Abstract
Phthalates are widely used as plasticizers in everyday products. Yet, studies on the effects of phthalates on female reproductive health are limited. In this study, pregnant C57/Bl6 mice were exposed via oral gavage to corn oil, 100, 500, or 1000mg/kg MEHP from gestational days 17–19. Reproductive lifespan was decreased by one month in the highest F1 exposure group (9.8±0.4 versus 11.1±0.6 months in control F1 females). F1 females exhibited delayed estrous onset at the two higher exposures and prolonged estrus was observed in all MEHP-exposed females. Serum FSH and estradiol were significantly elevated at the highest exposure and altered mRNA expression was found for the steroidogenic genes LHCGR, aromatase, and StAR. At one year of age, mammary gland hyperplasia was observed in high dose MEHP-exposed females. In summary, late gestational exposure to MEHP leads to multiple latent reproductive effects throughout murine life resulting in premature ovarian senescence and mammary hyperplasia.
Keywords: MEHP, ovary, premature ovarian failure, steroidogenesis
1. INTRODUCTION
Phthalate esters are used ubiquitously as plasticizers, with global production reaching over four million tons a year [1,2]. Di-ethyl-hexyl-phthalate (DEHP), the most widely used phthalate ester, is readily metabolized by esterases in the gut to mono-2-ethyl-hexyl-phthalate (MEHP). Exposure to DEHP can occur from a number of sources due to the widespread use of phthalates. Phthalate-containing plastics can leach phthalates readily into bodily fluids; consequently, food containers and medical devices such as blood bags and intravenous tubing can pose a significant route of human exposure to DEHP and other phthalates. Human exposure to phthalates has been established, as epidemiological studies show that an overwhelming majority of individuals have detectable levels of MEHP metabolites in their urine. High levels of the phthalate metabolite MEHP in the urine of pregnant women has been linked to cryptorchidism in their male offspring [2].
Phthalates have widely been considered to be male reproductive toxicants in rodents [3, 4] and epidemiological studies [5,6,7] and more recently xenotransplant models [8] suggest that exposure to plasticicizers in the human male results in reproductive abnormalities. Depending upon the phthalate ester (PE) and its route of exposure, in utero [3] or peripubertal [4], profound effects on the male reproductive system have been observed, including varying degrees of cryptorchidism, germ cell death, malformations of the epididymis, vas deferens, seminal vesicles, and prostate and decreased levels of testosterone [2,3,4]. In addition to the adverse reproductive effects observed in the male, studies have demonstrated that the ovary is also vulnerable to phthalate exposure [9]. High doses of DEHP have been shown to suppress serum estradiol levels and ovulation in adult female rats [10]. Another study found that exposure from conception through lactation in the rat leads to increased tertiary atretic follicles by adulthood but no other reproductive abnormalities were observed [11, 12]. Chronic exposure to di-butyl-phthalate (DBP) from gestation through pregnancy reduces fertility in rats [13]. In humans, a report on female workers in a phthalate processing plant found that women exhibited anovulatory cycles in response to high occupational exposure to phthalates [14].
We conducted the present study to determine the effects of MEHP on the female murine reproductive system because no studies to date have evaluated the adult reproductive effects in mice following in utero exposure to this metabolite. Importantly, administration of 500 mg/kg MEHP is within the dose range previously shown to lead to altered reproductive function in the female rodent [11]. The doses utilized in the present study are not considered physiologically relevant. The Agency for Toxic Substances and Disease Registry in 1993 reported a maximum daily intake of 2 mg in the general population. However, subsets of the population may receive levels significantly higher than 2mg per day [15]. Medical interventions with DEHP-containing devices or pharmaceuticals have been demonstrated to contribute to elevated levels of DEHP metabolites in urine and/or blood. In rodent models, phthalates have been shown to cross the placental barrier, with concentrations in the fetus within two-fold compared to those found in the mother [16]. An exposure window from GD17 to GD19 was selected for in utero exposure to MEHP. This period of exposure was selected because although it is after the ovary has been established, it is a key period of folliculogenesis. This is the critical period of germ cell nest breakdown and primordial follicle formation [17]. This process has been demonstrated to be sensitive to endocrine disruption in rodents [18] and was chosen as a narrow but relevant window of exposure. Based on prior studies [18,19], we hypothesized that late gestational exposure to MEHP effects the process of follicular recruitment in these animals resulting in adult life reproductive effects such as premature ovarian senescence and mammary gland hyperplasia.
2. Materials and Methods
2.1 Mice
Timed-pregnant C57/Bl6 mice were purchased from Charles River Laboratories (Shrewsbury, MA). Animals were kept in climate-controlled colonies (23.3±2 degrees Celsius; humidity 30–70%) with a 12 hour light/dark cycle. All procedures involving animals were performed in accordance with the guidelines of the Institutional Animal Care and Use committee of Brown University in compliance with the guidelines established by the National Institute of Health.
2.2 Exposure Paradigm
At gestational days 17, 18, and 19 (with day of plug detection considered GD1), pregnant dams were exposed via oral gavage to 100, 500, or 1000 mg/kg MEHP (TCI America) dissolved in corn oil, or an equivalent volume of corn oil as a vehicle control. Typical dosage volumes ranged from 40 μl to 60 μl. Seven dams were exposed per treatment group. Dams were monitored for changes in weight throughout the exposure period. Pups were counted at birth and weighed every other day from PND1 to 21 and weaned at PND 25. Whenever mice were necropsied, they were sacrificed on the first observed day of the estrous cycle to maintain consistency within and between treatment groups.
2.3 Reproductive Endpoints
Reproductive endpoints assessed included age at vaginal opening, age of first estrous cycle, and estrous cycle length and phase. Estrous cycles were determined via vaginal lavage cytology followed by staining with toluidine blue and recorded daily for 17-day spans from PND40 to PND56, and for mice not sacrificed at PND56, for at least 7 sequential days per month. All cycles were measured at approximately 10 A.M. daily. Assessments were completed following guidelines set by Pedersen [20].
2.4 Continuous-Breeding Assay
In order to assess the reproductive fitness of female mice, pregnant C57/Bl6 dams were exposed via oral gavage to 100, 500, or 1000 mg/kg MEHP dissolved in corn oil or an equivalent volume of corn oil as vehicle control from gestational days 17–19 as described above. The F1 female pups were then paired with healthy C57/Bl6 male mice for breeding starting at an age of 8 weeks. Mating pairs were placed in separate cages and inspected each morning. After a litter was weaned, the female was paired with a new male to avoid low reproducibility due to male reproductive dysfunction. If no litter was produced, the female was paired one more time with a healthy male to confirm female infertility. Litter size and cumulative number of progeny per female were recorded for each group and used to evaluate the reproductive fitness of female mice. The ages of the control and F1 exposed females at their final delivered live litters were recorded.
2.5 Mammary Gland Whole Mount Analysis
F1 females were sacrificed at PND365, following either continuous breeding or non-breeding isolation. At sacrifice, inguinal mammary glands from the fourth and ninth positions were removed, and mounted via the whole mount protocol described in Fenton et al, 2002 [21]. Briefly, mammary glands were placed on microscope slides and fixed in Carnoy’s fixative. Following rehydration they were stained using carmine solution, dehydrated via ethanol and xylene washes, and photographed via a dissecting microscope (Zeiss Stereo Discovery V.8, Carl Zeiss International).
2.6 Hormone Assessment
To measure serum FSH, estradiol, and LH levels, blood samples were collected by cardiac puncture from female mice at the first detected day of estrus. Mice were euthanized via carbon dioxide asphyxiation and all necropsies were performed within an hour of establishing estrus stage. The serum was stored at −80°C until assayed. FSH and LH levels were assayed by the University of Virginia Ligand Core Facility as previously described for radioimmunoassay of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (http://www.healthsystem.virginia.edu/internet/crr/ligand.cfm; Specialized Cooperative Center Program in Reproductive Research, National Institute of Child Health and Human Development/National Institutes of Health U54 HD28934). Estradiol levels were measured utilizing the Estradiol ELISA kit (11-ESTHU-E01) obtained from Alpco Immunoassays (Salem, NH) according to the manufacturer’s specifications.
2.7 RNA Isolation
Mice were necropsied at PND56, ovaries were then flash-frozen, and stored at −80°C until further processing. RNA was extracted from whole ovaries by homogenization with Tri-Reagent (Sigma-Aldrich, St. Louis, MO), followed by isolation using an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA quality and concentration was determined using a NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, DE). RNA Samples with concentrations less than 100 ng/μl or with a 260/280nm absorption ratio less than 1.95 were not included in further studies.
2.8 Gene Array
At PND56, RNA was isolated from the ovaries of different litters of F1 females exposed in utero to 1000 mg/kg MEHP (N=4 ovaries) and from the ovaries of different litters of F1 females exposed in utero to corn oil (N=3 ovaries). The ovarian RNA was then hybridized for gene array experiments. Microarray analysis was conducted using Affymetrix GeneChip Mouse Gene 1.0 ST Arrays (Affymetrix, Santa Clara, CA) following manufacturer’s protocols. Statistical analysis of the raw CEL files was performed with ParTek Genomics Suite Software (Partek, St. Louis, MO) using Robust Multiple Array (RMA) analysis with log2 transformation and gating to include only genes differentially expressed with a p-value of less than 0.01 and at least a 2-fold difference relative to controls.
2.9 Quantitative RT-PCR
Total RNA was treated with DNase-I (Invitrogen, Carlsbad, CA) and then reverse-transcribed with iScript cDNA Synthesis Kit (Bio-Rad) following the manufacturer’s protocols, and the cDNA templates were amplified with QuantiTect primers (Qiagen) using iQ SYBR Green Supermix (Bio-Rad) on an iCycler iQ Multicolor Real-time PCR Detection System (Bio-Rad). Each sample was run in triplicate, and mRNA levels were analyzed relative to hypoxanthine phosphoribosyltransferase (HPRT), a housekeeping gene unaltered by MEHP exposure [22, 23]. Log2-transformed relative expression ratios were calculated as described using the equation set forth by Pfaffl [24], in which efficiencies for both the gene of interest and the calibrator hypoxanthine phosphoribosyltransferase (HPRT) were used. To avoid litter effects, only one female per litter was used for RNA, with 7 litters per treatment group used for RT-PCR.
2.10 Follicle Counts
Ovaries were removed at sacrifice from PND56 or PND365 mice and fixed in Bouin’s Fixative (Sigma-Aldrich) for 24h, then processed and embedded into paraffin for sectioning. Selected ovaries were sectioned completely into 7μm sections. Every other section was counted and follicles classified as either primordial, primary, secondary, or antral. Primordial follicles were defined as those containing an oocyte with a single layer of flat granulosa cells. Follicles were classified as primary if they contained an oocyte surrounded by 7 or more cuboidal granulosa cells. Secondary follicles were classified as having two or more complete layers of granulosa cells. Antral follicles were classified as having a visible antrum with an area greater than that of the oocyte. Only follicles with visible nuclei were counted to prevent counting the same oocyte multiple times. Total ovary area per section was measured using Aperio Imagescope Software (Aperio Technologies, Vista, CA) and the number of follicles were divided by ovary area to compensate for possible differences in ovary size and section location. As with the RT-PCR, only one ovary per litter was selected for counting to avoid litter effects. Follicle counts were normalized to area counted.
2.11 KGN Cell Culture
The human granulosa-like KGN cell line [25], grown in DMEM/F-12 medium supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml) and FCS (10% v/v) from cells kindly provided by Richard Freiman (Brown University). Cells were maintained as monolayer cultures in 100 mm dishes at 37°C in 5% CO2. Cells were serum-starved for 24h, followed by 24h exposure to MEHP at 10 or 100 μM or EtOH vehicle control, in combination with either forskolin (25 μM) or additional EtOH, following the protocol established by Ohno et al [26]. Ethanol did not exceed 0.05% by volume in any treatment group. Following exposure, cells were harvested for RNA isolation and real-time RT-PCR as described above.
2.12 Statistical Analysis
All animal data are presented as the mean ± SEM. Data were analyzed using two-way ANOVA analysis (SigmaPlot software version 8). Each data point for animal experiments represents a single randomly selected individual from an in utero exposed litter. A p < 0.05 was considered statistically significant.
3. RESULTS
3.1 Maternal body and pup weights
Administration of MEHP at 100, 500, and 1000mg/kg from GD17–19 did not affect maternal body weight or pup growth. Maternal body and pup weights are shown in Table 1. Maternal body weight was recorded immediately prior to administering the first dose of MEHP (GD17) and at the time of final dosing of MEHP (GD19). Seven pregnant dams were examined per exposure group. A minimum of two pups were randomly selected from control and exposed litters and weighed at postnatal day 7. Again, we found no significant differences in pup weight between corn oil exposed and MEHP exposed pups (Table 1). This trend continued throughout the lifetime of the animal, including at PND28 (within 2–3 days of the mean day of vaginal opening for all treatment groups) and at PND56, the young adult sacrifice timepoint. There were no significant differences in animal weight between exposure groups when matched for age.
Table 1.
In utero exposure to MEHP does not induce overt maternal toxicity or changes in pup weight.
| Treatment | Weight (g) at PND7 | Changes in maternal weight during dosing (g) |
|---|---|---|
| Control | 3.1 ± 0.7 (n=14) | 2.0 ± 0.6 (n=7) |
| 100 mg/kg | 3.3 ± 0.9 (n=14) | 2.3 ± 1.0 (n=7) |
| 500 mg/kg | 3.6 ± 0.8 (n=14) | 1.7 ± 1.1 (n=7) |
| 1000 mg/kg | 3.2 ± 1.0 (n=14) | 1.6 ± 1.5 (n=7) |
3.2 Vaginal Opening and Estrous Cyclicity
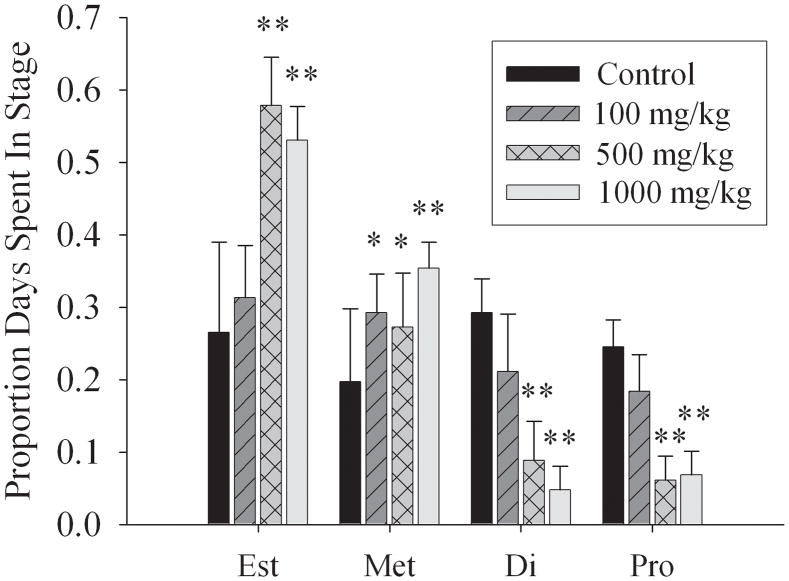
Beginning at 25 days of age, randomly selected F1 females from both control and MEHP-exposed litters were monitored daily for vaginal opening. A trend was found for delayed vaginal opening in both the 500mg/kg and 1000mg/kg MEHP exposure groups (Table 2). Following vaginal opening, F1 females were monitored for the onset of estrous (Table 2). At both the 500mg/kg and 1000mg/kg MEHP exposure groups, there was a four-day delay in estrous onset (p < 0.01). Body weights for these animals remained consistent across treatment groups (Table 1), suggesting that alterations in maturity are not related to general growth differences. In both the 500mg/kg and 1000mg/kg exposure groups, F1 females exhibited a prolonged estrous phase of approximately twice the length of control F1 females (p < 0.01) (Fig. 1). The duration of metestrous was also significantly increased relative to controls in the 500mg/kg and 1000mg/kg F1 adult females (Fig. 1). Adult F1 females in the 100mg/kg MEHP exposure group exhibited a similar pattern to the higher exposure groups; however, only the length of metestrous was significantly increased relative to control females (p< 0.05). A strong trend for an increase in the time spent in the estrous stage of the cycle was observed in F1 females in the 100mg/kg MEHP exposure group (p = 0.06) (Fig. 1). A total of 14 females from 7 random litters per group were examined. Moreover, altered estrous cyclicity was observed in exposed F1 females for up to one year.
Table 2.
Markers of sexual maturity in in utero exposed F1 females.
| Treatment | Vaginal Opening (PND) | Onset of Estrous (PND) |
|---|---|---|
| Control | 30.0 ± 1.3 (n=14) | 35.1 ± 1.9 (n=14) |
| 100 mg/kg | 30.0 ± 1.4 (n=14) | 35.6 ± 1.0 (n=14) |
| 500 mg/kg | 30.7 ± 2.7 (n=14) | 38.9 ± 1.8** (n=14) |
| 1000 mg/kg | 30.8 ± 2.5 (n=14) | 39.3 ± 1.9** (n=14) |
indicates significant difference from controls
p< 0.01.
Figure 1. Effects on the estrous cycle of F1 females exposed in utero to vehicle control, 100mg/kg, 500mg/kg, or 1000mg/kg MEHP.
Graph depicts the fraction of time spent in each stage by exposure group. Numerical values represent the percent of time spent per day in a particular stage of estrus; means + standard deviation are shown. * indicates significant difference from controls as determined by (1 or 2-way?) ANOVA * p < 0.05; ** p < 0.01.
3.3 Litter size and Reproductive Lifespan
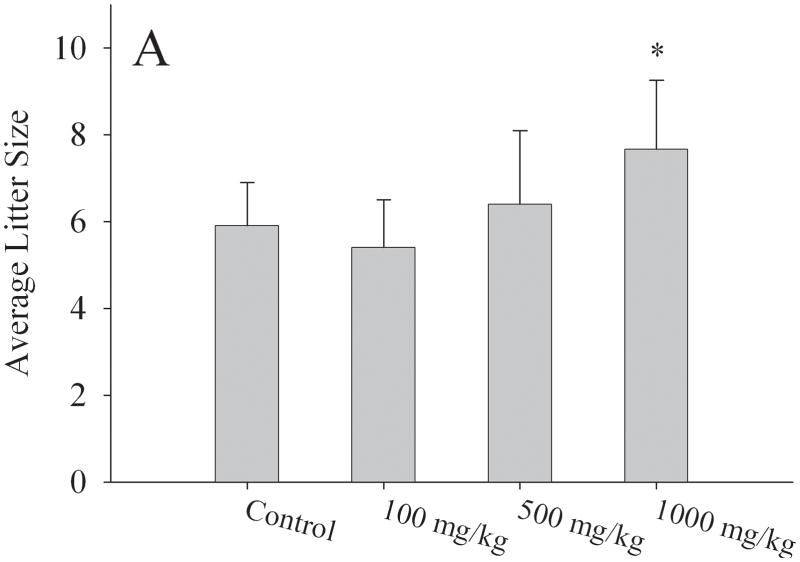
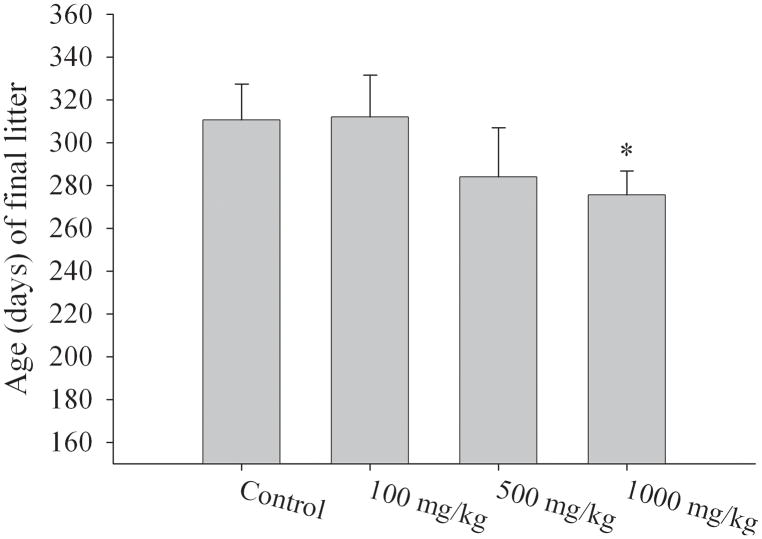
Adult F1 females exposed in utero to MEHP were housed with unexposed male mice of proven fertility. All breeding pairs successfully had 3 or more litters and litters were weaned at PND25. Initially, at 1000mg/kg MEHP, adult F1 females exhibited a significant increase in average litter size (7.6 ± 1.6) relative to control females (5.9 ± 1.0) (p < 0.05) (Fig. 2a). Since C57/Bl6 mice typically have litter sizes of 5–6 pups, the observed increase in litter size (7–9 pups per litter) prompted us to determine the reproductive lifespan of the in utero exposed F1 female generation. We found that over a ten month period, the total number of pups delivered per group did not differ significantly among the exposure groups (23.6±3.1 pups [corn oil]; 21.5±1.2 pups [100mg/kg MEHP]; 21.3±4.8 pups [500mg/kg MEHP]; and 23.0±2.6 pups [1000mg/kg MEHP]). However, continual breeding of exposed F1 females with males of proven fertility revealed a one month decrease in the overall reproductive lifespan of the F1 female generation in the highest exposure group relative to controls. Adult F1 females exposed in utero to 1000mg/kg MEHP delivered their final litter at 275.5 ± 11.4 days of age, compared to 310.5 ± 16.8 days of age in the controls (p < 0.05) (Fig. 2b).
Figure 2. Effects on fertility of F1 females exposed in utero to 0mg/kg, 100mg/kg, 500mg/kg, or 1000mg/kg MEHP.
F1 females were bred with unrelated male mice of proven fertility. (a) Total number of pups per litter were counted and averaged for all the exposure groups. N = 12 litters/4 breeding pairs (control); N= 8 litters/3 breeding pairs (100 mg/kg); N= 10 litters/3 breeding pairs (500 mg/kg); and 9 litters/4 breeding pairs (1000 mg/kg); means and standard deviation are shown. (b) Effect of in utero exposure to MEHP on F1 female reproductive lifespan. The graph depicts the age of F1 females at a given exposure at delivery of last live litter. All F1 female mice were sacrificed at day 365. Values on the figure are average ages and SD. indicates the mean differed from control as determined by ANOVA; * p <0.05.
3.4 Follicle counts
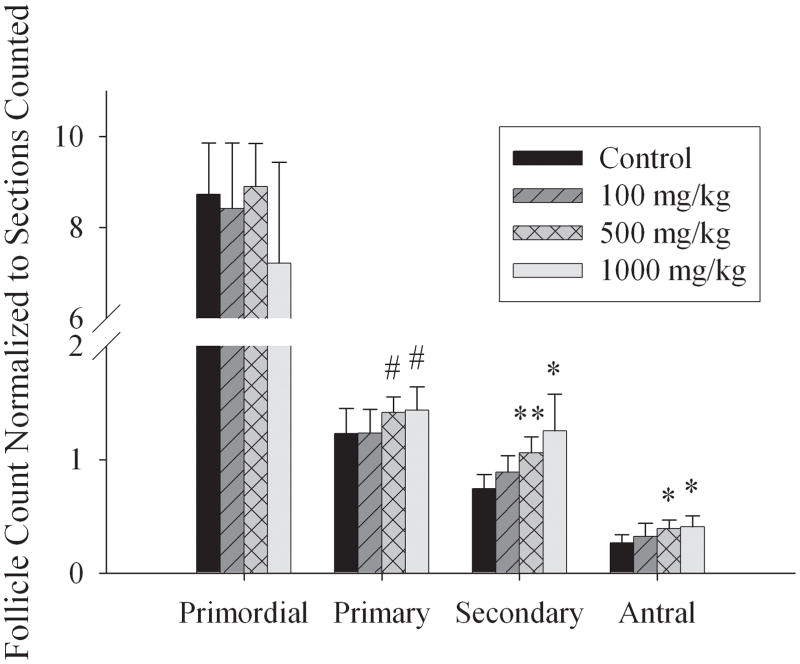
Figure 3 depicts the follicle counts of corn oil exposed and MEHP exposed ovaries from adult F1 females sacrificed on the first day of estrous. Follicle counts were determined in one ovary per mouse from one random female per litter (N=7 litters). To determine the number of follicles present, we counted the number of follicles per type per section, and normalized the number of follicles counted per section to the total ovary area of that section to control for possible differences in ovary size, orientation, or section depth. We found no significant differences in the number of primordial follicles between either the control or exposure groups at any of the doses of MEHP administered. Scoring of primary follicles revealed that in 500mg/kg/day MEHP (1.05 ± 0.14) and 1000 mg/kg/day MEHP (1.04 ± 0.16 mm2) exposure groups, there was a trend for higher numbers of primary follicles relative to the corn oil exposed group (0.90 ± 0.12 mm2) (Fig 3). We also observed a significant increase in the number of secondary follicles at 500 and 1000mg/kg/day MEHP. In the 500 mg/kg/day exposure group, there were 0.79 ± 0.13 secondary follicles per mm2 (p < 0.05) and at 1000mg/kg/day there were 0.91 ± 0.25 follicles per mm2 compared to 0.55 ± 0.12 follicles per mm2 in control ovaries (p < 0.05). The number of antral follicles was significantly increased at the higher exposure groups relative to control ovaries with 0.20 ± 0.07 antral follicles per mm2 in controls compared to 0.29 ± 0.06 at 500 mg/kg/day and 0.30 ± 0.07 follicles per mm2 at 1000 mg/kg/day (p < 0.05).
Figure 3. Effects on ovarian follicle counts in F1 females exposed in utero to 0mg/kg, 100mg/kg, 500mg/kg, or 1000mg/kg MEHP.
Ovarian follicle counts in females exposed in utero to MEHP, normalized to section area. Bars represent the mean and SD of ovaries from at least three separate litters for both F1 control and F1 exposed litters. indicates the mean differed from control as determined by ANOVA; * p < 0.05.
3.5 Mammary Gland Whole Mounts
Inguinal mammary glands (positions 4 and 9) removed from F1 females at 365 days of age were mounted, fixed, and stained for gross assessment. Figure 4 depicts the whole mount images of mammary glands from F1 virgin (Fig 4. c,d) and F1 breeding females (Fig. 4a,b) exposed to corn oil (Fig 4. a,c) or 1000mg/kg MEHP in utero (Fig 4. b,d). Regardless of breeding status, F1 females exposed to 1000 mg/kg MEHP in utero (Fig. 4. b,d) exhibited significant hyperplasia relative to corn oil exposed (Fig. 4a,c ) with more extensive branching and the presence of hyperplastic nodules (Fig 4c,d).
Figure 4. Effects of in utero exposure to 1000 mg/kg MEHP on mammary gland hyperplasia at PND365.
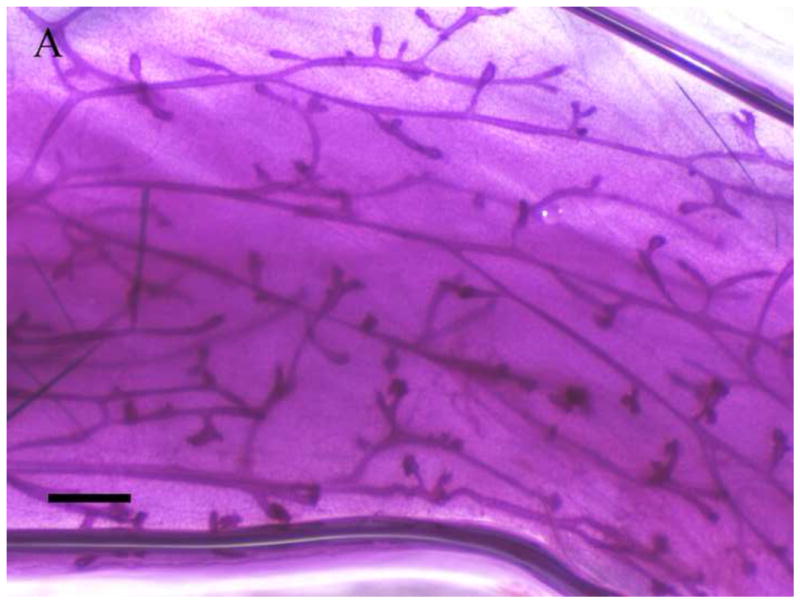
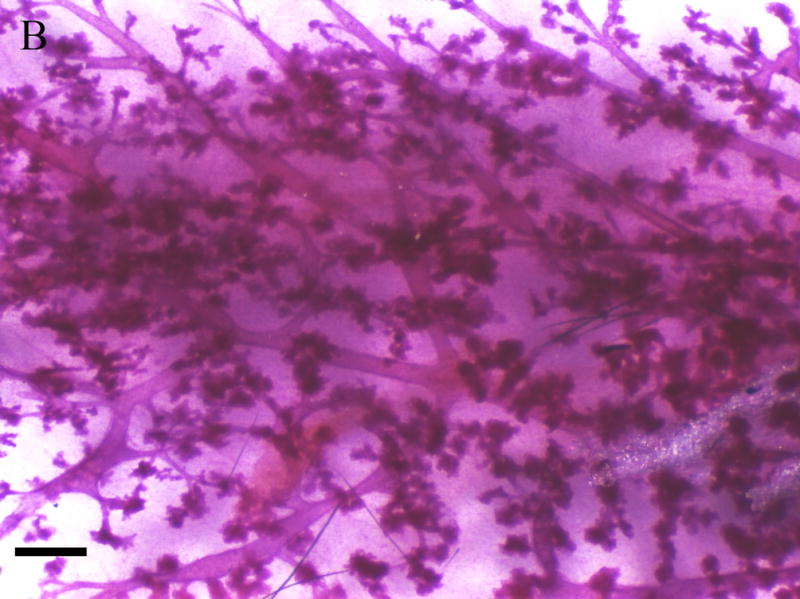
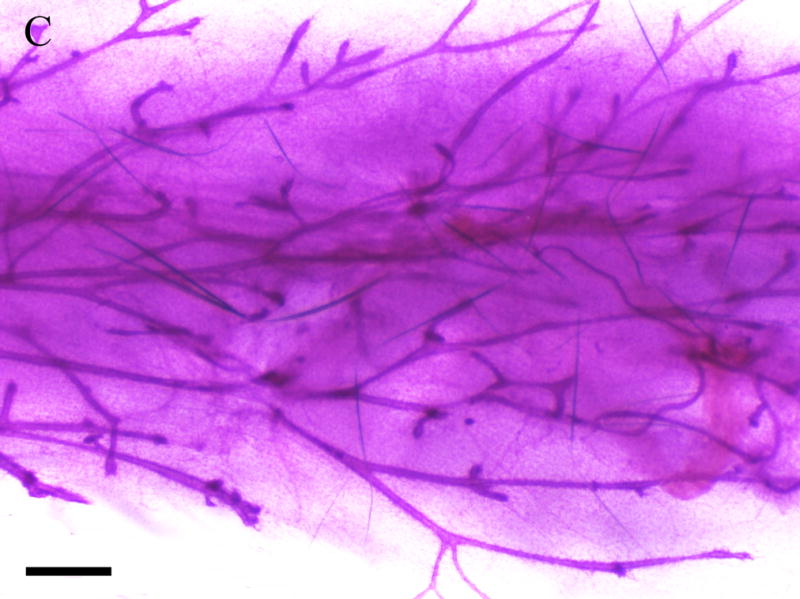
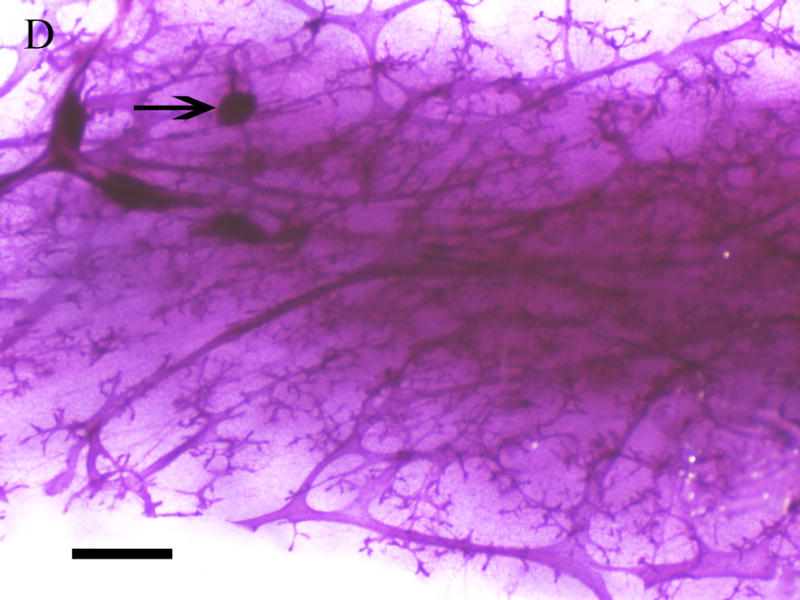
Mammary glands were removed and whole mounted and stained with carmine solution. A. Mammary gland of virgin control female. B. 1000 mg/kg in utero exposed virgin female. C. Mammary gland of breeding control dam. D. 1000 mg/kg in utero exposed breeding dam. Bar indicates 1 mm. Arrows indicate hyperplastic alveolar nodules.
3.6 Serum Hormone Levels
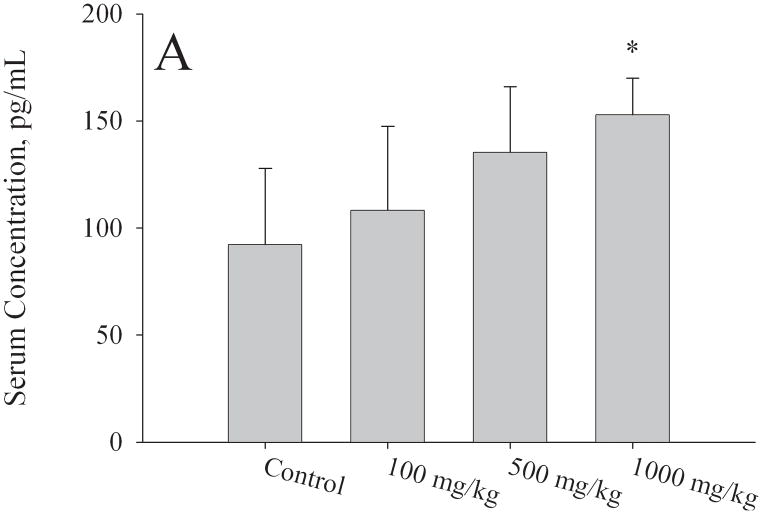
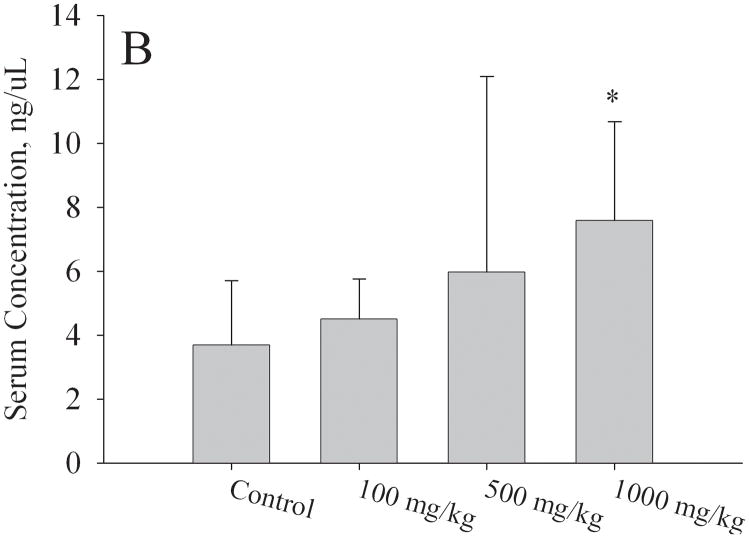
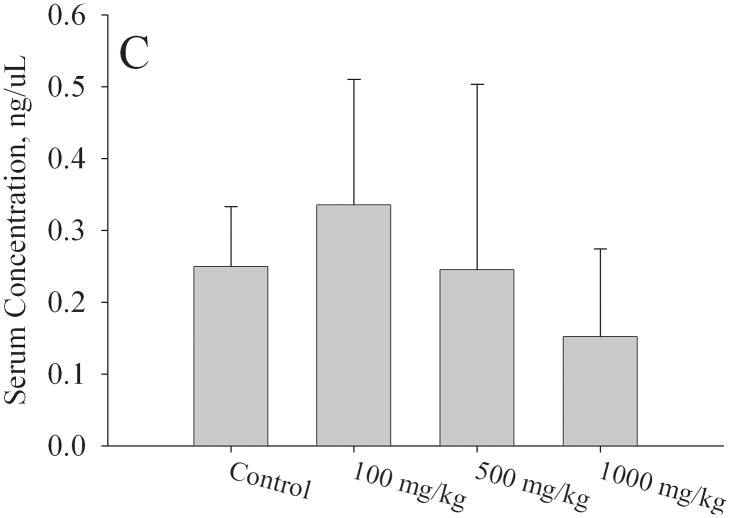
Levels of estradiol, FSH, and LH in serum isolated from F1 females at PND56 were measured by ELISA. All serum samples were obtained during the first day of estrous stage of the estrous cycle. Mean estradiol levels were significantly higher (p < 0.05) in the 1000mg/kg/day exposure group (153 ± 17 pg/mL) compared to controls (92 ± 37 pg/mL) (Fig. 5a). A strong trend for increased serum estradiol levels was detected at 500mg/kg (135 ± 31 pg/mL, p = 0.075). No significant difference was detected in the 100mg/kg/day group compared to control. For the estradiol assay, the numbers of mice utilized per group were the following: N=6 for control group and 1000 mg/kg, n=5 for 100 and 500 mg/kg. Serum FSH levels (Fig. 5b) were significantly elevated at 1000mg/kg (7.6 ± 3.1 ng/μL, p < 0.05) relative to control (3.7 ± 2.0 ng/μL). Serum LH levels (Fig. 5c) showed no significant differences at the 100 or 500mg/kg/day exposure groups; however, at 1000mg/kg/day there were lower levels of LH (0.15 ± 0.10 ng/μL) relative to controls (0.25 ± 0.08 ng/μL, p = 0.05). For the FSH and LH assays, the numbers of mice utilized per group were the following: N=6 controls, N=6 1000 mg/kg, N=5 500 mg/kg, N=4 100 mg/kg.
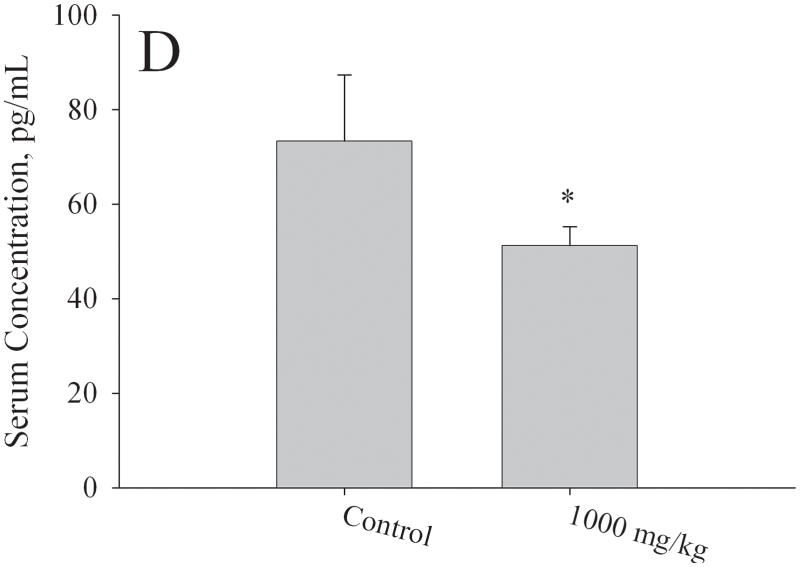
Figure 5. Effect of in utero to 0mg/kg, 100mg/kg, 500mg/kg, or 1000mg/kg MEHP on A. Estradiol at PND56; B. FSH; C. LH hormone production, and D. Estradiol at PND365.
Serum was collected via cardiac puncture at approximately two months of age during estrus and various hormone measurements were made by ELISA. A minimum of 3 litters each per control and per exposure group were examined. Each bar represents the mean and SD of serum from mice of at least 3–5 separate litters for both F1 control and F1 exposed litters. * indicates p < 0.05 relative to controls.
Additionally, 4 control mice and 4 1000 mg/kg exposed females were sacrificed at PND365 and the serum also analyzed for estradiol concentrations (Fig. 5d). In these animals, control mice exhibited significantly higher serum estradiol than the exposed mice (73.3 ± 14.0 pg/mL vs. 51.2 ± 4.1 pg/mL, respectively).
3.7 Affymetrix Gene Array and Quantitative RT-PCR
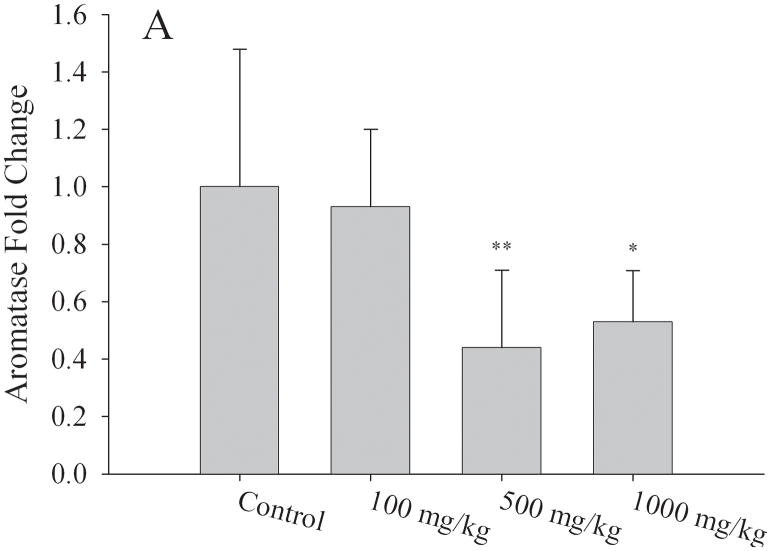
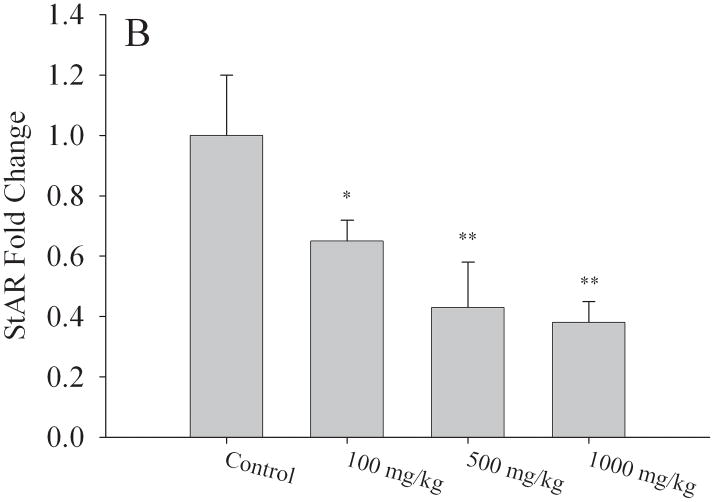
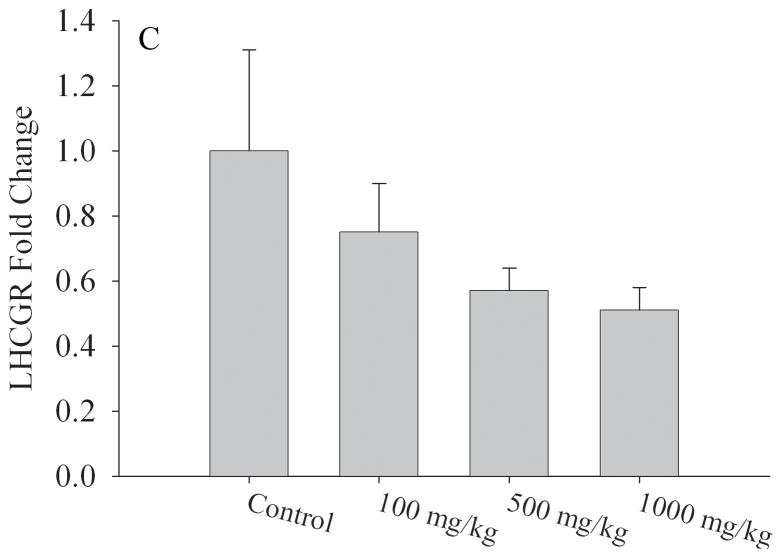
Table 3 depicts selected Affymetrix Gene array results performed on mRNA isolated from ovaries of different litters of corn oil exposed (N=3 litters) and 1000mg/kg/day MEHP exposed (N=4 litters) F1 adult females (PND56). Our results indicated 85 differentially expressed genes which exhibited both a 2-fold difference in expression and a p value of < 0.05 (Table S1). Genes of interest were selected because of their role(s) in hormone signaling and/or reproductive development and validated by quantitative RT-PCR. We found a strong correlation between our array results and the quantitative RT-PCR analyses (Fig 6). Aromatase mRNA expression was reduced by 50% relative to corn oil exposed controls at both the 500mg/kg/day (p < 0.01) and 1000mg/kg/day MEHP (p < 0.05) exposure groups (Fig. 6a). The most striking changes were observed for the steroidogenic acute regulatory protein (StAR) with decreased expression of 30% at 100mg/kg/day (p < 0.05), 50% at 500mg/kg/day (p < 0.01) and 60% at 1000mg/kg (p < 0.01) (Fig. 6b). Luteinizing hormone/choriogonadotropin receptor (LHCGR) mRNA expression was also reduced by approximately 50% in both the 500 and 1000mg/kg/day (p < 0.01) exposure groups relative to control litters (Fig. 6c).
Table 3.
Affymetrix gene array indicates a number of significantly altered genes of interest.
| ID | Gene Symbol | Gene Name | Fold Change | P value |
|---|---|---|---|---|
| NM_020278 | LGI1 | leucine-rich, glioma inactivated 1 | 4.840 | 1.94E–02 |
| NM_019779 | CYP11A1 | cytochrome P450, family 11, subfamily A, polypeptide 1 | −2.096 | 6.35E–03 |
| NM_181754 | GPR141 | G protein-coupled receptor 141 | −2.620 | 4.17E–02 |
| NM_013582 | LHCGR | luteinizing hormone/choriogonadotropin receptor | −2.776 | 9.81E–03 |
| NM_207683 | PIK3C2G | phosphoinositide-3-kinase, class 2, gamma polypeptide | −3.077 | 1.68E–04 |
| NM_011485 | STAR | steroidogenic acute regulatory protein | −3.405 | 5.71E–04 |
| NM_016668 | BHMT | betaine--homocysteine S-methyltransferase | −9.009 | 5.98E–04 |
Figure 6.
Effect of MEHP on A. Aromatase; B. StAR,; and C. LHCGR mRNA expression from in utero exposed and control F1 female ovaries. All expression was normalized to the house keeping gene, HPRT. Each bar represents the mean and standard deviation of ovaries from at least three separate litters for both F1 control and F1 exposed litters.* indicates significant difference from controls: * indicates p < 0.05; ** indicates p < 0.01.
3.8 KGN in vitro cell culture
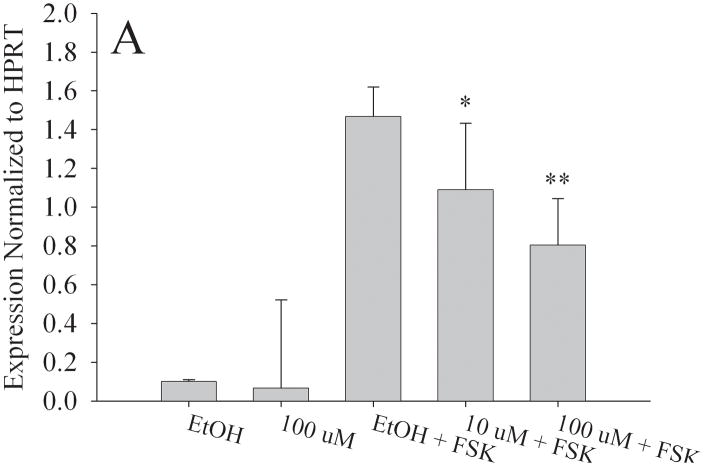
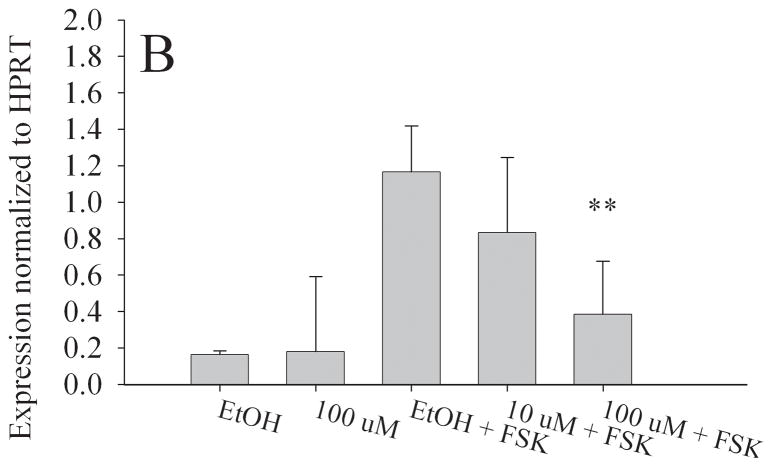
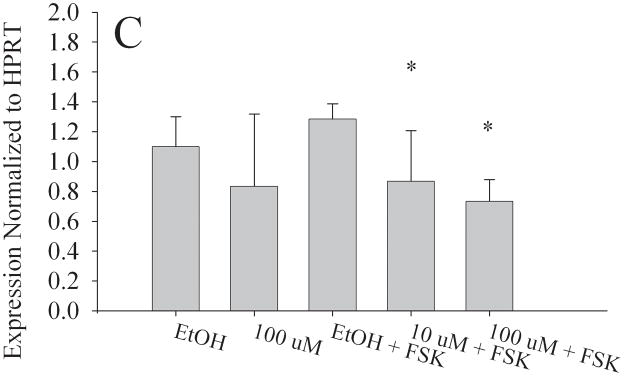
To determine if in utero exposure to MEHP targets the granulosa cell population, we examined the expression of StAR, Aromatase, LHCGR, and FSHR in the KGN cell line [25,26], a human granulosa-like cell line. We chose doses of both forskolin (FSK) and MEHP that did not significantly alter KGN cell viability (Fig. S2). RNA was then isolated from the KGN cell line for real time RT-PCR. Consistent with our in vivo observations, aromatase (Fig. 7a), StAR (Fig. 7b), LHCGR (Fig. 7c) and FSHR (Fig. 7d) expression were all significantly decreased at 100 μM MEHP relative to forskolin controls (p < 0.01 for Aromatase, LHCGR, and FSHR; p < 0.05 for StAR). At 10 μM MEHP both aromatase and LHCGR were significantly decreased (p < 0.05). No significant differences were detected between non-FSK controls and non-FSK MEHP-exposed KGN cells.
Figure 7.
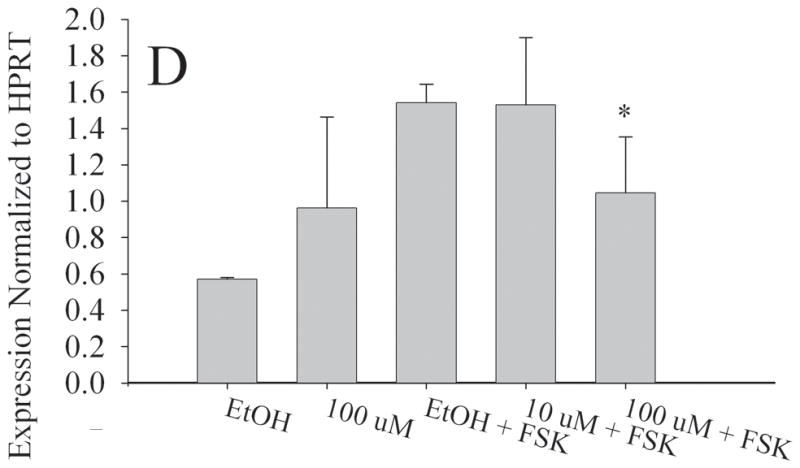
Effect of MEHP on A. Aromatase; B. StAR; C. LHCGR; and D. FSHR mRNA in a granulosa-like cell line, KGN. The KGN Granulosa-like cell line was cultured for 24 h with 100μm MEHP, 25 μM Forskolin (FSK), 10 μm MEHP, or 100 μm MEHP plus FSK or vehicle control (ethanol). A. Aromatase; B. StAR; C. LHCGR; and D. FSHR mRNA was measured using real time RT-PCR and normalized to HPRT. Each bar represents the mean and/SD of at least three separate experiments. * indicates p < 0.05 relative to forskolin control. ** indicates p < 0.01.
4. DISCUSSION
Our present study adds to the growing body of evidence that in utero or early life exposures to endocrine disrupting chemicals accelerates female reproductive senescence in the rodent [27,28]. The consequences of such exposures in humans remains to be determined. Epidemiological studies have demonstrated an association between EDC exposure and either anovulatory cycles or early menopause. For example, a group of female workers in a phthalate processing plant exhibited anovulatory cycles following high occupational exposure to phthalates [14]. Another study indicated women exposed in utero to diethylstilbesterol have increased risk of earlier onset of menopause [29]. Moreover, exposure to the endocrine disrupting chemicals dioxin and DDE (dichlorodiphenyltrichloro- ethane), a metabolite of DDT (dichlorodiphenyltrichloroethane) has been associated with early menopause [30, 31].
In the present study, we observed no overt maternal toxicity following in utero exposure to 100, 500, or 1000mg/kg/day MEHP. In utero exposure to MEHP did not significantly affect maternal body weight or pup growth at any of the doses examined (Table 1); however, we observed changes in the estrous cycle of exposed females at all doses examined (Table 2). We postulate that these changes may be due to alterations in normal hormonal signaling which extend to the uterine histology, as measured by vaginal smear cytology [32]. Altered estrous cyclicity has been reported in adult female rats following acute exposure to extremely high doses of MEHP [33]. Likewise, prolonged estrous has been observed in rats following in utero exposure to bisphenol A, another endocrine disrupting chemical [34].
We also observed an increase in the number of pups per litter in young adult F1 females exposed to the highest dose of MEHP (1000mg/kg/day), an intriguing finding since C57/Bl6 mice generally have small litters. Endocrine disrupting compounds such as phthalates are postulated to activate the primordial follicle pool [19] and our observations of increased numbers of mature follicles in adult females in the highest exposure group and the subsequent decrease in overall reproductive lifespan support this hypothesis.
In the present study, we found evidence for a reduced estrogenic effect, indicated by the late onset of sexual maturity and decreased aromatase expression. However, we also found evidence for estrogenic effects, as estradiol levels were significantly elevated and LH levels were suppressed by 1000mg/kg/day MEHP. We postulate that at this dose, larger estradiol- and testosterone-producing follicles and ovarian stroma remain functional, which may account for the higher estradiol levels. Our findings are consistent with a study of marmoset monkeys receiving DEHP at 1000 to 2000mg/kg, which showed increases in both blood estradiol levels and ovarian and uterine weight suggesting that DEHP led to accelerated development in the non-human primate [35]. The observation of elevated estradiol levels is reversed by PND365, at which point the controls exhibit higher levels than the 1000 mg/kg exposed animals. This is consistent with the understanding that at this high dose, the animals have entered a menopausal state before their control corn oil exposed counterparts.
Another intriguing finding of our study was the hyperplasia observed in the mammary glands of F1 females exposed to 1000 mg/kg MEHP regardless of breeding status. Mammary gland hyperplasia is associated with exposure to high serum estradiol levels following xenoestrogen exposure [36, 37] and may be the result of the elevated estradiol levels seen in the F1 females in our study. Based on our findings of higher estradiol levels at 1000mg/kg/day, we examined the expression of aromatase in vivo and in vitro. Reduced aromatase activity at the transcript level inhibits estradiol production, and previous studies have shown that in vitro exposure of either isolated rat antral follicles or rat granulosa cells to DEHP or MEHP at moderate to high doses suppresses aromatase mRNA expression [38]. Our experiments in the current study using the human granulosa-derived cell line KGN suggest that this is also the case in humans, as well as the suppression of FSHR, StAR, and LHCGR. Though the KGN cell line is granulosa-based and not thecal, it still expresses StAR [39]. However, while these data suggest the granulosa cell population is susceptible, this is not meant to indicate the sole target of MEHP because the time of exposure in our animal studies occurs before any large population of mature granulosa cells in the ovary. Furthermore we cannot speculate on the effects of MEHP on thecal cells based on the current findings, though the perturbation of estrogen signaling is apparent.
Similar to the findings of others, we found significant decreases in ovarian aromatase expression in both the 500 and 1000mg/kg/day MEHP exposure groups relative to controls. One possible explanation for high estradiol/low aromatase expression is that aromatase protein levels are altered in our system. A more intriguing possibility and one that deserves further investigation is that of reprogramming of the HGP axis. In previous studies involving MEHP or DEHP, the toxicant is administered directly and biological samples are examined immediately. In our study the exposure is in utero and the adult effects are examined months later, suggesting the potential for perinatal reprogramming. A recent study by Gore et al [27] demonstrates that early life exposure to methoxychlor leads to lifelong molecular reprogramming of the hypothalamus and premature reproductive aging similar to the premature reproductive aging observed in our study with MEHP.
It must be stated that the overwhelming majority of results seen in this study are found at doses of 500 and 1000 mg/kg, doses that are well beyond the scope of physiologically relevant doses. Interestingly, the only statistically significant effect seen at 100 mg/kg, a dose one or two orders of magnitude above that which might be considered physiologically relevant [14,15,40], is a decrease in StAR mRNA expression. In the absence of any other observed toxicological or physiological effects at this dose, and indeed without assessing StAR at the protein or transcript level, 100 mg/kg might be considered extremely close to the no observable adverse effect level (NOAEL).
Based on our study, we propose that exposure to MEHP activates primordial follicle recruitment; whether the mechanism involves reprogramming of the HPG axis remains to be determined. However, it is possible that the increased rate of follicle maturation results in higher estradiol levels due to the presence of active granulosa cells. From early adulthood, this suspected rapid recruitment of follicles continues, ultimately resulting in increased litter size. The large-litter phenotype, along with the prolonged estrus phenotype, continues until approximately 9 to 10 months of age, at which point the high dose-exposed females cease having litters, and by 1 year of age exhibit significantly lower serum estradiol levels relative to the control animals. This is consistent with the hypothesis that these animals are recruiting follicles at an accelerated rate. In agreement with these findings, MEHP-exposed F1 females sacrificed at approximately one year of age exhibit extensive mammary gland hyperplasia, an observation consistent with elevated estradiol levels throughout much of their lifetime.
In conclusion, our study demonstrates that an acute exposure to MEHP in utero from gestational days 17–19 leads to an initial increase in litter size, an increase in the number of mature follicles and increased estradiol levels in young adult F1 females (PND56), the consequence of which is decreased overall fertility later in life. Therefore, considering the differences between our study and previous studies, our data suggests that the timing, duration, route of exposure, and species used are all important variables that determine the effect of MEHP on steroid biosynthesis, ovarian and mammary gland biology, and reproductive lifespan.
Supplementary Material
Research Highlights.
In utero exposure to MEHP causes lifelong reproductive effects in the mouse.
By young adulthood, detectable changes in cyclicity, follicular recruitment.
At one year of age, induces premature loss of fertility, mammary hyperplasia.
Effects predominantly seen at high doses (500 mg/kg and greater)
Acknowledgments
We would like to acknowledge Dr. Christoph Schorl of Brown University for his expertise in the performing of gene array experiments and Dr. Jodie Pietruska for helpful insight in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gray LE, Jr, Barlow NJ, Howdeshell KL, Ostby JS, Furr JR, Gray CL. Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol Sci. 2009;110(2):411–25. doi: 10.1093/toxsci/kfp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martino-Andrade AJ, Chahoud I. Reproductive toxicity of phthalate esters. Mol Nutr Food Res. 2010;54(1):148–57. doi: 10.1002/mnfr.200800312. [DOI] [PubMed] [Google Scholar]
- 3.Noriega NC, Howdeshell KL, Furr J, Lambright CR, Wilson VS, Gray LE., Jr Pubertal administration of DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male Sprague-Dawley and Long-Evans rats. Toxicol Sci. 2009;111(1):163–78. doi: 10.1093/toxsci/kfp129. [DOI] [PubMed] [Google Scholar]
- 4.Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE., Jr A mixture of 5 phthalate esters inhibits fetal testosterone production in the Sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105(1):153–65. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 5.Durmaz E, Ozmert EN, Erkekoglu P, Giray B, Derman O, Hincal F, Yurdakök K. Plasma phthalate levels in pubertal gynecomastia. Pediatrics. 2010 Jan;125(1):e122–9. doi: 10.1542/peds.2009-0724. Epub 2009 Dec 14. [DOI] [PubMed] [Google Scholar]
- 6.Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect. 2006 Nov;114(11):1643–8. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki Y, Yoshinaga J, Mizumoto Y, Serizawa S, Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int J Androl. 2011 Jun 22; doi: 10.1111/j.1365–2605.2011.01190.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Mitchell RT, Childs AJ, Anderson RA, van den Driesche S, Saunders PT, McKinnell C, Wallace WH, Kelnar CJ, Sharpe RM. Do Phthalates Affect Steroidogenesis by the Human Fetal Testis? Exposure of Human Fetal Testis Xenografts to Di-n-Butyl Phthalate. J Clin Endocrinol Metab. 2012 Jan 11; doi: 10.1210/jc.2011-2411. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Eviron Health Perspect. 2003;111(2):139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol. 1994;128(2):216–23. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- 11.Grande SW, Andrade AJ, Talsness CE, Grote K, Golombiewski A, Sterner-Kock A, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult female offspring rats. Toxicology. 2007;229(1–2):114–22. doi: 10.1016/j.tox.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Grande SW, Andrade AJ, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicol Sci. 2006;91(1):247–54. doi: 10.1093/toxsci/kfj128. [DOI] [PubMed] [Google Scholar]
- 13.Gray LE, Jr, Laskey J, Ostby J. Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female Long Evans hooded rats. Toxicol Sci. 2006;93(1):189–95. doi: 10.1093/toxsci/kfl035. [DOI] [PubMed] [Google Scholar]
- 14.Aldyreva MV, Klimova TS, Iziumova AS, Timofeevskaia LA. The effect of phthalate plasticizers on the generative function. Gig Tr Prof Zabol. 1975;(12):25–9. [PubMed] [Google Scholar]
- 15.NTIS Publication Number PB/93/182400. Atlanta, GA: 1993. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicologica Profile for Di-(2-ethylhexyl) Phthalate, Update. [Google Scholar]
- 16.Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci. 2007 Jun;97(2):491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- 17.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001 Jun 15;234(2):339–51. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007 Aug;148(8):3580–90. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Breen K, Pepling ME. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol. 2009;202(3):407–17. doi: 10.1677/JOE-09-0109. [DOI] [PubMed] [Google Scholar]
- 20.Pedersen T. Follicle kinetics in the ovary of the cyclic mouse. Acta Endocrinol (Copenh) 1970;64:304–323. doi: 10.1530/acta.0.0640304. [DOI] [PubMed] [Google Scholar]
- 21.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestation and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67(1):63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 22.Takagi H, Shibutani M, Lee KY, Masutomi N, Fujita H, Inoue K, Mitsumori K, Hirose M. Impact of maternal dietary exposure to endocrine-acting chemicals on progesterone receptor expression in microdissected hypothalamic medial preoptic areas of rat offspring. Toxicol Appl Pharmacol. 2005;208(2):127–36. doi: 10.1016/j.taap.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Anderson D, Yu TW, Hinçal F. Effect of some phthalate esters in human cells in the comet assay. Teratog Carcinog Mutagen. 1999;19(4):275–80. doi: 10.1002/(sici)1520-6866(1999)19:4<275::aid-tcm4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, Takayanagi R, Kashimura Y, Haji M, Nawata H. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses follicle-stimulating hormone receptor. Endocrinology. 2001;142(1):437–45. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- 26.Ohno S, Yukinawa F, Noda M, Kakajin S. Mono-(2-ethylhexyl) phthalate induces NR4A subfamily and GIOT-1 gene expression, and suppresses CYP19 expression in human granulosa-like tumor cell line KGN. Toxicology Letters. 2009;129:353–359. doi: 10.1016/j.toxlet.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol. 2011 Dec;25(12):2157–68. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Z, Valdez KE, Ting AY, Franczak A, Gum SL, Petroff BK. Ovarian endocrine disruption underlies premature reproductive senescence following environmentally relevant chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Biol Reprod. 2007 Feb;76(2):198–202. doi: 10.1095/biolreprod.106.053991. Epub 2006 Oct 18. [DOI] [PubMed] [Google Scholar]
- 29.Hatch EE, Troisi R, Wise LA, Hyer M, Palmer JR, Titus-Ernstoff L, Strohsnitter W, Kaufman R, Adam E, Noller KL, Herbst AL, Robboy S, Hartge P, Hoover RN. Age at natural menopause in women exposed to diethylstilbestrol in utero. Am J Epidemiol. 2006 Oct 1;164(7):682–8. doi: 10.1093/aje/kwj257. [DOI] [PubMed] [Google Scholar]
- 30.Akkina J, Reif J, Keefe T, Bachand A. Age at natural menopause and exposure to organochlorine pesticides in Hispanic women. J Toxicol Environ Health A. 2004 Sep 24;67(18):1407–22. doi: 10.1080/15287390490483845. [DOI] [PubMed] [Google Scholar]
- 31.Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, Olive DL, Needham L, Patterson D, Jr, Mocarelli P. Serum dioxin concentrations and age at menopause. Environ Health Perspect. 2005 Jul;113(7):858–62. doi: 10.1289/ehp.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baranda-Avila N, Mendoza-Rodríguez CA, Morimoto S, Langley E, Cerbón M. Molecular mechanism of cell proliferation in rodent uterus during the estrous cycle. J Steroid Biochem Mol Biol. 2003;113(3–5):259–68. doi: 10.1016/j.jsbmb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Takai R, Hayashi S, Kiyokawa J, Iwata Y, Matsuo S, Suzuki M, Mizoguchi K, Chiba S, Deki T. Collaborative work on evaluation of ovarian toxicity. 10) Two- or four-week repeated dose studies and fertility study of di-(2-ethylhexyl) phthalate (DEHP) in female rats. J Toxicol Sci. 2009;34(Suppl 1):SP111–9. doi: 10.2131/jts.34.s111. [DOI] [PubMed] [Google Scholar]
- 34.Mendoza-Rodríguez CA, García-Guzmán M, Baranda-Avila N, Morimoto S, Perrot-Applanat M, Cerbón M. Administration of bisphenol A to dams during perinatal period modifies molecular and morphological reproductive parameters of the offspring. Reprod Toxicol. 2011;31(2):177–83. doi: 10.1016/j.reprotox.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Tomonari Y, Kurata Y, David RM, Gans G, Kawasuso T, Katoh M. Effect of di(2-ethylhexyl) phthalate (DEHP) on genital organs from juvenile common marmosets: I. morphological and biochemical investigation in 65-week toxicity study. J Toxicol Environ Health A. 2006;69(17):1651–72. doi: 10.1080/15287390600630054. [DOI] [PubMed] [Google Scholar]
- 36.Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, Newbold RR, Delclos KB. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reprod Toxicol. 2009;28(3):342–53. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Kleinberg DL, Ruan W. IGF-I, GH, and sex steroid effects in normal mammary gland development. J Mammary Gland Biol Neoplasia. 2008;13(4):353–60. doi: 10.1007/s10911-008-9103-7. [DOI] [PubMed] [Google Scholar]
- 38.Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol. 2010;242(2):224–30. doi: 10.1016/j.taap.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pierre P, Froment P, Nègre D, Ramé C, Barateau V, Chabrolle C, Lecomte P, Dupont J. Role of adiponectin receptors, AdipoR1 and AdipoR2, in the steroidogenesis of the human granulosa tumor cell line, KGN. Hum Reprod. 2009 Nov;24(11):2890–901. doi: 10.1093/humrep/dep292. Epub 2009 Aug 11. [DOI] [PubMed] [Google Scholar]
- 40.Gibson TP, Briggs WA, Boone BJ. Delivery of di-2-ethylhexyl phthalate to patients during hemodialysis. J Lab Clin Med. 1976 Mar;87(3):519–24. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.