Abstract
Postictal cerebrovascular dysfunction is an adverse effect of seizures in newborn piglets. The brain heme oxygenase (HO) provides protection against cerebrovascular dysfunction. We investigated the contribution of reactive oxygen species (ROS) to seizure-induced vascular damage and the mechanism of HO vasoprotection. In a bicuculline model of seizures, we addressed the hypotheses: (1) seizures increase brain ROS; (2) ROS contribute to cerebral vascular dysfunction; (3) ROS initiate a vasoprotective mechanisms by activating endogenous HO; and (4) HO products have antioxidant properties. As assessed by dihydroethidium oxidation (ox-DHE), seizures increased ROS in cerebral vessels and cortical astrocytes; ox-DHE elevation was prevented by tiron and apocynin. An HO inhibitor, tin protoporphyrin, potentiated, whereas an HO-1 inducer, cobalt protoporphyrin, blocked seizure-induced increase in DHE oxidation. Heme oxygenase products carbon monoxide (CO) (CORM-A1) and bilirubin attenuated ox-DHE elevation during seizures. Antioxidants tiron and bilirubin prevented the loss of postictal cerebrovascular dilations to bradykinin, glutamate, and sodium nitroprusside. Tiron and apocynin abrogated activation of the brain HO during seizures. Overall, these data suggest that long-term adverse cerebrovascular effects of seizures are attributed to oxidative stress. On the other hand, seizure-induced ROS are required for activation of the endogenous antioxidant HO/CO/bilirubin system that alleviates oxidative stress-induced loss of postictal cerebrovascular function in piglets.
Keywords: apocynin, bicuculline, CORM-A1, dihydroethidine, tiron
Introduction
Seizures, defined as excessive synchronous neuronal activity in the brain, frequently occur in premature neonates and may produce permanent neuronal damage followed by life-long neurologic problems. Neuronal damage caused by seizures has been well documented in animal models and in patients (Clancy, 2006; Meldrum, 2002; Patel, 2004; Pestana et al, 2010). The cerebral cortex is the primary element in the generation of epileptic seizures. Importantly, the vasculature of the cerebral cortex is also damaged during seizures. Bicuculline-induced seizures in newborn piglets produce sustained structural and functional damage to pial arterioles, major resistance vessels of the cerebral cortex (Carratu et al, 2003; Parfenova et al, 2005; Zimmermann et al, 2007). Functional and morphological evidence of blood–brain barrier (BBB) damage during seizures also has been reported (Marchi et al, 2011). The cerebral vascular endothelium is most vulnerable to seizure-induced damage that produces a loss of endothelium-dependent vasodilator functions during the long-term postictal period (Parfenova et al, 2005; Zimmermann et al, 2007).
Mechanisms of seizure-induced cerebral vascular complications remain largely unknown. Oxidative stress is a likely contributor to cerebrovascular dysfunction. Previously, we (Basuroy et al, 2006; Parfenova et al, 2006) showed that reactive oxygen species (ROS) are major contributors to apoptosis of cerebral vascular endothelial cells caused by glutamate and inflammatory cytokines, the main seizure-related injurious factors (Meldrum, 2002; Vezzani et al, 2008). In contrast to well-documented experimental evidence of neuronal oxidative stress during seizures (Patel, 2004; Pestana et al, 2010), no studies have focused on the effects of seizures in the cerebral vasculature.
The brain endothelial cells are characterized by a potent endogenous cytoprotective mechanism that involves heme oxygenase (HO). Previously, we provided in vivo evidence that HO-2, the constitutively active isoform, has an essential cerebroprotective role against seizure-induced loss of endothelial vasodilator function in newborn piglets (Carratu et al, 2003; Parfenova and Leffler, 2008). Heme oxygenase-1 is not expressed in the normal brain cortex and is not induced by seizures (Carratu et al, 2003; Parfenova et al, 2005). However, pharmacologically induced HO-1 also prevents occurrence of postictal cerebrovascular endothelial damage and dysfunction (Parfenova et al, 2005; Zimmermann et al, 2007).
The mechanisms involved in cytoprotective effects of HO in cerebral circulation during epileptic seizures remain elusive. In cerebral vascular endothelial cells exposed to seizure-related injurious excitatory and inflammatory mediators, the cytoprotective capacity of HO is defined by the antioxidant potencies of carbon monoxide (CO) and bilirubin, the end products of HO-mediated heme catabolism (Basuroy et al, 2006; Parfenova et al, 2006; Parfenova and Leffler, 2008). Therefore, oxidative stress appears to be a likely cause of cerebrovascular dysfunction following seizures. On the other hand, a signaling role of ROS in the regulation of endogenous HO activity in the newborn cerebral circulation cannot be excluded. Our study in the bicuculline model of epileptic seizures in newborn piglets in vivo has been designed to investigate the following hypotheses: (1) seizures increase ROS formation in the cerebral vasculature; (2) ROS and oxidative stress contribute to long-term postictal cerebral vascular dysfunction; (3) ROS also initiate a cerebroprotective mechanisms by activating endogenous HO; and (4) HO products, CO and bilirubin, exhibit antioxidant properties in cerebral circulation.
Materials and methods
Chemicals
Bicuculline was from Tocris (R&D Systems, Minneapolis, MN, USA). Pancuronium bromide was from Astra Pharmaceutical Products (Westborough, MA, USA). Dihydroethidium (DHE) was purchased from Invitrogen (Carlsbad, CA, USA). Apocynin was from Calbiochem (EMD Chemicals, Gibbstown, NJ, USA). CO-releasing molecule-A1 (CORM-A1) was from Dalton Pharma Services (Toronto, Canada). Tin protoporphyrin (SnPP) and cobalt protoporphyrin (CoPP) were purchased from Frontier Scientific (Logan, UT, USA). All other reagents were from Sigma (St Louis, MO, USA).
Animals
Newborn piglets (1 to 5 days old, 1.5. to 2.5 kg, either sex) purchased from a commercial breeder were used in all experiments. Piglets were housed in a specially designed pen with warmed floor and given continual access to commercial pig milk substitute. Veterinary care was provided by the Department of Comparative Medicine, whose staff includes four full-time veterinarians in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited program. All experimental protocols and procedures using animals were approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center in accordance with the National Institutes of Health guidelines for the care and use of animals in research. The manuscript is written in accordance with the ARRIVE (Animal Research: Reporting in vivo experiments).
Acute Seizure Protocol
This protocol was used for detection of the brain ROS production during seizures. Seizures were induced by bicuculline in anesthetized, ventilated, and instrumented newborn piglets (1 to 5 days old, 1.5 to 2.5 kg, either sex) as we described previously (Carratu et al, 2003; Parfenova et al, 2005). Body temperature was kept at 37°C to 38°C with a servo-controlled heating pad. A catheter was placed into a femoral vein for infusion of 5% dextrose saline (4 mL/kg per hour) and drugs. Another catheter was inserted into the abdominal aorta via a femoral artery for monitoring heart rate and mean arterial blood pressure (MABP) and for sampling of blood for pH and gases. Animals were paralyzed with pancuronium bromide (0.2 mg/kg, intravenously). Pancuronium attenuates the increase in MABP during seizures without affecting the cerebral blood flow response (Pourcyrous et al, 1992). Bicuculline (3 mg/kg, intravenously), a blocker of inhibitory GABAA (γ-aminobutyric acid) receptors on glutamatergic neurons, induced spontaneous seizures characterized by a sudden onset (in 1 to 2 minutes) of repetitive synchronous neuronal discharges and a robust elevation in EEG (electroencephalogram) amplitude and spectral power in α (8 to 13 Hz), β (>13 Hz), δ (<4 Hz), and θ (4 to 8 Hz) bands of over 1 hour duration (Parfenova et al, 2004). Neuronal activation is accompanied by a robust increase in heart rate (from ∼120 to 140 to ∼200 beats/min) sustained for the duration of seizures (Carratu et al, 2003; Parfenova et al, 2005; Zimmermann et al, 2007). Severe tachycardia is a common feature of generalized and nongeneralized seizures in patients (Greene et al, 2006; Opherk et al, 2002; van Elmpt et al, 2006; Shah et al, 2010; Zijlmans et al, 2002). The heart rate-based automatic monitoring approach has been proposed as a sensitive method for early detection of seizures in newborns (Greene et al, 2006; van Elmpt et al, 2006). In our experiments, elevated heart rate was used as a reliable indicator of ongoing seizures in piglets.
Isolation of Cerebral Vessels and Cortical Astrocytes
For fluorescence microscopy studies, pial vessels (50- to 200-μm branches of the middle cerebral artery) were dissected from the brain and cleaned of connective tissue. For fluorescence spectroscopy studies, cerebral vessels were isolated by consecutive differential filtration of the brain cortex homogenate through 300-, 60-, and 20-μm mesh nylon filters as we described previously (Leffler et al, 2006). Cerebral microvessels (300 to 60 μm) were retained on the 60-μm filter, whereas neurons and capillaries were largely retained on the 20-μm filter. The 20-μm filtrate of the brain cortex parenchyma was composed mainly of cortical astrocytes (>90% of cells) identified by the astrocyte-specific markers, glial fibrillary acidic protein (GFAP) and aquaporin-4 (Leffler et al, 2006).
Detection of Reactive Oxygen Species Production by Oxidation of Dihydroethidium in Cerebral Vessels and Astrocytes
Dihydroethidium, a brain-permeable oxidant-sensitive probe, is widely used for detection of ROS in the brain in vivo (Bindokas et al, 1996; Capone et al, 2011; Chan et al, 1998; Kooli et al, 2008; Kunz et al, 2007; Murakami et al, 1998; Hall et al, 2012). Two products of DHE oxidation (ox-DHE), ethidium (Eth) and EOH (2-hydroxyethidium), bind to the nuclear DNA forming a strong red fluorescent complex. 2-Hydroxyethidium is a product of DHE oxidation by superoxide, whereas Eth also detects H2O2-related products (Bindokas et al, 1996; Zhao et al, 2005). Recent studies in mice with genetically altered expression of superoxide dismutase demonstrated that ox-DHE fluorescence quantitatively correlates with the superoxide levels in the live brain (Hall et al, 2012). Therefore, in our experiments with freshly isolated brain tissue, ox-DHE fluorescence is presumably indicative of superoxide and, to a lesser extent, H2O2 levels. Dihydroethidium (1.5 mg/kg, intravenously) was administered immediately before bicuculline (seizure groups) or saline (control group); the brain was removed 1 hour after the administration to detect the level of nuclear-bound ox-DHE accumulated during a 1-hour period after saline or bicuculline administration in all experimental groups. For fluorescence microscopy of ox-DHE, pial arterioles were excised under a dissecting microscope. In another approach to ox-DHE fluorescence imaging, the cerebral cortex was immediately placed in cold PBS (phosphate-buffered saline), and tangential slices (200 μm) were cut from the cortical surface. Cerebral vessels and the brain slices were immediately examined with a Nikon Diaphot fluorescent microscope (Nikon Inc., Garden City, NY), and the images were collected by IPLab Spectrum software (Spectra Services Inc., Ontario, NY) using identical microscope settings.
For quantitative measurements of ox-DHE levels, we used fluorescence spectroscopy because it allows: (1) extraction of the nuclear-bound ox-DHE, (2) comparison of the samples obtained from different animals over an extended period of time, and (3) straightforward quantitative data analysis. Tissue samples from animals treated with the antioxidants apocynin and tiron were used to verify the reliability of ROS quantitation by DHE oxidation. To extract the nuclear-bound ox-DHE, freshly isolated cerebral vessels and astrocytes were disrupted by sonication in PBS, and the tissue lysates were cleared by centrifugation. The ox-DHE fluorescence (excitation/emission, 485/590 nm) that represents cumulative Eth and EOH products was detected by a Synergy HT microplate reader (BioTek Instruments, Winooski, VT, USA) and normalized to the protein amount. To ensure quantitative detection of ROS by ox-DHE fluorescence, we used tiron, a ROS scavenger, and apocynin, a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibitor (Genovese et al, 2011; Jackman et al, 2009; Tang et al, 2008). Tiron (2 g/kg, intravenously) and apocynin (20 mg/kg, intravenously) were administered 30 minutes before bicuculline. Additional control experiments were conducted to verify that in the absence of tissue lysates, the ox-DHE baseline fluorescence was not altered by any of the agents used in the study.
Detection of Serum Bilirubin
The serum bilirubin level was detected by the difference in maximal (450 nm) and minimal (530 nm) absorbances (Davies and Keohane, 1973) using an Ultraspec 2100-Pro spectrophotometer (Amersham Biosciences, Piscataway, NJ, USA) and quantified based on the indirect bilirubin standard curve values.
Survival Protocol for Detection of Long-Term Cerebrovascular Functional Outcome of Seizures
This protocol that minimizes surgical interventions was used to test the long-term effects of seizures as was described in greater detail elsewhere (Parfenova et al, 2005; Zimmermann et al, 2007). The body temperature was maintained at 37°C to 38°C. Seizures were induced by bicuculline (3 mg/kg, intraperitoneally) in animals paralyzed with pancuronium bromide (0.2 mg/kg, intravenously). In addition to the intact control group, three seizure survival groups were studied: (1) saline control (5 mL saline, intraperitoneally, 30 minutes before bicuculline); (2) bilirubin-treated (5 mg/kg, intraperitoneally, 30 minutes before bicuculline); and (3) tiron-treated (1 g/kg, intraperitoneally, 30 minutes before bicuculline). Piglets were kept on the ventilator for 2 to 3 hours until the seizure activity subsided. When fully conscious, piglets were transferred to the animal care facility and kept in warmed cages with food and water ad libitum for 2 days.
Intravital Microscopy for Detection of Cerebral Vascular Reactivity
Cerebral vascular reactivity was assessed 2 days after the seizures. The animals were anesthetized with ketamine/acepromazine/α-chloralose, ventilated with room air, and equipped with catheters for recording MABP, heart rate, and blood sampling as we previously described (Parfenova et al, 2005). A closed cranial window was implanted for intravital microscopy of pial arterioles as we previously described (Parfenova et al, 2005). The space under the window was filled with artificial cerebrospinal fluid (aCSF) (in mM): 3.0 KCl, 1.5 MgCl2, 1.5 CaCl2, 132 NaCl, 6.6 urea, 3.7 dextrose, and 24.6 NaHCO3 equilibrated with 6% CO2–6% O2–88% N2 to pH 7.3 to 7.35 at 37°C. Pial arteriolar diameter was measured with a videomicrometer coupled to a television camera. To test cerebral vascular reactivity, we used the physiologically relevant endothelium-dependent (bradykinin, 10−7 mol/L, and glutamate, 10−5 mol/L), and -independent (sodium nitroprusside (SNP); 10−6 mol/L) vasodilators. All dilators were topically applied to the cerebral surface, and changes in pial arteriolar diameter were repeatedly recorded; the stable diameter achieved between 5 to 10 minutes was taken as the response.
Detection of Carbon Monoxide Production by Gas Chromatography/Mass Spectrometry
To detect endogenous HO-catalyzed CO formation, freshly isolated cerebral vessels and astrocytes were incubated for 1 hour at 37°C in a Krebs buffer in the presence of the standard 13C18O (Sigma-Aldrich, St Louis, MO, USA), and the headspace gas was collected for GC/MS (gas chromatography/mass spectrometry) (Leffler et al, 2006). To detect CO levels in the brain in vivo, samples of cortical periarachnoid CSF (pCSF) were collected from the brain surface before and during seizures. Periarachnoid CSF (0.4 mL) was collected from under the closed cranial window in 10-minute intervals into sealed vials containing 13C18O in Krebs buffer (Leffler et al, 2006; Zimmermann et al, 2007). Carbon monoxide in the headspace gas was detected using a Varian Saturn 3 GC/MS (Varian, Palo Alto, CA, USA). Carbon monoxide concentration was calculated based on the peak areas with the mass-to-charge ratios corresponding to 12C16O and 13C18O and normalized to protein content. The results were expressed as picomoles of CO released into the headspace per mg of protein per hour.
Statistical Analysis
Values are presented as mean values±s.e. of absolute values or percentage of control. Data were analyzed by repeated measures analysis of variance. A level of P<0.05 was considered significant.
Results
Effects of Seizures on Systemic Circulatory Parameters
Severe tachycardia up to 200 b.p.m., a sensitive test for detection of seizures (Greene et al, 2006; Opherk et al, 2002; van Elmpt et al, 2006; Shah et al, 2010; Zijlmans et al, 2002), was recorded in ictal piglets (Table 1). No significant differences in the heart rate were observed among all experimental groups (Table 1), suggesting that all compounds tested in our experiments (antioxidants, modulators of HO activity/expression, CORM-A1, and bilirubin) did not alter the course of seizures. Seizures also caused a moderate increase in MABP in all groups (Table 1), as in our previous experiments (Carratu et al, 2003; Parfenova et al, 2004, 2010). No significant changes in arterial blood gases and pH among experimental groups were observed, with the exception of tiron and CoPP, which produced a slight acidosis (Table 1).
Table 1. Systemic circulatory parameters in experimental groups of newborn piglets.
| Group | n | HR, beats/min | MABP, mm Hg | Arterial PCO2, mm Hg | Arterial PO2, mm Hg | pH |
|---|---|---|---|---|---|---|
| Intact control (no seizures) | 50 | 137±4 | 76±2 | 31±1 | 105±2 | 7.46±0.01 |
| Seizures control | 50 | 193±4* | 89±2* | 34±1 | 99±3 | 7.43±0.02 |
| Seizures + tiron | 4 | 205±7* | 98±9* | 38±3 | 94±8 | 7.34±0.05* |
| Seizures + apocynin | 4 | 197±4* | 93±2* | 36±2 | 99±7 | 7.51±0.06 |
| Seizures + SnPP | 5 | 194±7* | 92±8* | 34±1 | 102±2 | 7.42±0.04 |
| Seizures + CoPP | 6 | 191±7* | 90±4* | 36±2 | 105±7 | 7.39±0.03* |
| Seizures + CORM-A1 | 9 | 205±12* | 86±4* | 34±1 | 103±7 | 7.41±0.02 |
| Seizures + bilirubin | 10 | 182±8* | 82±2* | 37±2 | 99±8 | 7.39±0.02 |
CoPP, cobalt protoporphyrin; HR, heart rate; MABP, mean arterial blood pressure; SnPP, tin protoporphyrin.
Data represent average values of systemic parameters taken from each animal 5, 10, 30, and 60 minutes after injection of saline (intact control group) or bicuculline (seizures groups).
*P<0.05 compared with intact control values.
Effects of Seizures on Reactive Oxygen Species Production
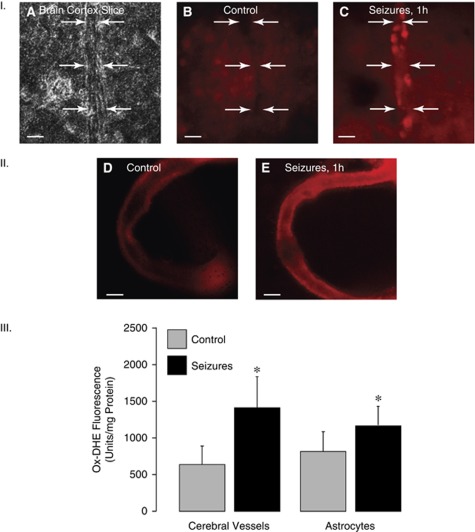
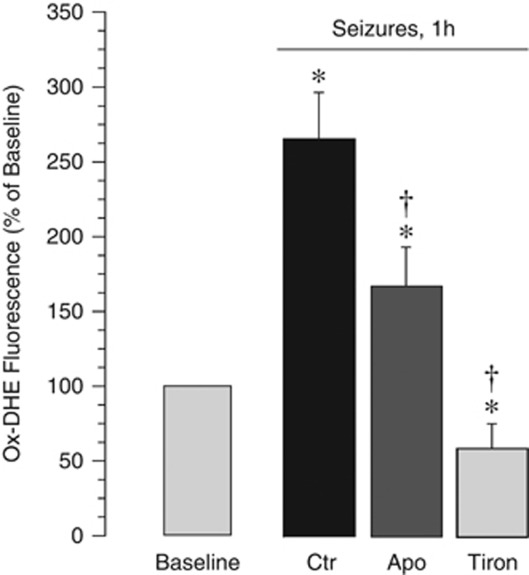
Seizures caused an increase in ox-DHE formation in cerebral microvessels and the astrocyte-enriched cerebral cortex parenchyma, as detected by ox-DHE fluoromicrography in the fresh brain slices (Figure 1, panel I) and excised pial arterioles (Figure 1, panel II). Quantitative detection of ox-DHE was performed by fluorescence spectroscopy (Figure 1, panel III). Both cerebral microvessels and cortical astrocytes are the sites of increased ROS production (ox-DHE increase during seizures, 2.6±0.4- and 1.5±0.3-fold over the baseline in cerebral vessels and astrocytes, respectively; P<0.05, N=8 animals in each group). Antioxidants tiron and apocynin inhibited seizure-induced ox-DHE fluorescence in cerebral microvessels (Figure 2), verifying the reliability of quantitative detection of ROS by ox-DHE spectroscopy. Tiron (2 g/kg, intravenously), a ROS scavenger, completely prevented ox-DHE elevation in cerebral microvessels during seizures (Figure 2). Apocynin (20 mg/kg, intravenously), an antioxidant that inhibits NADPH oxidase activity (Genovese et al, 2011; Jackman et al, 2009; Tang et al, 2008), decreased seizure-evoked ox-DHE fluorescence in cerebral vessels by 40% to 50% (Figure 2), suggesting that NADPH oxidase contributes to seizure-induced ROS production in the cerebral vasculature.
Figure 1.
Effects of seizures (1 hour) on reactive oxygen species (ROS) in cerebral vessels and cortical astrocytes as detected by dihydroethidium (DHE) oxidation (ox-DHE). Panel I: Ox-DHE fluoromicrography of the brain cortex slices. (A) A phase contrast image of a brain cortex slice with a penetrating vessel; (B) a fluorescence image, control brain; (C) a fluorescence image, seizing brain (representative pictures; bar, 200 μm). Arrows indicate the outer borders of the vessel wall. Panel II: Ox-DHE fluoromicrography of pial arterioles excised from control (D) and seizing (E) piglets 1 hour after saline (D) or bicuculline (E) injection (representative pictures; bar, 200 μm). Panel III: Detection of ox-DHE by fluorescence spectroscopy of cortical cerebral microvessels (60 to 300 μm) and cortical astrocytes (N=8 animals in each group). *P<0.05 compared with control values.
Figure 2.
Effects of apocynin (20 mg/kg, intravenously) and tiron (2 g/kg, intravenously) on seizure-evoked reactive oxygen species (ROS) production in cerebral vessels as detected by ox-dihydroethidium (DHE) fluorescence spectroscopy (N=5 animals in each group). *P<0.05 compared with the baseline value. †P<0.05 compared with seizure saline control values.
Antioxidant Properties of Constitutive Heme Oxygenase-2
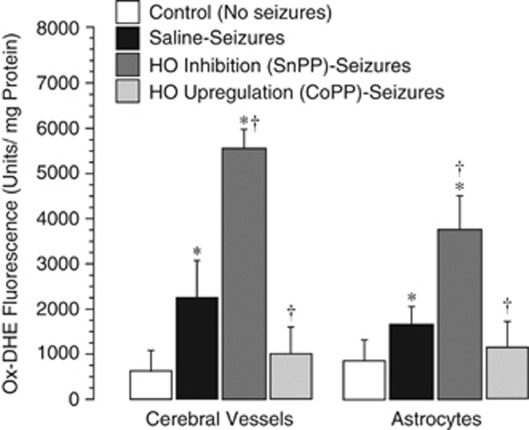
Our previous findings demonstrate that constitutive HO-2 is the only HO isoform expressed in cerebral vessels and the brain parenchyma in control and ictal animals (Carratu et al, 2003; Parfenova et al, 2005). To investigate the contribution of HO-2 to acute endogenous antioxidant defense in the seizing brain, we used SnPP, which effectively inhibits HO-mediated CO production under resting conditions and during seizures (Carratu et al, 2003). Tin protoporphyrin (3 mg/kg, intravenously) administered 30 minutes before seizures greatly enhanced the accumulation of seizure-induced ox-DHE-detectable ROS in cerebral microvessels and astrocytes (Figure 3). These data indicate that the products of HO-2 activity are endogenous antioxidants in cerebral vessels and astrocytes.
Figure 3.
Effects of the brain heme oxygenase (HO) activity inhibition by tin protoporphyrin (SnPP) (3 mg/kg, intravenously; 30 minutes) and of HO-1 upregulation by cobalt protoporphyrin (CoPP) (25 mg/kg, intraperitoneally; 24 hours) on seizure-evoked reactive oxygen species (ROS) production in cerebral vessels and cortical astrocytes as detected by dihydroethidium oxidation (ox-DHE) fluorescence spectroscopy (N=4 animals in each group). *P<0.05 compared with baseline values. †P<0.05 compared with corresponding saline seizure values.
Antioxidant Properties of Pharmacologically Induced Heme Oxygenase-1
We induced HO-1 expression in the brain using systemically administered CoPP as in our previous studies (Parfenova et al, 2005, 2010; Parfenova and Leffler, 2008). Cobalt protoporphyrin (25 mg/kg, intraperitoneally) induced HO-1 expression/activity in 24 hours, as indicated by a greatly increased CO production in cerebral vessels and astrocytes. Following 24 hours after CoPP injection, CO production was increased from 240±40 to 480±70 pmol/mg protein/h and from 330±50 to 1020±80 pmol/mg protein/h in cerebral vessels and cortical astrocytes, respectively (P<0.05, N=4 animals in each group). In piglets with upregulated HO-1 activity, seizures failed to increase ROS production in cerebral microvessels and the brain parenchyma (Figure 3). These data suggest that HO-1 activity provides effective antioxidant protection in cerebral vessels and astrocytes in a seizing brain.
Antioxidant Effects of CORM-A1 During Seizures
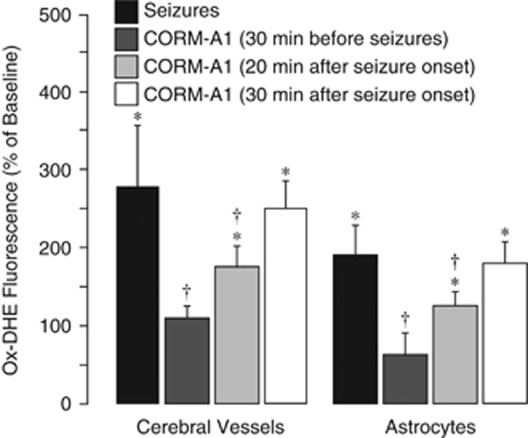
Systemically administered CORM-A1, a slow CO releaser at physiological pH, provides a convenient tool for pharmacological delivery of CO to the brain (Zimmermann et al, 2007). CORM-A1 (2 mg/kg, intraperitoneally), administered 30 minutes before seizures, completely prevented the ox-DHE elevation in cerebral vessels and cortical astrocytes during seizures (Figure 4; P<0.05, N=5). Remarkably, CORM-A1 administered at 20 minutes after seizure onset also reduced the ox-DHE level in cerebral vessels and in astrocytes, although less effectively than in a preventive protocol (Figure 4; P<0.05). However, when administered 30 minutes after seizure onset, CORM-A1 had no antioxidant effects (Figure 4; P>0.05).
Figure 4.
Effects of CORM-A1 (2 mg/kg, intraperitoneally) applied 30 minutes before or 20 and 30 minutes after seizure onset on reactive oxygen species (ROS) production in cerebral vessels and cortical astrocytes as detected by dihydroethidium oxidation (ox-DHE) fluorescence spectroscopy (N=5 animals in each group). *P<0.05 compared with baseline control values. †P<0.05 compared with seizure saline control values.
Antioxidant Effects of Bilirubin During Seizures
In control piglets, the serum bilirubin level was 2.9±0.5 mg/100 mL (N=9), which is slightly above the normal level in human neonates (<1 mg/100 mL; Stocker, 2004). Bilirubin (5 mg/kg, intravenously) increased the serum level to 16.2±2.5 mg/100 mL (N=9), which corresponded to the newborn jaundice level (15 mg/100 mL). The highest increase in serum bilirubin was observed during the first 30 minutes after injection, followed by a slow progressive decline. The serum bilirubin remained greatly elevated above the baseline level even at 2 hours after administration.
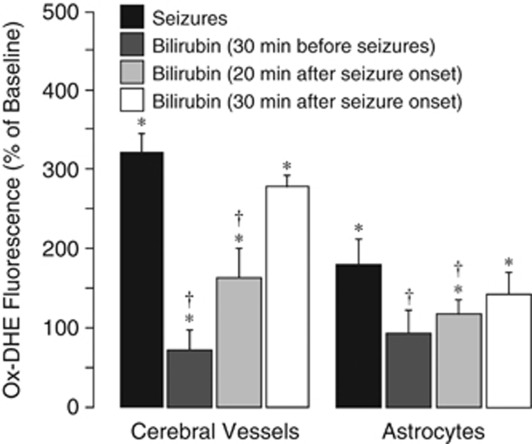
Pretreatment with bilirubin (5 mg/kg, intravenously, 30 minutes before seizures) prevented seizure-induced ROS production by cerebral microvessels (75% reduction in ox-DHE level; Figure 5) and, to a lesser extent, by cortical astrocytes (45% reduction in ox-DHE level; Figure 5). Importantly, bilirubin also had antioxidant effects in cerebral vessels and in the parenchyma when administered during the advanced ictal period (at 20 minutes after seizure onset; Figure 5). However, bilirubin administered 30 minutes after seizure onset was not effective in reducing the seizure-induced ox-DHE-detectable ROS production (Figure 5).
Figure 5.
Effects of bilirubin (5 mg/kg, intravenously) applied 30 minutes before or 20 and 30 minutes after seizure onset on reactive oxygen species (ROS) production in cerebral vessels and cortical astrocytes as detected by dihydroethidium oxidation (ox-DHE) fluorescence spectroscopy (N=5 animals in each group). *P<0.05 compared with baseline control values. †P<0.05 compared with seizure saline control values.
Long-Term Effects of Antioxidants on Postictal Cerebral Vascular Function
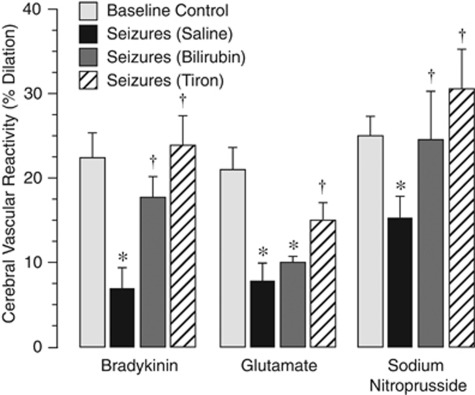
We detected long-term postictal cerebral vascular functions in control and antioxidant-treated piglets (Figure 6). Postictal cerebral vascular responses to physiologically relevant stimuli that produce dilation via distinct cell- and signal-mediated mechanisms were tested 2 days after seizures in control piglets and in piglets pretreated with bilirubin or tiron, which block the seizure-induced ROS increase in vessels and astrocytes (Figures 2 and 5). Postictal cerebral vascular responses to bradykinin (10−7 mol/L), glutamate (10−5 mol/L), and SNP (10−6 mol/L) were significantly reduced compared with the control responses in intact piglets (Figure 6). When animals were pretreated with bilirubin (5 mg/kg, intraperitoneally, 30 minutes before seizures), the loss of cerebral vascular reactivity to bradykinin and SNP was completely prevented, whereas the responses to glutamate remained suppressed (Figure 6). Tiron (1 g/kg, intraperitoneally, 30 minutes before seizures) completely prevented long-term loss of postictal cerebral vascular responses to all tested dilators (Figure 6).
Figure 6.
Effects of antioxidants bilirubin and tiron on long-term effects of seizures on cerebral vasodilator function. Cerebral vascular responses to topical bradykinin (10−7 mol/L), glutamate (10−5 mol/L), and sodium nitroprusside (10−6 mol/L) were detected under baseline conditions (baseline control) and 48 hours after seizures. Piglets were untreated (saline control) or pretreated with bilirubin (5 mg/kg, intravenously) or tiron (1 g/kg, intraperitoneally) 30 minutes before seizure onset (N=4 animals in each group). *P<0.05 compared with baseline control values. †P<0.05 compared with seizure saline control values.
Effects of Antioxidants on Brain Carbon Monoxide Production/Heme Oxygenase Activity
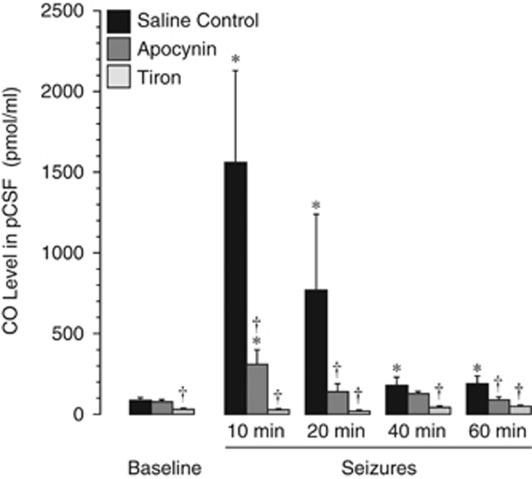
Seizures rapidly increase the enzymatic activity of the brain HO as indicated by SnPP-inhibited elevation of CO in periarachnoid cortical cerebrospinal fluid (pCSF) collected from under the closed cranial window (Carratu et al, 2003). We investigated whether exogenous antioxidants may alter HO activity that catalyzes production of endogenous antioxidant products. The time-dependent accumulation of CO in pCSF was quantitatively detected by GC/MS. Confirming our earlier findings (Carratu et al, 2003), seizures produced immediate and sustained elevation of CO in pCSF (Figure 7). The maximal increase in CO level was 7- to 15-fold above the baseline at 10 to 20 minutes after seizure onset. Apocynin (20 mg/kg, intravenously, 30 minutes before seizures) greatly reduced, whereas tiron (1 g/kg, intraperitoneally, 30 minutes before seizures) completely blocked HO activation (Figure 7). These data suggest that HO activation during seizures occurs in a ROS-sensitive manner.
Figure 7.
Effects of antioxidants on carbon monoxide (CO) level in cortical periarachnoid cerebrospinal fluid (pCSF) before and during seizures. Apocynin (20 mg/kg, intravenously) or tiron (2 g/kg, intravenously) were administered 30 minutes before seizure onset. pCSF samples were collected from under the cranial window before and during seizures. CO level in pCSF was determined by gas chromatography/mass spectrometry (N=4 animals in each group). *P<0.05 compared with baseline control values. †P<0.05 compared with seizure saline control values.
Discussion
Our new findings in the newborn brain are (1) seizures elevate ROS in the cerebral vasculature and astrocytes; (2) constitutive HO-2 and induced HO-1 provide potent antioxidant protection in cerebral vessels and astrocytes; (3) HO activation during seizures occurs in an oxidant-sensitive manner; and (4) the products of HO-catalyzed heme breakdown, CO and bilirubin, administered before or during seizures, act as potent antioxidants that account for cerebroprotective properties of HO. Overall, these data suggest that ROS elevation in the seizing brain has dual effects. On one hand, seizure-induced ROS cause oxidative stress and sustained postictal cerebrovascular damage. On the other hand, ROS activate the endogenous antioxidant cerebroprotective HO/CO/bilirubin system that alleviates the damaging effects of seizures on cerebral vascular functions.
We now demonstrate that seizures increase ROS production in the cerebral vasculature and in cortical astrocytes. The increase in ROS may produce oxidative stress and vascular tissue damage. Apoptotic changes in cerebral vessels, appearance of brain-derived endothelial cells in peripheral blood, and long-term loss of endothelial dilator function, resulting from epileptic seizures in newborn piglets (Carratu et al, 2003; Parfenova et al, 2005, 2010; Zimmermann et al, 2007) appear to be potential functional consequences of seizure-mediated oxidative stress. Recent evidence suggests that oxidative stress is a likely contributor to seizure-related neuronal damage (Pestana et al, 2010; Güneş et al, 2009). In this study, we focused on nonneuronal components of the cerebral cortex, namely on cerebral blood vessels and astrocytes. Vulnerability of the cerebral vasculature to oxidative stress most likely accounts for the cerebrovascular injury and long-term loss of cerebrovascular endothelial dilator function in the neonatal brain (Carratu et al, 2003; Parfenova et al, 2005, 2010; Zimmermann et al, 2007). Indeed, antioxidants and ROS scavengers prevent apoptosis of cultured cerebral vascular endothelial cells exposed to seizure-related excitotoxic and inflammatory mediators (Basuroy et al, 2006, 2009; Parfenova et al, 2006).
Constitutive HO-2 and pharmacologically induced HO-1 provide a potent cerebroprotective mechanism that alleviates loss of cerebrovascular function caused by seizures (Carratu et al, 2003; Parfenova et al, 2005). Inhibition of HO activity by SnPP during seizures aggravated postictal cerebral vascular dysfunction (Carratu et al, 2003; Parfenova et al, 2005, 2010; Zimmermann et al, 2007). We have now demonstrated that inhibition of constitutive HO-2 activity greatly potentiated, whereas induction of HO-1 blocked, ROS production in cerebral vessels and cortical astrocytes during seizures, indicating antioxidant properties of HO in the cerebral circulation. Furthermore, the antioxidants tiron and bilirubin alleviated the loss of postictal cerebrovascular dilator function. These data suggest that the elevated levels of ROS in the seizing brain produce oxidative stress that is a main contributor to detrimental effects of seizures on cerebrovascular functions.
Systemically administered CORM-A1, that delivers CO to the brain and prevents sustained loss of cerebral vascular endothelial dilator function (Parfenova et al, 2010; Zimmermann et al, 2007), completely blocked the burst of ROS production when administered before seizures. This correlates with the antioxidant potencies of CORM-A1 in cerebral vascular endothelial cells stimulated by glutamate or tumor necrosis factor (TNF)-α (Basuroy et al, 2006; Parfenova et al, 2006). Importantly, CORM-A1 also had antioxidant effects in cerebral vessels when administered during 20 minutes into the ictal period, indicating a potential therapeutic window for this compound. In addition to its antioxidant effects in cerebral vessels, antioxidant properties of CORM-A1 were observed in cortical astrocytes. We found that the antioxidant properties of CORM-A1 in TNF-α-stimulated cerebral vascular endothelial cells are due to inhibition of Nox4 NADPH oxidase-derived ROS (Basuroy et al, 2009). In addition, the antioxidant actions of CO could be due to its ability to inhibit mitochondria-derived ROS and initiate mitochondrial pro-survival pathways (Sandouka et al, 2005).
The excitatory neurotransmitter glutamate that evokes abnormal bursts of neuronal activation during seizures in patients and in animal models appears to be the major factor causing seizure-induced oxidative neuronal injury (Meldrum, 2002). Cerebral vascular endothelial cells are among nonneuronal target cell fro glutamate excitotoxicity (Parfenova et al, 2006; Parfenova and Leffler, 2008). Glutamate, via a glutamate receptor-mediated mechanism, increases ROS and causes ROS-mediated apoptosis in cerebral endothelial cells (Parfenova et al, 2006). The proinflammatory cytokine, TNF-α, is another seizure-related ROS-elevating factor in cerebral vasculature (Basuroy et al, 2006, 2009). The molecular sources of ROS in cerebral vasculature exposed to seizure-related injurious factors include NADPH oxidase and mitochondria (Basuroy et al, 2006, 2009).
We provide evidence of beneficial antioxidant and cerebroprotective effects of low concentrations of bilirubin during cerebrovascular insult. Endogenous bilirubin is formed from biliverdin, the end product of HO-catalyzed heme degradation, by the action of biliverdin reductase (Stocker, 2004). Bilirubin from the bloodstream can penetrate through either a normal or a compromised BBB (Hansen, 2002). Elevated bilirubin in the blood has long been considered a cytotoxic compound for the developing brain. In healthy newborn babies, the normal plasma bilirubin level is <1 mg/dL; but in jaundiced newborns, bilirubin can be elevated over 15 mg/dL (Stocker, 2004). In rare cases, at concentrations above 25 mg/dL in severely jaundiced newborns, bilirubin can form deposits in the brain that may be involved in development of neurologic dysfunction and encephalopathy (kernicturus) (Belanger et al, 1997; Hansen, 2002). However, observations in newborn babies provide evidence that moderately elevated bilirubin may be beneficial for newborn babies because it increases the antioxidant capacity of plasma, provides a lower incidence of oxidative stress injury, and protects against retinopathy (Belanger et al, 1997; Hegyi et al, 1994). We now demonstrate that bilirubin administered before or during seizures at a concentration producing only a moderate increase in the serum level (16±2 mg/dL) acts as a potent antioxidant in cerebral vessels and in cortical astrocytes. A therapeutic window of opportunity for antioxidant potencies of bilirubin and CORM-A1 was limited to a 20-minute period after seizure onset. The ability of bilirubin to effectively block seizure-induced ROS in the parenchymal astrocytes indicates that bilirubin permeates the BBB, supporting the findings of other investigators (Hansen, 2002). Importantly, bilirubin alleviated loss of endothelium-dependent and -independent cerebrovascular dilator responses during postictal period. These observations suggest that bilirubin as a potent brain-permeable antioxidant greatly reduces cerebrovascular complications of neonatal seizures.
The antioxidant properties of bilirubin are based on enzymatic and nonenzymatic reactions. In enzymatic redox cycling, bilirubin is oxidized to biliverdin and then is recycled back to bilirubin catalyzed by biliverdin reductase (Baranano et al, 2002; Sedlak and Snyder, 2004). In the nonenzymatic reaction, bilirubin may quench ROS by donating a reactive hydrogen atom, thus forming a bilirubin radical (Stocker, 2004). In cerebral vascular endothelial cells stimulated by TNF-α, bilirubin (0.1 to 1 μmol/L) blocked NADPH oxidase-mediated ROS formation and prevented death by apoptosis (Basuroy et al, 2009). The beneficial role of low concentrations of bilirubin in cell survival has been also reported in glutamate-stimulated neurons (Baranano et al, 2002) and in cerebral vascular endothelial cells challenged with glutamate (Parfenova et al, 2006) and TNF-α (Basuroy et al, 2006).
What are the functional consequences of brain oxidative stress during seizures? We have demonstrated previously that seizures produce a sustained loss of cerebrovascular dilator function extended for at least 2 days of the postictal period (Carratu et al, 2003; Parfenova et al, 2005, 2010; Zimmermann et al, 2007). If oxidative stress is the leading cause of cerebrovascular damage, then reducing the level of ROS in the brain during seizures should alleviate postictal loss of cerebrovascular function. We tested postictal cerebral vascular reactivity using physiologically relevant stimuli that cause vasodilation via distinct cell-mediated mechanisms. Bradykinin and SNP act directly on the cerebral vasculature (endothelial and smooth muscle cells, respectively), whereas the endothelium-dependent vasodilator effect of glutamate also involves the astrocyte component of the neurovascular unit (Leffler et al, 2006). Systemically administered tiron and bilirubin, that blocked the ROS increase in cerebral vessels during seizures, prevented loss of cerebral vascular dilator responses to endothelium-dependent and -independent stimuli during long-term postictal period. CORM-A1, which was highly effective in reducing the brain ROS during seizures, also preserved postictal cerebrovascular function (Zimmermann et al, 2007; Parfenova et al, 2010). Furthermore, reduction of the brain ROS during seizures by HO-1 upregulation prevented, whereas potentiation of oxidative stress by HO inhibition aggravated the loss of cerebral vascular function (Parfenova et al, 2005, 2010). Overall, these finding indicate that: (1) ROS cause postictal cerebral vascular dysfunction and (2) reducing ROS, either by activation of endogenous HO, or by systemic antioxidants, alleviates long-term cerebral vascular complications of seizures.
Endothelial dysfunction and disruption of the BBB properties is intimately linked to neuronal activity in the seizing brain (Marchi et al, 2011). Whereas seizures may lead to BBB dysfunction, BBB failure may also lead to seizures. In our model, seizures are initiated by bicuculline, which targets the glutamatergic neurons, thus initiating a burst of neuronal activation within 1 to 2 minutes and a corresponding increase in cerebral blood flow that supports neuronal metabolism (Parfenova et al, 2004). Cerebrovascular damage leading to a loss of endothelium-dependent and -independent dilator functions occurs not earlier than 2 to 4 hours after seizure onset (Carratu et al, 2003; Parfenova et al, 2010). Therefore, in our experimental model, seizures are the cause, and postictal cerebral vascular dysfunction is the consequence of epileptic seizures.
Which cellular targets in the neurovascular unit define the cerebroprotective effects of antioxidants? Previously, we demonstrated that antioxidants protect cultured cerebral vascular endothelial cells from damage by seizure-related injurious factors (Basuroy et al, 2006, 2009; Parfenova et al, 2006, 2010). We addressed the possibility that cerebroprotective effects of antioxidants may result from their potential anticonvulsant actions. The question of whether antioxidants have anticonvulsant potencies has not yet been addressed in the literature. Previously, we demonstrated that SnPP did not affect the basal brain electrical activity and had no effect on EEG amplitude or the duration of bicuculline-induced seizures in newborn piglets (Parfenova et al, 2004). Because SnPP greatly increases the brain ROS during seizures, these data suggest that oxidants do not have anticonvulsant effects. In our present experiments, we used seizure-induced tachycardia as a well-established approach for detection of seizure activity that correlates with the dynamics of neuronal discharges (Greene et al, 2006; Opherk et al, 2002; Parfenova et al, 2004; van Elmpt et al, 2006; Shah et al, 2010; Zijlmans et al, 2002). We found no significant differences in the heart rate in antioxidant-treated (tiron, apocynin, CoPP, CORM-A1, and bilirubin) or prooxidant-treated (SnPP) groups, suggesting that ROS have no effects on neuronal activity during seizures.
We present novel data that constitutive HO-2 activity in the neonatal brain is activated via a ROS-dependent posttranslational mechanism. Seizures cause a rapid increase in the endogenous CO production in cerebral circulation via activation of constitutive HO-2 without increasing HO-2 protein or inducing HO-1 expression (Carratu et al, 2003; Parfenova et al, 2005). Posttranslational activation of HO-2 by glutamate is also observed in isolated cerebral vessels and cortical astrocytes (Leffler et al, 2006; Parfenova and Leffler, 2008). The antioxidants tiron and apocynin blocked elevation of the brain CO production during seizures, suggesting that activation of cerebroprotective HO-2 during seizures is redox-dependent.
Overall, ROS elevation in the seizing neonatal brain has dual effects on cerebrovascular functions. On one hand, ROS have damaging effects and cause long-term loss of postictal cerebral vasodilator functions. On the other hand, ROS activate endogenous HO-2 thus initiating a cerebroprotective mechanism. Antioxidants products of HO activity, CO and bilirubin, prevent long-term loss of cerebrovascular dilator functions during the postictal period. Because promoting cerebrovascular health is critical for preserving normal neuronal function by delivering nutrients and oxygen and protecting the brain from blood-borne hazardous substances, these findings suggest therapeutic potential of antioxidants in the treatment of seizures. Understanding the mechanism of cerebral vascular oxidative stress in the seizing brain may lead to the development of effective antioxidant therapeutic strategies to prevent the cerebral vascular injury and loss of cerebral blood flow regulation that may aggravate neuronal damage.
The authors declare no conflict of interest.
References
- Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-a in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2009;296:C422–C432. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. Heme oxygenase-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2006;291:C897–C808. doi: 10.1152/ajpcell.00032.2006. [DOI] [PubMed] [Google Scholar]
- Belanger S, Lavoie JC, Chessex P. Influence of bilirubin on the antioxidant capacity of plasma in newborn infants. Biol Neonate. 1997;71:233–238. doi: 10.1159/000244422. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Jordan J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: selective imaging with hydroethidine. J Neurosci. 1996;16:1324–1336. doi: 10.1523/JNEUROSCI.16-04-01324.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H397–H407. doi: 10.1152/ajpheart.00679.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carratu P, Pourcyrous M, Fedinec A, Leffler CW, Parfenova H. Endogenous heme oxygenase prevents impairment of cerebral vascular functions caused by seizures. Am J Physiol Heart Circ Physiol. 2003;285:H1148–H1157. doi: 10.1152/ajpheart.00091.2003. [DOI] [PubMed] [Google Scholar]
- Chan PH, Kawase M, Murakami K, Chen SF, Li Y, Calagui B, Reola L, Carlson E, Epstein CJ. Overexpression of SOD1 in transgenic rats protects vulnerable neurons against ischemic damage after global cerebral ischemia and reperfusion. J Neurosci. 1998;18:8292–8299. doi: 10.1523/JNEUROSCI.18-20-08292.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy RR. Summary proceedings from the neurology group on neonatal seizures. Pediatrics. 2006;117:S23–S27. doi: 10.1542/peds.2005-0620D. [DOI] [PubMed] [Google Scholar]
- Davies RE, Keohane SJ. Early changes in light-irradiated solutions of bilirubin: a spectrophotometric analysis. Photochem Photobiol. 1973;17:303–312. doi: 10.1111/j.1751-1097.1973.tb06358.x. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Paterniti I, Esposito E, Bramanti P, Cuzzocrea S. Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res. 2011;1372:92–102. doi: 10.1016/j.brainres.2010.11.088. [DOI] [PubMed] [Google Scholar]
- Greene BR, de Chazal P, Boylan G, Reilly RB, O'Brien C, Connolly S. Heart and respiration rate changes in the neonate during electroencephalographic seizure. Med Biol Eng Comput. 2006;44:27–34. doi: 10.1007/s11517-005-0001-5. [DOI] [PubMed] [Google Scholar]
- Güneş S, Dirik E, deg; U, Seçkin E, Kuralay F, Köse S, Unalp A. Oxidant status in children after febrile seizures. Pediatr Neurol. 2009;40:47–49. doi: 10.1016/j.pediatrneurol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Hall DJ, Han SH, Chepetan A, Inui EG, Rogers M, Dugan LL. Dynamic optical imaging of metabolic and NADPH oxidase-derived superoxide in live mouse brain using fluorescence lifetime unmixing. J Cereb Blood Flow Metab. 2012;32:23–32. doi: 10.1038/jcbfm.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TW. Mechanisms of bilirubin toxicity: clinical implications. Clin Perinatol. 2002;29:765–778. doi: 10.1016/s0095-5108(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Hegyi T, Goldie E, Hiatt M. The protective role of bilirubin in oxygen-radical diseases of the preterm infant. J Perinatol. 1994;14:296–300. [PubMed] [Google Scholar]
- Jackman KA, Miller AA, De Silva TM, Crack PJ, Drummond GR, Sobey CG. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br J Pharmacol. 2009;156:680–688. doi: 10.1111/j.1476-5381.2008.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooli A, Kermorvant-Duchemin E, Sennlaub F, Bossolasco M, Hou X, Honoré JC, Dennery PA, Sapieha P, Varma D, Lachapelle P, Zhu T, Tremblay S, Hardy P, Jain K, Balazy M, Chemtob S. Arachidonic acids induce a heme oxygenase-dependent vasorelaxation of cerebral microvasculature. Free Radic Biol Med. 2008;44:815–825. doi: 10.1016/j.freeradbiomed.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Kunz A, Anrather J, Zhou P, Orio M, Iadecola C. Cyclooxygenase-2 does not contribute to postischemic production of reactive oxygen species. J Cereb Blood Flow Metab. 2007;27:545–551. doi: 10.1038/sj.jcbfm.9600369. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol. 2006;291:H2897–H2904. doi: 10.1152/ajpheart.00722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Tierney W, Alexopoulos AV, Puvenna V, Granata T, Janigro D. The etiological role of blood--brain barrier dysfunction in seizure disorders. Cardiovasc Psychiatry Neurol. 2011;2011:482415. doi: 10.1155/2011/482415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS. Concept of activity-induced cell death in epilepsy: historical and contemporary perspectives. Prog Brain Res. 2002;135:3–11. doi: 10.1016/S0079-6123(02)35003-9. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res. 2002;52:117–127. doi: 10.1016/s0920-1211(02)00215-2. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol. 2006;290:C1399–C1410. doi: 10.1152/ajpcell.00386.2005. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Carratu P, Tcheranova D, Fedinec A, Pourcyrous M, Leffler CW. Epileptic seizures cause extended postictal cerebral vascular dysfunction that is prevented by HO-1 overexpression. Am J Physiol Heart Circ Physiol. 2005;288:H2843–H2850. doi: 10.1152/ajpheart.01274.2004. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Daley ML, Carratu P, Leffler CW. Heme oxygenase inhibition reduces neuronal activation evoked by bicuculline in newborn pigs. Brain Res. 2004;1014:87–96. doi: 10.1016/j.brainres.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Parfenova H, Leffler CW. Cerebroprotective functions of HO-2. Curr Pharm Des. 2008;14:443–453. doi: 10.2174/138161208783597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenova H, Leffler CW, Tcheranova D, Basuroy S, Zimmermann A. Epileptic seizures increase circulating endothelial cells in peripheral blood as early indicators of cerebral vascular damage. Am J Physiol Heart Circ Physiol. 2010;298:H1687–H1698. doi: 10.1152/ajpheart.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. 2004;37:1951–1962. doi: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Pestana RR, Kinjo ER, Hernandes MS, Britto LR. Reactive oxygen species generated by NADPH oxidase are involved in neurodegeneration in the pilocarpine model of temporal lobe epilepsy. Neurosci Lett. 2010;484:187–191. doi: 10.1016/j.neulet.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Pourcyrous M, Leffler CW, Bada HS, Korones SB, Stidham GL, Busija DW. Effects of pancuronium bromide on cerebral blood flow changes during seizures in newborn pigs. Pediatr Res. 1992;31:636–639. doi: 10.1203/00006450-199206000-00019. [DOI] [PubMed] [Google Scholar]
- Sandouka A, Balogn E, Foresti R, Mann BE, Johnson TR, Tayem Y, Green CJ, Fuller B, Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) modulate respiration in isolated mitochondria. Cell Mol Biol (Noisy-le-grand) 2005;51:425–32. [PubMed] [Google Scholar]
- Sedlak TW, Snyder SH. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics. 2004;113:1776–1782. doi: 10.1542/peds.113.6.1776. [DOI] [PubMed] [Google Scholar]
- Shah DK, Zempel J, Barton T, Lukas K, Inder TE. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–106. doi: 10.1203/PDR.0b013e3181bf5914. [DOI] [PubMed] [Google Scholar]
- Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 2004;6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- Tang XN, Cairns B, Cairns N, Yenari MA. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience. 2008;154:556–562. doi: 10.1016/j.neuroscience.2008.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elmpt WJ, Nijsen TM, Griep PA, Arends JB. A model of heart rate changes to detect seizures in severe epilepsy. Seizure. 2006;15:366–375. doi: 10.1016/j.seizure.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22:797–780. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: prevalence and definition of an objective clinical sign. Epilepsia. 2002;43:847–854. doi: 10.1046/j.1528-1157.2002.37801.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann A, Leffler CW, Tcheranova D, Fedinec AL, Parfenova H. Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol. 2007;293:H2501–H2507. doi: 10.1152/ajpheart.00354.2007. [DOI] [PubMed] [Google Scholar]