Abstract
Objectives
To assess the feasibility of two patient-reported health related quality of life (HRQOL) instruments, CARE and SF-12, as tools for evaluating HRQOL outcome consequences following renal surgery, and to determine which domains of these HRQOL instruments are most sensitive to HRQOL outcome effects of renal surgery.
Methods
Patients completed CARE and SF-12 preoperatively (baseline) and at 2, 4, 12 and 24 weeks after surgery. Clinical data, patient response rate, HRQOL changes over time, and likelihood of patient return to baseline HRQOL were evaluated.
Results
Seventy-one patients were enrolled. Sixty patients completed the baseline and at least one follow-up set of questionnaires. The CARE pain, gastrointestinal (GI) and activity domain scores and the SF-12 physical composite score (PCS) were sensitive to changes in HRQOL (all p<0.05), whereas other domain subscores of these instruments did not change from pre-surgical baseline to post-surgical follow-up. Postsurgical HRQOL effects detected by the CARE pain, GI, and activity domains, and SF-12 PCS were most evident at 2 weeks (all p<0.001). The CARE composite score demonstrated 74% and 50% of patients returned to within 90% of baseline 4 weeks after radical and partial nephrectomy respectively.
Conclusion
Evaluation of patient-reported HRQOL outcomes after renal surgery is feasible, our findings suggest that the activity, pain, and GI domains of CARE and PCS subscore of the SF-12 are sensitive measures of HRQOL outcome consequences of renal surgery and represent appropriate measures of either care quality or comparative effectiveness analyses of robotic, laparoscopic, and open renal surgery.
Keywords: renal cell carcinoma, nephrectomy, quality of life, outcomes
Introduction
The need to re-evaluate comparative health related quality of life (HRQOL) expectations after kidney surgery has been fueled by refinement of contemporary surgical techniques, including nephron-sparing (partial nephrectomy), laparoscopic, and robotic-assisted laparoscopic approaches. These less invasive approaches offer the possibility of smaller incisions, less pain, and shorter recovery at the expense of a longer surgical learning curve, potentially more complications in inexperienced hands, and increased costs;1,2 however, validated metrics for evaluating patient-reported outcomes in this setting have not been clearly identified. Accordingly, although several retrospective single and multi-institutional studies have evaluated the short-term clinical benefits of laparoscopy in kidney surgery, studies evaluating patientreported HRQOL outcomes using surgery-specific HRQOL instruments are lacking.3
Many of the initial HRQOL assessments after kidney cancer surgery have been biased with lack of baseline HRQOL assessment, low response rates, evaluation of only open surgery, limited evaluation during the perioperative phase, or lack of a surgery-specific validated instrument.3,4 Prospective assessment of HRQOL using validated instruments is necessary to facilitate evidence-based decisions regarding therapy and set appropriate patient expectations. One such instrument is the Convalescence and Recovery Evaluation (CARE).5 The CARE is a 27-item validated short term health instrument that was designed specifically to measure changes common to all patients after abdominal and pelvic surgery and has four domains (Pain, Gastrointestinal, Cognitive, and Activity). Prior studies have been limited to measures of general HRQOL (e.g. SF-36), which may be insensitive to changes observed following surgery, Moreover, the instruments used in these studies were not developed for use in evaluating surgical outcomes, and have not been validated for such a purpose.3,4
Prior to widespread adoption of minimally invasive treatments for kidney cancer, robotic and laparoscopic approaches should demonstrate comparative effectiveness to standard open surgery. Additionally, straightforward methods of evaluating care quality in surgical patients may be a necessary component of future health care policy. Therefore, it will be necessary to evaluate the feasibility of administering validated HRQOL instruments to kidney surgery patients and determine the instrument domains that are appropriate measures for comparative effectiveness analysis and care quality measurement. We provide a feasibility study of an internet-based system to administer CARE to patients undergoing renal surgery and an evaluation of whether CARE is sensitive to detecting changes in HRQOL outcomes following various approaches to renal surgery, including laparoscopic, robotic, and open partial and radical nephrectomy.
Materials and Methods
Patient Population
All English speaking patients who underwent surgery for renal cancer at our institution between July 2009 and September 2010 were invited to participate in the study. All surgical procedures were either open, standard laparoscopic or robotic-assisted. Minimally invasive surgery was performed transperitoneal and open surgery was performed either through a flank or thoracoabdominal incision. The decision to perform radical versus partial nephrectomy for small renal masses were based on the AUA guidelines, surgeon, and patient preference.6 Clinical and quality of life data was collected prospectively and postoperative complications were collected retrospectively. Complications were recorded using the Clavien classification system.7 Eligibility for analysis included signed consent and completion of baseline and at least one follow-up questionnaire. This study was approved by our institution’s Institutional Review Board (IRB) and was compliant according to the Health Insurance Portability and Accountability Act (HIPAA).
Measures
We used the CARE and SF-12 to evaluate various HRQOL domains.5,8 Domain scores for CARE range from 0 to 100, with higher scores corresponding to a better health state and domain scores are combined to generate a composite score.8 SF-12 is an abbreviated version of the SF-36 and consists of 12 items and measures two domains including mental and physical component summaries (MCS, PCS), respectively. The SF-12 was chosen because it is more succinct, validated, and easier to complete for patients than the SF-36.9,10 Scores for each scale range from 0 to 100 with higher scores indicating higher function or well-being. Patients received surveys at preoperative (baseline) visit and at 2, 4, 12 and 24 weeks after surgery. Patients were given the choice of an Internet or paper version of the instruments. All preoperative and postoperative data was collected by a research assistant not participating in the care of patients. Patients were contacted via phone or email on up to 3 separate occasions if the survey was not complete 1 week after due date.
Statistical Methods
Descriptive statistics were used to characterize the clinical characteristics of the study cohort at baseline and are shown in Table 1. A Mantel-Haenszel Chi-square metric was used to compare the distributions of baseline categorical clinical characteristics across treatment modalities. In cases where the sample size was small (N<5), a Fisher’s exact test was used. For the continuous covariates medians and their distributions were compared using a Wilcoxon Two-Sample Test across surgical procedures. Two-sided P-values < .05 were considered statistically significant. SAS version 9.2 (SAS Institute, Cary, North Carolina) was used for all calculations. We made no attempt to evaluate laparoscopic patients separate from open surgical patients as the number of open patients was small in all groups.
Table 1.
Patient Demographics
| Characteristic | Partial N=36 |
Radical N=35 |
p- value |
|---|---|---|---|
| Male gender – cases (%) | 27 (75%) | 26 (74%) | 1.00 |
| Age (year) - Median (IQR) | 59 (44, 67) | 58 (50, 67) | 0.80 |
| ASA Score – cases (%) | 0.02 | ||
| 2 | 25 (69%) | 14 (40%) | |
| 3–4 | 11 (31%) | 21 (60%) | |
| Clinical Stage (TNM, 2002) – cases (%) | <0.01 | ||
| cT1a | 22 (67%) | 4 (14%) | |
| cT1b | 9 (27%) | 4 (14%) | |
| cT2 | 0 ( 0%) | 4 (14%) | |
| cT3a | 2 ( 6%) | 15 (52%) | |
| cT3b-c | 0 ( 0%) | 2 ( 7%) | |
| Clinical Stage N0 – cases (%) | 25 (69%) | 17 (50%) | 0.09 |
| Clinical Stage M0 – cases (%) | 35 (97%) | 25 (71%) | <0.01 |
| Surgery Type – cases (%) | 0.54 | ||
| Open | 8 (22%) | 5 (14%) | |
| Laparoscopic | 28 (78%) | 30 (86%) | |
| Pathologic Tumor Size - Median (IQR) | 2.6 (1.8, 4.0) | 7.1 (5.6, 11.0) | <0.01 |
| Pathology – cases (%) | |||
| Benign | 5 (14%) | 6 (17%) | 0.75 |
| Renal Cell Carcinoma | 31 (86%) | 29 (83%) | |
| Operative Time - Median (IQR) | 246 (210, 294) | 182 (144, 213) | <0.01 |
| Estimated Blood Loss - Median (IQR) | 125 (100, 300) | 70 ( 50, 100) | <0.01 |
| Hospital Stay - Median (IQR) | 3.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.04 |
| Complications Clavien Grade | 10 (28%) | 6 (17%) | 0.77 |
| I | 8 (22%) | 4 (11%) | |
| II | 1 ( 3%) | 1 ( 3%) | |
| IIIa | 1 ( 3%) | 1 ( 3%) |
Clinical Stages N0 = negative lymph node status, M0 = non-cytoreductive surgery; cT3b-c = pts falling under both cT3b and cT3c; Pathology Benign = oncocytoma, angiomyolipoma, other benign; Pathology RCC = clear cell carcinoma, papillary, chromophobe, collecting duct; IQR = interquartile range
Results
A total of 82 patients underwent renal surgery for renal cancer during the study period. Of these, 71 (86.6%) patients completed the baseline questionnaires and 60 (85%) completed at least one CARE and SF-12 questionnaires during the 24 week perioperative period and comprise the study cohort. Non-responders were classified as patients who completed a baseline questionnaire but failed to complete any additional questionnaire. There was no statistical difference between the demographics of responders and non-responders. Table 1 summarizes the clinical and pathologic features with majority of patients undergoing laparoscopic vs. open renal surgery (81% vs. 19%), respectively. A total of 28 patients underwent minimally invasive partial nephrectomy, with 15 (54%) and 13 (46%) of those undergoing robotic partial and pure laparoscopic partial nephrectomy respectively. The overall complication rate was 23% (28% for partial nephrectomies and 17% for radical nephrectomies).
51% of surveys were completed online and 49% were completed by traditional paper format. Response rate was similar for the two versions. The average time to complete the web-based (12 minutes) was less than time required for the written version (16 minutes). 95% of patients reported that the survey was either “No trouble whatsoever” or “Easy enough to complete” for both versions.
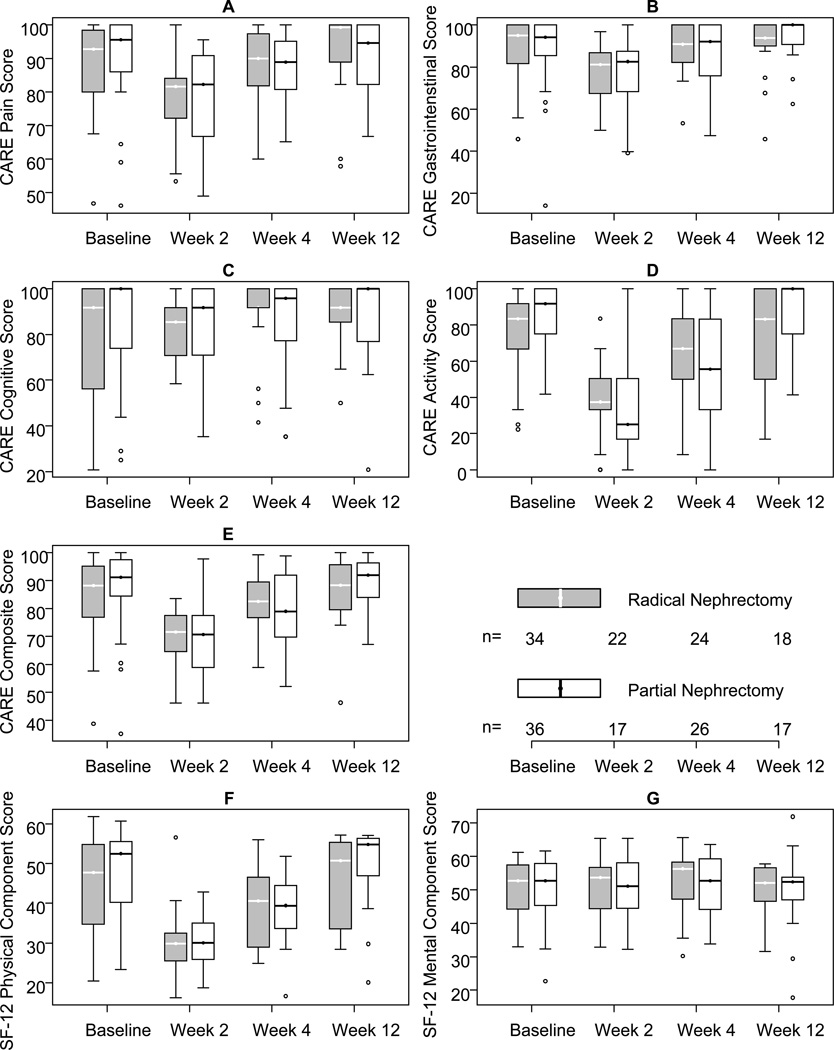
HRQOL results from CARE and SF-12 are demonstrated in Figure 1. The results of both radical and partial nephrectomy groups are displayed by post operative time interval and stratified into the the domains of both CARE and SF-12. The 24 week interval is not demonstrated as it did not differ significantly from the 12 week results. We found HRQOL decreased significantly at the 2 weeks with average recovery at 4 and 12 weeks to near baseline. Changes in CARE and SF-12 raw scores and percent changes from baseline are demonstrated in Table 2. The CARE composite score, as well as the activity, pain, GI, and SF-12 PCS domains demonstrated significant changes from baseline following kidney surgery in all groups at 2 weeks (P<0.001). The CARE activity and SF-12 PCS domains showed significant changes from baseline at the 4 week interval in both partial and radical nephrectomy groups (P<0.02). The CARE composite score was significantly decrease from baseline at 4 weeks only in partial nephrectomy patients (P<0.001).
Figure 1.
Changes in quality of life after kidney surgery. The graphs demonstrate unadjusted changes in mean quality of life scores over time for each domain, stratified by either partial or radical nephrectomy. The middle of the box is the median, the top and bottom are upper and lower quartiles, the line ranges are within 1.5 times the interquartile range of the upper and lower quartiles, and data points above these represent outliers (points beyond 1.5 times the interquartile range)
Table 2.
Raw changes and percent changes in CARE and SF-12 scores during follow-up compared to baseline
| Survey Instrument |
Domain | Interval | Radical | Partial | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw Δ | p- value |
% Δ | p- value |
Raw Δ | p- value |
% Δ | p- value |
|||
| CARE | Pain | 2 wks | −10.48 | 0 | −10.54 | 0 | −14.62 | 0 | −17.03 | 0 |
| 4 wks | −0.53 | 0.827 | 1.96 | 0.497 | −5.52 | 0.018 | −7.88 | 0.004 | ||
| 12 wks | 3.68 | 0.169 | 7.43 | 0.022 | −0.96 | 0.723 | −2.66 | 0.415 | ||
| GI | 2 wks | −11.12 | 0 | −11.18 | 0.004 | −12.14 | 0 | −13.26 | 0.002 | |
| 4 wks | 2.20 | 0.429 | 6.46 | 0.095 | −3.82 | 0.155 | −5.56 | 0.135 | ||
| 12 wks | 3.53 | 0.255 | 9.42 | 0.029 | 2.88 | 0.359 | 2.23 | 0.608 | ||
| Cognition | 2 wks | 2.46 | 0.493 | 11.84 | 0.087 | −0.99 | 0.803 | 3.77 | 0.616 | |
| 4 wks | 10.46 | 0.003 | 25.77 | 0 | −0.96 | 0.778 | −1.95 | 0.876 | ||
| 12 wks | 10.88 | 0.005 | 28 | 0 | 2.46 | 0.535 | 3.66 | 0.626 | ||
| Activity | 2 wks | −38.27 | 0 | −47.79 | 0 | −48.31 | 0 | −56.35 | 0 | |
| 4 wks | −14.16 | 0.067 | 1.34 | 0.905 | −27.16 | 0 | −32.03 | 0.003 | ||
| 12 wks | −2.88 | 0.640 | 27.32 | 0.028 | −1.63 | 0.794 | 1.19 | 0.924 | ||
| Composite | 2 wks | −14.18 | 0 | −15.12 | 0 | −19.32 | 0 | −22.32 | 0 | |
| 4 wks | −0.065 | 0.978 | 3.60 | 0.282 | −9.68 | 0 | −12.13 | 0 | ||
| 12 wks | 4.04 | 0.439 | 10.21 | 0.006 | 0.67 | 0.804 | 3.05 | 0.453 | ||
| SF-12 | PCS | 2 wks | −14.98 | 0 | −24.87 | 0 | −17.58 | 0 | −33.46 | 0 |
| 4 wks | −5.59 | 0.01 | −5.99 | 0.277 | −8.22 | 0 | −16.54 | 0.002 | ||
| 12 wks | 1.60 | 0.506 | 13.32 | 0.027 | 2.61 | 0.282 | 7.30 | 0.229 | ||
| MCS | 2 wks | 0.39 | 0.801 | 1.51 | 0.664 | 0.41 | 0.809 | 3.10 | 0.425 | |
| 4 wks | 2.29 | 0.133 | 5.85 | 0.087 | 0.89 | 0.547 | 3.33 | 0.308 | ||
| 12 wks | 1.10 | 0.515 | 4.52 | 0.244 | 1.18 | 0.493 | 2.69 | 0.490 | ||
The percentage of patients that returned to >90% of baseline score reported by CARE and SF-12 are depicted in Table 3. Of radical nephrectomy patients at 4 weeks, 78% and 48% returned to baseline (RTB) for CARE pain and activity domains, respectively, and 74% of patients RTB according to the CARE composite score. All of these numbers improved by 12 weeks. In the partial nephrectomy groups, fewer patients experienced RTB with 58% and 35% RTB for CARE pain and activity at 4 weeks, respectively. The RTB results using SF-12 PCS were similar.
Table 3.
The percentage of patients that returned to >90% of baseline score reported by CARE and SF-12
| Survey Instrument |
Variable | % |
|||
|---|---|---|---|---|---|
| 2 wks | 4 wks | 12 wks | 24 wks | ||
| CARE | Radical nephrectomy (N) | 22 | 23 | 17 | 10 |
| Partial nephrectomy (N) | 17 | 26 | 17 | 13 | |
| Pain | |||||
| Radical nephrectomy | 45 | 78 | 82 | 90 | |
| Partial nephrectomy | 41 | 58 | 77 | 85 | |
| Gastrointestinal | |||||
| Radical nephrectomy | 50 | 78 | 88 | 70 | |
| Partial nephrectomy | 41 | 69 | 94 | 92 | |
| Cognition | |||||
| Radical nephrectomy | 77 | 96 | 100 | 50 | |
| Partial nephrectomy | 82 | 81 | 82 | 77 | |
| Activity | |||||
| Radical nephrectomy | 17 | 48 | 77 | 60 | |
| Partial nephrectomy | 6 | 35 | 71 | 85 | |
| CARE Composite | |||||
| Radical nephrectomy | 41 | 74 | 88 | 90 | |
| Partial nephrectomy | 18 | 50 | 94 | 92 | |
| SF-12 | PCS | ||||
| Radical nephrectomy | 27 | 57 | 88 | 80 | |
| Partial nephrectomy | 12 | 27 | 82 | 85 | |
| MCS | |||||
| Radical nephrectomy | 73 | 96 | 94 | 80 | |
| Partial nephrectomy | 71 | 73 | 82 | 100 | |
N = number of patients; PCS = physical component summary; MCS = mental component summary
Comment
Our study shows that SF-12 and CARE demonstrated temporal changes in convalescence after renal surgery, and therefore both surveys appear to be responsive to meaningful differences in postoperative recovery across variety of domains. Approximately half of our patients elected to receive the surveys via the Internet and the vast majority of patients (95%) did not find the survey troublesome. Our relatively straightforward process yielded an acceptable response rate of 85%.
Although our study was not designed or powered to compare different surgical procedures to each other, certain recovery trends can be gleaned from our data and this information can be used to help counsel patients, keeping in mind that most of our patients underwent minimally invasive surgery. First and most importantly, our results demonstrated that average CARE activity, pain, GI and composite scores, as well as the SF-12 PCS scores were most sensitive to changes after surgery at the 2 week time point for both radical and partial nephrectomy groups. The mean CARE activity and SF-12 PCS were also significantly decreased from baseline at 4 weeks suggesting a slightly longer recovery than might be assumed considering most of these patients underwent laparoscopic surgery. In radical nephrectomy patients, the CARE cognition score was increased at 4 weeks. This unexpected result may be a result of pre-operative anxiety, depression, or uncertainty. Just under half of our radical nephrectomy patients (11/35) had metastatic disease and underwent cytoreductive nephrectomy. Future studies will be designed to evaluate baseline HRQOL and recovery in this subset of patients. Using the CARE composite score as a measure of overall recovery, we found patients recovered more quickly after radical nephrectomy than partial nephrectomy (p=0.006). Our study was not designed to evaluate this comparison and these results should be considered hypothesis generating. Direct comparisons between surgical groups, with both short and longer term follow-up, will be one the aims of a future study.
In addition to evaluating temporal recovery trends in the first 12 weeks after surgery, we evaluated the likelihood of patient return to baseline (RTB). According to the CARE composite score, overall HRQOL recovered by 4 weeks in 74 and 50% of radical and partial nephrectomy patients respectively, however activity was much slower to recover: in that only 48 and 35% of radical and partial patients returned to baseline activity at 4 weeks. Most patients (>80%) returned to baseline by 12 weeks according to both CARE and SF-12. These subtleties in recovery by domain are crucial for patients to understand prior to surgery and should be emphasized in preoperative counseling. In the era of minimally invasive surgery there are ever increasing patient expectations regarding recovery. Many patients have been given the sense that their recovery from minimally invasive urologic surgery will be very simple, and unfortunately hospital and practice web sites are frequently misleading in this regard.11
Others have described HRQOL after surgery for renal cancer, however, the studies lacked either baseline HRQOL, suffered from low response rates or used generic HRQOL surveys.4,12,13 Generic surveys such as the SF-36 have demonstrated varying degrees of sensitivities across different procedures.14,15 CARE was developed primarily to evaluate domains common to all patients undergoing abdominal and pelvic surgery with a particular emphasis on perioperative convalescence.5 Novarra et al. evaluated mid term recovery using the SF-36 at 6 and 12 months in 129 patients.3 They found a significant number of patients (20–50%) who did not reach baseline at 12 months. Although this recovery appears slower than that from our cohort, most of their patients underwent open surgery. Moreover, comparing recovery trends across institutions is challenging because different postoperative care pathways and cultural expectations can influence recovery.3 Poulakis et al evaluated HRQOL using SF-36, EORTC QLQ-C30 (a cancer specific survey) and individual items evaluating stress and fear of cancer recurrence in 416 patients, 51 of whom were evaluated prospectively.4 Patient comorbidities, type of operation (partial versus radical), female sex, and tumor size had the most significant impact on HRQOL. At 12 months, radical nephrectomy patients had lower role physical and role emotional scores than partial nephrectomy patients. Longer term follow of our patients might reveal differences in general HRQOL between partial and radical nephrectomy patients representing the importance and impact of overall renal function. Our data collection was straightforward and demonstrates that the particular domains of pain and activity and composite score from CARE as well as SF-12 PCS can be used to evaluate short term surgical outcomes or to evaluate healthcare quality after kidney cancer surgery.
Our study has several limitations. It is a single institution, non-randomized study. Although our overall response rate was relatively high (85%), there were patients that did not respond to every timepoint, possibly influencing results. We did investigate whether there were any differences in the non-responding patients with regard to complications or demographics and found no differences. Although Dillman has previously proposed responses rates above 75% to be suitable when reporting survey outcomes,16 it is possible that non-responders had worse HRQOL outcomes as suggested in a prior study.3 Our follow up is relatively short and does not represent long term outcomes, which will be of interest in evaluating some of the differences between nephron sparing surgery versus radical nephrectomy. Moreover our study was not powered to stratify recovery by patient demographic variables such as age, tumor location, tumor size, or comorbidities, factors which others have found important in determining long term HRQOL.4 We did not use an organ or disease specific instrument in the current study. We used CARE which has been previously validated for a variety of abdominal and pelvic surgeries with particular usefulness in the perioperative period.5 Furthermore, we utilized the SF-12 to compare the physical domains measured by CARE and use of a more robust instrument such as SF-36 might have elucidated other postoperative changes.
Conclusion
Obtaining patient reported HRQOL information after renal surgery is feasible with a high response rate using either Internet or paper based instruments. The CARE and SF-12 instruments are useful for evaluating the impact of HRQOL for patients undergoing surgery for renal cancer. The domains most sensitive to postoperative convalescence appear to be the CARE pain, activity, and GI domains as well as SF-12 PCS. Most patients returned to their baseline HRQOL by 12 weeks after surgery. Our results provide useful information regarding perioperative expectations for patients undergoing surgery for renal cancer. Future studies can use this technique to perform comparative effectiveness analysis of different surgical techniques, in particular compare open to minimally invasive techniques for both radical and partial nephrectomy or to compare different approaches to minimally invasive surgery. Additional studies might also use these instruments to measure and compare care quality among health care delivery systems.
Acknowledgment
We wish to thank Mr. Andrew Percy for his assistance with data entry, editing, and statistical advice with this manuscript.
Financial Support: This project was supported by a joint Developmental Award from the Dana Farber/Harvard Cancer Center Kidney Cancer SPORE and the Kidney Cancer Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J. Urol. 2007;178(1):41–46. doi: 10.1016/j.juro.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67(2):254–259. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 3.Novara G, Secco S, Botteri M, et al. Factors predicting health-related quality of life recovery in patients undergoing surgical treatment for renal tumors: prospective evaluation using the RAND SF-36 Health Survey. Eur. Urol. 2010;57(1):112–120. doi: 10.1016/j.eururo.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Poulakis V, Witzsch U, de Vries R, Moeckel M, Becht E. Quality of life after surgery for localized renal cell carcinoma: comparison between radical nephrectomy and nephron-sparing surgery. Urology. 2003;62(5):814–820. doi: 10.1016/s0090-4295(03)00687-3. [DOI] [PubMed] [Google Scholar]
- 5.Hollenbeck BK, Dunn RL, Wolf JS, Jr, et al. Development and validation of the convalescence and recovery evaluation (CARE) for measuring quality of life after surgery. Qual Life Res. 2008;17(6):915–926. doi: 10.1007/s11136-008-9366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J. Urol. 2009;182(4):1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedgepeth RC, Wolf JS, Jr, Dunn RL, Wei JT, Hollenbeck BK. Patient-reported recovery after abdominal and pelvic surgery using the Convalescence and Recovery Evaluation (CARE): implications for measuring the impact of surgical processes of care and innovation. Surg Innov. 2009;16(3):243–248. doi: 10.1177/1553350609342075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 10.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of healthrelated quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 11.Mulhall JP, Rojaz-Cruz C, Müller A. An analysis of sexual health information on radical prostatectomy websites. BJU Int. 2010;105(1):68–72. doi: 10.1111/j.1464-410X.2009.08762.x. [DOI] [PubMed] [Google Scholar]
- 12.Clark PE, Schover LR, Uzzo RG, et al. Quality of life and psychological adaptation after surgical treatment for localized renal cell carcinoma: impact of the amount of remaining renal tissue. Urology. 2001;57(2):252–256. doi: 10.1016/s0090-4295(00)00927-4. [DOI] [PubMed] [Google Scholar]
- 13.Onishi T, Nishikawa K, Hasegawa Y, et al. Assessment of health-related quality of life after radiofrequency ablation or laparoscopic surgery for small renal cell carcinoma: a prospective study with medical outcomes Study 36-Item Health Survey (SF-36) Jpn. J. Clin. Oncol. 2007;37(10):750–754. doi: 10.1093/jjco/hym107. [DOI] [PubMed] [Google Scholar]
- 14.Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287(3):321–328. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 15.Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273(2):129–135. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 16.Hoddinott SN, Bass MJ. The dillman total design survey method. Can Fam Physician. 1986;32:2366–2368. [PMC free article] [PubMed] [Google Scholar]