Abstract
Important advances have led to a better understanding of the biology and pathobiology of corneal myofibroblasts and their generation after surgery, injury, infection and disease. Transforming growth factor (TGF) beta, along with platelet-derived growth factor (PDGF) and interleukin (IL)-1, has been shown to regulate myofibroblast development and death in in-vitro and in-situ animal models. The myofibroblast precursor cells regulated by these cytokines include both keratocyte-derived and bone marrow-derived cells. Cytokines that promote and maintain myofibroblasts associated with late haze after photorefractive keratectomy are modulated in part by the epithelial basement membrane functioning as barrier between the epithelium and stroma. Structural and functional defects in the basement membrane likely lead to prolonged elevation of TGFβ, and perhaps other cytokine, levels in the stroma necessary to promote differentiation of myofibroblasts. Conversely, repair of the epithelial basement membrane likely leads to a decrease in stromal TGFβ levels and apoptosis of myofibroblasts. Repopulating keratocytes subsequently reorganize the associated fibrotic extracellular matrix deposited in the anterior stroma by the myofibroblasts. Investigations of myofibroblast biology are likely to lead to safer pharmacological modulators of corneal wound healing and transparency.
Keywords: myofibroblast, cornea, fibrocytes, bone marrow-derived cells, haze, surface irregularity, transforming growth factor β, platelet-derived growth factor, PRK, stroma, wound healing
I. Introduction
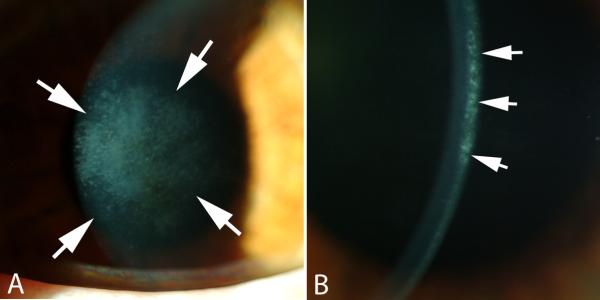
Injury to the cornea, and resultant loss of vision, threatens the survival of animals dependent on sight. The myofibroblast is a cell particularly suited to restore the integrity of the cornea after a penetrating injury, for example, because of its ability to contract wounds, secrete extracellular matrix, and generate adhesion structures with the surrounding substrate. Myofibroblast generation, and contraction produced by these cells, is, therefore, likely to be a beneficial contributor to the processes that restore the integrity of the eye after traumatic corneal laceration, even iatrogenic ones such as radial keratotomy incisions (Garana, et al., 1992), although unpredictability in their generation is likely a contributor to variability of the surgical result between different patients. Similarly, these cells tend to be beneficial contributors to wound strength at the donor-recipient interface after penetrating keratoplasty and the flap edge in laser insitu keratomileusis (LASIK) (Netto, et al., 2007), especially in eyes that don't require flap lift for retreatment. The development of corneal myofibroblasts after other surgeries, however, is considered a pathological response to injury. For example, the development of clinically significant superficial stromal opacity one to three months after photorefractive keratectomy (PRK), termed “late haze” as seen in the slit lamp images in Fig. 1A and 1B, leads to persistent corneal opacity, regression of the intended effect of surgery and development of irregular astigmatism (Lipshitz, et al., 1997; Mohan, et al., 2003). It is important to distinguish pathological late haze associated with myofibroblasts from the mild haze that occurs in the first few weeks to months in nearly all corneas that have PRK, including those with perfect clinical outcomes. The more common, clinically insignificant haze is not attributable to myofibroblasts, and the excessive extracellular matrix they produce, but likely to corneal fibroblasts that are opaque due to decreased corneal crystallin production (Jester, et al., 1999b). This common haze increases proportionally with increasing stromal keratectomy depth (Møller-Pedersen, et al., 1998a).
Fig. 1.
Corneal stromal opacity (haze). A shows haze that overlies the area of ablation with the excimer laser that occurred at three months after PRK for -7-diopters of myopia when no mitomycin C treatment was applied at the time of surgery. Magnification 20X. B. Slit lamp examination shows that the haze is localized in the anterior stroma immediately beneath the epithelial basement membrane. Magnification 40X.
Myofibroblasts also may contribute to interface opacity and graft failure following modern corneal endothelial replacement surgeries such as Descemet's stripping automated endothelial keratoplasty (DSAEK) and Descemet's membrane endothelial keratoplasty (DMEK), although limited studies have been performed to characterize their role in the wound healing response to these surgeries (Heindl, et al., 2011). They are also likely contributors to persistent scars that occur following corneal alkali burns and infections caused by bacteria, fungi and viruses, such as herpes simplex virus.
Ideally, the development and persistence of myofibroblasts in the cornea could be regulated pharmacologically to optimize the wound healing response—augmenting their development when their functions are beneficial and inhibiting their development when their functions are detrimental to the goals of treatment or surgery. In order to approach this ideal, a detailed understanding of the biology of corneal myofibroblasts is needed. The purpose of this review article is to highlight what is currently known about myofibroblast development, disappearance and function in the cornea.
II. Myofibroblast characteristics and functions in the cornea
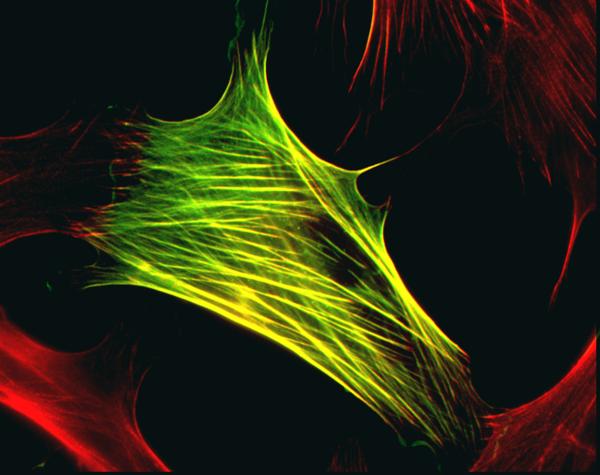
Myofibroblasts are fibroblastic cells that have ultrastructural and physiological characteristics of smooth muscle cells, such as prominent intracellular microfilament bundles (stress fibers) and contractile responses to smooth muscle agonists (Luttrull, Smith and Jester, 1985; Jester, et al., 1999a) as can be seen in Fig. 2, where a myofibroblast cell with stress fibers was stained for alpha-smooth muscle actin. These cells also have altered proteoglycan expression compared to keratocytes (Funderburgh, et al., 2001). Myofibroblasts are not detectible in normal unwounded corneas (Ishizaki, et al., 1993; Chaurasia, et al., 2009). However, depending on the type and extent of injury, differentiation of these cells from precursors within the stroma leads to transforming growth factor beta (TGFβ)-regulated intracellular expression of alpha-smooth muscle actin (αSMA) and associated contractility that facilitates wound closure (Jester, et al., 1999a; Chaurasia, et al., 2009). Thus, immunocytochemical detection of αSMA is the most common marker used to detect myofibroblasts in-vitro and in-situ, although mature myofibroblasts also express vimentin and desmin (Chaurasia, et al., 2009). In addition to αSMA, myofibroblasts also express fibronectin receptor α5β1 and/or αvβ3 integrins that localize to focal adhesions involved in the assembly of fibronectin fibrils (Jester, et al, 1999a). These molecules are a part of a cellular apparatus that allows myofibroblasts to exert mechanical force and participate in wound matrix organization and wound contraction (Jester, et al., 1995).
Fig. 2.
Myofibroblast cell with stress fibers stained for alpha-smooth muscle actin. Green is FITC-anti-alpha smooth muscle actin. Red is Alex 543 phalloidin. Magnification 600x. Image graciously provided by James V. Jester, Ph.D., The Gavin Herbert Eye Institute, University of Irvine, California.
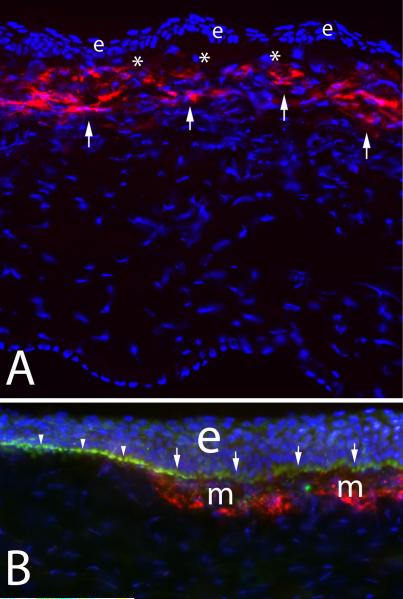
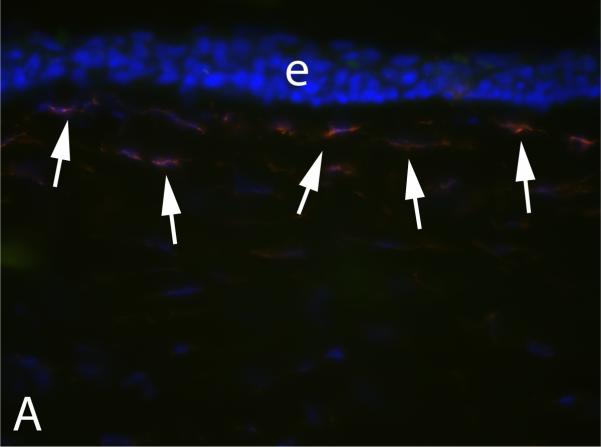
After incisional wounds like radial keratotomy, myofibroblasts are generated within the wound with intracellular stress fibers becoming aligned parallel to the long axis of the wound to facilitate contraction (Petroll, Cavanagh and Jester, 1998). The functional importance of the myofibroblasts in these wounds seems obvious. After surface ablation surgeries like PRK, however, myofibroblasts tend to be generated in the anterior stroma immediately beneath the epithelial basement membrane (Mohan, et al., 2003). This sub-epithelial basement membrane localization can be noted in a central corneal section from a cornea that had photorefractive keratectomy for high myopia, which underwent immunohistochemical staining for alpha-smooth muscle actin (Fig. 3A). What function is served by myofibroblasts in this location is not well understood, especially when they develop after a low laser ablation to correct myopia, which sometimes occurs, and in this situation they are thought to be more of a pathophysiological wound healing response to the surgical injury.
Fig. 3.
Corneal myofibroblasts. A. Myofibroblasts (arrows) beneath the basement membrane in the anterior stroma after PRK for -9 diopters of myopia in a rabbit cornea. e indicates epithelium. * is artifactual breaks between the epithelium and stroma that occur during tissue sectioning. 200X magnification. B. At higher magnification with double staining for the α-smooth muscle actin maker for myofibroblasts (red) and integrin beta-4 (green) decorating the epithelial basement membrane in a rabbit cornea that had -9 diopter PRK, it can be seen that the basement membrane appears more irregular (arrows) overlying the myofibroblasts (m) than it does in the adjacent area free of myofibroblasts (arrowheads). e indicates the epithelium. The blue is DAPI staining for all cell nuclei. Magnification 600X. B reprinted by permission from Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp. Eye Res. 2006;82:788-97.
Myofibroblasts in all tissues excrete large quantities of extracellular matrix, including type 1 collagen and fibronectin (Gao, et al., 2008; Karamichos, et al., 2010). This excretion may serve a tissue regenerative function, but also contributes to stromal opacity in the transparent cornea, in addition to the decreased transparency of the myofibroblasts themselves that is attributable to diminished crystallin protein production compared to keratocytes (Jester, et al., 1999b, Jester, et al., 2012). The anomalous, opaque extracellular matrix laid down my myofibroblasts often contributes to corneal opacity long after myofibroblasts have disappeared from the site of an injury and must be reabsorbed by keratocytes, and possibly other cells, in order to restore normal transparency.
III. Myofibroblast generation in the cornea: Precursors and cytokines
A. Corneal myofibroblast precursors
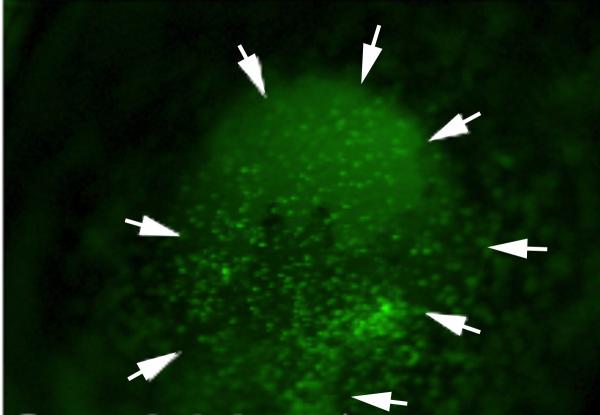
Until recently, dogma has been that all myofibroblasts that appeared in the cornea arose from corneal precursor cells—namely the keratocytes or corneal fibroblasts derived from keratocytes. This presumption was primarily based on the finding that corneal fibroblasts give rise to myofibroblasts when they are treated with transforming growth factor (TGF) β in-vitro (Masur, et al., 1996; Barry-Lane, et al., 1997; Petridou, et al., 2000; Kaur, et al., 2009a). In other organs, however, such as skin, liver and lung, studies have shown that bone marrow-derived cells serve as precursors for myofibroblasts (Bhawan and Majno, 1989; Direkze, et al., 2003; Hashimoto, et al., 2004). Following injury to the corneal epithelium, thousands of bone marrow-derived cells migrate into the corneal stroma from the limbus (Wilson, et al., 2004; Barbosa, et al., 2010a). This is best seen after the corneal epithelium is injured by scrape with a scalpel blade in chimeric animals in which the bone marrow-derived cells express green fluorescent protein (GFP) (Fig. 4). Recent studies used such chimeric mice that had total body irradiation and bone marrow transplanted from green fluorescent donor mice—all cells of which express GFP (Barbosa, et al., 2010a). Thus, in the engineered chimeric mice, only the bone marrow-derived cells, and not other cells such as keratocytes, corneal epithelial cells, or corneal endothelial cells, expressed GFP. When the corneas of these chimeric mice subsequently underwent haze-generating irregular phototherapeutic keratectomy, a significant proportion of myofibroblasts in the corneas were simultaneously GFP+ and αSMA+, conclusively demonstrating that many of the myofibroblasts originated from bone marrow-derived cells. That study could not exclude the possibility that some of the myofibroblasts generated in situ were also derived from keratocytes, or their daughter corneal fibroblasts, since it was not possible to produce 100% chimerization of the mice because irradiation levels became lethal in attempting to achieve greater than 90 to 95% donor bone marrow cells. On average, however, more than nine times as many αSMA+ myofibroblasts were GFP+, rather than GFP-, in the central, mid-peripheral or peripheral cornea. Thus, in that model, the majority of myofibroblasts originated from bone marrow-derived cells rather than keratocytes or their daughter cells. Animal studies in rabbits also support bone marrow-derived cell origin of corneal myofibroblasts (Santhiago, et al, 2011). In that study, PRM−151, a recombinant form of human pentraxin-2 (PTX-2, also referred to as serum amyloid P, hSAP), that inhibits differentiation of circulating monocytes into fibrocytes and profibrotic macrophages (Castano, et al., 2009; Wynn, 2007), significantly decreased myofibroblasts generation in rabbit corneas when given by subconjunctival injection after haze-generating PRK.
Fig. 4.
At 24 hours after corneal epithelial scrape injury in a chimeric mouse in which bone marrow-derived cells express green fluorescent protein, hundreds of labeled cells can be seen entering the cornea. The area of the scrape is delineated by the arrows. Magnification 30X.
In-vitro studies using corneal fibroblasts and bone marrow-derived cells isolated from both normal and green fluorescent mice demonstrated conclusively that both bone marrow-derived cells and corneal fibroblasts could transform into myofibroblasts in-vitro (Singh, et al., 2012). In those experiments, GFP+ bone marrow-derived cells were co-cultured with GFP-stromal fibroblasts, and visa versa. In either co-culture experiment, transformation of the GFP+ precursor into GFP+ myofibroblasts was augmented by the presence of the other GFP- cell type. Thus, more SMA+GFP+ myofibroblasts were generated from GFP+ corneal fibroblasts when GFP- bone marrow-derived cells were present in the cultures, and more SMA+GFP+ myofibroblasts were generated from GFP+ bone marrow-derived cells when GFP- corneal fibroblasts were present in the cultures. In Transwell System experiments included in these studies, anti-human LAP (TGF-β1) antibody that binds mouse LAP and/or transforming growth factor-β type I receptor kinase inhibitor (LY) added to the co-culture inhibited the generation of SMA+ myofibroblasts from either precursor cell type when the alternative GFP- cell type was cultured in the other well. These data suggest that TGFβ modulates the generation of myofibroblasts from either corneal fibroblast or bone marrow-derived cell precursors and that juxtacrine interaction between the two cell types could be important in the generation of myofibroblasts in situ.
The identity of the specific bone marrow-derived cells that give rise to myofibroblasts in the cornea remains to be elucidated. “Fibrocytes,” bone marrow-derived mesenchymal progenitor cells that express cell surface markers related to hematopoietic stem cells, monocytes and fibroblasts, appear to be good candidates (Abe, et al., 2001; Quan, Cowper and Bucala, 2006; Wynn, 2007). These cells have been shown to differentiate into fibroblasts and myofibroblasts in a variety of organs (Bucala, et al., 1994; Barth and Westhoff, 2007; Bellini and Mattoli, 2007; Strieter, et al., 2009). Preliminary studies have noted that some SMA+ corneal myofibroblasts retain expression of CD11b, CD34 and CD45 antigens, but not Thy1.2, CD19 or Ly6G antigens suggesting monocyte or macrophage origin, consistent with fibrocyte origin (M. Lin, F.L. Barbosa and S.E. Wilson, unpublished data, 2011), but further investigation is needed conclusively identify the specific cells. Inhibition of myofibroblast generation after PRK in rabbits by PRM−151, an inhibitor of differentiation of circulating monocytes into fibrocytes and pro-fibrotic macrophages (Santhiago, et al., 2011) supports a role for these cells in the cornea.
Our working hypothesis is that corneal myofibroblasts can be generated from either keratocyte-derived or bone marrow-derived precursor cells, and which precursor predominates in a particular cornea may be related to the type of injury, genetic factors, and other unknown influences. It is also unknown whether the origin of the myofibroblast in any way determines altered function of the myofibroblasts. In other words, do myofibroblasts that arise in the cornea from keratocytes have altered functions from those that arise from bone marrow-derived cells. Additional studies on myofibroblasts derived from alternative precursors will likely lead to a better understanding of myofibroblast phenotype and functions.
There are clinical observations that suggest cellular differences between patients with corneal haze. When visually significant late haze develops in humans two to three months after photorefractive keratectomy (PRK), the haze is “corticosteroid-responsive” in 10% to 15% of patients (Wilson and Salomao, 2009). In these patients, frequent topical application of prednisolone acetate 1% corticosteroid results in rapid resolution of the corneal opacity and associated change in refractive error. In the other 85% to 90% of patients, the corticosteroids have no effect on the corneal opacity or the change in refractive error. These two groups of haze patients cannot be distinguished by the appearance of the haze at the slit lamp or the level of regression of the intended effect of PRK, or any other known features: the topical corticosteroids must be given to all patients with haze for one week to distinguish whether the haze is steroid-responsive or steroid-resistant. In all of these patients, if both eyes had PRK, both eyes are either steroid-responsive or steroid-resistant. There must be corneal cellular differences that underlie these physiological differences in haze in different patients. Our working hypothesis is that the majority of myofibroblasts in corneas with late haze that are steroid-responsive are derived from bone marrow-derived precursors that retain steroid sensitivity, whereas the majority of myofibroblasts in corneas with late haze that are steroid-resistant are derived from corneal fibroblasts or other keratocyte-derived daughter cells. At present, there is no way to test this hypothesis at the cellular level in human corneas.
In some organs, studies have suggested that myofibroblasts can be generated from epithelial cells through a process termed “epithelial-to-mesenchymal transformation or transition,” in some cases mediated by TGFβ (Willis, duBois, and Borok, 2006; Hills and Squires, 2010; Davies, et al., 2005; Thiery, et al., 2009; Guarino, Tosoni and Nebuloni, 2009). Some studies demonstrate that epithelial-to-mesenchymal transition has a role in the normal physiology of the cornea (Kawakita, et al., 2005) and in subepithelial fibrosis associated with limbal stem cell deficiency (Kawashima, et al., 2010). It is unknown whether this process contributes to myofibroblasts associated with stromal opacity after corneal injury, surgery, or infection.
C. Myofibroblast development from progenitor cells
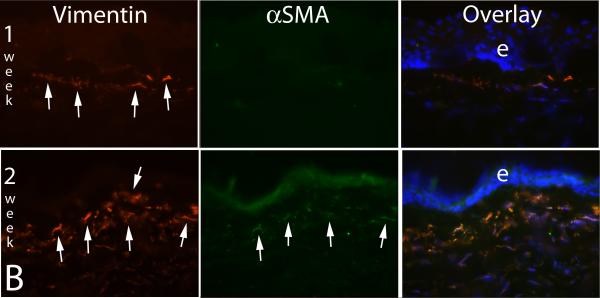
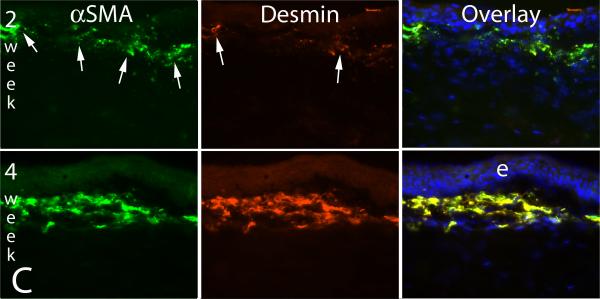
A common clinical observation in humans who develop severe late haze after PRK is that these corneas have high transparency and appear to have had excellent outcomes during the early postoperative period (Wilson and Salomao, 2009), but then rapidly develop the characteristic subepithelial opacity (Fig. 1) two to three months after surgery. A similar lag between PRK for high myopia or irregular phototherapeutic keratectomy (PTK) surgery and the development of corneal opacity occurs in rabbits or mice, but the time period is usually shorter, typically being three to four weeks (Mohan, et al., 2003; Mohan, et al., 2008), which is similar to the length of time required for the appearance of αSMA+ myofibroblasts in the corneas of these species. What is the biological basis of this lag? Are the myofibroblast precursors generated immediately after surgery and take time to develop into mature myofibroblasts or are the precursor cells generated or enter the cornea later because of a delayed signal? A recent time course study of intermediate filaments in rabbit corneas that had haze-generating -9 diopter PRK provided important insights into these questions (Chaurasia, et al., 2009). In that study, the expression of vimentin (V), α-smooth muscle actin (A), and desmin (D) in unwounded control corneas and corneas that had PRK were monitored by immunocytochemistry. All normal keratocytes of rabbit or cat corneas express vimentin that can be detected by immunocytochemistry (Ishizaki, et al., 1993; Jester, et al., 1994). We discovered, however, if the anti-vimentin antibodies are serially diluted, a subepithelial population of keratocytes that express higher levels of vimentin than most other keratocytes can be detected in rabbit corneas. This can be seen at high magnification in Fig. 5A. In the unwounded cornea these cells do not express αSMA or desmin. At one week after -9 diopter PRK, many more vimentin+ cells are detected in this same location in the anterior stroma, but still no αSMA+ or desmin+ cells are detected (Fig. 5B, middle upper panel). By two weeks after PRK, some of the now even more highly vimentin+ cells in the anterior stroma also become αSMA+ (Fig. 5B, middle bottom panel), but many are not yet desmin+ (Fig. 5C, middle upper panel). At three to four weeks after PRK in the rabbits, when corneal haze has become prominent at the slit lamp, vimentin+ cells in the anterior stroma are also highly αSMA+ and desmin+, as can be seen in the bottom panels of Fig 5C. The developing myofibroblasts in the anterior corneal stroma undergo an orderly differentiation from V+A-D- precursor cells to V+A+D- more mature cells to V+A+D+ mature myofibroblasts that begins immediately after injury. Thus, the V+A-D- precursor cells are present in the anterior stroma immediately after PRK, even with low corrections, but do not produce significant opacity visible at the slit lamp. In some corneas, once V+A+D+ myofibroblasts become numerous in the anterior stroma at three to four weeks after surgery, corneal opacity becomes prominent at the slit lamp and persists for an extended period of time.
Fig. 5.
Myofibroblast development in the cornea. A. Vimentin detected in anterior keratocytes of the unwounded rabbit cornea (arrows) by immunocytochemistry with low primary antibody concentration. e is the epithelium. Magnification 400X. With a higher primary antibody concentration all keratocytes are found to express vimentin (not shown). B. At one week after -9 diopter PRK in a rabbit, many more vimentin+ myofibroblasts (arrows) are detected in the anterior stroma, but none of the cells are αSMA+. By two weeks after -9 diopter PRK in a rabbit, many of the vimentin+ (red, arrows left panel) myofibroblasts are also αSMA+ (green, arrows center panel, and in overlay). e is epithelium. Magnification 200X. C. At two weeks after -9 diopter PRK in the rabbit, some of the αSMA+ myofibroblasts (green, arrows in left panel) are also desmin+ (red, arrows center panel), but many are desmin-. By four weeks after PRK, almost all cells are αSMA+desmin+. Staining for vimentin shows all these αSMA+desmin+ myofibroblasts are also vimentin+ (not shown) and, therefore, these are V+A+D+ myofibroblasts. Magnification 200X. Reprinted with permission from Caurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res., 2009;89:133-9.
This myofibroblast developmental pathway appears to be relevant to keratocyte-derived precursors. It is not known whether corneal myofibroblasts that develop from bone marrow-derived cells undergo a similar developmental sequence, but these developmental details will be explored in the future using chimeric animals.
C. TGFβ, PDGF and IL-1 in myofibroblast generation and death
TGFβ receptor activation is critical for the development of myofibroblasts from corneal fibroblasts (Masur, et al., 1996; Barry-Lane, et al., 1997; Jester, et al., 2002; Kaur, et al., 2009a) and is also a likely crucial contributor to the development of myofibroblasts from bone marrow-derived cells since nucleic acid vectors that interfere with TGFβ signaling decrease myofibroblast generation (Singh, et al., 2011) in a mouse PTK model in which bone marrow-derived cells have been shown to be precursor for most myofibroblasts (Barbosa, et al., 2010a). However, comparatively little is known about myofibroblasts development from bone marrow-derived precursors. Therefore, this section will necessarily focus primarily on myofibroblast development from corneal fibroblast precursor cells.
Three isoforms of TGFβ are expressed in human corneas and other animal corneas examined (Tuli SS, et al., 2006; Huh, et al., 2009) Many studies have demonstrated that TGFβ1 contributes to myofibroblast generation and corneal scarring in several species (Carrington, et al., 2006; Huh, et al., 2009; Singh, et al., 2011; Karamichos, et al., 2010; Karamichos, Hutcheon and Zieske, 2011; Reneker, et al., 2010). Fini and coworkers (Stramer, et al., 2003; Fini and Stramer, 2005; LaGier, et al, 2007) have also implicated TGFβ2 in the corneal stromal myofibroblastic and fibrotic response. TGFβ3, and to a lesser extent TGFβ2, have been associated scarless wound healing in the cornea and other organs (Waddington, et al., 2010; Karamichos, et al., 2010; Karamichos, Hutcheon and Zieske, 2011). Less is known about the expression patterns of the TGFβ receptors in the cornea and fibrocytes (Tuli, et al., 2006; Hong et al., 2007; Bakhshayesh, et al., 2011), although temporal and spatial variations in expression of these receptors and inhibitors, and the receptors of other cytokines, is clearly important to the regulation of corneal healing (Li, Lee and Tseng, 1999; Nakamura, et al., 2006; Carrington, et al., 2006; Hong et al., 2007; Huh, Chang, and Jung, 2009; Bakhshayesh, et al., 2011).
The effects of TGFβ in the cornea are known to be mediated by Smad-dependent and Smad-independent signaling pathways depending upon cellular responses and microenvironment (Petridou, et al., 2000; Kawakita, et al, 2005; Huh, et al., 2009; Tandon, et al., 2009). Regulation of Smad and other downstream modulators, such as p38MAPK, has the potential to control the fibrotic corneal healing response (Saika, et al., 2010). Further investigation should be directed to characterization and modulation of these TGFβ signal transduction pathways in both keratocyte-derived and bone marrow-derived cells that contribute to both the development of corneal myofibroblasts and stromal opacities.
TGFβ and interleukin (IL)-1 have opposing effects on corneal myofibroblast viability (Kaur, et al., 2009a). TGFβ is a critical driver of myofibroblast development from corneal fibroblasts in-vitro, and also blocks IL-1α– or IL-1β-stimulated myofibroblast apoptosis. If TGF-β1 concentrations are sufficiently high, myofibroblasts survive even high levels of IL-1 in the culture medium (Kaur, et al., 2009a). However, as TGF-β1 levels fall, myofibroblasts become susceptible to IL-1-triggered cell death.
IL-1α and IL-1β are expressed in both SMA+ myofibroblasts and αSMA- stromal cells in situ after haze-generating injury (Barbosa, et al., 2010b). Thus, stromal cells such as corneal fibroblasts, keratocytes, or even inflammatory cells, such as monocytes, may produce IL-1 that acts in paracrine fashion to trigger myofibroblast apoptosis when TGFβ-stimulation of myofibroblasts diminishes. However, some SMA+ myofibroblasts themselves produce IL-1, suggesting that myofibroblast viability could also be regulated via autocrine mechanisms when TGFβ levels decline in the anterior stroma (Barbosa, et al., 2010b). The importance of the IL-1 system was confirmed in IL-1 receptor I knockout mice that had irregular PTK to generate corneal haze (Barbosa, et al., 2012). SMA+ myofibroblast density was higher and cell apoptosis in the anterior stroma was lower in the IL-1RI knockout mice compared to control mice at one month, three and six months after irregular PTK.
Other cytokines also likely make an important contribution to the development of myofibroblasts from keratocyte-derived or bone marrow cell-derived precursors, and to myofibroblast viability once they are generated. Corneal myofibroblasts express platelet-derived growth factor (PDGF) receptor-alpha in situ (Kaur, et al., 2009b). Jester and Ho-Chang (2003) found that fibroblast growth factor-2 and PDGF induced fibroblast differentiation, with focal adhesion and fibronectin assembly and significant extracellular matrix contraction, but did not trigger SMA+ myofibroblast generation. However, SMA- myofibroblast precursor cells would not have been detected in that study. In an insitu study of myofibroblast generation in rabbit corneas (Kaur, et al., 2009c), stromal PDGF-B blockade during the early postoperative period following haze-generating PRK decreased stromal SMA+ myofibroblast generation. A subsequent parallel in situ study in mice (Singh, et al., 2011) where both PDGF-B and TGFβ receptor functions could be blocked individually or simultaneously with plasmid vectors expressing receptor fragments linked to the KDEL endoplasmic reticulum retention signaling sequence following haze-generating irregular phototherapeutic keratectomy (PTK) confirmed that TGFβ receptor II or PDGF-B receptor blockade decreased SMA+ myofibroblast generation. TGFβ receptor blockade, but not PDGF-B blockade, decreased vimentin+ cells in the corneal stroma early after the injury. However, TGFβ blockade or PDGF-B blockade decreased the generation of αSMA+ myofibroblasts at one month after opacity-generating corneal injury. These results provided evidence that PDGF-B receptor modulates the V+A- myofibroblast precursor to V+A+ myofibroblast transition during myofibroblast development, whereas TGFβ modulates both an increase in V+A- myofibroblast precursors and more mature V+A+ myofibroblasts.
What are the sources of TGFβ and PDGF that modulate myofibroblast development in the corneal stroma? The unwounded or wounded corneal epithelium produces high levels of both cytokines (Wilson, et al., 1994; Kim, et al., 1999; Tuli, et al., 2006). Tuli and coworkers (2006) have shown that high levels of TGFβ1 protein are present in the basal epithelial cells during the corneal wound healing response in rats. Both TGFβ and PDGF bind with high affinity to basement membranes because of their affinity for collagen type IV and proteoglycans, respectively (Paralkar, Vukicevic and Reddi, 1991, García-Olivas, et al., 2003; Kim, et al., 1999), and these cytokines, therefore, likely do not penetrate into the corneal stroma in unwounded corneas at sufficient levels to modulate myofibroblast development. Damage or removal of the basement membrane produced by injury increases TGFβ and PDGF penetration into the underlying stroma. After injury, corneal stromal cells, including invading monocytes (Grotendorst, Smale and Pencev, 1989) and corneal fibroblasts (Kaji, et al., 2001), also express TGFβ, but this expression fades rapidly as the wound healing response is completed. Thus, these stromal sources of TGFβ do not appear to be sufficient to modulate myofibroblast viability and function one to three months or even years after PRK—the times at which mature myofibroblasts have been shown to develop and persist in corneas that develop PRK-related late haze. Long after surrounding stromal cells such as keratocytes have returned to quiescence and immune cells have disappeared, the healed corneal epithelium continues to produce high levels of TGFβ and PDGF. However, the penetration of these cytokines into the stroma would be limited by the regenerated epithelial basement membrane, unless there were persistent structural or functional defects in this important barrier layer.
IV. The importance of the epithelium, extracellular matrix, integrins and basement membrane in myofibroblast generation and persistence
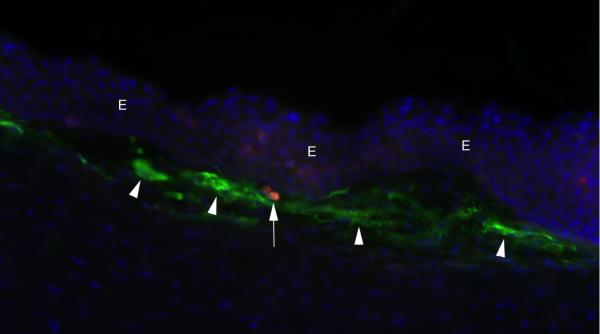
When late haze occurs after PRK, the opacity is always noted with the slit lamp immediately beneath the epithelium (Fig. 1b). When sections from corneas with haze undergo immunocytochemistry for αSMA, most myofibroblasts are located immediately beneath the epithelial basement membrane. Also, even at the light microscopic level, the overlying basement membrane often appears to be structurally abnormal at high magnification in immunohistological staining where the basement membrane is highlighted, for example by staining the tissue for integrin beta-4, as can be seen in Fig. 3B. This proximity of myofibroblasts to the epithelium in situ suggested epithelial-stromal interactions were involved in myofibroblast development. To confirm this interaction, ectopic epithelium from a donor rabbit eye was placed within a lamellar stromal LASIK incision in a recipient rabbit eye (Wilson, et al., 2003). Corneas in which epithelial tissue was introduced into the interface, but not control corneas with lamellar stromal incision alone, had αSMA+ myofibroblasts develop adjacent to the ectopic epithelium in the stroma anterior and posterior to the interface at one week and one month after surgery. These experiments supported the hypothesis that epithelial-stromal interactions were important in the development of corneal myofibroblasts.
Clinical observations on patients who developed late haze after PRK prior to the use of mitomycin C provided insights into other factors leading to myofibroblast development in the cornea. Late stromal haze was much more common after PRK for correction of higher levels of myopia (greater than -6 diopters) and rarely occurred after corrections less than -5 diopters (Lipshitz, et al., 1997; Kuo, Lee and Hwang, 2004). There were numerous theories advanced to explain the increase in late haze with higher PRK corrections of myopia, most of which were not published. These included the effects of increased heat associated with longer ablations required to correct higher levels of myopia with the excimer laser and a differing phenotype of posterior keratocytes leading to these cells being more prone to myofibroblast generation and opacity production. It was also noted that some excimer laser models were much more likely to have late haze as a complication of PRK, and these lasers tended to produce more surface irregularity when they ablated the cornea. The Summit excimer laser was especially prone to late haze (Wilson SE, unpublished data, 1996). The pattern of the opening laser diaphragm was actually imprinted on the surface of the ablated cornea with the Summit laser and this irregularity could be observed even with the relatively poor resolution of the operating microscope used with this laser. P. Vinciguerra and D. Epstein (unpublished data, 2001) reported that performing smoothing phototherapeutic keratectomy after delivery of the PRK ablation to correct high myopia markedly reduced the incidence of late haze. Also, surface irregularities detected with videokeratography, such as central islands and peninsulas, were found to be more common after high PRK corrections for myopia (McGhee and Bryce, 1996). To test the hypothesis that corneal surface irregularity was related to myofibroblast generation and late haze, a method was developed to apply the excimer laser to the cornea through a fine mesh screen in a rabbit model (Netto, et al., 2006a). In each eye, -4.5 diopters of PRK was applied after removal of the epithelium with a blade—an amount of laser ablation insufficient to trigger myofibroblast generation and late haze. However, in three other groups, the screen was placed in the path of the excimer laser beam for the last 10%, 30% or 50% of the terminal pulses of the PRK ablation, respectively, producing graduated and reproducible levels of corneal surface irregularity. This study found that myofibroblast density in the anterior stroma and the intensity of corneal haze increased directly in proportion to the degree of surface irregularity. Also, in another group that had -4.5 diopter PRK with 50% screening, followed by PTK with a smoothing agent to remove the induced surface irregularity, the myofibroblast density and corneal haze was reduced to the same level as -4.5 diopter PRK alone (Netto, et al., 2006a). That study conclusively established a relationship between surface irregularity and myofibroblast generation and late haze after PRK. Another important finding in this study was that structural abnormalities were detected in the epithelial basement membrane of corneas with surface irregularities that developed late haze (Fig. 3C) but not in corneas that did not develop haze.
Based on this study of surface irregularity and haze (Netto, et al., 2006a), it was proposed that the normally functioning epithelial basement membrane critically modulates myofibroblast development through its barrier function preventing persistent penetration of epithelial TGFβ and PDGF into the stroma at sufficient levels to drive myofibroblast development and, perhaps more importantly, maintain viability once mature myofibroblasts are generated. This hypothesis holds that stromal surface irregularity after PRK leads to structural and functional defects in the regenerated epithelial basement membrane, which increases and prolongs penetration of TGFβ and PDGF into the anterior corneal stroma to modulate myofibroblast development from either keratocyte-derived or bone marrow-derived precursor cells. It seems likely that the myofibroblast developmental program begins in the cornea after all PRK procedures, even corrections for low myopia, but that the precursors and immature myofibroblasts fail to develop into mature αSMA+ myofibroblasts when the basement membrane regenerates normally and stromal TGFβ and PDGF levels fall in the corneal stroma. Our working hypothesis is that the epithelial basement membrane is an integral corneal regulatory structure that limits the fibrotic response in the stroma by regulating the availability of epithelium-derived TGFβ, PDGF, and perhaps other growth factors and extracellular matrix components, to stromal cells, including myofibroblast precursors.
It is also possible that injury to the tissue and basement membrane increases bioavailability or function of integrins or integrin-linked kinases that have a critical role in the development of myofibroblasts, although the specific mechanisms of these proteins involvement in cell adhesion and adhesive signaling remains poorly understood (Masur, et al., 1999; Jester, et al., 2002; Liu, et al., 2010; Blumbach, et al., 2010). A study in human corneal fibroblasts suggested that alpha 11 beta 1 integrin was regulated by cell/matrix stress involving activin A, a multifunctional cytokine of the transforming growth factor-beta superfamily of growth factors, and Smad3, and that alpha 11 beta 1 integrin regulated myofibroblast differentiation (Carracedo, et al., 2010). Another study demonstrated that alpha 5 beta 1 integrin was important in myofibroblast transformation (Jester, et al., 1994).
Other factors besides surface irregularity also likely contribute to myofibroblast generation. It seems possible that genetic factors are also involved in myofibroblast generation and late haze development, especially in patients where the complication develops after normal PRK for low levels of myopia. In these cases, rarely occurring after treatments as low as -2 diopters of myopia, late haze is always bilateral, as it is in the more common variant associated with high myopia corrections. It may be that there are genetic abnormalities of the epithelial basement membrane increase the permeability to TGFβ and PDGF after injury, but no genetic associations or familial occurrences of late haze have been reported. Specifically, no studies have reported an association between anterior basement membrane dystrophy and late haze after PRK, but further investigation would be of interest.
There has been a report of an association between ultraviolet light exposure after PRK and development of haze (Nagy, et al., 1997). The mechanism of UV-B light augmentation of haze, however, appears to be unrelated to an increase in myofibroblast development since histologically the rabbit corneas treated with UV-B light after PRK developed anterior stromal extracellular vacuolization.
V. Disappearance of the myofibroblast and resolution of corneal haze
Many human corneas that develop late haze after PRK show slow resolution of the opacity accompanied by restoration of the refractive correction between one and three years after the original surgery. This appears to be mediated via a two-step process: 1) disappearance of the myofibroblasts and 2) reabsorption of the abnormal extracellular matrix and restoration of normal stromal structure associated with transparency.
Disappearance of the myofibroblasts could be mediated by trans-differentiation of the cells back to keratocytes or by apoptosis. Maltseva and coworkers (2001) found that fibroblast growth factor (FGF)-1 or FGF-2 reversed the myofibroblast phenotype to αSMA-corneal fibroblasts in-vitro. There has been no demonstration of myofibroblast to corneal fibroblast trans-differentiation occurring in corneas in situ.
Myofibroblast apoptosis is detected with the TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay as early as one month after -9 diopter PRK for myopia (Fig. 6) in rabbit corneas in situ (Wilson, Chaurasia and Medeiros, 2007). Since myofibroblast density and associated haze peaks at one month after PRK for high myopia in the rabbit, it appears likely that the balance between myofibroblast generation and apoptosis in a cornea determines myofibroblast density at a particular point in time. Our working hypothesis is that return of normal epithelial basement membrane structure and function leads to a drop in the penetration of epithelium-derived TGFβ (Netto, et al., 2006) in the anterior stroma and apoptosis of developing myofibroblasts mediated by either autocrine or paracrine IL-1 (Kaur, et al, 2009a). In corneas where there is a rapid restoration of basement membrane structure and function, for example with PRK for low myopia in rabbits or humans, apoptosis of myofibroblasts probably outstrips myofibroblast generation from progenitor cells during the first few weeks after surgery and, therefore, very few myofibroblasts survive to produce disorganized extracellular matrix. Conversely, when basement membrane structural and functional defects persist for an extended time after PRK, myofibroblast generation probably exceeds myofibroblast apoptosis and a high density of extracellular matrix-producing myofibroblasts becomes established in the subepithelial stroma. A rare TUNEL+ myofibroblast was detected in a patch of subepithelial myofibroblasts at one month after PRK for high myopia in a rabbit in Fig. 6. Even when myofibroblast density rises to high levels and haze is severe, in most corneas the basement membrane is eventually repaired, TGFβ levels drop, and myofibroblasts are cleared from the subepithelial stoma by apoptosis.
Fig. 6.
Myofibroblast apoptosis detected at one month after -9 diopter PRK for myopia in a rabbit cornea. Simultaneous staining for αSMA in subepithelial myofibroblasts (green, arrowheads) and the TUNEL assay detects a single myofibroblast (red, arrow) undergoing apoptosis. Blue is the DAPI stain for cell nuclei and E indicates the epithelium. Magnification 400X. Reprinted with permission from Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response, Exp. Eye Res. 2007;85:305-11.
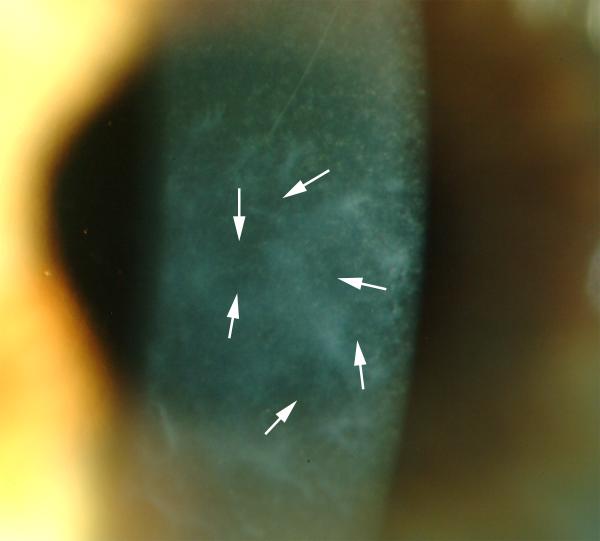
Even when all myofibroblasts disappear from the anterior stroma by apoptosis and/or trans-differentiation, the disorganized extracellular matrix produced by these cells remains and must be removed to restore transparency. This function is likely carried out by keratocytes that repopulate the anterior stroma when the myofibroblasts disappear. The process of clearing is usually signaled by the appearance of clear areas, called lacunae—that can be noted throughout the haze in a human cornea that had photorefractive keratectomy in Fig. 7. Over a period of months or even years, depending on the patient, these lacunae expand and coalesce until the entire cornea is restored to transparency.
Fig. 7.
Lacunae (arrows) of transparent corneal stroma are beginning to appear within the dense haze of a patient 1.5 years after PRK complicated by severe late haze. Magnification 40X.
Late haze that rarely occurs in corneas that had PRK despite mitomycin C prophylactic treatment (breakthrough haze) often shows much less tendency to resolve even years after the original surgery compared with late haze that develops after PRK without mitomycin C treatment. This is thought to be attributable to diminished keratocyte repopulation of the anterior stroma of the cornea due to the prolonged mitomycin C effect of inhibiting mitosis of stromal cells (Netto, et al., 2006b). Thus, if the density of keratocytes is reduced or their function is inhibited long-term, the extracellular matrix may persist despite the disappearance of the myofibroblasts that produced the disorganized matrix.
VI. Pharmacological interventions to block myofibroblast generation and haze formation
The only pharmacological treatment that is widely used for the prevention and treatment of haze after PRK and other stromal surface ablation procedures is prophylactic or therapeutic application of mitomycin C as an early promoter of anterior stromal cell apoptosis immediately after treatment and a long-term, non-specific inhibitor of corneal stromal cellular mitosis—likely including the progenitor cells that give rise to myofibroblasts—for months after treatment (Raviv, et al., 2000; Netto, et al., 2006b). This treatment has been shown to be highly effective in preventing clinically significant late haze when used after PRK (Raviv, et al., 2000). Few complications of corneal mitomycin C treatment have been reported, other than short-term decreases in keratocyte density (Netto, et al., 2006b; de Benito-Llopis, et al., 2012), but follow-up of large numbers of treated eyes is limited to approximately ten years and concerns remain about the long-term effects of the treatment twenty to sixty years later. It is important to note that normalization of keratocyte density in the stroma monitored with histologic methods or confocal microscopy does not preclude late functional abnormalities of the repopulating stroma cells.
A better approach to prevention of haze after PRK and other surgeries would be a more targeted prevention of myofibroblast development from precursors that does not affect other stromal cells. Unfortunately, despite ongoing investigations, no effective pharmacological agent has been developed for clinical use. Several approaches, however, have been explored in animal models. Møller-Pedersen and coworkers (1998b) found that neutralizing antibodies to TGFβ could inhibit stromal haze after PRK. Santhiago and coworkers (2011) found that a monocyte development inhibitor, PRM-151, decreased corneal myofibroblast generation after PRK in rabbits, but the treatment was not effective when given topically but only when delivered by subconjunctival injection for several days after surgery. Sharma and coworkers (2009) found that Trichostatin A (TSA), a histone deacetylase inhibitor that suppresses TGF-beta-induced fibrogenesis, inhibited haze after PRK in rabbits. None of these treatments have been tested in humans after PRK. Further study of corneal myofibroblast biology is likely to lead to effective pharmacologic agents to more safely inhibit or promote myofibroblast generation, depending on the clinical situation, and allow more precise modulation of the corneal wound healing response to injury, infection, disease and surgery in the cornea.
Highlights.
Corneal myofibroblasts and the matrix they produce causes corneal opacity
The corneal epithelium and basement membrane have key roles in generation of myofibroblasts
Corneal myofibroblasts can be generated from either keratocyte-derived or bone marrow-derived precursors
Acknowledgements
Supported in part by US Public Health Service grants EY10056 and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary interest statement: The author has no proprietary or financial interest in the topics discussed in this manuscript
References
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Bakhshayesh M, Soleimani M, Mehdizadeh M, Katebi M. Effects of TGF-β and b-FGF on the Potential of Peripheral Blood-Borne Stem Cells and Bone Marrow-Derived Stem Cells in Wound Healing in a Murine Model. Inflammation. doi: 10.1007/s10753-011-9298-4. in press. in press. [DOI] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia S, Cutler A, Asosingh K, Kaur H, de Medeiros F, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010a;91:92–6. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Chaurasia S, Kaur H, de Medeiros FW, Agrawal V, Wilson SE. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp. Eye Res. 2010b;91:456–61. doi: 10.1016/j.exer.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa FL, Santhiago MR, Singh V, Agrawal V, Wilson SE. Interleukin-1 receptor role in the viability of corneal myofibroblasts. Exp. Eye Res. 2012 doi: 10.1016/j.exer.2011.12.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry-Lane PA, Wilson SE, Cavanagh HD, Petroll WM, Jester JV. Characterization of SV40-transfected cell strains from rabbit keratocytes. Cornea. 1997;16:72–8. [PubMed] [Google Scholar]
- Barth PJ, Westhoff CC. CD34+ fibrocytes: morphology, histogenesis and function. Curr Stem Cell Res Ther. 2007;2:221–7. doi: 10.2174/157488807781696249. [DOI] [PubMed] [Google Scholar]
- Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab. Invest. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- Bhawan J, Majno G. The myofibroblast. Possible derivation from macrophages in xanthogranuloma. Am J Dermatopathol. 1989;11:255–8. doi: 10.1097/00000372-198906000-00010. [DOI] [PubMed] [Google Scholar]
- Blumbach K, Zweers MC, Brunner G, Peters AS, Schmitz M, Schulz JN, Schild A, Denton CP, Sakai T, Fässler R, Krieg T, Eckes B. Defective granulation tissue formation in mice with specific ablation of integrin-linked kinase in fibroblasts - role of TGFβ1 levels and RhoA activity. J. Cell Sci. 2010;123:3872–83. doi: 10.1242/jcs.063024. [DOI] [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol. Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Carracedo S, Lu N, Popova SN, Jonsson R, Eckes B, Gullberg D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J. Biol. Chem. 2010;285:10434–45. doi: 10.1074/jbc.M109.078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest. Ophthalmol. Vis. Sci. 2006;47:1886–94. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- Castano A, Lin SL, Surowy T, Nowlin BT, Turlapati SA, Patel T, Singh A, Li S, Lupher ML, Jr., Duffield JS. Serum amyloid P inhibits fibrosis through FcgammaR-dependent monocyte/macrophage regulation in vivo. Science Translational Medicine. 2009;1:5ra13. doi: 10.1126/scitranslmed.3000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009;89:133–9. doi: 10.1016/j.exer.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J. Cell Biochem. 2005;95:918–31. doi: 10.1002/jcb.20458. [DOI] [PubMed] [Google Scholar]
- de Benito-Llopis L, Cañadas P, Drake P, Hernández-Verdejo JL, Teus MA. Keratocyte density 3 months, 15 months, and 3 years after corneal surface ablation with mitomycin C. Am J Ophthalmol. 2012;153:17–23. doi: 10.1016/j.ajo.2011.05.034. [DOI] [PubMed] [Google Scholar]
- Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–20. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms impacting on surgical outcomes. Cornea. 2005;24(suppl 8):S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta-induced keratocyte-myofibroblast transdifferentiation. J. Biol. Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Li J, Huang H, Li X. Connective tissue growth factor stimulates renal cortical myofibroblast-like cell proliferation and matrix protein production. Wound Repair Regen. 2008;16:408–15. doi: 10.1111/j.1524-475X.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest. Ophthalmol. Vis. Sci. 1992;33:3271–82. [PubMed] [Google Scholar]
- García-Olivas R, Hoebeke J, Castel S, Reina M, Fager G, Lustig F, Vilaró S. Differential binding of platelet-derived growth factor isoforms to glycosaminoglycans. Histochem. Cell Biol. 2003;120:371–82. doi: 10.1007/s00418-003-0576-6. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J. Cell Physiol. 1989;140:396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- Guarino M, Tosoni A, Nebuloni M. Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition. Hum. Pathol. 2009;40:1365–76. doi: 10.1016/j.humpath.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J. Clin. Invest. 2004;113:243–52. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindl LM, Schlötzer-Schrehardt U, Cursiefen C, Bachmann BO, Hofmann-Rummelt C, Kruse FE. Myofibroblast metaplasia after descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2011;151:1019–1023. doi: 10.1016/j.ajo.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Hills CE, Squires PE. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am. J. Nephrol. 2010;31:68–74. doi: 10.1159/000256659. [DOI] [PubMed] [Google Scholar]
- Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007;282:22910–20. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- Huh MI, Chang Y, Jung JC. Temporal and spatial distribution of TGF-beta isoforms and signaling intermediates in corneal regenerative wound repair. Histol. Histopathol. 2009;24:1405–16. doi: 10.14670/HH-24.1405. [DOI] [PubMed] [Google Scholar]
- Huh MI, Kim YH, Park JH, Bae SW, Kim MH, Chang Y, Kim SJ, Lee SR, Lee YS, Jin EJ, Sonn JK, Kang SS, Jung JC. Distribution of TGF-beta isoforms and signaling intermediates in corneal fibrotic wound repair. J. Cell Biochem. 2009;108:476–88. doi: 10.1002/jcb.22277. [DOI] [PubMed] [Google Scholar]
- Ishizaki M, Guang Z, Haseba T, Shafer SS, Kao WWY. Expression of collagen I, smooth muscle a-actin, and vimentin during the healing of alkali burned and lacerated corneas. Invest. Ophthalmol. Vis. Sci. 1993;34:3320–3328. [PubMed] [Google Scholar]
- Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest. Ophthalmol. Vis. Sci. 1994;35:730–43. [PubMed] [Google Scholar]
- Jester JV, Brown D, Pappa A, Vasilou V. Myofibroblast Differentiation Modulates Keratocyte Crystallin Protein Expression, Concentration and Cellular Light Scattering. Invest. Ophth. Vis. Sci. 2012 doi: 10.1167/iovs.11-9092. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–92. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor (beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999a;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGF beta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGF beta, PDGF and integrin signaling. Exp. Eye Res. 2002;75:645–57. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J. Cell Sci. 1999b;112:613–22. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Temporal, 3-dimensional, cellular anatomy of corneal wound tissue. J. Anat. 1995;186:301–311. [PMC free article] [PubMed] [Google Scholar]
- Kaji Y, Soya K, Amano S, Oshika T, Yamashita H. Relation between corneal haze and transforming growth factor-beta1 after photorefractive keratectomy and laser in situ keratomileusis. J. Cataract Refract. Surg. 2001;27:1840–6. doi: 10.1016/s0886-3350(01)01141-5. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AE, Zieske JD. Human corneal fibrosis: an in vitro model. Invest. Ophthalmol. Vis. Sci. 2010;51:1382–8. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Hutcheon AE, Zieske JD. Transforming growth factor-β3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J. Tissue Eng. Regen. Med. 2011;5:e228–38. doi: 10.1002/term.429. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Wilson SE. Corneal myofibroblast viability: Opposing effects of IL-1 and TGF beta-1. Exp. Eye Res. 2009a;89:152–8. doi: 10.1016/j.exer.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, Agrawal V, Wilson SE. Expression of PDGF receptor-alpha in corneal myofibroblasts in situ. Exp. Eye Res. 2009b;89:432–4. doi: 10.1016/j.exer.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, de Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SE. Corneal stroma PDGF blockade and myofibroblast development. Exp. Eye Res. 2009c;88:960–5. doi: 10.1016/j.exer.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol. 2005;167:381–93. doi: 10.1016/S0002-9440(10)62983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Hornia A, Yeh LK, Ouyang J, Liu CY, Tseng SC. Keratocan expression of murine keratocytes is maintained on amniotic membrane by down-regulating transforming growth factor-beta signaling. J. Biol. Chem. 2005;280:27085–92. doi: 10.1074/jbc.M409567200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima M, Kawakita T, Higa K, Satake Y, Omoto M, Tsubota K, Shimmura S, Shimazaki J. Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium. Mol. Vis. 2010;16:2727–32. [PMC free article] [PubMed] [Google Scholar]
- Kim W-J, Mohan RR, Mohan RR, Wilson SE. Effect of PDGF, IL-1 alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest. Ophthalmol. Vis. Sci. 1999;40:1364–72. [PubMed] [Google Scholar]
- Kuo IC, Lee SM, Hwang DG. Late-onset corneal haze and myopic regression after photorefractive keratectomy (PRK). Cornea. 2004;23:350–5. doi: 10.1097/00003226-200405000-00007. [DOI] [PubMed] [Google Scholar]
- LaGier AJ, Yoo SH, Alfonso EC, Meiners S, Fini ME. Inhibition of Human Corneal Epithelial Production of Fibrotic Mediator TGF-β2 by Basement Membrane-Like Extracellular Matrix. Invest. Ophthalmol. Vis. Sci. 2007;48:1061–1071. doi: 10.1167/iovs.06-0772. [DOI] [PubMed] [Google Scholar]
- Li DQ, Lee SB, Tseng SC. Differential expression and regulation of TGF-beta1, TGF-beta2, TGF-beta3, TGF-betaRI, TGF-betaRII and TGF-betaRIII in cultured human corneal, limbal, and conjunctival fibroblasts. Curr. Eye Res. 1999;19:154–61. doi: 10.1076/ceyr.19.2.154.5321. [DOI] [PubMed] [Google Scholar]
- Lipshitz I, Loewenstein A, Varssano D, Lazar M. Late onset corneal haze after photorefractive keratectomy for moderate and high myopia. Ophthalmology. 1997;104:369–73. doi: 10.1016/s0161-6420(97)30306-6. [DOI] [PubMed] [Google Scholar]
- Liu S, Xu SW, Blumbach K, Eastwood M, Denton CP, Eckes B, Krieg T, Abraham DJ, Leask A. Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J. Cell Sci. 2010;123:3674–82. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- Luttrull JK, Smith RE, Jester JV. In vitro contractility of avascular corneal wounds in rabbit eyes. Invest Ophthalmol. Vis. Sci. 1985;26:1449–1452. [PubMed] [Google Scholar]
- Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest. Ophthalmol. Vis. Sci. 2001;42:2490–5. [PubMed] [Google Scholar]
- Masur SK, Conors RJ, Jr, Cheung JK, Antohi S. Matrix adhesion characteristics of corneal myofibroblasts. Invest. Ophthalmol. Vis. Sci. 1999;40:904–10. [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–23. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee CN, Bryce IG. Natural history of central topographic islands following excimer laser photorefractive keratectomy. J. Cataract Refract. Surg. 1996;22:1151–8. doi: 10.1016/s0886-3350(96)80063-0. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AEK, Choi R, Hong J-W, Lee J-S, Mohan RR, Ambrósio R, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp. Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Stapleton WM, Sinha S, Netto MV, Wilson SE. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser. Exp. Eye Res. 2008;86:235–40. doi: 10.1016/j.exer.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998a;17:627–39. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGF beta modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr. Eye Res. 1998b;17:736–47. [PubMed] [Google Scholar]
- Nagy ZZ, Hiscott P, Seitz B, Shlötzer-Schrehardt U, Simon M, Jr., Süveges I, Naumann GO. Ultraviolet-B enhances corneal stromal response to 193-nm excimer laser treatment. Ophthalmology. 1997;104:375–80. doi: 10.1016/s0161-6420(97)30305-4. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Siddiqui SS, Shen X, Malik AB, Pulido JS, Kumar NM, Yue BY. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol. Vis. 2004;10:703–11. [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Medeiros FW, Dupps WJ, Sinha S, Krueger RR, Stapleton WM, Rayborn M, Suto C, Wilson SE. Femtosecond laser and microkeratome LASIK flaps: Comparative effects on wound healing and inflammatory infiltration. J. Ref. Surg. 2007;23:667–676. doi: 10.3928/1081-597x-20070901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006a;82:788–97. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, proliferation, haze, and keratocyte density. J. Ref. Surgery. 2006b;22:562–574. doi: 10.3928/1081-597x-20060601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar VM, Vukicevic S, Reddi AH. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev. Biol. 1991;143:303–8. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- Petridou S, Maltseva O, Spanakis S, Masur SK. TGF-beta receptor expression and Smad2 localization are cell density dependent in fibroblasts. Invest. Ophthalmol. Vis. Sci. 2000;41:89–95. [PubMed] [Google Scholar]
- Petroll WM, Cavanagh HD, Jester JV. Assessment of stress fiber orientation during healing of radial keratotomy wounds using confocal microscopy. Scanning. 1998;20:74–82. doi: 10.1002/sca.1998.4950200202. [DOI] [PubMed] [Google Scholar]
- Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr. Rheumatol. Rep. 2006;8:145–50. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- Raviv T, Majmudar PA, Dennis RF, Epstein RJ. Mytomycin-C for post-PRK corneal haze. J Cataract Refract Surg. 2000;26:1105–6. doi: 10.1016/s0886-3350(00)00625-8. [DOI] [PubMed] [Google Scholar]
- Reneker LW, Bloch A, Xie L, Overbeek PA, Ash JD. Induction of corneal myofibroblasts by lens-derived transforming growth factor beta1 (TGFbeta1): a transgenic mouse model. Brain Res Bull. 2010;81:287–96. doi: 10.1016/j.brainresbull.2009.10.019. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Sumioka T, Okada Y, Miyamoto T, Shirai K, Kitano A, Tanaka S. Transforming growth factor beta signal transduction: a potential target for maintenance/restoration of transparency of the cornea. Eye Contact Lens. 2010;36:286–9. doi: 10.1097/ICL.0b013e3181eef01c. [DOI] [PubMed] [Google Scholar]
- Santhiago MR, Singh V, Barbosa FL, Agrawal V, Wilson SE. Monocyte development inhibitor PRM-151 decreases corneal myofibroblast generation in rabbits. Exp. Eye Res. 2011;93:786–9. doi: 10.1016/j.exer.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Mehan MM, Sinha S, Cowden JW, Mohan RR. Trichostatin a inhibits corneal haze in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2009;50:2695–701. doi: 10.1167/iovs.08-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Agrawal V, Santhiago MR, Wilson SE. Stromal fibroblast-bone marrow-derived cell interactions: Implications for myofibroblast development in the cornea. Exp. Eye Res. 2012 doi: 10.1016/j.exer.2012.03.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Santhiago MR, Barbosa FL, Agrawal V, Singh N, Ambati BK, Wilson SE. Effect of TGFβ and PDGF-B blockade on corneal myofibroblast development in mice. Exp. Eye Res. 2011;93:810–7. doi: 10.1016/j.exer.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest. Ophthalmol. Vis. Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J. Leukoc. Biol. 2009;86:1111–8. doi: 10.1189/jlb.0309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 2010;10:565–78. doi: 10.2174/1566524011009060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tuli SS, Liu R, Chen C, Blalock TD, Goldstein M, Schultz GS. Immunohistochemical localization of EGF, TGF-alpha, TGF-beta, and their receptors in rat corneas during healing of excimer laser ablation. Curr. Eye Res. 2006;31:709–19. doi: 10.1080/02713680600837390. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Crossley R, Sheard V, Howe SJ, Buckley SM, Coughlan L, Gilham DE, Hawkins RE, McKay TR. Gene delivery of a mutant TGFβ3 reduces markers of scar tissue formation after cutaneous wounding. Mol. Ther. 2010;18:2104–11. doi: 10.1038/mt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc. Am. Thorac. Soc. 2006;3:377–82. doi: 10.1513/pats.200601-004TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp. Eye Res. 2007;85:305–11. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Hutcheon AEK, Mohan RR, Ambrósio R, Zieske JD, Hong JW, Lee J-S. Effect of ectopic epithelial tissue within the stroma on keratocyte apoptosis, mitosis, and myofibroblast transformation. Exp. Eye Res. 2003;76:193–201. doi: 10.1016/s0014-4835(02)00277-4. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Netto MV, Perez V, Possin D, Huang J, Kwon R, Alekseev A. RANK, RANKL, OPG, and M-CSF expression in stromal cells during corneal wound healing. Invest. Ophthalmol. Vis. Sci. 2004;45:2201–2211. doi: 10.1167/iovs.03-1162. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Salomao MQ. Corneal molecular and cellular biology update for the refractive surgeon. J. Ref Surgery. 2009;25:459–466. doi: 10.3928/1081597x-20090422-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Schultz GS, Chegini N, Weng J, He Y-G. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp. Eye Res. 1994;59:63–72. doi: 10.1006/exer.1994.1081. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]