We also thank Drs. John J. Rossi and Hua Yu for providing reagents, Drs. Susan Kane, Mei Kong, Toshifumi Tomoda, and Takahiro Maeda and members of the Division of Tumor Cell Biology for valuable comments, as well as the Analytical Cytometry Core, Light Microscopy Digital Imaging Core, Bioinformatics Core, Biostatistics Core, Pathology Core and Animal Facility for professional services.
Grant Support
The project described was supported by the National Cancer Institute (NCI) Grant Number R00 CA125892 (SEW) and P30 CA033572, and by the National Natural Science Foundation of China Grant Number 30872986 (XR) and 81171983 (HL).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 2.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18(5):460–6. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 4.Kuperwasser C, Chavarria T, Wu M, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101(14):4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melgarejo E, Medina MA, Sanchez-Jimenez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009;41(5):998–1001. doi: 10.1016/j.biocel.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Strieter RM, Wiggins R, Phan SH, et al. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- 7.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med. 2010;16(3):133–44. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6(6):R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 10.Kadesch T. Notch signaling: a dance of proteins changing partners. Exp Cell Res. 2000;260(1):1–8. doi: 10.1006/excr.2000.4921. [DOI] [PubMed] [Google Scholar]
- 11.Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121(4):751–8. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 12.Aagaard L, Amarzguioui M, Sun G, et al. A facile lentiviral vector system for expression of doxycycline-inducible shRNAs: knockdown of the pre-miRNA processing enzyme Drosha. Mol Ther. 2007;15(5):938–45. doi: 10.1038/sj.mt.6300118. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Yu Y, Tsuyada A, et al. Transforming growth factor-beta regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene. 2011;30(12):1470–80. doi: 10.1038/onc.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu L, Lau SK, Loera S, Somlo G, Kane SE. Protein kinase A activation confers resistance to trastuzumab in human breast cancer cell lines. Clin Cancer Res. 2009;15(23):7196–206. doi: 10.1158/1078-0432.CCR-09-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 16.Chang HY, Nuyten DS, Sneddon JB, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci U S A. 2005;102(10):3738–43. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell States in the breast. Cell. 2011;145(6):926–40. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cicalese A, Bonizzi G, Pasi CE, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138(6):1083–95. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 20.Potula HS, Wang D, Quyen DV, et al. Src-dependent STAT-3-mediated expression of monocyte chemoattractant protein-1 is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. J Biol Chem. 2009;284(45):31142–55. doi: 10.1074/jbc.M109.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song L, Ara T, Wu HW, et al. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J Clin Invest. 2007;117(9):2702–12. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Elf SE, Miyata Y, et al. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4(1):37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peacock CD, Wang Q, Gesell GS, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104(10):4048–53. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueno T, Toi M, Saji H, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6(8):3282–9. [PubMed] [Google Scholar]
- 25.Kellermann MG, Sobral LM, da Silva SD, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44(5):509–17. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Kojima Y, Acar A, Eaton EN, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107(46):20009–14. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian DZ, Rademacher BL, Pittsenbarger J, et al. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. Prostate. 2010;70(4):433–42. doi: 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 29.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267(2):271–85. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Saji H, Koike M, Yamori T, et al. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92(5):1085–91. doi: 10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 31.Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009;284(42):29087–96. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vasconcellos JF, Laranjeira AB, Zanchin NI, et al. Increased CCL2 and IL-8 in the bone marrow microenvironment in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(4):568–77. doi: 10.1002/pbc.22941. [DOI] [PubMed] [Google Scholar]
- 34.Molloy AP, Martin FT, Dwyer RM, et al. Mesenchymal stem cell secretion of chemokines during differentiation into osteoblasts, and their potential role in mediating interactions with breast cancer cells. Int J Cancer. 2009;124(2):326–32. doi: 10.1002/ijc.23939. [DOI] [PubMed] [Google Scholar]
- 35.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11(5):338–51. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 36.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 37.Yashiro-Ohtani Y, He Y, Ohtani T, et al. Pre-TCR signaling inactivates Notch1 transcription by antagonizing E2A. Genes Dev. 2009;23(14):1665–76. doi: 10.1101/gad.1793709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng W, Swenson LL, Fitzgibbon MJ, et al. Structure of mitogen-activated protein kinase-activated protein (MAPKAP) kinase 2 suggests a bifunctional switch that couples kinase activation with nuclear export. J Biol Chem. 2002;277(40):37401–5. doi: 10.1074/jbc.C200418200. [DOI] [PubMed] [Google Scholar]
- 39.Page JL, Wang X, Sordillo LM, Johnson SE. MEKK1 signaling through p38 leads to transcriptional inactivation of E47 and repression of skeletal myogenesis. J Biol Chem. 2004;279(30):30966–72. doi: 10.1074/jbc.M402224200. [DOI] [PubMed] [Google Scholar]
- 40.Yu Y, Wang Y, Ren X, et al. Context-dependent bidirectional regulation of the MutS homolog 2 by transforming growth factor beta contributes to chemoresistance in breast cancer cells. Mol Cancer Res. 2010;8(12):1633–42. doi: 10.1158/1541-7786.MCR-10-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang SE, Narasanna A, Whitell CW, Wu FY, Friedman DB, Arteaga CL. Convergence of p53 and transforming growth factor beta (TGFbeta) signaling on activating expression of the tumor suppressor gene maspin in mammary epithelial cells. J Biol Chem. 2007;282(8):5661–9. doi: 10.1074/jbc.M608499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SE, Narasanna A, Perez-Torres M, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell. 2006;10(1):25–38. doi: 10.1016/j.ccr.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 43.Farmery MR, Tjernberg LO, Pursglove SE, Bergman A, Winblad B, Naslund J. Partial purification and characterization of gamma-secretase from post-mortem human brain. J Biol Chem. 2003;278(27):24277–84. doi: 10.1074/jbc.M211992200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.