Abstract
We have previously shown that β-endorphin plays a functional role in the rewarding effect of acute cocaine. Considering that β-endorphin has high affinity for the μ opioid receptor, we determined the role of this receptor in the rewarding action of acute cocaine. For comparison, we assessed the role of the μ opioid receptor in the rewarding effect of acute morphine. We also examined the effect of intracerebroventricular (i.c.v.) administration of β-funaltrexamine (β-FNA), an irreversible μ opioid receptor antagonist, on the rewarding action of acute cocaine as well as that of morphine. Using the conditioned place preference (CPP) paradigm as an animal model of reward, we first assessed the rewarding action of cocaine in mice lacking β-endorphin or the μ opioid receptor and their respective wild-type littermates/controls. Mice were tested for preconditioning place preference on day 1, conditioned once daily with saline/cocaine (30 mg/kg, i.p.) or cocaine/saline on days 2 and 3, and then tested for postconditioning place preference on day 4. We next studied the rewarding action of acute morphine in μ knockout mice and their wild-type controls. The CPP was induced by single alternate-day saline/morphine (10 mg/kg, s.c.) or morphine/saline conditioning. We finally determined the effect of β-FNA on CPP induced by cocaine or morphine in wild-type mice, in which mice were treated with saline or β-FNA (9ug/3 μl; i.c.v.) a day prior to the preconditioning test day. Our results revealed that morphine induced a robust CPP in wild-type mice but not in mice lacking the μ opioid receptor or in wild-type mice treated with β-FNA. In contrast, cocaine induced CPP in μ knockout mice as well as in wild-type mice treated with β-FNA. On the other hand, cocaine failed to induce CPP in mice lacking β-endorphin. These results illustrate that β-endorphin is essential for the rewarding action of acute cocaine, but the μ opioid receptor may not mediate the regulatory action of endogenous β-endorphin.
Keywords: Cocaine, Morphine, β-FNA, Conditioned Place Preference (CPP), β-endorphin/μ opioid receptor system, Knockout mouse
1. Introduction
Addiction is a chronic relapsing brain disorder that results from neuronal adaptive changes along numerous brain circuits. These alterations are thought to lead to aberrant behaviors characterized by compulsive drug-seeking and drug-taking behaviors despite negative consequences associated with such behaviors. The mesolimbic dopaminergic neurons mediate the acute rewarding and reinforcing actions of cocaine and other addictive drugs. In fact, cocaine and other drugs abused by humans have been shown to increase extracellular dopamine in the nucleus accumbens in rodents (Di Chiara and Imperato, 1988). Chronic use of these drugs also brings about neuronal adaptive changes along the mesolimbic dopaminergic neurons. These alterations are thought to play a critical role in the initiation, development and maintenance of drug dependency [for a review, see (Thomas et al., 2008)].
The endogenous opioid system has also been implicated in the rewarding and reinforcing actions of cocaine and other addictive drugs. For example, blockade of opioid receptors by different approaches has been reported to reduce the rewarding action of cocaine (Bilsky et al., 1992; Gerrits et al., 1995; Heidbreder et al., 1996; Houdi et al., 1989; Kim et al., 1997; Menkens et al., 1992; Rademacher and Steinpreis, 2002; Schroeder et al., 2007; Thomas et al., 2008). However, the role of each endogenous opioid peptide and their cognate receptor in the rewarding and reinforcing action of acute cocaine is not fully characterized although β-endorphin has been implicated in the rewarding action of acute cocaine. For example, cocaine has been shown to cause the release of β-endorphin in the nucleus accumbens (Olive et al., 2001), a brain region where local administration of opioids has been shown to induce reward (Kelsey et al., 1989; Wise, 1989; Wise and Hoffman, 1992). Furthermore, we have previously shown that cocaine-induced conditioned place preference (CPP), an animal model of reward (Bardo and Bevins, 2000), was reduced in mice lacking β-endorphin (Marquez et al., 2008a). However, which opioid receptor mediates the regulatory actions of endogenous β-endorphin on CPP induced by acute cocaine is unknown. Given that β-endorphin is considered as an endogenous ligand of the μ opioid receptors, the present study determined the role of the μ opioid receptor in the regulatory actions of β-endorphin in CPP induced by single conditioning with cocaine. Considering that compensatory developmental changes may occur in knockout mice, we also assessed the effect of β-funaltrexamine (β-FNA), an irreversible μ opioid receptor antagonist (Portoghese et al., 1980), on cocaine CPP. For comparison, we examined the role of the μ opioid receptor in CPP induced by single conditioning with morphine, which is known to exert its rewarding action via the μ opioid receptor (Matthes et al., 1996; Nguyen et al., 2012). The selection of single conditioning and doses of morphine and cocaine was based on our previous studies showing that single conditioning with such a dose of morphine (10 mg/kg) or cocaine (30 mg/kg) induces a robust CPP in wild-type mice (Marquez et al., 2008a; Marquez et al., 2009; Marquez et al., 2008b).
2. Materials and Methods
2.1. Subjects
Male mice lacking μ opioid receptor (Matthes et al., 1996) or β-endorphin (Rubinstein et al., 1996) and their respective wild-type littermates/controls, aging 2–3 months, fully backcrossed on a C57BL/6 mouse background were bred in-house from heterozygous breeding pairs and used for all experiments. Mice were maintained in a temperature (21 ± 3°C)-controlled room with free access to water and food. All experiments were carried out during the light phase of a 12-h light/12-h dark cycle and approved by the Institutional Animal Care and Use Committee at Western University of Health Sciences (Pomona, California, USA).
2.2. Drugs
Cocaine hydrochloride and morphine sulfate, obtained from Sigma (St. Louis, MO), were dissolved in normal saline and injected intraperitoneally (i.p.) and subcutaneously (s.c.), respectively, in a volume of 0.1 ml per 10 g of body weight. β-FNA was generously supplied by the National Institute on Drug Abuse Drug Supply program (Research Triangle Park, NC).
2.3. Experimental procedures
2.3.1. The role of endogenous β-endorphin in CPP induced by acute cocaine
The CPP procedure was performed according to our published results (Marquez et al., 2008a) and conducted over a 4-day period. Briefly, mice were tested for baseline preference toward the CPP chambers on day 1. On days 2 and 3, mice received single alternate-day saline/cocaine or cocaine/saline conditioning. On day 4, mice were tested for postconditioning preference toward the CPP chambers. On test days, each mouse was placed in the central gray chamber of the CPP apparatus and allowed to freely explore the CPP chambers via this central chamber for 15 min. The amount of time that the mouse spent in each chamber was recorded. On the conditioning days, mice were treated with either saline or cocaine (30 mg/kg, i.p.) and confined respectively to the vehicle- or cocaine-paired chamber for 30 min. The following day, mice were injected with the alternate treatment and confined to the opposite conditioning chamber for 30 min. The assignment of mice to the conditioning chambers was counterbalanced. The choice of the dose of cocaine, and duration of conditioning were based on our previous studies (Marquez et al., 2008a; Marquez et al., 2008b).
2.3.2. The role of μ opioid receptor in CPP induced by cocaine
Mice lacking the μ opioid receptor and their wild-type littermates/controls were tested for preconditioning place preference on day 1, received conditioning on days 2 and 3, and then tested for postconditioning place preference on day 4, as described above. For comparison, the role of the μ opioid receptor in the rewarding action of acute cocaine was also studied. Naïve mice lacking the μ opioid receptor and their wild-type littermates/controls were tested for baseline place preference, as described above. Mice were then conditioned with morphine (10 mg/kg, s.c.) for 60 min. The choice of the dose of morphine, and the duration of conditioning were based on our previous study (Marquez et al., 2009).
2.3.3. Effects of β-FNA on cocaine-induced CPP
Wild-type mice were injected with saline or β-FNA (9 μg/3 μl; i.c.v.), according to earμier reports (Marquez et al., 2009; Yoburn et al., 1990; Yoburn et al., 1991), and tested for preconditioning place preference 24 h later. The following day, mice then received single alternate-day saline/cocaine (30 mg/kg, i.p.) or cocaine/saline conditioning, and then tested for postconditioning place preference 24 h later. For comparison, the effect of i.c.v. administration of β-FNA on CPP induced by morphine (10 mg/kg, s.c.) was also assessed. The choice of β-FNA dose was based on our pilot studies demonstrating that this dose of β-FNA (9 μg per 3 μl; i.c.v.) abolished the analgesic effect of morphine in the hot plate test in mice (Borse and Lutfy, unpublished data).
2.4. Data Analysis
Data are presented as mean (±S.E.M.) and analyzed using repeated measures analysis of variance (ANOVA). The between factors were genotype or treatment (saline vs. β-FNA) and the amount of time that mice spent in the drug-paired chamber on the pre- and postconditioning test days. The within factor was pre- vs. postconditioning test day. The post-hoc Bonferroni multiple comparison test was used to reveal significant differences between various groups. P<0.05 was considered significant.
3. Results
3.1. Cocaine-induced CPP was attenuated in β-endorphin deficient mice
Figure 1 illustrates the amount of time that mice lacking β-endorphin and their wild-type littermates spent in the cocaine-paired chamber on the test days. Repeated measures ANOVA revealed a significant interaction between genotype and the amount of time that mice spent in the conditioning chambers on the test days (F1,14 = 6.49; P<0.05). Post-hoc analysis of the data showed that wild-type mice spent more time in the drug-paired chamber on the post- compared to preconditioning test day (P<0.001). However, β-endorphin deficient mice spent a comparable amount of time in the cocaine-paired on the pre- and post-conditioning test days (P>0.05). This result suggests that acute cocaine failed to induce CPP in β-endorphin null mice at a dose that induced a significant CPP in their wild-type littermates.
Fig. 1.
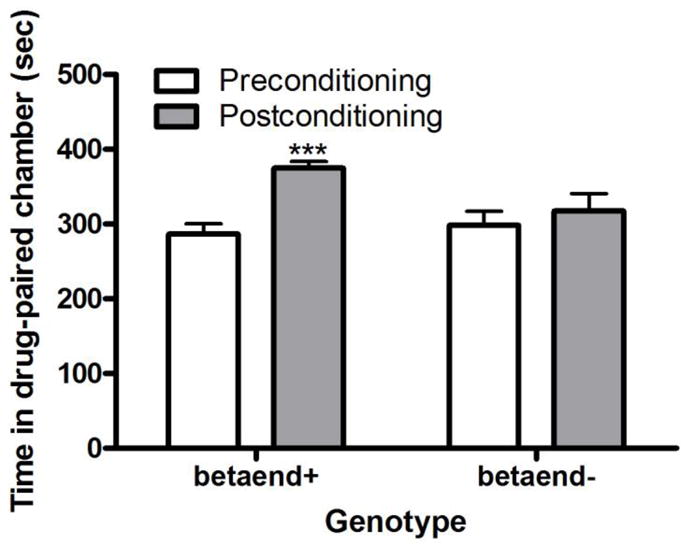
Cocaine induced CPP was blunted in mice lacking β-endorphin (betaend-) compared to their wild-type littermates (betaend+). The data represent mean (±S.E.M.) of the amount of time that mice (n = 8 mice per genotype) spent in the drug-paired chamber on pre- and postconditioning test day. ***P<0.001 compared to its respective preconditioning test day.
3.2. CPP induced by cocaine was not altered in mice lacking the μ opioid receptor
Figure 2 illustrates the amount of time that wild-type and μ null mice spent in the cocaine-paired chamber on the pre- and postconditioning test days. Repeated measures ANOVA revealed no significant interaction between genotype and time (F1,14 = 0.15; P>0.05); but, there was a significant effect of time (F1,12 = 48.08; P<0.001). Post-hoc analysis of the data showed that both wild-type and knockout mice spent more time in the cocaine-paired chamber on the post- compared to preconditioning test day. This result illustrates that cocaine induced a significant CPP in both wild-type and μ knockout mice.
Fig. 2.
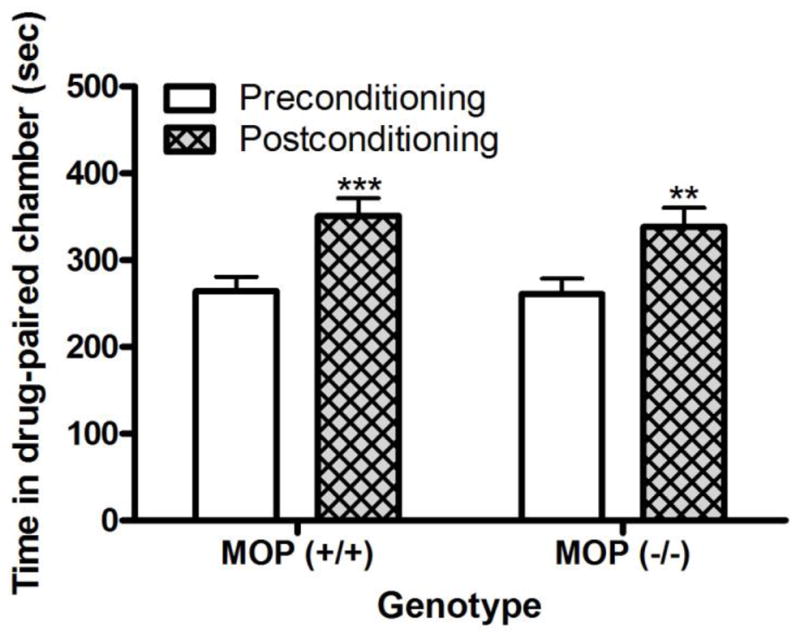
Cocaine-induced CPP was not altered in mice lacking the μ opioid receptor [MOP (−/−)] compared to their wild-type littermates/controls [MOP (+/+)]. The data represent mean (±S.E.M.) of the amount of time that mice (n = 7 mice per genotype) spent in the drug-paired chamber on each test day. **P<0.01; ***P<0.001 compared to its respective preconditioning test day.
3.3. Morphine-induced CPP was abolished in mice lacking the μ opioid receptor
The amount of time that mice lacking the μ opioid receptor and their wild-type littermates/controls spent in the morphine-paired chamber on the pre- and postconditioning test days is shown in figure 3. Repeated measures ANOVA revealed a significant interaction between genotype and time (F1,12 = 5.19; P<0.05). Post-hoc analysis of the data demonstrated that wild-type mice spent significantly more time in the morphine-paired chamber on the post- compared to preconditioning test day (P<0.05). However, mice lacking the μ opioid receptor spent approximately the same amount of time in the morphine-paired on the pre- and postconditioning test days (P>0.05). This finding suggests that morphine induced a significant CPP in wild-type but not μ knockout mice.
Fig. 3.
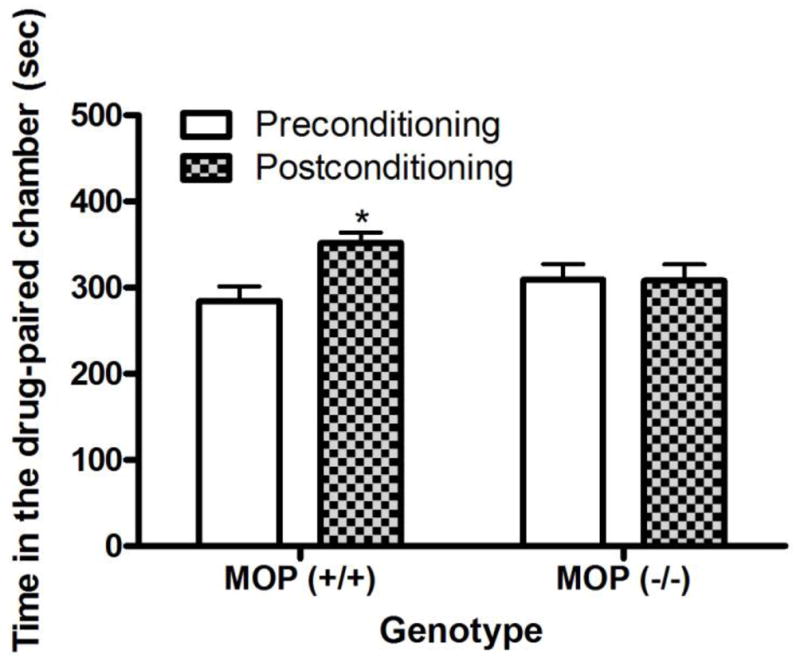
Morphine-induced CPP was blunted in mice lacking the μ opioid receptors [MOP (−/−)] compared to their wild-type littermates/controls [MOP (+/+)]. The data represent mean (±S.E.M.) of the amount of time that mice (n = 7 mice per genotype) spent in the drug-paired chamber on each test day. *P<0.05 compared to its respective preconditioning test day.
3.4. The rewarding action of acute cocaine was not altered in wild-type mice treated with β-FNA
Figure 4 illustrates the amount of time that mice treated with saline or β-FNA (9 μg/3 μl; i.c.v.) spent in the cocaine-paired chamber prior to and following single alternate-day saline/cocaine (30 mg/kg) or cocaine/saline conditioning. Repeated measures ANOVA revealed no significant time by treatment interaction (F1,13 = 0.01; P>0.05); but, there was a significant effect of time (F1,13 = 14.25; P<0.01). Post-hoc analysis of the data showed that mice spent significantly more time in the cocaine-paired chamber on post- compared to preconditioning test day (P<0.05). However, this response was not altered in mice treated with β-FNA. This result reveals that cocaine induced CPP but this response was not altered by β-FNA.
Fig. 4.
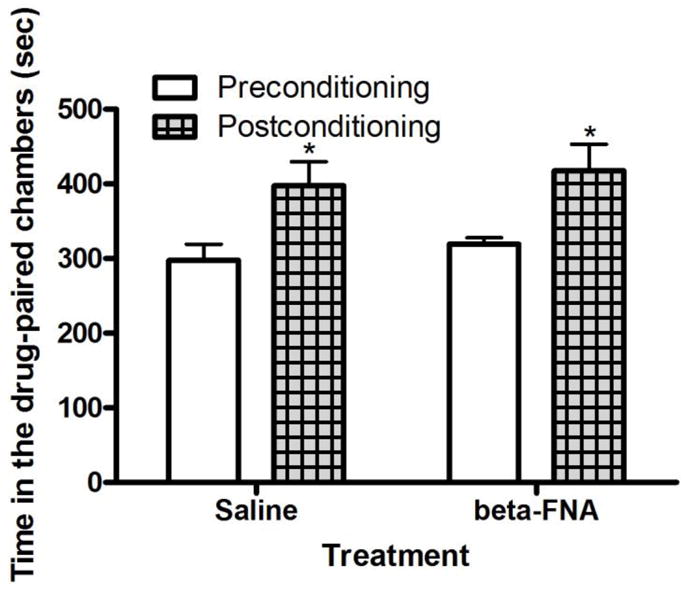
Cocaine induced a comparable CPP in wild-type mice treated with β-FNA and their vehicle-treated controls. The data represent mean (±S.E.M.) of the amount of time that mice (n = 7–8 mice per treatment) spent in the drug-paired chamber on each test day. *P<0.05 compared to its respective preconditioning test day.
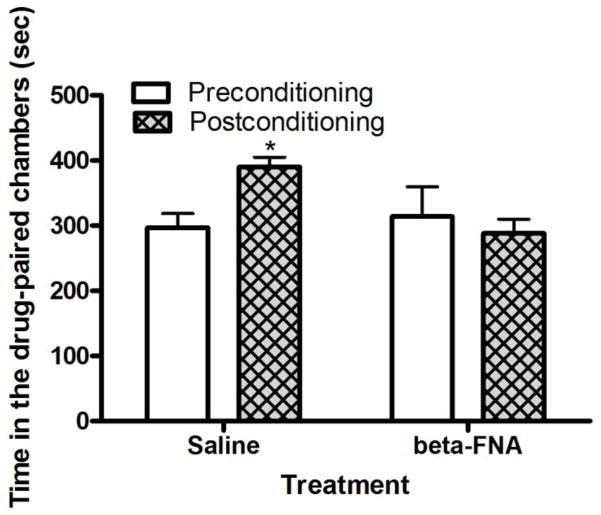
3.5. The rewarding action of acute morphine was blunted in wild-type mice treated with β-FNA
The amount of time that mice spent in the morphine-paired chamber on the pre- and postconditioning test days is depicted in figure 5. Repeated measures ANOVA revealed a significant effect of time X treatment interaction (F1,14 = 5.24; P<0.05). Post-hoc analysis of the data showed that mice treated with vehicle and conditioned with morphine spent significantly more time in the morphine-paired chamber on the post- compared to preconditioning test day (P<0.05). However, this response was blunted in mice treated with β-FNA 48–72 h prior to morphine conditioning. This result suggests that single conditioning with morphine induced CPP but this response was blocked by β-FNA, an irreversible μ opioid receptor antagonist.
Fig. 5.
Morphine-induced CPP was abolished in wild-type mice treated with β-FNA. The data represent mean (±S.E.M.) of the amount of time that mice (n = 8 mice per treatment) spent in the drug-paired chamber on each test day. *P<0.05 compared to its respective preconditioning test day.
4. Discussion
The main finding of the present study is that CPP induced by acute cocaine was attenuated in β-endorphin deficient mice but not in mice lacking the μ opioid receptor or in wild-type mice treated with β-FNA, an irreversible μ opioid receptor antagonist. On the other hand, the rewarding action of acute morphine was abolished in mice lacking the μ opioid receptor as well as in wild-type mice treated with β-FNA. Together, the present results reveal that β-endorphin plays a functional role in the rewarding action of acute cocaine, but deletion or blockade of the μ receptor was without a significant effect on this action of cocaine.
Previous studies have implicated the endogenous opioids in the rewarding and addictive effects of cocaine. For example, we have previously shown that cocaine-induced CPP was significantly reduced in mice lacking β-endorphin (Marquez et al., 2008a), suggesting that endogenous β-endorphin plays a functional role in the rewarding actions of acute cocaine. In the present study, we confirmed these findings and demonstrated that CPP induced by single cocaine conditioning was blunted in β-endorphin deficient mice. This observation corroborates previous findings implicating endogenous β-endorphin in the rewarding and reinforcing actions of acute cocaine (Marquez et al., 2008a; Olive et al., 2001; Roth-Deri et al., 2006; Roth-Deri et al., 2004).
Given that β-endorphin is considered as an endogenous agonist of the μ opioid receptor (Akil et al., 1984), we tested the possibility that the regulatory action of endogenous β-endorphin on cocaine CPP would be mediated via the μ opioid receptor. Thus, we assessed CPP induced by acute cocaine in mice lacking the μ opioid receptor and their wild-type littermates. Considering that compensatory developmental changes could occur in knockout mice, we also examined the effect of β-FNA, an irreversible μ opioid receptor antagonist (Portoghese et al., 1980), on cocaine CPP. As a positive control, we assessed whether morphine CPP would be altered in mice lacking the μ opioid receptor compared to their wild-type littermates or in wild-type mice treated with β-FNA compared to their saline-treated controls. Our results illustrated that cocaine induced a comparable CPP in mice lacking the μ opioid receptor and their wild-type littermates/controls. Furthermore, we found a comparable cocaine CPP in mice treated with β-FNA and their saline-treated controls. In contrast, as expected, morphine failed to induce CPP in mice lacking the μ opioid receptor or in wild-type mice treated with β-FNA, provide further support that the rewarding action of morphine is mediated via the μ opioid receptor (Matthes et al., 1996; Nguyen et al., 2012). These results also confirm that the μ opioid receptor was not functional in μ knockout mice or wild-type mice treated with β-FNA because morphine failed to induce CPP in either group of mice.
The results of the present study appear in contrast with a number of reports showing that the μ opioid receptor is involved in the rewarding and reinforcing actions of cocaine (Becker et al., 2002; Hall et al., 2004; Hummel et al., 2004; Hummel et al., 2006; Mathon et al., 2005; Rademacher and Steinpreis, 2002; Schroeder et al., 2007). However, one should interpret the data with caution because we used a higher dose of cocaine compared to previous studies (Becker et al., 2002; Hall et al., 2004). Interestingly, cocaine CPP induced by a lower (5 mg/kg), but not higher (10 mg/kg), dose of cocaine was blunted in μ-deficient mice compared to wild-type controls (Becker et al., 2002). On the other hand, Hall and colleagues reported a blunted CPP in μ knockout mice with either dose of cocaine (Hall et al., 2004); but, these authors used a biased CPP paradigm. Nevertheless, the motor stimulatory effect of cocaine (20 and 40 mg/kg) was not altered in mice lacking the μ opioid receptor compared to their wild-type controls (Becker et al., 2002). Likewise, the ability of this dose of cocaine (20 mg/kg) to increase dopamine was not altered in mice lacking the μ opioid receptor compared to their wild-type controls (Chefer et al., 2004). The other variable between the present and previous studies (Becker et al., 2002; Hall et al., 2004) was the duration of conditioning. We utilized single alternate day conditioning with a relatively higher dose of cocaine because we have previously shown that single conditioning with lower doses of cocaine fail to induce CPP in wild-type mice (Marquez et al., 2008a; Marquez et al., 2008b). Thus, the present data illustrating that CPP induced by acute cocaine was reduced in mice lacking β-endorphin, but not in μ knockout mice, suggest that β-endorphin functions as a facilitator of the rewarding action of acute cocaine. However, the μ opioid receptor may not be essential for the development of this response. An alternative explanation may be that β-endorphin interacts with a subtype of μ opioid receptor that may be still functional in mice lacking the μ opioid receptor or in wild-type mice treated with β-FNA. Although our finding that the rewarding action of morphine was abolished in μ receptor knockout mice as well as in wild-type mice treated with β-FNA argues against this possibility, there is evidence showing that the effect of morphine and β-endorphin was differentially affected by pertussis toxin (Tseng and Collins, 1995). Furthermore, the ability of β-endorphin to stimulate GTP-γ-S binding is reduced but not completely abolished in mice lacking the μ opioid receptors (Mizoguchi et al., 2002). Additionally, β-endorphin is known to exert some of its actions via receptor other than μ opioid receptors (Tseng, 2001). Thus, further research is needed to characterize the receptor mechanism involved in the regulatory action of endogenous β-endorphin on cocaine reward.
5. Conclusion
The results of the current study illustrate that the rewarding action of acute cocaine was attenuated in β-endorphin but not in μ opioid receptor knockout mice or in wild-type mice treated with β-FNA. In contrast, the rewarding action of acute morphine was abolished in mice lacking the μ opioid receptor as well as in wild-type mice treated with β-FNA. Collectively, the present results reveal that endogenous β-endorphin plays a modulatory role in the acute rewarding actions of cocaine, a response that may be mediated via receptor other than the μ opioid receptor.
Acknowledgments
The author would like to thank Emelia Aghazada, Marisol Rivas and Duc Le for technical support. The present study was supported in part by an intramural grant from Western University of Health Sciences and in part by a MIDARP Grant R24 DA017298-02 to T.C.F. and in part by a NIDA Grant DA016682-03 to KL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Hollt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Montegut MJ, Delong CL, Reid LD. Opioidergic modulation of cocaine conditioned place preferences. Life sciences. 1992;50:PL85–90. doi: 10.1016/0024-3205(92)90105-x. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Kieffer BL, Shippenberg TS. Contrasting effects of mu opioid receptor and delta opioid receptor deletion upon the behavioral and neurochemical effects of cocaine. Neuroscience. 2004;127:497–503. doi: 10.1016/j.neuroscience.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MA, Patkina N, Zvartau EE, van Ree JM. Opioid blockade attenuates acquisition and expression of cocaine-induced place preference conditioning in rats. Psychopharmacology (Berl) 1995;119:92–98. doi: 10.1007/BF02246059. [DOI] [PubMed] [Google Scholar]
- Hall FS, Goeb M, Li XF, Sora I, Uhl GR. mu-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Brain Res Mol Brain Res. 2004;121:123–130. doi: 10.1016/j.molbrainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Shoaib M, Shippenberg TS. Differential role of delta-opioid receptors in the development and expression of behavioral sensitization to cocaine. European journal of pharmacology. 1996;298:207–216. doi: 10.1016/0014-2999(95)00815-2. [DOI] [PubMed] [Google Scholar]
- Houdi AA, Bardo MT, Van Loon GR. Opioid mediation of cocaine-induced hyperactivity and reinforcement. Brain Res. 1989;497:195–198. doi: 10.1016/0006-8993(89)90989-x. [DOI] [PubMed] [Google Scholar]
- Hummel M, Ansonoff MA, Pintar JE, Unterwald EM. Genetic and pharmacological manipulation of mu opioid receptors in mice reveals a differential effect on behavioral sensitization to cocaine. Neuroscience. 2004;125:211–220. doi: 10.1016/j.neuroscience.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Hummel M, Schroeder J, Liu-Chen LY, Cowan A, Unterwald EM. An antisense oligodeoxynucleotide to the mu opioid receptor attenuates cocaine-induced behavioral sensitization and reward in mice. Neuroscience. 2006;142:481–491. doi: 10.1016/j.neuroscience.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Kelsey JE, Carlezon WA, Jr, Falls WA. Lesions of the nucleus accumbens in rats reduce opiate reward but do not alter context-specific opiate tolerance. Behav Neurosci. 1989;103:1327–1334. doi: 10.1037//0735-7044.103.6.1327. [DOI] [PubMed] [Google Scholar]
- Kim HS, Park WK, Jang CG, Oh KW, Kong JY, Oh S, Rheu HM, Cho DH, Kang SY. Blockade by naloxone of cocaine-induced hyperactivity, reverse tolerance and conditioned place preference in mice. Behav Brain Res. 1997;85:37–46. doi: 10.1016/s0166-4328(96)00162-3. [DOI] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Dabaja I, Gajawada N, Lutfy K. The role of beta-endorphin in the acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology (Berl) 2008a;197:443–448. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Bebawy D, Lelievre V, Coute AC, Evans CJ, Waschek JA, Lutfy K. The role of endogenous PACAP in motor stimulation and conditioned place preference induced by morphine in mice. Psychopharmacology (Berl) 2009;204:457–463. doi: 10.1007/s00213-009-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology. 2008b;54:564–568. doi: 10.1016/j.neuropharm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathon DS, Lesscher HM, Gerrits MA, Kamal A, Pintar JE, Schuller AG, Spruijt BM, Burbach JP, Smidt MP, van Ree JM, Ramakers GM. Increased gabaergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience. 2005;130:359–367. doi: 10.1016/j.neuroscience.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Menkens K, Bilsky EJ, Wild KD, Portoghese PS, Reid LD, Porreca F. Cocaine place preference is blocked by the delta-opioid receptor antagonist, naltrindole. European journal of pharmacology. 1992;219:345–346. doi: 10.1016/0014-2999(92)90319-y. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Wu HE, Narita M, Loh HH, Nagase H, Tseng LF. Loss of mu-opioid receptor-mediated G-protein activation in the pons/medulla of mice lacking the exons 2 and 3 of mu-opioid receptor gene. Neurosci Lett. 2002;335:91–94. doi: 10.1016/s0304-3940(02)01171-0. [DOI] [PubMed] [Google Scholar]
- Nguyen AT, Marquez P, Hamid A, Lutfy K. The role of mu opioid receptors in psychomotor stimulation and conditioned place preference induced by morphine-6-glucuronide. European journal of pharmacology. 2012;682:86–91. doi: 10.1016/j.ejphar.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese PS, Larson DL, Sayre LM, Fries DS, Takemori AE. A novel opioid receptor site directed alkylating agent with irreversible narcotic antagonistic and reversible agonistic activities. J Med Chem. 1980;23:233–234. doi: 10.1021/jm00177a002. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Steinpreis RE. Effects of the selective mu(1)-opioid receptor antagonist, naloxonazine, on cocaine-induced conditioned place preference and locomotor behavior in rats. Neurosci Lett. 2002;332:159–162. doi: 10.1016/s0304-3940(02)00950-3. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Mayan R, Yadid G. A hypothalamic endorphinic lesion attenuates acquisition of cocaine self-administration in the rat. Eur Neuropsychopharmacol. 2006;16:25–32. doi: 10.1016/j.euroneuro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Schindler CJ, Yadid G. A critical role for beta-endorphin in cocaine-seeking behavior. Neuroreport. 2004;15:519–521. doi: 10.1097/00001756-200403010-00027. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japon M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1996;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JA, Hummel M, Simpson AD, Sheikh R, Soderman AR, Unterwald EM. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology (Berl) 2007;195:265–272. doi: 10.1007/s00213-007-0883-z. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng LF. Evidence for epsilon-opioid receptor-mediated beta-endorphin-induced analgesia. Trends in pharmacological sciences. 2001;22:623–630. doi: 10.1016/s0165-6147(00)01843-5. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Collins KA. Pretreatment with pertussis toxin blocks morphine- but not beta-endorphin-induced antinociception in the mouse. European journal of pharmacology. 1995;294:345–348. doi: 10.1016/0014-2999(95)00687-7. [DOI] [PubMed] [Google Scholar]
- Wise RA. Opiate reward: sites and substrates. Neuroscience and biobehavioral reviews. 1989;13:129–133. doi: 10.1016/s0149-7634(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Wise RA, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse. 1992;10:247–263. doi: 10.1002/syn.890100307. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Lutfy K, Azimuddin S, Sierra V. Differentiation of spinal and supraspinal opioid receptors by morphine tolerance. Life sciences. 1990;46:343–350. doi: 10.1016/0024-3205(90)90013-h. [DOI] [PubMed] [Google Scholar]
- Yoburn BC, Lutfy K, Candido J. Species differences in mu- and delta-opioid receptors. European journal of pharmacology. 1991;193:105–108. doi: 10.1016/0014-2999(91)90207-7. [DOI] [PubMed] [Google Scholar]