Abstract
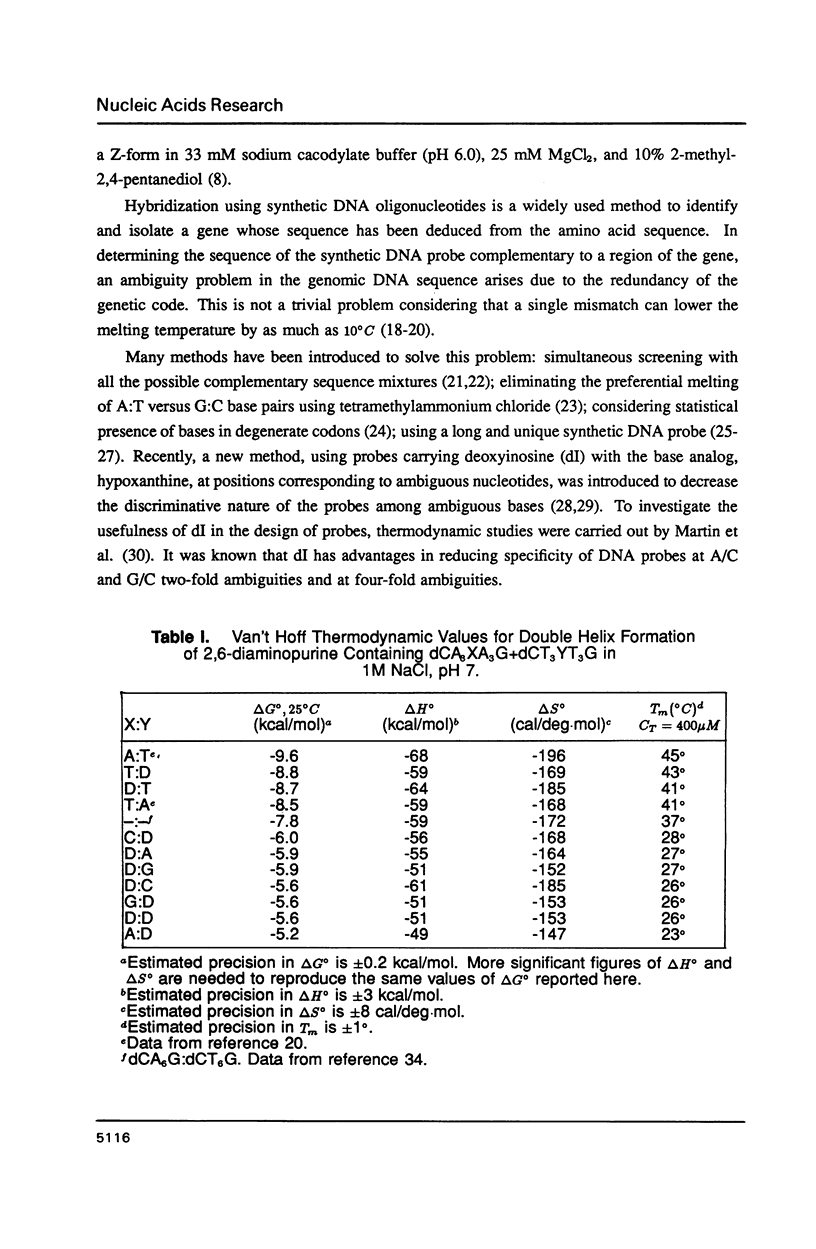
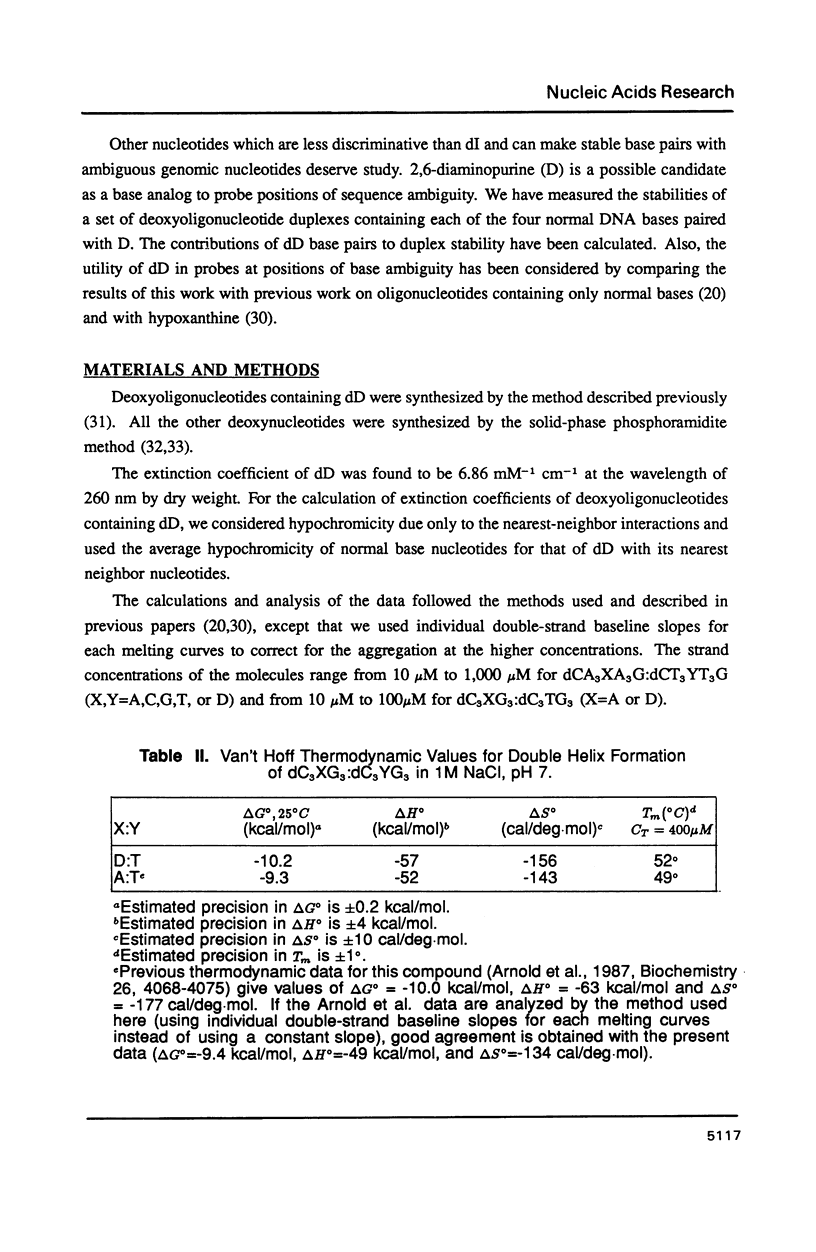
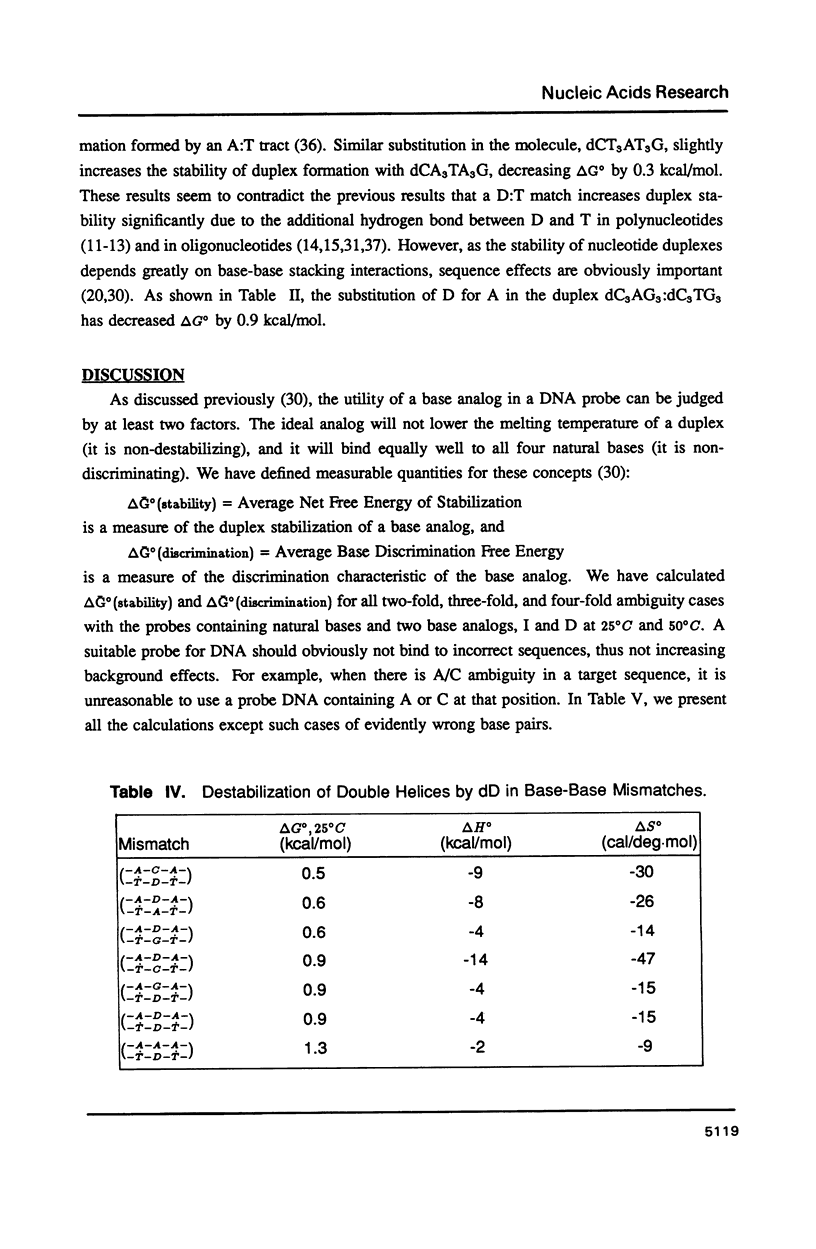
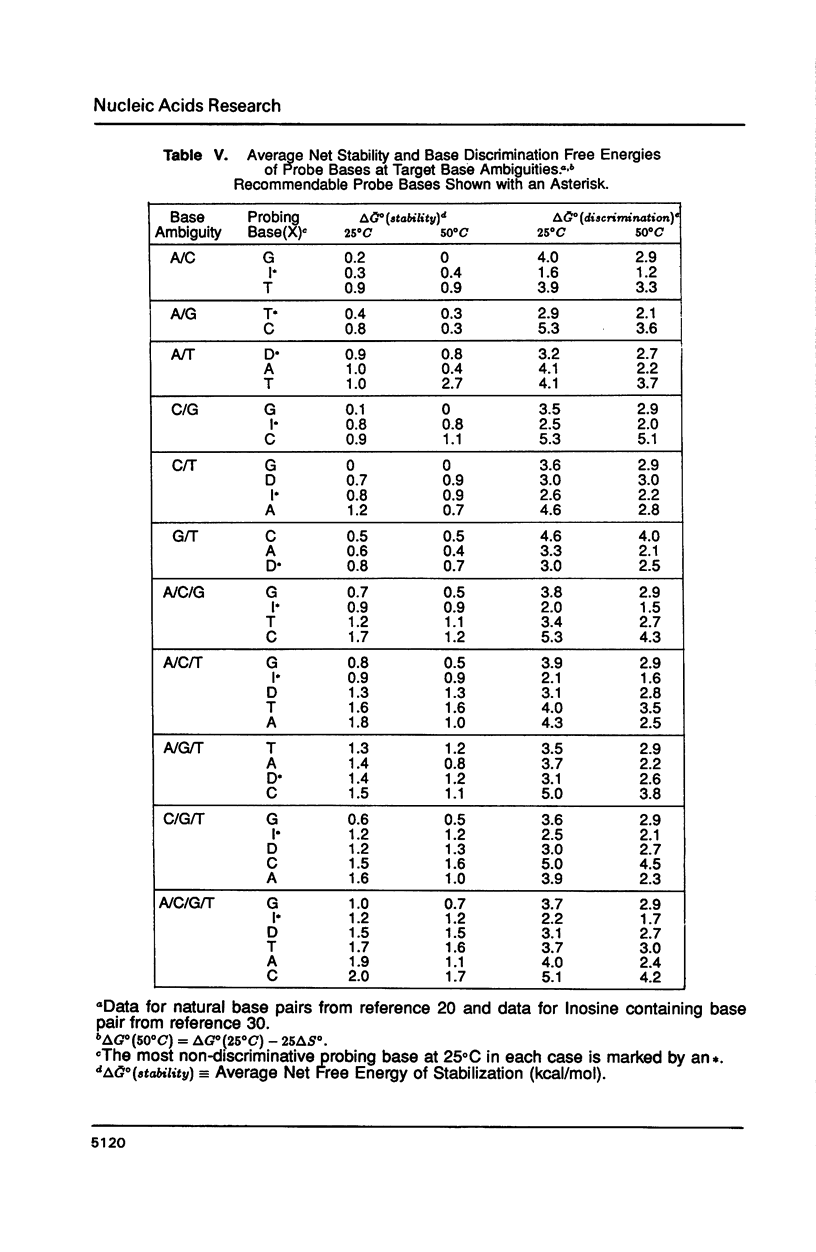
The thermal stabilities of oligodeoxyribonucleotide duplexes containing 2,6-diaminopurine (D) matched with each of the four normal DNA bases were determined by optical melting techniques. Comparison of optical melting curves yielded relative stabilities for the D-containing standard base pairs in an otherwise identical base-pair sequence. The D:T pair was found to be more stable than the A:T pair in dC3DG3:dC3TG3, as stable as the A:T in dCT3DT3G:dCA3TA3G, and less stable than the A:T in dCA3DA3G:dCT7G. The order of stabilities for X:Y in the DNA duplex dCA3XA3G:dCT3YT3G is: (A:T) greater than (T:D) congruent to (D:T) greater than or equal to (T:A) greater than (C:D) congruent to (D:A) congruent to (D:G) greater than or equal to (D:C) congruent to (G:D) congruent to (D:D) greater than or equal to (A:D). Implications of these results for design of DNA oligonucleotide probes are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Kingston I. B. Isolation of a genomic clone for bovine pancreatic trypsin inhibitor by using a unique-sequence synthetic DNA probe. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6838–6842. doi: 10.1073/pnas.80.22.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borah B., Cohen J. S., Howard F. B., Miles H. T. Poly(d2NH2A-dT): two-dimensional NMR shows a B to A conversion in high salt. Biochemistry. 1985 Dec 3;24(25):7456–7462. doi: 10.1021/bi00346a064. [DOI] [PubMed] [Google Scholar]
- Coll M., Wang A. H., van der Marel G. A., van Boom J. H., Rich A. Crystal structure of a Z-DNA fragment containing thymine/2-aminoadenine base pairs. J Biomol Struct Dyn. 1986 Oct;4(2):157–172. doi: 10.1080/07391102.1986.10506337. [DOI] [PubMed] [Google Scholar]
- Gaffney B. L., Marky L. A., Jones R. A. The influence of the purine 2-amino group on DNA conformation and stability. Synthesis and conformational analysis of d[T(2-aminoA)]3. Nucleic Acids Res. 1982 Jul 24;10(14):4351–4361. doi: 10.1093/nar/10.14.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Yelverton E., Ullrich A., Heyneker H. L., Miozzari G., Holmes W., Seeburg P. H., Dull T., May L., Stebbing N. Human leukocyte interferon produced by E. coli is biologically active. Nature. 1980 Oct 2;287(5781):411–416. doi: 10.1038/287411a0. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. B., Frazier J., Miles H. T. A new polynucleotide complex stabilized by 3 interbase hydrogen bonds, poly-2-aminoadenylic acid + polyuridylic acid. J Biol Chem. 1966 Sep 25;241(18):4293–4295. [PubMed] [Google Scholar]
- Howard F. B., Frazier J., Miles H. T. Poly(2-aminoadenylic acid): interaction with poly(uridylic acid). Biochemistry. 1976 Aug 24;15(17):3783–3795. doi: 10.1021/bi00662a022. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Miles H. T. Poly(2-aminodeoxyadenylic acid): circular dichroism and thermal stability of its complexes and their relevance to phage DNA in which a is replaced by 2NH2A. Biopolymers. 1983 Feb;22(2):597–600. doi: 10.1002/bip.360220204. [DOI] [PubMed] [Google Scholar]
- Janion C., Scheit K. H. The effect of thioketo substitution on uracil-2-aminopurine and uracil-2, 6-diaminopurine interactions in polynucleotides. Biochim Biophys Acta. 1976 May 3;432(2):192–198. doi: 10.1016/0005-2787(76)90161-1. [DOI] [PubMed] [Google Scholar]
- Jaye M., de la Salle H., Schamber F., Balland A., Kohli V., Findeli A., Tolstoshev P., Lecocq J. P. Isolation of a human anti-haemophilic factor IX cDNA clone using a unique 52-base synthetic oligonucleotide probe deduced from the amino acid sequence of bovine factor IX. Nucleic Acids Res. 1983 Apr 25;11(8):2325–2335. doi: 10.1093/nar/11.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudyakov I. Y., Kirnos M. D., Alexandrushkina N. I., Vanyushin B. F. Cyanophage S-2L contains DNA with 2,6-diaminopurine substituted for adenine. Virology. 1978 Jul 1;88(1):8–18. doi: 10.1016/0042-6822(78)90104-6. [DOI] [PubMed] [Google Scholar]
- Kirnos M. D., Khudyakov I. Y., Alexandrushkina N. I., Vanyushin B. F. 2-aminoadenine is an adenine substituting for a base in S-2L cyanophage DNA. Nature. 1977 Nov 24;270(5635):369–370. doi: 10.1038/270369a0. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Martin F. H., Castro M. M., Aboul-ela F., Tinoco I., Jr Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 1985 Dec 20;13(24):8927–8938. doi: 10.1093/nar/13.24.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka M., Miles H. T., Howard F. B. Copolymers of adenylic and 2-aminoadenylic acids. Effect of progressive changes in hydrogen bonding and stacking on interaction with poly(uridylic acid). Biochemistry. 1980 May 27;19(11):2429–2439. doi: 10.1021/bi00552a022. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Martin F. H., Tinoco I., Jr DNA and RNA oligomer thermodynamics: the effect of mismatched bases on double-helix stability. Biopolymers. 1981 Dec;20(12):2509–2531. doi: 10.1002/bip.1981.360201204. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Orkin S. H., Daddona P. E., Shewach D. S., Markham A. F., Bruns G. A., Goff S. C., Kelley W. N. Molecular cloning of human adenosine deaminase gene sequences. J Biol Chem. 1983 Nov 10;258(21):12753–12756. [PubMed] [Google Scholar]
- Rackwitz H. R., Scheit K. H. The stereochemical basis of template function. Eur J Biochem. 1977 Jan 3;72(1):191–200. doi: 10.1111/j.1432-1033.1977.tb11239.x. [DOI] [PubMed] [Google Scholar]
- Rinkel L. J., Mellema J. R., van der Marel G. A., van Boom J. H., Altona C. Influence of 2-aminoadenosine, A', on the conformational behaviour of d(T-A'-T-A'). A one-dimensional proton NMR study at 300 MHz and 500 MHz. Eur J Biochem. 1986 Jan 15;154(2):259–265. doi: 10.1111/j.1432-1033.1986.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Roy K. B., Miles H. T. Binding isotherms of nucleosides and polynucleotides measured by continuous ultrafiltration at constant volume: a rapid and precise technique. Biochemistry. 1982 Jan 5;21(1):57–62. doi: 10.1021/bi00530a011. [DOI] [PubMed] [Google Scholar]
- Scheit K. H., Rackwitz H. R. Synthesis and physicochemical properties of two analogs of poly(dA): poly(2-aminopurine-9-beta-D-deoxyribonucleotide) and poly 2-amino-deoxyadenylic acid. Nucleic Acids Res. 1982 Jul 10;10(13):4059–4069. doi: 10.1093/nar/10.13.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kato K., Hayashizaki Y., Wakabayashi T., Ohtsuka E., Matsuki S., Ikehara M., Matsubara K. Molecular cloning of the human cholecystokinin gene by use of a synthetic probe containing deoxyinosine. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1931–1935. doi: 10.1073/pnas.82.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z. K., Ikuta S., Huang T., Dugaiczyk A., Itakura K. Solid-phase synthesis of polynucleotides. VIII: A simplified synthesis of oligodeoxyribonucleotides. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):383–391. doi: 10.1101/sqb.1983.047.01.045. [DOI] [PubMed] [Google Scholar]
- Tibanyenda N., De Bruin S. H., Haasnoot C. A., van der Marel G. A., van Boom J. H., Hilbers C. W. The effect of single base-pair mismatches on the duplex stability of d(T-A-T-T-A-A-T-A-T-C-A-A-G-T-T-G) . d(C-A-A-C-T-T-G-A-T-A-T-T-A-A-T-A). Eur J Biochem. 1984 Feb 15;139(1):19–27. doi: 10.1111/j.1432-1033.1984.tb07970.x. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]


