Abstract
We investigated mechanisms by which circulating factors during hyperglycemic (HG) stroke affect cerebrovascular function and the role of peroxynitrite in stroke outcome. Middle cerebral arteries (MCAs) were isolated from male Wistar rats and perfused with plasma from rats that were hyperglycemic for 5 to 6 days by streptozotocin and underwent either MCA occlusion (HG MCAO) or Sham surgery (HG Sham) compared with MCA perfused with physiologic saline (No plasma). Myogenic responses and endothelial function were compared in untreated MCA (n=8/group) or with inhibitors of NADPH oxidase (apocynin; n=8), peroxynitrite (FeTMPyP; n=8) or endothelin-1 (ET-1)A (BQ-123; n=8). Finally, animals were treated in vivo before reperfusion after mild (<68% cerebral blood flow (CBF) decrease) or severe (>68% CBF decrease) MCAO with FeTMPyP (n=12) or vehicle (n=12) and CBF and infarction measured. The HG MCAO plasma increased tone in MCA versus No plasma (P<0.05) that was reversed by FeTMPyP, but not by apocynin or BQ-123. The HG Sham plasma also increased tone in MCA (P<0.05) that was reversed by BQ-123 only. In vivo, FeTMPyP was neuroprotective during mild, but not severe ischemia. These results show that circulating factors in plasma can affect cerebrovascular function through peroxynitrite generation and ET-1. In addition, peroxynitrite decomposition improves stroke outcome acutely during mild, but not severe HG ischemia.
Keywords: circulating factors, hyperglycemic stroke, middle cerebral artery, peroxynitrite, rats, vascular tone
Introduction
Hyperglycemia is present in up to 40% of ischemic stroke patients, often without a preexisting diagnosis of diabetes (Williams et al, 2002). Elevated blood glucose, and not necessarily diabetic complications, is associated with significantly worsened outcome including larger infarction, edema formation, and a higher risk of mortality (Capes et al, 2001; Alvarez-Sabin et al, 2003). The mechanisms by which hyperglycemia worsens stroke outcome are likely multifactorial. Hyperglycemia has direct effects on the vasculature with vasoactive mediators and second messengers produced under hyperglycemic (HG) conditions that can worsen stroke outcome. For example, plasma levels of endothelin-1 (ET-1), a potent vasoconstrictor, are elevated in streptozotocin-induced diabetic rats (Makino and Kamata, 1998) as well as in patients and animal models after ischemic stroke (Barone et al, 1994; Ziv et al, 1992). Hyperglycemia is also associated with increased oxidative stress that exacerbates cerebral injury (Bemeur et al, 2007), possibly due to protein kinase C activation that enhances vasoreactivity in addition to promoting oxidative stress (Cipolla et al, 2011).
One largely unexplored influence on stroke injury is the production and release of proinflammatory circulating factors. Clinical stroke and experimental cerebral ischemia elicit an inflammatory response that involves activation and release of cytokines, chemokines, endothelial-leukocyte adhesion molecules, and proteolytic enzymes that exacerbate ischemic injury (Denes et al, 2010). Circulating levels of proinflammatory mediators such as interleukin-6, tumor necrosis factor-α, and interferon gamma have been shown to be increased in plasma from stroke patients and cerebral ischemic animals, which could enhance brain infarct volume (Fassbender et al, 1994; Offner et al, 2006). The effect of these proinflammatory factors has been largely studied in brain tissue; however, because they are also vasoactive and circulating, they may influence vascular function and cerebral blood flow (CBF) in nonischemic tissue. Thus, the first goal of this study was to determine the influence of circulating factors during HG stroke on vascular reactivity and endothelial function by perfusing nonischemic middle cerebral arteries (MCA) intraluminally with plasma from acutely HG rats that underwent MCA occlusion (MCAO) for 2 hours with 2 hours of reperfusion or Sham surgery.
In addition to circulating factors produced in response to ischemia and reperfusion, the hypoxic brain vascular tissue synthesizes proinflammatory cytokines that promote adhesion of circulating neutrophils to endothelial cells and generation of reactive oxygen and nitrogen species such as superoxide, nitric oxide (NO), and peroxynitrite (Shreeniwas et al, 1992). Superoxide can impair vascular NO-mediated relaxation (Jimenez-Altayo et al, 2006) and damage cell membranes by inducing lipid peroxidation (Bromont et al, 1989). Further, excessive NO produced by NO synthase (NOS) isoforms during ischemia combines rapidly with superoxide during reperfusion to form peroxynitrite. Peroxynitrite decreases the effectiveness of NO and interacts with lipids, DNA, and proteins to cause damage to the vasculature (Iadecola, 1997; Maneen et al, 2006). Reactive oxygen and nitrogen species such as superoxide and peroxynitrite are important therapeutic targets for ischemic stroke, especially during hyperglycemia when oxidative stress is high (Bemeur et al, 2007). Thus, another goal of this study was to investigate the role of reactive oxygen and nitrogen species as underlying mechanisms by which MCAO plasma is vasoactive by using pharmacological inhibitors of NAD(P)H oxidase and peroxynitrite. We hypothesized that proinflammatory circulating factors present in MCAO plasma would interact with the recipient endothelium to produce superoxide that readily combines with basal NO to form peroxynitrite, a potent and vasoactive reactive oxygen and nitrogen species (Maneen et al, 2006). Finally, we also determined if treatment of HG MCAO animals in vivo with 5,10,15,20-tetrakis (N-methyl-4′-pyridyl) porphinato iron (III) chloride (FeTMPyP), a peroxynitrite decomposition catalyst, could improve reperfusion CBF and infarction.
Materials and methods
Animal Model of Transient Focal Ischemia and Plasma Samples
All procedures were approved by the Institutional Animal Care and Use Committee and complied with the NIH guidelines for the care and use of laboratory animals. Male Wistar rats (∼300 g) were used for all experiments. The MCAO model of focal ischemia was used in HG rats to obtain plasma, as previously described (Cipolla and Godfrey, 2010; Cipolla et al, 2011). Animals were made HG by a single intraperitoneal injection of streptozotocin (50 mg/kg) 5 to 6 days before MCAO, as previously described (Cipolla and Godfrey, 2010). Glucose was measured on the day of the surgery by a commercially available glucose monitor (Freestyle Lite; Abbott, Abbott Park, IL, USA). Ischemic animals were exposed to 2 hours of ischemia and 2 hours of reperfusion by suture removal. The HG Sham control animals underwent anesthesia and a midline incision without filament occlusion. To determine the effect of hyperglycemia on vascular function, another set of experiments was conducted with plasma from normoglycemic (NG) Sham animals and NG näive animals (no surgery). Animals were anesthetized with isoflurane in oxygen and blood gases were maintained within normal ranges. Laser Doppler was used to measure changes in CBF from baseline, including both the decrease in CBF during occlusion and the extent of reperfusion after suture removal.
Plasma from NG naïve, NG Sham, HG Sham, and HG MCAO animals was collected for perfusion of nonischemic MCA during isolated vessel experiments. Plasma was obtained from trunk blood and collected into vacutainer tubes containing heparin. Blood was centrifuged at 1,400 to 1,600 g, the plasma removed, aliquoted, pooled from five animals and then frozen at −80°C until experimentation.
Preparation of Cerebral Vessels and Pressurized Arteriograph System
To investigate the effect of plasma on vascular function, we used MCA from animals that were normoglycemic and nonischemic. Animals were anesthetized with inhaled isoflurane (3% in air), decapitated, and the brain quickly removed and placed in cold, oxygenated physiologic saline solution (PSS). MCA was taken from the M2 region, mounted on glass cannulas in an arteriograph chamber and perfused with PSS for control vessels (No plasma, n=8), plasma (20% in PSS) obtained from HG Sham animals (HG Sham, n=8) or HG MCAO animals (HG MCAO, n=8). Separate sets of MCA were perfused with NG naïve plasma (NG naive, n=8) or NG Sham plasma (NG Sham, n=8). Isolated vessel experiments were conducted as previously described (Cipolla and Curry, 2002; Cipolla et al, 2011). Briefly, MCA was equilibrated for 2 hours at 75 mm Hg with plasma or PSS in the lumen. Intravascular pressure was then increased to 175 mm Hg in 25 mm Hg increments and diameter recorded at each pressure once stable. Once active pressure-diameter curves were obtained, pressure was decreased to 75 mm Hg for the rest of the experiment. The NOS inhibitor nitro-L-arginine (L-NNA, 0.1 mmol/L) was added to the bath and the vasoconstriction recorded once stable. Finally, papaverine (0.1 mmol/L) and diltiazem (10 μmol/L) were added to the bath to fully relax the vessels. Pressure was then increased to 175 mm Hg and decreased in 25 mm Hg increments to 50 mm Hg and then in 10 mm Hg increments to 5 mm Hg to obtain passive diameter measurements at each point and to calculate percent tone.
To determine the effect of plasma on receptor-mediated endothelial function, another set of experiments was conducted using acetylcholine (ACh) instead of L-NNA. Reactivity to ACh was determined by commutative addition of ACh (0.1 to 10.0 μmol/L) to the arteriograph bath and measurement of lumen diameter at each concentration once stable.
To investigate the role of ET-1, superoxide, and peroxynitrite in mediating vascular changes in response to plasma, separate sets of MCA were perfused with specific inhibitors of ET-1A receptors (BQ-123), ET-1B receptors (BQ-788), NAD(P)H oxidase (apocynin), or a peroxynitrite decomposition catalyst (FeTMPyP) in addition to the plasma. The above protocol was then repeated with MCA perfused with HG Sham plasma+BQ-123 (1 μmol/L, n=8), HG Sham plasma+FeTMPyP (50 μmol/L, n=6), HG MCAO plasma+apocynin (100 μmol/L, n=8), HG MCAO plasma+FeTMPyP (50 μmol/L, n=8), HG MCAO plasma+BQ-788 (1 μmol/L, n=8), or HG MCAO plasma+BQ-123 (1 μmol/L, n=6) and compared with untreated HG MCAO plasma in the lumen of MCA. For information on specificity and efficacy of these inhibitors, and the concentrations used, please see Supplementary Materials.
Peroxynitrite Decomposition Catalyst Treatment In Vivo During MCAO and Measurement of CBF and Brain Infarct Volume
Separate sets of HG animals underwent MCAO for measurement of acute injury volume using 2,3,5-triphenyltetrazolium chloride staining. All animals underwent 2 hours of ischemia and 2 hours of reperfusion. Ten minutes before reperfusion, animals were infused intravenously via femoral catheter with 10 mg/kg FeTMPyP to decompose peroxynitrite or with vehicle (saline), as previously described (Cipolla et al, 2011). Blood gases and pH were maintained within normal ranges (Table 1). CBF was continuously measured using laser Doppler flowmetry (Perimed Periflux 5010 Laser Doppler System, North Royalton, OH, USA), as previously described (Cipolla et al, 2001; Cipolla and Godfrey, 2010). Briefly, the flow probe was affixed over a thinned area of skull posterior to the coronal suture and lateral to the sagittal suture over the MCA perfusion domain. At the end of the reperfusion period, the animals were decapitated and the brains removed for measurement of acute infarction. Briefly, brains were removed and sliced into 2 mm coronal sections. The brain slices were incubated in 2% 2,3,5-triphenyltetrazolium chloride in 1 × phosphate-buffered saline for 30 minutes at 36.5°C to stain for infarction. The brain slices were subsequently fixed in 3.7% phosphate-buffered saline-buffered formalin for 30 minutes at 4°C for imaging. Images were captured using a digital scanner and analyzed for acute injury volume corrected for brain edema with ImageJ software (NIH, Bethesda, MD, USA).
Table 1. Physiological characteristics of animals for in-vivo FeTMPyP treatment.
| Mild ischemia HG MCAO vehicle (n=6) | Mild ischemia HG MCAO 10 mg/kg FeTMPyP (n=6) | Severe ischemia HG MCAO vehicle (n=6) | Severe ischemia HG MCAO 10 mg/kg FeTMPyP (n=6) | |
|---|---|---|---|---|
| Blood glucose (mg/dL) | 310±24 | 327±24 | 252±13 | 248±25 |
| Weight (g) | 326±16 | 340±15 | 329±13 | 336±18 |
| CBF % decrease versus basal | −47±3 | −51±5 | −80±4 | −77±3 |
| CBF % recovery versus basal | 129±83 | 78±50 | −10±23 | 5±23 |
| Arterial blood gases start | ||||
| pH | 7.51±0.02 | 7.50±0.02 | 7.49±0.01 | 7.49±0.02 |
| pCO2 (mm Hg) | 27.7±1.7 | 27.8±2.2 | 26.4±0.9 | 27.6±1.4 |
| pO2 (mm Hg) | 119±11 | 174±21 | 110±6 | 107±8 |
| Arterial blood gases middle | ||||
| pH | 7.46±0.02 | 7.45±0.02 | 7.43±0.02 | 7.45±0.02 |
| pCO2 (mm Hg) | 34.2±1.4 | 37.7±3.5 | 39.3±2.4 | 36.9±2.5 |
| pO2 (mm Hg) | 103±5 | 101±17 | 100±10 | 97±5 |
| Arterial blood gases end | ||||
| pH | 7.37±0.04 | 7.39±0.03 | 7.42±0.03 | 7.39±0.02 |
| pCO2 (mm Hg) | 47.6±3.9 | 41.7±3.0 | 39.7±2.9 | 41.8±3.1 |
| pO2 (mm Hg) | 109±10 | 102±14 | 117±8 | 113±7 |
CBF, cerebral blood flow; HG MCAO, hyperglycemic middle cerebral artery occlusion.
Drugs and Solutions
In-vitro experiments were conducted in a bicarbonate-based PSS, the ionic composition was (mmol/L): NaCl 119.0, NaHCO3 24.0, KCl 4.7, KH2PO4 1.18, MgSO4.7H2O 1.17, CaCl2 1.6, EDTA 0.026, and glucose 5.5. PSS was made each week and stored without glucose at 4°C. Glucose was added to the PSS before each experiment. The PSS was aerated with 5% CO2, 10% O2, and 85% N2 to maintain pH. L-NNA, ACh, papaverine, apocynin, BQ-788, 2,3,5-triphenyltetrazolium chloride, and formalin were purchased from Sigma (St Louis, MO, USA). BQ-123 was purchased from Tocris (Ellisville, MO, USA), FeTMPyP from Calbiochem (La Jolla, CA, USA), and diltiazem from MP Biomedicals (Solon, OH, USA).
Data Calculations and Statistical Analysis
Percent tone and constriction to L-NNA were calculated as previously described (Cipolla and Curry, 2002; Cipolla and Godfrey, 2010). Reactivity to ACh was calculated as a percent dilation from baseline diameter with tone. Reperfusion CBF was determined from laser Doppler units as a percent change from baseline CBF. Acute infarct volume was corrected for brain edema and calculated as previously described (Shimakura et al, 2000). Results are presented as mean±s.e.m. Differences between individual groups were determined using one-way analysis of variance with a post hoc Student–Newman–Keuls test for multiple comparisons, where appropriate. For in-vivo analysis of infarction and reperfusion blood flow, two-way analysis of variance was used to compare two independent variables: FeTMPyP treatment and severity of ischemia and their interaction. Differences were considered significant when P<0.05.
Results
Effect of HG MCAO Plasma Perfusate on Vascular Tone and Endothelial Function in Nonischemic MCA
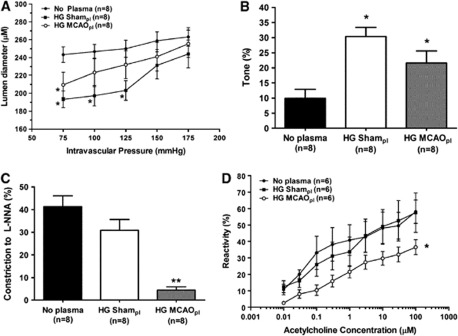
Because circulating factors during cerebral ischemia may be vasoactive and influence vascular tone of normal tissue outside of the ischemic territory, we investigated the effect of HG MCAO plasma on vascular function in nonischemic MCA. Figure 1A shows changes in active lumen diameter over a range of pressures in MCA perfused with PSS alone (No plasma) or with plasma from HG rats that underwent either Sham (HG Sham plasma) or MCAO procedure (HG MCAO plasma). Compared with No plasma, arteries perfused with either HG MCAO or HG Sham plasma had smaller lumen diameters at pressures within the myogenic pressure range (75 to 125 mm Hg). Figure 1B shows that the smaller lumen diameters in HG Sham and HG MCAO plasma vessels were due to an increase in basal myogenic tone, suggesting that circulating factors present in the plasma were vasoactive and increased tone in vitro.
Figure 1.
Effect of hyperglycemic middle cerebral artery occlusion (HG MCAO) plasma perfusion on vascular tone and endothelial function in nonischemic middle cerebral arteries (MCA). (A) Active lumen diameter versus pressure of nonischemic MCA perfused with physiologic saline solution (PSS) (No plasma), HG Sham or HG MCAO plasma (pl). Arteries perfused with either plasma type had smaller lumen diameters actively compared with No plasma. (B) Graph showing percent tone of nonischemic MCA perfused with PSS (No plasma), HG Sham, or HG MCAO plasma. HG Sham and HG MCAO plasma vessels had significantly increased myogenic tone compare with No plasma, suggesting that the smaller lumen diameters was due to increased tone. (C) Percent constriction to a single concentration of the nitric oxide synthase (NOS) inhibitor (L-NNA, 0.1 mmol/L) in nonischemic MCA perfused with PSS (No plasma), HG Sham, or HG MCAO plasma. Constriction to L-NNA was decreased in the HG MCAO plasma group only compared with No plasma. (D) Graph showing percent reactivity to acetylcholine (ACh) (0.1 to 10.0 μmol/L) under the same experimental conditions described above. ACh-induced vasodilation was decreased only in the HG MCAO plasma group, suggesting that this plasma was increasing tone by decreasing the influence to nitric oxide (NO) on tone. *P<0.05 and **P<0.01 versus No plasma. L-NNA, NOS inhibitor nitro-L-arginine.
Because plasma was perfused intraluminally only, we determined if the increase in myogenic tone found in arteries perfused with HG Sham and HG MCAO plasma was due to circulating factors that affected the endothelium by inhibiting NO. Both the contractile response to a single concentration of the NOS inhibitor L-NNA (0.1 mmol/L) and the dilation to commutative doses of ACh (0.1 to 10.0 μmol/L) were compared in response to the different plasmas. Despite increased tone in both plasma groups, constriction to L-NNA (Figure 1C) and ACh-induced vasodilation (Figure 1D) were decreased only in the HG MCAO plasma group, suggesting that this plasma was increasing tone by decreasing the vasodilatory influence to NO on tone. However, HG Sham plasma increased tone in MCA despite similar responses to L-NNA and ACh as No plasma, suggesting that the increased tone present in MCA perfused with HG Sham plasma was due a vasoconstrictor present or produced in response to the plasma.
Effect of Hyperglycemia on Vascular Function in MCA
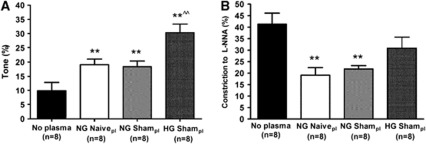
To determine if vasoactive circulating factors produced during exsanguination were responsible for the increase in tone found in MCA perfused with either type of HG plasma, we measured the effect of NG Sham plasma and NG naïve plasma (no surgery) on vascular function in nonischemic MCA. Figure 2A shows that arteries perfused with either NG Sham or NG naïve plasma also had increased tone compared with No plasma, but not to the same extent as HG Sham plasma. Moreover, the percent constriction to L-NNA was decreased with both NG naïve and NG Sham plasmas (Figure 2B), a result that was distinct from HG Sham plasma. Thus, factors produced during exsanguination or the presence of plasma proteins may contribute to some of the increase in tone in response to HG Sham plasma, but there are clearly unique effects of this plasma not due to exsanguination.
Figure 2.
Effect of hyperglycemia on vascular tone and endothelial function in nonischemic middle cerebral arteries (MCA). (A) Graph showing percent tone of nonischemic MCA perfused with physiologic saline solution (PSS) (No plasma), normoglycemic (NG) naïve (no surgery), NG Sham, or hyperglycemic (HG) Sham plasma (pl). All vessels perfused with plasma had significantly increased tone compared with No Plasma. However, HG Sham plasma had significantly increased tone versus both NG naïve and NG Sham plasmas. (B) Percent constriction to a single concentration of the nitric oxide synthase (NOS) inhibitor (L-NNA, 0.1 mmol/L) under the same experimental conditions described in panel A. Constriction to L-NNA was decreased with NG naïve and NG Sham plasmas only compared with No plasma. **P<0.01 versus No plasma; ^^P<0.01 versus NG naïve and NG Sham plasmas. L-NNA, NOS inhibitor nitro-L-arginine.
Role of Endothelin-1 on Vascular Tone of MCA Perfused with HG Sham Plasma
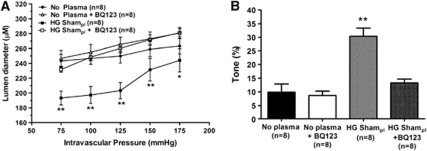
Because the increase in tone with HG Sham plasma did not appear to be associated with endothelial dysfunction or exsanguination, we investigated whether there was a vasoconstrictor present in the plasma that was increasing tone. To determine if the increase in myogenic tone found in the HG Sham plasma group was caused by the potent vasoconstrictor ET-1 that has been shown to be elevated during hyperglycemia and diabetes (Makino and Kamata, 1998), we perfused arteries with HG Sham plasma+1 μmol/L BQ-123, a selective ET-1A receptor antagonist. Figures 3A and 3B show that inhibition of ETA receptors in HG Sham plasma restored active diameters and myogenic tone to No plasma levels, suggesting that circulating ET-1, through activation of ETA receptors, is responsible for the increase in tone after exposure to HG Sham plasma. BQ-123 had no effect on tone in vessels perfused with PSS alone (No plasma), suggesting that ET-1 does not appear to be basally produced in normal MCA.
Figure 3.
Influence of endothelin-1 (ET-1) on active diameters and myogenic tone in middle cerebral arteries (MCA) perfused with hyperglycemic (HG) Sham plasma. (A) Active lumen diameter versus pressure of nonischemic MCA perfused with physiologic saline solution (PSS) (No plasma), No plasma+1 μmol/L BQ-123, HG Sham plasma (pl), and HG Sham plasma+1 μmol/L BQ-123. Inhibition of ET-1A receptors with BQ-123 had no effect on MCA perfused with PSS alone (No plasma), but restored diameter to that of No plasma when added to HG Sham plasma. (B) Graph showing percent of tone of nonischemic MCA in the same groups as in panel A. BQ-123 prevented the increased in myogenic tone caused by the HG Sham plasma, suggesting that activation of ET-1A receptors by either circulating or stimulated ET-1 in HG Sham plasma was responsible for the increased tone. *P<0.05 and **P<0.01 versus No plasma.
To determine whether the increase in tone with HG Sham plasma was related to a direct effect of glucose, nonischemic MCA was perfused with NG Sham plasma+300 mg/dL glucose (the concentration similar to in-vivo levels). We found that intraluminal exposure to glucose significantly increased tone compared with NG Sham plasma alone. The percent tone in NG Sham plasma was 18±2% versus 48±7% in NG Sham plasma+300 mg/dL glucose (n=8/group; P⩽0.001). However, the underlying mechanism by which glucose increased tone was different than with the HG Sham plasma because the increase in tone could not be prevented by BQ-123. The percent in tone in NG Sham plasma+300 mg/dL glucose+1 μmol/L BQ-123 was 45±3% (n=7; P>0.05). Thus, while high glucose exposure acutely increased tone, it appears there are other vasoactive effects of plasma when hyperglycemia is systemic.
Role of Superoxide and Peroxynitrite in Mediating Changes in Vascular Function of MCA Perfused with HG MCAO Plasma
The HG MCAO plasma was associated with endothelial dysfunction (e.g., decreased ACh dilation) that could be responsible for the increased tone in those vessels. Previous studies have shown that HG cerebral ischemia results in increased oxidative stress (Bemeur et al, 2007) that can decreases the bioavailability of NO through production of superoxide to form peroxynitrite (Chrissobolis et al, 2011). We therefore investigated the role of superoxide and peroxynitrite in mediating reactivity changes found in MCA perfused intraluminally with HG MCAO plasma. Because superoxide and peroxynitrite are highly reactive and not circulating, we hypothesized that circulating factors present in plasma stimulate recipient endothelium to produce superoxide and peroxynitrite. Further, because vascular production of superoxide is mainly via NAD(P)H oxidase (Griendling et al, 2000), we treated arteries with the NAD(P)H inhibitor apocynin in addition to HG MCAO plasma. Perfusion of arteries with HG MCAO plasma+apocynin (100 μmol/L) did not prevent the decrease in lumen diameter (Figure 4A) or the increase in tone (Figure 4C) in response to plasma. Figure 4D shows that apocynin also did not affect the response to L-NNA, suggesting that NAD(P)H oxidase-generated superoxide does not have an important role in the increased tone of MCA perfused with HG MCAO plasma. In contrast, perfusion of MCA with HG MCAO plasma+50 μmol/L FeTMPyP, a peroxynitrite decomposition catalyst, prevented the decrease in lumen diameter (Figure 4B) and the increase in tone (Figure 4C) in response to HG MCAO plasma. However, FeTMPyP only partially restored the contraction to L-NNA despite decreasing tone, suggesting that peroxynitrite generated in response to HG MCAO plasma may act as a vasoconstrictor.
Figure 4.
Influence of superoxide and peroxynitrite on reactivity changes and endothelial dysfunction in middle cerebral arteries (MCA) perfused with hyperglycemic MCA occlusion (HG MCAO) plasma. (A) Active lumen diameter versus pressure of nonischemic MCA perfused with physiologic saline solution (PSS) (No plasma), HG MCAO plasma (pl) or HG MCAO plasma+100 μmol/L apocynin. Apocynin did not prevent the decrease in lumen diameter induced by HG MCAO plasma. (B) Active lumen diameter versus pressure of nonischemic MCA perfused with PSS (No plasma), HG MCAO plasma (pl), or HG MCAO plasma+50 μmol/L FeTMPyP. FeTMPyP completely reversed the decrease in lumen diameter induced by HG MCAO plasma. (C) Graph showing percent tone of nonischemic MCA perfused with PSS (No plasma), HG MCAO plasma (pl), HG MCAO plasma+100 μmol/L apocynin, or HG MCAO plasma+50 μmol/L FeTMPyP. Apocynin had little effect on myogenic tone; however, FeTMPyP prevented the increase in tone in response to HG MCAO plasma. (D) Graph showing percent tone in response to 0.1 mmol/L L-NNA under the same experimental conditions described in panel C. Neither apocynin nor FeTMPyP restored the response to L-NNA to No plasma levels, suggesting that the effect of FeTMPyP on decreasing tone in response to HG MCAO plasma may due to inhibiting vasoconstricting properties of peroxynitrite. *P<0.05 versus No plasma; **P<0.01 versus No plasma and ^P<0.05 versus HG MCAO plasma. L-NNA, NOS inhibitor nitro-L-arginine.
Because FeTMPyP was effective at ameliorating the increase in tone by HG MCAO plasma, we also tested whether this effect was specific to HG MCAO plasma or was involved in the increase in tone in arteries perfused with HG Sham plasma. Thus, we perfused MCA with HG Sham plasma+50 μmol/L FeTMPyP. We found that FeTMPyP did not affect the increase in tone induced by HG Sham plasma, suggesting that peroxynitrite is not involved in the response to HG Sham plasma. Percent tone in HG Sham plasma was 30±3% versus 34±5% for HG MCAO plasma+50 μmol/L FeTMPyP (n=8 and 6, respectively; P>0.05).
The improved myogenic tone in response to HG MCAO plasma with peroxynitrite decomposition suggests there were factors present in this plasma that were generating peroxynitrite through either superoxide or NO production. Because increased ET-1 levels in plasma have been shown in patients and animal models after ischemic stroke (Barone et al, 1994; Ziv et al, 1992) and endothelial ETB receptor activation induces NO-dependent relaxation (Kohan et al, 2011), we hypothesized that NO production induced by ETB receptor activation by HG MCAO plasma may be combining with superoxide to form peroxynitrite. Thus, we perfused arteries with HG MCAO plasma+1 μmol/L BQ-788, a selective ETB receptor antagonist. However, inhibition of ETB receptors did not affect myogenic tone or endothelial function, suggesting that ETB receptors do not have an important role in generating peroxynitrite with HG MCAO plasma. Percent tone in HG MCAO plasma was 22±4% versus 20±6% for HG MCAO plasma+1 μmol/L BQ-788 (n=8/group; P>0.05). Percent constriction to L-NNA in HG MCAO plasma was 5±2 versus 11±3 in HG MCAO plasma+1 μmol/L BQ-788 (P>0.05). We also tested whether ET-1 through activation of ETA receptors (Kohan et al, 2011) was involved in the increase in tone with HG MCAO plasma. Thus, we also perfused arteries with HG MCAO plasma+1 μmol/L BQ-123. BQ-123 did not ameliorate the increase in tone induced by HG MCAO plasma but increased it. The percent tone in HG MCAO plasma was 22±4% versus 41±3% for HG MCAO plasma+1 μmol/L BQ-123 (n=8 and 6, respectively; P<0.01). This result suggests that ET-1A receptors are not involved in increasing myogenic tone after exposure to HG MCAO plasma.
Peroxynitrite Decomposition During Hyperglycemic Middle Cerebral Artery Occlusion on Cerebral Blood Flow and Acute Brain Infarction
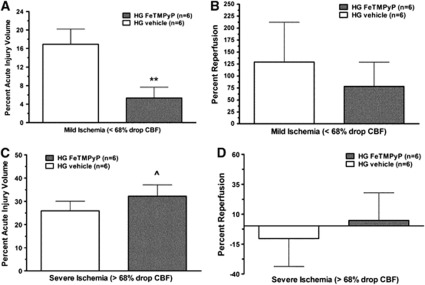
The in-vitro studies above suggested that peroxynitrite generation is an important contributor that increased myogenic tone in response to HG MCAO plasma in nonischemic MCA. Because increased tone may cause greater ischemia or decreased reperfusion within the peri-infarct region or altered perfusion in nonischemic brain regions, we next determined if peroxynitrite produced in vivo during MCAO affected stroke outcome. Thus, we treated HG MCAO animals after 2 hours ischemia with 10 mg/kg FeTMPyP 10 minutes before a 2-hour reperfusion. We also assessed whether FeTMPyP affected reperfusion blood flow that may improve stroke outcome. Our initial analysis found that treatment with FeTMPyP caused significant variability in infarct size. However, when we more closely analyzed the data, we found there was a threshold of ischemia that existed for acute infarction. For example, FeTMPyP was neuroprotective and decreased acute injury volume only in animals in which occlusion produced <68% decrease in CBF (mild ischemia; Figure 5A) but was not protective in animals where the occlusion induced >68% decrease in CBF causing more severe ischemia (severe ischemia; Figure 5C). Two-way analysis of variance revealed that the effect of FeTMPyP treatment on acute injury volume during mild ischemia was significantly different from that during severe ischemia such that infarct was considerably less with treatment only with mild ischemia. To determine if the neuroprotective effect of FeTMPyP was related to the degree of reperfusion, we measured the change in CBF after suture removal as a percent change from baseline blood flow before occlusion. Figure 5B shows that during mild ischemia, there was significant reperfusion CBF. However, FeTMPyP treatment did not affect reperfusion in this group, suggesting that the neuroprotective effects of FeTMPyP were not related to the extent of reperfusion. However, during severe ischemia where acute infarction was greater and FeTMPyP was not effective at reducing injury, there was little to no reperfusion (Figure 5D).
Figure 5.
Effect of FeTMPyP on reperfusion cerebral blood flow (CBF) and acute infarct volume during mild and severe hyperglycemic (HG) stroke. Graphs showing percent infarct volume in vehicle or FeTMPyP-treated animals after mild ischemia (<68% decrease; A) and severe ischemia (>68% decrease; C). FeTMPyP was neuroprotective and decreased infarct volume after mild ischemia but was not protective after more severe ischemia. Percent reperfusion (recovery of CBF calculated from baseline CBF using laser Doppler flowmetry) after 2 hours of ischemia and 2 hours of reperfusion after mild ischemia (<68% decrease; B) and severe ischemia (>68% decrease; D). There was considerable reperfusion after mild ischemia that was not affected by FeTMPyP treatment, suggesting that the neuroprotective effect of this compound shown in panel A was independent of an effect on CBF. However, after more severe ischemia in which FeTMPyP treatment was not effective at preventing acute brain injury, there was little to no reperfusion regardless of treatment, suggesting that the lack of effect in this group may be due to prevention of the compound reaching the target tissue. **P<0.01 versus HG vehicle; ^P<0.05 versus HG FeTMPyP after mild ischemia.
Discussion
The present study investigated the effect of circulating factors in plasma from HG rats that underwent MCAO for 2 hours with 2 hours of reperfusion on vascular function of nonischemic MCA. We found that arteries perfused intraluminally with HG MCAO plasma had increased myogenic tone and endothelial dysfunction compared with arteries perfused with PSS only, suggesting that circulating factors present in plasma in response to ischemia and reperfusion are vasoactive in nonischemic MCA. The significance of this vasoconstricting effect of stroke plasma in normal vasculature is not clear, but may contribute to expanding infarction or dysregulation of CBF at later time periods. However, scavenging peroxynitrite abolished the increase in myogenic tone and reduced acute infarction during mild, but not severe ischemia, suggesting that the neuroprotective effect of FeTMPyP depends of the severity of brain injury, at least in the acute setting. During mild ischemia, peroxynitrite decomposition did not change the extent of reperfusion CBF, suggesting that FeTMPyP improves stroke outcome through a neuroprotective mechanism independent of CBF. However, during more severe ischemia in which FeTMPyP was ineffective at preventing stroke damage, there was little reperfusion blood flow in either group, suggesting that the lack of a beneficial effect may be due to prevention of the agent from reaching the ischemic brain tissue.
It has been shown that cerebral ischemia and reperfusion, especially when combined with hyperglycemia, evokes generation of nitrogen and oxygen-free radicals that contribute to cerebral damage (Bemeur et al, 2007; Cipolla et al, 2011). Nitric oxide reacts with superoxide to produce peroxynitrite and most of the cytotoxic effects of NO are due to peroxynitrite (Pacher et al, 2007). Superoxide has also been shown to be important in the pathophysiology of ischemic brain damage (Chan et al, 1994); however, our results in vitro suggest that NAD(P)H oxidase-generated superoxide does not contribute significantly to the vasoconstricting effects caused by HG MCAO plasma. The lack of an effect of apocynin on MCA perfused with plasma may be due to different mechanisms of superoxide production than NAD(P)H oxidase, such as xanthine oxidase or mitochondrial production (Margaill et al, 2005). Alternatively, the concentration of apocynin used in these experiments may not have been high enough to be effective, although other studies have shown this concentration was able to effectively inhibit superoxide production from cerebral arteries (Mayhan et al, 2009). Despite apocynin not affecting plasma-induced vasoreactivity, generation of peroxynitrite appears to be an important contributor to increased myogenic tone in response to HG MCAO plasma. Because peroxynitrite decomposition did not completely reverse the decreased constriction to L-NNA, it is possible that this oxidant acts directly on the smooth muscle to induce vasoconstriction. In support of this, we previously reported that low-to-moderate concentrations of peroxynitrite caused constriction of cerebral arteries (Maneen et al, 2006).
A limitation of this study is that we did not determine what is circulating in stroke plasma that generates peroxynitrite by the recipient endothelium. The half-life of peroxynitrite is short, and thus it is not likely that peroxynitrite is present or circulating in the plasma, but that circulating factors present in the plasma are stimulating the recipient endothelial cells to generate superoxide, NO or both that then readily combine to produce peroxynitrite. Because ETB receptor activation induces NO-dependent relaxation (Kohan et al, 2011), we investigated the role of the ETB receptor as a possible NO source to form peroxynitrite. However, treatment with an ETB receptor antagonist did not have significant effect on vascular changes induced by HG MCAO plasma, suggesting that ETB receptors are not involved in increasing myogenic tone or endothelial dysfunction after exposure to HG MCAO plasma. There are other factors present in stroke plasma that can stimulate endothelial NO or superoxide, including proinflammatory cytokines (tumor necrosis factor-α, interleukin-6, and interferon gamma), growth factors (vascular endothelial growth factor), and hormones such as glucocorticoids (Kruyt et al, 2010; Duckles and Miller, 2010). Future studies are needed to determine exactly what factors are present in plasma that are involved in peroxynitrite generation in normal MCA.
Despite finding that FeTMPyP was effective at ameliorating the increase in myogenic tone induced by HG MCAO plasma, administration of FeTMPyP after 2 hours of MCAO and 10 minutes before reperfusion did not affect reperfusion CBF, but was able to decrease acute infarction in vivo after mild ischemia, suggesting that its beneficial effect was independent of changes in CBF. This apparent discrepancy may be because reperfusion blood flow was measured in the ischemic MCA territory, whereas the vasoconstricting effect of plasma was in nonischemic MCA. Increased production of peroxynitrite in the peri-infarct and core infarct regions have been reported after ischemic stroke, especially after reperfusion (Fukuyama et al, 1998). However, production of peroxynitrite from healthy endothelium after interacting with circulating factors may be through different or additional mechanisms than production in response to reperfusion within the infarcted territory. In addition, because we measured infarction after 2 hours of reperfusion, an acute time period, it is unclear from these studies what the final infarct may evolve into. Thus, we cannot exclude that the protection seen with the peroxynitrite scavenger was only temporary and that the effect of circulating factors on normal cerebral vascular tissue contributes to longer term outcome.
Several studies have used peroxynitrite decomposition catalysts during MCAO and found conflicting results. Thiyagarajan et al (2004), using a rat MCAO model, showed that treatment with the peroxynitrite decomposition catalysts with FeTMPyP or FeTPPS decreased infarct volume, brain edema, and neurologic deficits when administered at 2 and 6 hours after MCAO. Furthermore, Suofu et al (2010) found a neuroprotective role of FeTMPyP after cerebral ischemia and showed that peroxynitrite contributes to the neurovascular injury through matrix metalloproteinase activation. In contrast, one study showed that FeTPPS was ineffective at reducing infarct volume during MCAO (Kunz et al, 2007). From the results of the current study, it is possible that the apparent discrepancy between these studies with the effect of FeTMPyP on infarct volume is due to the severity of ischemia. Peroxynitrite decomposition catalysts may be beneficial as treatment for stroke if the degree of cerebral injury is not too severe, but may not be beneficial when the severity of damage increases and other mechanisms are involved in stroke damage. In any case, studies assessing the long-term outcome with FeTMPyP are needed.
The HG Sham plasma also increased myogenic tone in MCA, but through an apparently different mechanism than the HG MCAO plasma. Endothelin-1 via the activation of ETA receptors expressed in smooth muscle cells (Kohan et al, 2011) appears to be important for the increased tone in the HG Sham plasma group because BQ-123 prevented the increase in tone after perfusion with HG Sham plasma. Enhanced ET-1 release has been shown in endothelial cells exposed to high glucose (Yamauchi et al, 1990) and in HG animals treated with streptozotocin (Makino and Kamata, 1998). Other studies have shown enhanced ET-1 induced vasoconstriction during hyperglycemia and diabetes (Alabadi et al, 2004; Matsumoto et al, 2004); however, this is the first study that we know of showing a specific effect of HG plasma. A limitation of this study is that we did not measure ET-1 in plasma and thus do not know if ET-1 is coming from the recipient endothelium, the donor endothelium, or both.
It is worth noting that although BQ-123 prevented the increase in tone with HG Sham plasma, it did not have a similar effect on tone when vessels were perfused with HG MCAO plasma, despite both plasmas being from HG animals. In fact, BQ-123 increased tone with HG MCAO plasma. The major difference between the plasmas appears to be a circulating factor in the HG MCAO plasma—that is absent in the HG Sham plasma—that stimulates the recipient endothelium to produce peroxynitrite. It is possible that peroxynitrite interferes with ET-1A receptors, which might explain why BQ-123 was effective only with HG Sham plasma. However, the finding that BQ-123 increased tone with HG MCAO plasma is more difficult to explain at this time and further studies will be needed to understand this result. It is also worth noting that another limitation of this study is that we have not determined which factor is increasing tone in response to NG plasma. It is possible that it is related to ET-1A receptor activation as well, but to a lesser extent than in the HG animals where it would be expected to be increased (Makino and Kamata, 1998).
In summary, circulating factors present in plasma in response to HG stroke increased myogenic tone and impaired endothelial function in nonischemic MCA. Peroxynitrite decomposition prevented the increased tone with HG MCAO plasma, suggesting that factors present in plasma are interacting with the recipient endothelium to produce peroxynitrite. Scavenging peroxynitrite in vivo was neuroprotective acutely during mild, but not severe HG ischemia, which was unrelated to reperfusion blood flow. Thus, treatment to decrease the detrimental effects of peroxynitrite during HG stroke appear to be limited to conditions in which stroke is not severe. In addition, how circulating factors during HG stroke, which are vasoactive to normal vasculature, influence overall outcome is not clear, but may contribute to dysregulation of CBF and possibly evolving infarction at later time points.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by NIH, NINDS Grants RO1 NS043316 and RO1 NS045940, NHLBI Grant PO1 HL095488, and the Totman Medical Research Trust.
Supplementary Material
References
- Alabadi JA, Miranda FJ, Llorens S, Centeno JM, Marrachelli VG, Alborch E. Mechanisms underlying diabetes enhancement of endothelin-1-induced contraction in rabbit basilar artery. Eur J Pharmacol. 2004;486:289–296. doi: 10.1016/j.ejphar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, Codina A, Quintana M. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator-treated patients. Stroke. 2003;34:1235–1241. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- Barone FC, Globus MY, Price WJ, White RF, Storer BL, Feuerstein GZ, Busto R, Ohlstein EH. Endothelin levels increase in rat focal and global ischemia. J Cereb Blood Flow Metab. 1994;14:337–342. doi: 10.1038/jcbfm.1994.41. [DOI] [PubMed] [Google Scholar]
- Bemeur C, Ste-Marie L, Montgomery J. Increased oxidative stress during hyperglycemic cerebral ischemia. Neurochem Int. 2007;50:890–904. doi: 10.1016/j.neuint.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Bromont C, Marie C, Bralet J. Increased lipid peroxidation in vulnerable brain regions after transient forebrain ischemia in rats. Stroke. 1989;20:918–924. doi: 10.1161/01.str.20.7.918. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Chan PH, Epstein CJ, Kinouchi H, Kamii H, Imaizumi S, Yang G, Chen SF, Gafni J, Carlson E. SOD-1 transgenic mice as a model for studies of neuroprotection in stroke and brain trauma. Ann NY Acad Sci. 1994;738:93–103. doi: 10.1111/j.1749-6632.1994.tb21794.x. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Miller AA, Drummond GR, Kemp-Harper BK, Sobey CG. Oxidative stress and endothelial dysfunction in cerebrovascular disease. Front Biosci. 2011;16:1733–1745. doi: 10.2741/3816. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: the threshold duration of reperfusion for myogenic activity. Stroke. 2002;33:2094–2099. doi: 10.1161/01.str.0000020712.84444.8d. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Godfrey JA. Effect of hyperglycemia on brain penetrating arterioles and cerebral blood flow before and after ischemia/reperfusion. Transl Stroke Res. 2010;1:127–134. doi: 10.1007/s12975-010-0014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Huang Q, Sweet JG. Inhibition of protein kinase C{beta} reverses increased blood-brain barrier permeability during hyperglycemic stroke and prevents edema formation in vivo. Stroke. 2011;42:3252–3257. doi: 10.1161/STROKEAHA.111.623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Lessov N, Hammer ES, Curry AB. Threshold duration of ischemia for /myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke. 2001;32:1658–1664. doi: 10.1161/01.str.32.7.1658. [DOI] [PubMed] [Google Scholar]
- Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav Immun. 2010;24:708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Arch. 2010;459:841–851. doi: 10.1007/s00424-010-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Rossol S, Kammer T, Daffertshofer M, Wirth S, Dollman M, Hennerici M. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J Neurol Sci. 1994;122:135–139. doi: 10.1016/0022-510x(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Fukuyama N, Takizawa S, Ishida H, Hoshiai K, Shinohara Y, Nakazawa H. Peroxynitrite formation in focal cerebral ischemia-reperfusion in rats occurs predominantly in the peri-infarct region. J Cereb Blood Flow Metab. 1998;18:123–129. doi: 10.1097/00004647-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20:132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Jimenez-Altayo F, Briones AM, Giraldo J, Planas AM, Salaices M, Vila E. Increased superoxide anion production by interleukin-1beta impairs nitric oxide-mediated relaxation in resistance arteries. J Pharmacol Exp Ther. 2006;316:42–52. doi: 10.1124/jpet.105.088435. [DOI] [PubMed] [Google Scholar]
- Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91:1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145–155. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- Kunz A, Park L, Abe T, Gallo EF, Anrather J, Zhou P, Iadecola C. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Kamata K. Elevated plasma endothelin-1 level in streptozotocin-induced diabetic rats and responsiveness of the mesenteric arterial bed to endothelin-1. Br J Pharmacol. 1998;123:1065–1072. doi: 10.1038/sj.bjp.0701704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneen MJ, Hannah R, Vitullo L, DeLance N, Cipolla MJ. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Stroke. 2006;37:894–899. doi: 10.1161/01.STR.0000204043.18592.0d. [DOI] [PubMed] [Google Scholar]
- Margaill I, Plotkine M, Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radic Biol Med. 2005;39:429–443. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yoshiyama S, Kobayashi T, Kamata K. Mechanisms underlying enhanced contractile response to endothelin-1 in diabetic rat basilar artery. Peptides. 2004;25:1985–1994. doi: 10.1016/j.peptides.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Arrick DM, Sharpe GM, Sun H. Nitric oxide synthase-dependent responses of the basilar artery during acute infusion of nicotine. Nicotine Tob Res. 2009;11:270–277. doi: 10.1093/ntr/ntn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakura A, Kamanaka Y, Ikeda Y, Kondo K, Suzuki Y, Umemura K. Neutrophil elastase inhibition reduces cerebral ischemic damage in the middle cerebral artery occlusion. Brain Res. 2000;858:55–60. doi: 10.1016/s0006-8993(99)02431-2. [DOI] [PubMed] [Google Scholar]
- Shreeniwas R, Koga S, Karakurum M, Pinsky D, Kaiser E, Brett J, Wolitzky BA, Norton C, Plocinski J, Benjamin W, Burns DK, Goldstein A, Stern D. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest. 1992;90:2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suofu Y, Clark J, Broderick J, Wagner KR, Tomsick T, Sa Y, Lu A. Peroxynitrite decomposition catalyst prevents matrix metalloproteinase activation and neurovascular injury after prolonged cerebral ischemia in rats. J Neurochem. 2010;115:1266–1276. doi: 10.1111/j.1471-4159.2010.07026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiyagarajan M, Kaul CL, Sharma SS. Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br J Pharmacol. 2004;142:899–911. doi: 10.1038/sj.bjp.0705811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59:67–71. doi: 10.1212/wnl.59.1.67. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Ohnaka K, Takayanagi R, Umeda F, Nawata H. Enhanced secretion of endothelin-1 by elevated glucose levels from cultured bovine aortic endothelial cells. FEBS Lett. 1990;267:16–18. doi: 10.1016/0014-5793(90)80276-o. [DOI] [PubMed] [Google Scholar]
- Ziv I, Fleminger G, Djaldetti R, Achiron A, Melamed E, Sokolovsky M. Increased plasma endothelin-1 in acute ischemic stroke. Stroke. 1992;23:1014–1016. doi: 10.1161/01.str.23.7.1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.