Abstract
Oxygen sensing in hypoxic neurons has been classically attributed to cytochrome c oxidase and prolyl-4-hydroxylases and involves stabilization of transcription factors, hypoxia-inducible factor-1α (Hif-1α) and nuclear factor erythroid 2-related factor 2 (Nrf2) that mediate survival responses. On the contrary, release of cytochrome c into the cytosol during hypoxic stress triggers apoptosis in neuronal cells. We, here advocate that the redox state of neuroglobin (Ngb) could regulate both Hif-1α and Nrf2 stabilization and cytochrome c release during hypoxia. The hippocampal regions showing higher expression of Ngb were less susceptible to global hypoxia-mediated neurodegeneration. During normoxia, Ngb maintained cytochrome c in the reduced state and prevented its release from mitochondria by using cellular antioxidants. Greater turnover of oxidized cytochrome c and increased utilization of cellular antioxidants during acute hypoxia altered cellular redox status and stabilized Hif-1α and Nrf2 through Ngb-mediated mechanism. Chronic hypoxia, however, resulted in oxidation and degradation of Ngb, accumulation of ferric ions and release of cytochrome c that triggered apoptosis. Administration of N-acetyl-cysteine during hypoxic conditions improved neuronal survival by preventing Ngb oxidation and degradation. Taken together, these results establish a role for Ngb in regulating both the survival and apoptotic mechanisms associated with hypoxia.
Keywords: antioxidants, hypoxia, hypoxia-inducible factor, neuroglobin, nuclear factor erythroid 2-related factor 2
Introduction
Decreased oxygen delivery to the brain during global hypoxia causes irreversible cellular damage, through release of cytochrome c from the mitochondria and activation of executor caspases. On the contrary, hypoxic stress is also known to trigger endogenous neuroprotective responses that could have a role in neuronal survival. Though the fate of neurons subjected to hypoxic injury is often associated with the recruitment of modulators that regulate transcriptional and posttranscriptional events promoting repair mechanisms, the master regulator that determines the preponderance of a cell toward death or survival mechanisms remains an enigma.
With the discovery of neuroglobin (Ngb), a highly conserved oxygen-binding neuronal protein with prospective neuroprotective properties, it has emerged as an important molecule in hypoxia-mediated cellular signaling. Ngb, a monomeric protein with a predicted molecular mass of ∼17 kDa, is widely expressed in the central nervous system and particularly more abundant in the cerebral cortex, hippocampus, thalamus, hypothalamus, and cerebellum of rat brain (Reuss et al, 2002; Wystub et al, 2003). Although Ngb shares only 21% to 25% sequence homology with vertebrate hemoglobin and myoglobin, it conserves the key amino-acid residues that are required for hemoglobin and myoglobin function (Burmester et al, 2000). Previous studies have shown exacerbation of stroke on infusion of antisense oligodeoxynucleotides directed against Ngb and amelioration of mitochondrial disruption and oxidative stress during hypoxia/reoxygenation of cultured neurons overexpressing Ngb (Sun et al, 2003; Yu et al, 2009a, 2009b). Redox studies on Ngb showed occurrence of a rapid reaction between ferrous Ngb and ferric cytochrome c that led to the assumption that neuroprotection by Ngb arises from its intervention in the intrinsic pathway of apoptosis (Fago et al, 2006). This hypothesis has been substantiated by findings of Raychaudhuri et al (2010) on ability of Ngb to block the intrinsic pathway of apoptosis in cells supplemented with BH3 mimetic HA14-1. Furthermore, overexpression of Ngb in a transgenic mouse model reduced cerebral infarct size after middle cerebral artery occlusion (Khan et al, 2006). The signaling mechanisms pertaining to Ngb-mediated neuroprotection and its possible role in neurodegeneration during hypoxic stress, however, remain to be investigated.
Much importance in hypoxic signaling has been attributed to the hypoxia-inducible factors (Hifs), which regulate the expression of several target genes involved in oxygen homeostasis (Semenza, 1998, 2004). Hypoxia-inducible factor-1 is a phosphorylation-dependent and redox-sensitive transcription factor that regulates neuroprotection during hypoxic conditions (Fan et al, 2009). Since O2 availability appeared to be rate limiting for Hif-prolyl-4-hydroxylase (PHD) activity, which regulates Hif-1α stability, these enzymes were purported to act as bona fide oxygen sensors (Hirsila et al, 2003; Epstein et al, 2001). However, the dependency of PHD activity on the availability of ascorbate and cysteine (Gerald et al, 2004; Myllyla et al, 1978) and depletion of the enzyme activity on supplementation of desferrioxamine (DFO), an iron chelator (Zaman et al, 1999), or production of reactive oxygen species (ROS) during cytokine signaling (BelAiba et al, 2004) indicate regulation of Hif-1α stabilization by redox status of the cell. Oxidation of Fe2+ to Fe3+ in PHDs leading to generation of ROS may promote Hif-1α buildup via inactivation of these enzymes under physiological conditions and in cancer (Chandel et al, 1998). In addition, ferroportin responsible for transport of Fe3+ from the cytosol to the extracellular space (Ganz and Nemeth, 2006) has also been implicated in iron homeostasis. However, the role of ferroportin in regulating Hif-1α expression and the mechanism of Hif-1 activation during hypoxia in light of the cellular redox status remain to be investigated. Besides Hif-1α, nuclear factor erythroid 2-related factor 2 (Nrf2) is another transcription factor that is translocated into the nucleus under conditions of stress and induces several detoxifying and antioxidant genes through antioxidant response element (Li et al, 2007). A number of studies have shown the crucial role of Nrf2 in cell survival (Barhwal et al, 2009a). Nrf2 normally resides in the cytosol in association with Kelch-like- erythroid-cell-derived protein with CNC homology-associated protein 1 (Keap1) and is degraded by ubiquitination mediated by Cullin-3.
During the present study, an attempt has been made to investigate Ngb-mediated regulation of neuroprotective and neurodegenerative mechanisms in hippocampal neurons during hypoxic stress. We, hypothesized that Ngb could be the master switch that regulates neuronal survival or cell death during global hypoxia and ischemia by modulating the redox status of the cells. In this study, we discovered that Ngb maintained cytochrome c in the Fe2+ state during hypoxia through utilization of intracellular antioxidants. Subsequent depletion of antioxidants resulted in activation of Nrf2- and Hif-1α-mediated survival response. Oxidation and degradation of Ngb on prolonged hypoxic exposure, however, resulted in decreased Nrf2 and Hif-1α and release of pro-apoptotic Fe3+ cytochrome c into the cytosol.
Materials and methods
Experimental Models
The study was approved by the animal ethics committee of the Defence Institute of High Altitude Research along the guidelines of ‘Committee for the Purpose of Control and Supervision of Experiments on Animals' of Government of India. The experiments were conducted in both in vivo and in vitro. Adult male Sprague-Dawley rats (220±10 g) were used for in-vivo studies in accordance with the guidelines published in NIH Guide for the Care and Use of Laboratory Animals. The animals were housed in hygienic conditions with day and night cycle of 12 hours each and food and water were made available ad libitum. For in-vitro studies, N2a cells were cultured in Dulbecco's minimum essential medium (DMEM; Sigma Chemicals, St Louis, MO, USA) supplemented with penicillin and streptomycin (Sigma Chemicals) and 10% fetal calf serum (FCS; Sigma) at 37°C in 5% CO2. Cells were incubated at 37°C, 5% CO2, and 1% O2 for the hypoxic group while cells of the same preparation were maintained at 37°C, 21% O2, and 5% CO2 for 48 hours and served as normoxic. The experimental procedures used have been summarized in Supplementary Table S1.
Experimental Procedures In Vivo
Hypoxic exposure and drug administration
For exposure to hypobaric hypoxia, animals (n=40 per group) were inducted to a simulated altitude of 7,600 m (25,000 ft, 282 mm Hg) in a specially designed animal decompression chamber having provisions for regulation of temperature, pressure, humidity, and light intensity for a period ranging from 1 to 7 days (Barhwal et al, 2009a; Hota et al, 2010). The temperature and humidity in the chamber were maintained at 32±2°C and 63±3%, respectively. The rate of ascent and descent to the simulated altitude was maintained at 300 m/min. Fresh air was continuously flushed at a rate of 8 L/min to prevent accumulation of carbon dioxide within the chamber. The pressure of the decompression chamber was brought down to sea level every day for a 15–20-minute interval for replenishment of food and water. N-acetyl-cysteine (NAC) was supplemented to the drug-treated animals (n=32 per group) at a daily oral dose of 150 mg/kg body weight (dissolved in distilled water) at the same time. The normoxic animals were kept under similar temperature and humidity conditions in a separate chamber maintained at 12 hours day and night cycle and 760 mm barometric pressure. After the stipulated period of normoxic or hypoxic exposure, the animals were killed and biochemical (n=6 per group), histological (n=6 per group), western blot (n=6 per group), electrophoretic mobility shift assay (EMSA) (n=6 per group), high-performance liquid chromatography (n=6 per group), and electron microscopy (n=3 per group) were performed along with protein oxidation and ubiquitination assay (n=6 per group) (Supplementary Figure 1).
Neurodegeneration by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay
For histological studies, animals were perfused and fixed with 4% paraformaldehyde and brains were cryoprotected in sucrose for 48 hours before sectioning. Serial cryosections of 30 μm were obtained and sections between Bregma −4.16 and −4.30 mm were picked up at random from each group. DNA fragmentation, a marker of apoptosis was studied by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay using ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (Chemicon International, Inc., Temecula, CA, USA). Propidium iodide (0.5 μg/mL) was used as a counterstain and TUNEL-positive cells between guard zones of 10 μm in six random fields of 0.1 mm2 in the CA1, CA2, CA3, CA4, and dentate gyrus regions were visualized under confocal microscope. The TUNEL-positive cells were scored using the Stereo Investigator software and the results were expressed in terms of percentage of cells undergoing apoptosis.
Immunofluorescence staining
Brain cryosections were washed briefly in phosphate-buffered saline (PBS), permeabilized by 0.25% Triton, and blocked with 1% bovine serum albumin at 4°C. Sections were then incubated with anti-Ngb antibody (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) for 4 hours, washed with PBS containing 0.1% Tween, and incubated with Alexa Fluor 488-conjugated secondary antibody (Molecular Probes, Eugene, OR, USA) for 1 hour at 37°C. After appropriate washings, antibody complexes were detected under fluorescent microscope (Olympus IX71). Sections incubated only with Alexa Fluor 488-conjugated secondary antibody in the absence of primary antibody served as negative control. Cells between guard zones of 10 μm in six random fields of 0.1 mm2 were counted and Ngb-positive cells were scored using Stereo Investigator software. Results were expressed in terms of percentage considering normoxic controls to be 100%.
Perl's staining
Brain iron was determined by Perls' staining as described by Hill and Switzer (1984). In brief, fixed sections were incubated in 1% sodium borohydride (Sigma) for 30 minutes, washed with PBS, and incubated in 30 μg/mL proteinase K (Sigma), with 0.1% Triton X-100, for 20 minutes. Sections were then incubated in Perls' solution (1% HCl/1% potassium ferrocyanide) for 30 minutes and then rinsed in PBS. For the intensification of Perls' reaction, sections were incubated in 0.5% 3,3' diaminobenzidine solution in 0.05 mol/L Tris-HCl (pH 7.6), containing 30% hydrogen peroxide (2 μL/mL), for 15 minutes in the dark. The reaction was stopped by rinsing in deionized water for 20 minutes. Control slides were carried through the 3,3' diaminobenzidine intensification without preincubation with Perls' solution. No positive staining was found in any such control slides.
Electron microscopy
For visualization of ferric iron deposition in the brain, animals were first perfused with 0.005 mol/L PBS (pH 7.4) followed by a fixative containing 1% glutaraldehyde and 4% paraformaldehyde in 0.005 mol/L PBS. The animals were perfused with Perl's fixative (pH 0.8 to 1.0) containing 1% potassium ferrocyanide and 4% paraformaldehyde in saline as described by Meguro et al (2007). Animals were then perfused with saline to remove excess Perl's fixative and thereafter, the hippocampus was isolated and postfixed with 0.5% glutaraldehyde in PBS. The sections were cut at 40 μm thickness and were treated with 0.3% H2O2 and 0.065% sodium azide for 15 minutes and then washed with 0.005 mol/L PBS. The sections were then treated with 0.025% 3,3′ Diaminobenzidine-HCl (Sigma, USA) in 0.01 mol/L PBS containing 0.04% nickel. For electron microscopy, sections were postfixed with 1% osmium tetraoxide for 1 hour, washed and dehydrated with graded ethanol series, treated with propylene oxide and Epon mixture, and then embedded in Epon mixture for 1 hour. Ultrathin section were made (80–100 nm) and visualized under the electron microscope.
Estimation of ferrous/ferric ratio
The ferrous-to-ferric ratio was also estimated in the hippocampal homogenate using iron assay kit (Biovision, Milpitas, CA, USA) according to manufacturer's instructions. In brief, ferric carrier protein dissociated ferric into the solution in the presence of acid buffer. After reduction to the Fe2+ form, iron reacts with Ferene S to produce a stable colored complex and give absorbance at 593 nm. A specific chelate chemical was also included in the buffer to block copper ion interference. The concentration of Fe2+ and Fe3+ ion was determined from the standard curve.
Estimation of hydroxyl radical
Two major ROS metabolites 2,5-DHBA and 2,3-DHBA (dihydrobenzoic acid) released into the supernatant were analyzed by high-performance liquid chromatography with electrochemical detection (Waters, Milford, MS, USA) using DHBA-250 column (Chiueh et al, 1992). Since hydroxyl radical is highly reactive, short lived, and cannot be preserved, it needs to be trapped with the trapping agent, i.e., salicylic acid (2.5 mM) which reacts with the hydroxyl radical to produce DHBAs. The isocratic system consisted of a pump, an autosampler, and a Coulochem III detector. The mobile phase comprising 50 mM sodium acetate, 50 mM citric acid, 25% methanol, 5% isopropanol, pH 2.5 with phosphoric acid and the flow rate was maintained at 0.5 mL/min. Samples were prepared by homogenizing 100 mg of hippocampal tissue in 0.1 mol/L PBS (pH 7.4) and extracted with diethyl ether. Ether phase was collected and evaporated to dryness in rotavapor evaporator. The residual fraction was reconstituted in 100 μL of mobile phase and 10 μL of the samples was injected into autosampler. The level of hydroxyl radical production in response to hypoxic exposure was compared with that of normoxic samples. The results obtained were based on the quantities of increased 2,3-DHBA.
Western blot for protein expression
Expression of Ngb, Nrf2 (Santa Cruz Biotechnology), ferroportin (Cell Signaling, Danvers, MA, USA), cytochrome c, caspase 3, and Hif-1α (Sigma) was studied by immunoblotting of cytosolic and nuclear protein extracts from hippocampal tissue as described previously (Barhwal et al, 2009a, 2009b). In brief, the hippocampal tissue was homogenized in 1 × lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 0.1 mol/L dithiothreitol, protease inhibitor cocktail, 10 mM KCl). The homogenate was centrifuged at 10,000 to 11,000 × g for 20 minutes at 4°C and the supernatant thus obtained was aliquoted as ‘cytosolic fraction'. The crude nuclei pellet was resuspended in extraction buffer (20 mM HEPES, pH 7.9 with 1.5 mM MgCl2, 0.42 mol/L NaCl, 0.2 mol/L EDTA, 25% v/v glycerol) containing 0.1 mol/L dithiothreitol and protease inhibitor. After homogenization, the suspension was shaken gently for 30 minutes and then centrifuged at 20,000 to 21,000 × g for 5 minutes. The supernatant thus obtained was collected as ‘nuclear extract'. In all, 50 μg of sample protein was resolved by SDS–PAGE and transferred onto nitrocellulose membranes as described elsewhere (Hota et al, 2010). The membranes were blocked with 5% nonfat milk, washed with PBST (0.01 mol/L PBS, pH 7.4, 1 mL Tween-20) and probed for the desired proteins using suitable primary and secondary antibodies at appropriate dilutions. The antibody-treated membranes were developed using chemiluminescent kit (Sigma Chemicals) and the bands thus obtained on X-ray films were quantitated by densitometry. Glyceraldehyde phosphate dehydrogenase and lamin (Molecular Probes; Invitrogen, Carlsbad, CA, USA) were used as loading control for cytosolic and nuclear proteins, respectively.
Protein carbonyl and ubiquitination assay
Protein oxidation was studied using the protein carbonyl immunoblot kit (Cell Biolabs, San Diego, CA, USA) according to the procedure suggested by the manufacturer. Protein bands were developed on X-ray films using the chemiluminescence method and quantified by densitometry. Oxidation of Ngb and ferroportin was studied after immunoprecipitation and elution of the proteins using a suitable immobilized primary antibody using immunoprecipitation kit from Pierce (Thermo Fisher Scientific, Rockford, IL, USA). The eluent was resolved by 10% SDS–PAGE and transferred onto PVDF membrane followed by probing for protein oxidation.
For isolating ubiquitinated Ngb and ferroportin, ubiquitinated proteins affinity beads comprising a GST-fusion protein containing ubiquitin-associated sequence bound to glutathione agarose were used (Calbiochem, San Diego, CA, USA). The polyubiquitinated proteins from tissue lysates were enriched and the ubiquitinated proteins were identified by loading the beads directly onto SDS–PAGE and then immunoblotted with anti-Ngb (Santa Cruz) or anti-ferroportin (Cell Signaling).
Coimmunoprecipitation for neuroglobin and cytochrome c interaction
Coimmunoprecipitation of Ngb and cytochrome c was performed using coimmunoprecipitation kit from Pierce (Thermo Fisher Scientific) according to manufacturer's instruction. In brief, Ngb monoclonal antibodies immobilized on amine-reactive resin were used as capture antibodies. The tissue homogenates were incubated with the immobilized antibodies in the spin column overnight at 4°C. Oxidized Ngb and oxidized cytochrome c prepared from rat hippocampus served as negative control. The columns were centrifuged at 1,000 × g for 10 minutes followed by two subsequent washings with wash buffer and the eluent was resolved by 12% SDS–PAGE. The protein expression was probed with cytochrome c antibody (Sigma). Purified cytochrome c (Sigma) and prestained molecular marker were used as loading control for identification of bands specific for cytochrome c. Optical density of the bands denoted the extent of colocalization of Ngb and cytochrome c in the samples.
Experimental Procedures In-Vitro
Neuroglobin overexpression
Vector expressing human Ngb was generated using human cDNA clone from Origene (Rockville, MD, USA), subcloned into the KpnI and XbaI sites of pcDNA3.1 and the construct was verified by direct sequencing. Plasmids were propagated using DH5α-competent cells, according to the standard protocols, and purified using EndoFree Maxi Prep kits (Qiagen, Hilden, Germany). N2a cells were plated at a density of 1 × 106 onto the culture dishes and transfected with pcDNA3.1 or NGB_pcDNA3.1 using Lipofectamine (Invitrogen), according to manufacturer's instructions. Sixteen hours after transfection, cells were washed with serum-free medium, and cultured in fresh medium for 48 hours for transgene expression. The medium was then replaced with selection medium containing G418 (400 μg/mL; Invitrogen). Eight to twelve clones from each transfection were selected after 4 weeks of selection. Based on screening for Ngb expression using anti-Ngb, three stable clonal cell lines were selected for further analysis.
Neuroglobin silencing
Neuroglobin siRNA expression vectors (named Ngb-siRNA) targeted to different regions of mouse Ngb cDNA (gi:18999491) were constructed using a GFP-expressing siRNA vector (p-Genesil-1; Wuhan Genesil Biotechnology, Wuhan, China), as described previously (Li et al, 2007). The efficiency of Ngb-siRNA plasmids was confirmed by cotransfecting Ngb-siRNA and p-Ngb-EGFP plasmids at a ratio of 4:1 into N2a cells using Lipofectamine reagent. All constructs were confirmed by DNA sequencing.
N2a cells were transfected with Ngb-siRNA plasmids or control vectors, and stable cell lines were screened using a neomycin antibiotic (Sigma, USA) starting at a concentration of 800 μg/mL. The concentration of neomycin antibiotic was gradually reduced to a final concentration of 200 μg/mL within 2 weeks. Stable N2a cell lines expressing neomycin-resistant Ngb-siRNA (N2a/Ngb-siRNA) or control plasmids (N2a/vec) were obtained by subcloning. The cells were maintained in N2a medium plus neomycin antibiotic (200 μg/mL), and the medium was changed every 2 or 3 days. N2a cells within 20 passages were used in all experiments. Silencing of Ngb was confirmed by both immunocytochemistry and western blotting using anti-Ngb antibody.
Estimation of oxidized and reduced cytochrome c
Mitochondria were isolated from N2a cells using mitochondria isolation kit (MITO-ISO1; Sigma) as per manufacturer's instructions and suspended in KCl buffer. Partial permeabilization of the outer membrane was achieved using digitonin (200 μg/250 μg mitochondria). The oxidized and reduced state of cytochrome c was estimated spectrophotometrically using a double beam spectrophotometer with wavelength pair of 530/550 nm (Basu et al, 2010; Pasdois et al, 2011). In all, 1 mM GSH or 7 mM Na2S2O4 was added to maintain cytochrome c in the reduced or oxidized state, respectively. The absorbance was calculated and ratio between the absorbance denoted the proportion of oxidized and reduced cytochrome c.
Assessment of apoptosis by flow cytometry and Hoechst staining
N2a cells were resuspended in binding buffer (10 mM HEPES NaOH, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2) and incubated for 5 minutes with 1 mL of Annexin V-FITC (Santa Cruz) at room temperature in the dark. The cells were then subjected to flow cytometric analyses (Becton Dickinson FACS Calibur, San Jose, CA, USA), using FlowJo (TreeStar, Inc., Ashland, OR, USA) software.
For assessment of chromatin condensation, N2a cells were fixed with 4% paraformaldehyde for 30 minutes and Hoechst staining was performed as previously reported (Hota et al, 2010). After being washed twice with PBS, Hoechst 33258 was used to stain the nuclei for 5 minutes at room temperature. Apoptotic nuclei were scored under fluorescent microscope (Olympus BX-51) in five randomly chosen fields and expressed as percentage of cells showing chromatin condensation.
Subcellular protein fractionation
Cytosolic and nuclear extracts were prepared from N2a cells using subcellular protein fractionation kit according to manufacturer's instructions (Pierce, Rockford, IL, USA). In brief, the cells were harvested with trypsin-EDTA and centrifuged at 500 × g for 5 minutes. The pellet was suspended in ice-cold PBS and 1 to 10 × 106 cells were transferred in a microcentrifuge tube and centrifuged at 500 × g for 2 to 3 minutes. The cytoplasmic extraction buffer containing protease inhibitor was then added to the cell pellet, incubated at 4°C for 10 minutes and centrifuged at 500 × g for 5 minutes. The supernatant thus obtained served as ‘cytosolic extract'. To the pellet, ice-cold nuclear extraction buffer containing protease inhibitor was added, vortexed for 15 seconds and incubated at 4°C for 30 minutes. The contents were then centrifuged at 500 × g for 5 minutes and the supernatant obtained served as ‘nuclear extract'.
DNA binding assay of hypoxia-inducible factor-1α and nuclear factor erythroid 2-related factor 2 for in-vivo and in-vitro samples
Extent of binding of Nrf2 and Hif-1α with DNA was studied by EMSA as described earlier (Hota et al, 2010). The DNA binding sequence used for Nrf2 was 5′-TTTTATGCTGTGTCATGGTT-3′. A typical double-stranded consensus oligonucleotide for Hif-1 (5′-TCTGTACGTGACCACACTCACCTC-3′) and a mutant DNA sequence (5′-TCTGTAAAAGACCACACTCACCTC-3′) (Wang and Semenza, 1993) were purchased from Santa Cruz Biotech Inc. Nuclear extracts were prepared from in-vivo and in-vitro samples as described above. The probes were biotin end-labeled using lightshift chemiluminescent EMSA kit (Pierce). Nuclear fractions incubated with immobilized primary antibodies for Hif-1α and Nrf2 were considered as negative controls. Bands were developed using chemiluminescent substrate as suggested by the manufacturer.
Western blot for protein expression in N2a cells
Cytosolic and nuclear fractions obtained from the N2a cells were resolved by SDS–PAGE and the proteins were transferred onto nitrocellulose membrane as described earlier (Hota et al, 2010). The membranes were blocked with 5% nonfat milk, washed with PBST and probed overnight with appropriate dilutions of primary antibody. The membranes were then washed, incubated in suitable secondary antibody, and developed with chemiluminescent kit. The optical density of the bands was determined. Glyceraldehyde phosphate dehydrogenase and lamin were used as loading control for cytosolic and nuclear proteins, respectively.
Statistical Analysis
No mortality of animals occurred during the experiment. All data were expressed as mean±s.e.m. of three separate experiments. Analysis of variance with Dunnett's multiple comparison and paired as well as unpaired t test or Kruskall–Wallis test with Dunn's multiple comparison and Mann–Whitney test were used wherever appropriate to assess statistical differences between groups. P values <0.01 were considered statistically significant.
Results
Hypobaric Hypoxia-Mediated Neurodegeneration Is Associated with Neuroglobin Expression in Hippocampus
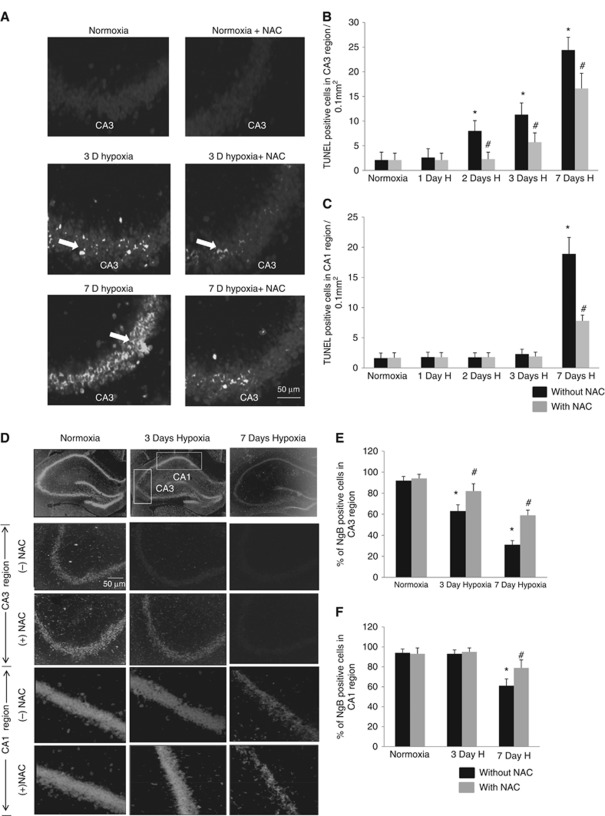
Rats exposed to simulated altitudes of 25,000 ft showed increased TUNEL-positive neurons in the CA1 and CA3 regions of the hippocampus when compared with the normoxic group. Up to 11.3±5.7 cells/0.1 mm2 (P<0.001) in the CA3 region showed DNA fragmentation after 3 days hypoxic exposure (Figures 1A and 1B), while only 2.8±0.6 cells/0.1 mm2 showed DNA fragmentation in the CA1 region. No significant difference was observed between the number of TUNEL-positive cells in the CA1 region of normoxic and 3-day hypoxic animals. DNA fragmentation in the CA1 region, however, increased to 18.9±3.2 cells/0.1 mm2 (P<0.001) after 7 days of exposure to hypobaric hypoxia (Figure 1C). Oral administration of NAC at a dose of 150 mg/kg body weight during hypoxic exposure, however, resulted in amelioration of neurodegeneration to 8.1±0.8 cells/0.1 mm2 (P<0.001) in the CA1 region and 16.6±3.1 cells/0.1 mm2 (P<0.01) in the CA3 region after 7 days hypoxic exposure as shown in Figures 1B and 1C. Since we hypothesized that redox state of Ngb could have a role in regulating apoptosis in the hippocampal neurons, the expression of Ngb was studied by immunocytochemistry. Our results showed higher number of Ngb-positive puncta in the CA1 and CA3 regions of normoxic animals when compared with those exposed to chronic hypobaric hypoxia for 7 days (Figures 1D and 1E). The quantitative analysis of immunofluorescence staining showed increased expression of Ngb in the CA1 region when compared with CA3 neurons of both normoxic and 3-day hypoxic rats (Figures 1E and 1F). Administration of NAC during hypoxic exposure also resulted in increased Ngb expression in both CA1 and CA3 regions when compared with the hypoxic rats without antioxidant supplementation.
Figure 1.
Hypobaric hypoxia induced neurodegeneration in CA1 and CA3 regions of the hippocampus. (A) Representative images of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)-positive cells in the CA3 region of the hippocampus. Graphs denote mean±s.e.m. of number of TUNEL-positive cells in random fields of 0.1 mm2 in each group (n=6) of (B) CA3 region (C) CA1 region. *Denotes P<0.01 when compared with normoxic group and # denotes P<0.01 when compared with corresponding N-acetyl-cysteine (NAC)-untreated group. (D) Representative images of the neuroglobin (Ngb)-positive cells in random fields of 0.1 mm2 in the CA1 and CA3 regions of hippocampus. Graph depicting mean±s.e.m. of percentage of Ngb-positive cells in (E) CA3 and (F) CA1 regions of the hippocampus. *Denotes P<0.01 when compared with normoxic group and # denotes P<0.01 when compared with corresponding NAC-untreated group.
Hypoxic Insult Causes Increased Ferric Ion Deposition and Oxidative Stress
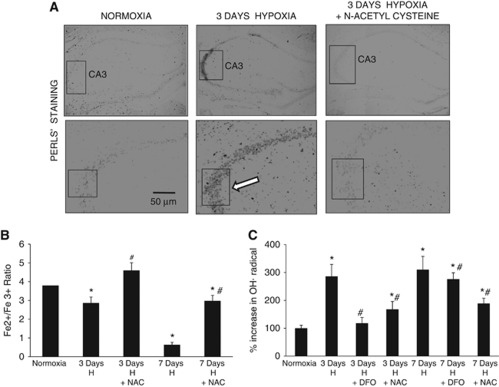
Since depletion of intracellular antioxidants and subsequent oxidation of Ngb during hypoxia could lead to dissociation of ferric ion from the globin moiety of Ngb, ferric ion deposition in the cytosol was investigated by Perl's staining and electron microscopy. The results showed increased ferric-positive reaction in the CA3 region of hypoxic animals when compared with the normoxic and NAC supplemented animals (Figure 2A). Electron micrographs of CA3 region also revealed intense staining in the cytosol of hypoxic neurons as compared with the normoxic, NAC supplemented hypoxic, and 3,3' diaminobenzidine control samples (Figure 2B). Biochemical estimation of Fe2+/Fe3+ ratio in cytosolic samples showed increased ferric ion concentration and progressive decrease in Fe2+/Fe3+ ratio from 3.8±0.46 in normoxic animals to 0.64±0.13 (P<0.001) in 7-day hypoxic animals which was prevented by NAC administration (Figure 2C). A concomitant increase in 2,3-DHBA was also observed from 100±11% in normoxic animals to 310±48% (P<0.001) in the 7-day hypoxic animals, indicating increased ROS generation. The NAC administration during hypoxic exposure, however, significantly decreased 2,3-DHBA levels (Figure 2C). Administration of DFO during hypoxic exposure also resulted in decreased ROS concentration after 3 days hypoxic exposure, indicating occurrence of fenton reaction and Fe3+-mediated free radical generation during hypoxic stress. Interestingly, no significant change in ROS generation was observed on DFO administration during prolonged hypoxic exposure of 7 days, which could be due to depletion of cellular antioxidants or activation of neuroimmune responses.
Figure 2.
Hypoxia results in Fe3+ deposition and hydroxyl radical generation. (A) Representative images of Perl's staining in the CA1 and CA3 regions of hippocampus. Scale bars shown in μm. (B) Graph showing Fe2+/Fe3+ ratio in hippocampal samples as estimated biochemically. The concentration of Fe2+/Fe3+ was determined from a standard curve. (C) Graph depicting concentration of hydroxyl radical as determined by high-performance liquid chromatography. Concentration of reactive oxygen species (ROS) was determined indirectly by calculating the area of plot for the ROS metabolite 2,3-DHBA. *Denotes P<0.01 when compared with normoxia and # denotes P<0.01 when compared with corresponding NAC-untreated group. H, hypoxia; NAC, N-acetyl cysteine; DFO, desferrioxamine; DHBA, dihydrobenzoic acid.
Neuroglobin Oxidation and Ubiquitination Regulates Neuronal Apoptosis in Hypobaric Hypoxia
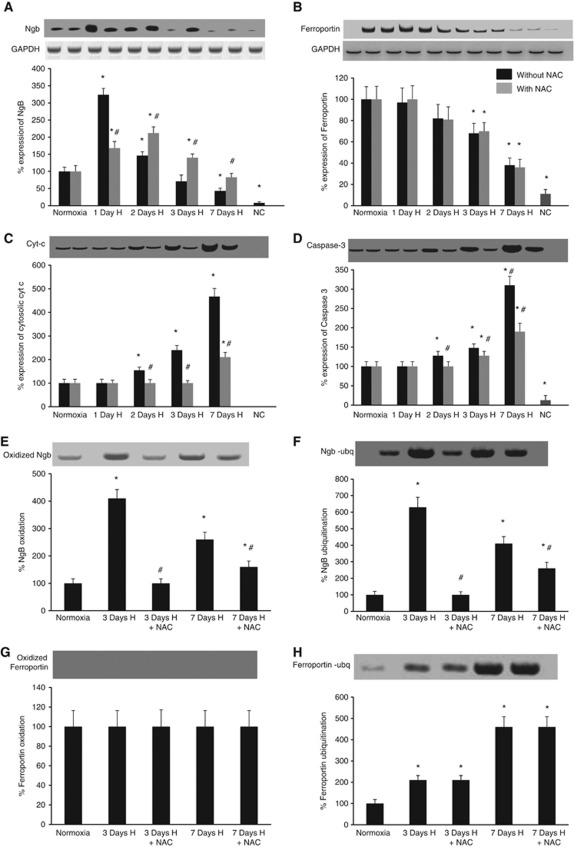
Western blots for Ngb expression showed upregulation after 1 day of hypoxic exposure, which however decreased progressively with increase in duration of exposure for 7 days (Figure 3A). On the contrary, administration of antioxidant during hypobaric hypoxia resulted in decreased expression of Ngb during the initial phase but increased Ngb expression on prolonged exposure when compared with the untreated group as shown in Figure 3A. Ferroportin, an iron exporter in neurons also showed decreased expression in the hypoxic animals, which was independent of NAC supplementation (Figure 3B). In addition, there was an increase in expression of cytosolic cytochrome c and caspase 3 after hypoxic exposure, which corresponded to the decrease in Ngb expression (Figures 3C and 3D).
Figure 3.
Hypoxic exposure mediates neuroglobin (Ngb) oxidation and ferroportin ubiquitination leading to decreased Ngb and ferroportin expression along with release of cytochrome c (Cyt c). Representative western blots of (A) Ngb, (B) ferroportin, (C) Cyt c, (D) caspase–3, (E) oxidized Ngb, (F) ubiquitinated Ngb, (G) oxidized ferroportin, and (H) ubiquitinated ferroportin. Glyceraldehyde phosphate dehydrogenase (GAPDH) was considered as loading control for western blots. Optical density of the protein bands was determined and percentage of protein expression was calculated assuming normoxic values without NAC treatment to be 100%. Samples from which the protein of interest was immune-precipitated before western blotting were loaded to gels as negative control. *Denotes P<0.01 when compared with 0 day control and # denotes P<0.01 when compared with corresponding NAC-untreated group. H, hypoxia; NAC, N-acetyl cysteine; NC, negative control.
Protein oxidation and ubiquitination studies were also performed to investigate the posttranslational modifications leading to degradation of both Ngb and ferroportin, which could contribute to ferric ion accumulation in the neurons during hypoxia. We observed an increase in Ngb oxidation and ubiquitination in the hippocampus of hypoxic animals when compared with the normoxic animals and those administered with NAC during hypoxic exposure (Figures 3E and 3F). No expression of oxidized ferroportin was detected in the hippocampal samples of both the normoxic and hypoxic animals (Figure 3G). Ferroportin, however, showed progressive increase in ubiquitination after exposure to hypobaric hypoxia, which was independent of antioxidant supplementation (Figure 3H).
Oxidation of Neuroglobin in Hypoxia Results in Release of Ferric Cytochrome c to Cytosol
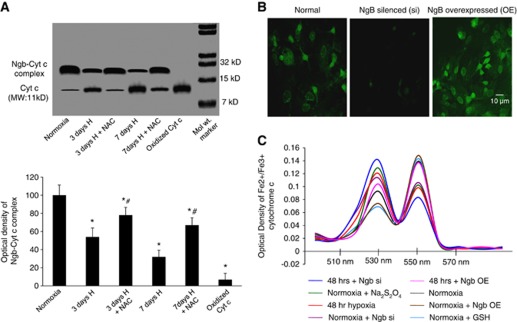
Ferrous Ngb is known to interact with ferric cytochrome c raising speculations on its role in maintaining ferrous state of cytochrome c. While ferrous cytochrome c is retained in the mitochondria, ferric cytochrome c is reported to be released into the cytosol triggering apoptosis. We, therefore, investigated the interaction of Ngb with cytochrome c after hypoxic exposure. Coimmunoprecipitation of the proteins showed decreased interaction between Ngb and cytochrome c as shown in Figure 4A.
Figure 4.
Hypoxia reduces Ngb–Cyt c interaction. (A) Western blot depicting Ngb–Cyt c interaction using monoclonal antibodies for Cyt c. Oxidized Ngb and oxidized Cyt c obtained from normoxic samples treated with H2O2 were loaded as negative control. The corresponding graph depicts optical density of the protein bands in terms of percentage of protein expression assuming normoxic values without drug treatment to be 100%. *Denotes P<0.01 when compared with normoxia and # denotes P<0.01 when compared with corresponding NAC-untreated group. (B) Representative images of normal, Ngb-silenced, and Ngb upregulated N2a cells. (C) Graph denotes mean value of six observations of absorbance at 530 nm for oxidized Cyt c and 550 nm for reduced Cyt c concentration. In all, 1 mM GSH or 7 mM Na2S2O4 was added to maintain Cyt c in the reduced or oxidized state, respectively. Cyt c, cytochrome c; H, hypoxia; NAC, N-acetyl cysteine; Ngb, neuroglobin; Si, silenced; OE, overexpressed.
To validate the reduced interaction between Ngb and ferric cytochrome c during hypoxia, further studies were performed in vitro using Ngb overexpressed, Ngb-silenced and normal N2a cells (Figure 4B). Estimation of ratio of ferrous and ferric cytochrome c in mitochondria isolated from hypoxic N2a cells showed increased ferric cytochrome c as evident from increase in absorbance at 550 nm when compared with the normoxic cells that had greater absorbance at 530 nm (Figure 4C). During hypoxia, overexpression of Ngb in N2A cells resulted in increased ferrous cytochrome c while silencing of Ngb led to increased ferric cytochrome c. Silencing of Ngb in normoxic cells also resulted in increased ferric cytochrome c as evident from increased absorbance at 550 nm.
Neuroglobin Overexpression During Hypoxia Delays Apoptosis
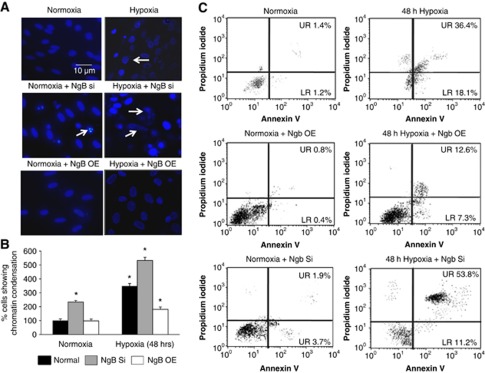
Neuroglobin silencing in N2a cells resulted in increased chromatin condensation under both normoxic and hypoxic conditions when compared with normal N2a cells (Figure 5A). Similar increase in chromatin condensation was also observed in the normal N2a cells exposed to hypoxia. Neuroglobin overexpressing N2a cells, however, showed decreased chromatin condensation after hypoxic exposure when compared with hypoxic N2a cells expressing Ngb at normal levels (Figure 5B). These findings on role of Ngb in delaying neuronal apoptosis were further substantiated by flow cytometric estimation of Annexin V expression, which was maximum in Ngb-silenced hypoxic N2a cells (Figure 5C).
Figure 5.
Neuroglobin (Ngb) upregulation decreases chromatin condensation and apoptosis in N2a cells exposed to hypoxia. (A) Representative images of normoxic, normoxic+Ngb-silenced, normoxic+Ngb overexpressed, hypoxic, hypoxic+Ngb-silenced, and hypoxic+Ngb overexpressed N2a cells. Scale bar denotes 10 μm. (B) Corresponding graph denotes percentage of cells showing chromatin condensation that was determined by counting mean±s.e.m. of number of cells with chromatin condensation and considering normoxic values to be 100%. *Denotes P<0.01 when compared with normoxic cells. (C) Representative flow cytometric images of normoxic, normoxic+Ngb overexpressed, normoxic+Ngb-silenced, hypoxic, hypoxic+Ngb overexpressed, and hypoxic+Ngb-silenced N2a cells. Apoptosis was determined based on Annexin V and necrosis was determined by propidium iodide staining. NAC, N-acetyl cysteine; Si, silenced; OE, overexpressed.
Neuroglobin Upregulation Stabilizes Hypoxia-Inducible Factor-1α and Nuclear Factor Erythroid 2-Related factor 2 in Hypoxia
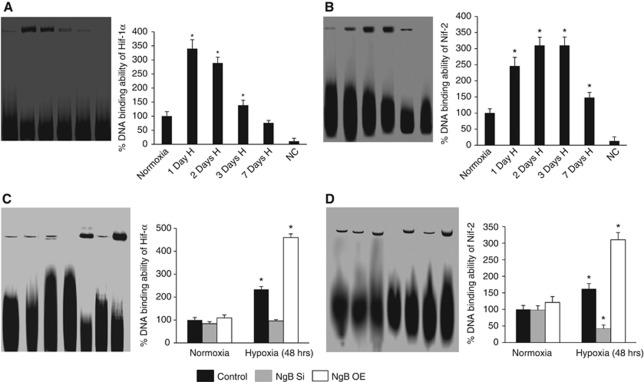
Both Hif-1α and Nrf2 are known to mediate neuroprotection during conditions of hypoxia and oxidative stress. We hypothesized that oxidized Ngb could lead to release of ferric iron resulting in oxidative stress-mediated upregulation of Nrf2. Ngb–cytochrome c cycle that required utilization of antioxidants could also contribute to depletion of cellular antioxidants resulting in inhibition of PHD activity and stabilization of Hif-1α. We therefore investigated the possible correlation between Ngb and these transcription factors. The EMSA showed increased DNA binding of Hif-1α after day 1 of hypoxic exposure, which progressively declined with increase in duration of exposure (Figure 6A). DNA binding ability of Nrf2, however, was maximum on second and third days of hypoxic exposure (Figure 6B). In-vitro studies on Ngb-silenced and overexpressed N2a cells revealed decreased DNA binding of Hif-1α and Nrf2 in Ngb-silenced hypoxic cells, which were even lower than the normoxic cultures. The Ngb overexpressed hypoxic cultures, however, showed increased DNA binding of both Hif-1α and Nrf2 as shown in Figures 6C and 6D.
Figure 6.
Hypoxia alters DNA binding ability of hypoxia-inducible factor-1α (Hif-1α) and nuclear factor erythroid 2-related factor 2 (Nrf2) through neuroglobin (Ngb)-mediated signaling. Nuclear fraction of proteins was prepared from in-vivo and in-vitro cell lysates and incubated with suitable probes for Hif-1α and Nrf2. Panels (A, B) and corresponding graphs showing representative electrophoretic mobility shift assay (EMSA) for DNA binding ability of Hif-1α and Nrf2 in hippocampus of normoxic and hypoxic rats. Panels (C, D) and corresponding graphs showing representative EMSA for DNA binding ability of Hif-1α and Nrf2 in N2a cells. Nuclear fractions incubated with immobilized primary antibodies for Hif-1α and Nrf2 were considered as negative controls. Graphs denote percentage of DNA binding as determined by optical density of the bands, considering normoxic control values to be 100%. *Denotes P<0.01 when compared with normoxic controls. H, hypoxia; NAC, N-acetyl cysteine; Si, silenced; OE, overexpressed; NC, negative control.
Neuroglobin Delays Apoptosis by Prolonging Survival Mechanisms in Hypoxia
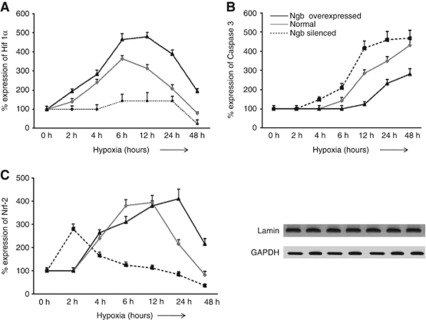
Since Ngb expression was correlated with both caspase 3 expression and Hif-1α and Nrf2 expression, we were interested in determining the sequence of events that decided cell survival or apoptosis during hypoxia. Hence, western blots were performed in N2a cells for expression of Hif-1α, Nrf2, and caspase 3 at different durations of hypoxic exposure. Expression of both Hif-1α and Nrf2 was maximum at 6 hours and started declining after 12 hours of exposure to hypoxia (Figures 7A and 7B). On the contrary, expression of caspase 3 increased logarithmically after 6 hours exposure and continued to remain high on 48 hours of exposure to hypoxia (Figure 7C). Interestingly, Ngb overexpressed N2a cells showed upregulation of both Hif-1α and Nrf2 up to 24 hours of hypoxia with a concomitant decrease in caspase 3 expression. Silencing of Ngb, however, resulted in persistent decrease in Hif-1α and Nrf2 expression along with increase in caspase 3 expression.
Figure 7.
Neuroglobin (Ngb) overexpression delays apoptosis in N2a cells exposed to hypoxia. Ngb-silenced, Ngb overexpressed, and normal N2a cells were exposed to different durations of hypoxia. Western blots were performed with nuclear fractions to study the expression of hypoxia-inducible factor-1α (Hif-1α) and nuclear factor erythroid 2-related factor 2 (Nrf2) while the cytosolic fraction was used to study caspase 3 expression. Protein expression was calculated in percentage, considering optical density of protein bands at 0 hour hypoxia to be 100%. Points on the graph depict mean±s.e.m. of six individual western blot observations at a particular duration of exposure to hypoxia. Graphs depict percentage protein expression of (A) Hif-1α, (B) Nrf2, and (C) caspase 3 following different durations of exposure to hypoxia. Lamin and glyceraldehyde phosphate dehydrogenase (GAPDH) were used as loading controls for nuclear and cytosolic proteins, respectively.
Discussion
Reduced oxygen supply to the brain during hypobaric hypoxia is known to cause neurodegeneration in the hippocampal neurons resulting in memory impairment (Barhwal et al, 2009a,2009b; Hota et al, 2010). The cellular response to oxygen deficiency in different regions within the hippocampus has however been less studied. Concomitant to the findings of Rybnikova et al (2006) in conditions of severe hypobaric hypoxia (180 Torr for 3 hours) (Rybnikova et al, 2006), the present study also reveals increased susceptibility of CA3 region to hypoxia-mediated neurodegeneration, which could be attributed to the altered cellular antioxidant status and decreased Ngb expression. On the contrary, Petito et al (1987) have reported increased susceptibility of CA1 pyramidal neurons to transient global ischemia, which could be due to the difference in duration and degree of oxygen deficiency when compared with the present experimental conditions. Though Ngb has been classically implicated to be an oxygen carrier for neurons, recent findings indicate its role in several signaling mechanisms (Fago et al, 2004; Yu et al, 2009a, 2009b). The dependency of redox state of Ngb on its ability to interact with other proteins indicates its role in sensing the cellular redox state (Raychaudhuri et al, 2010). Previous studies by Fago et al (2006) have shown increased ability of ferrous Ngb to bind to ferric cytochrome c. In other words, Ngb in its ferric state could trigger signaling mechanisms, which may be distinct from those of its ferrous state. The stability of ferric Ngb in an oxidizing milieu, however, remains to be explored. We have shown previously that hypoxic exposure leads to oxidative stress and depletion in antioxidant status (Hota et al, 2010; Barhwal et al, 2009a, 2009b). Interestingly, the depletion of Ngb in such an oxidizing environment establishes a relationship with myoglobin and indicates to destabilization of the heme and globin moieties (Capece et al, 2009). This is supported by the present findings on deposition of ferric iron in the cytosol and decreased ferric–ferrous ratio in hippocampus of animals exposed to hypobaric hypoxia. The ferric ions in turn could trigger Fenton reactions to generate free radicals. Since the free radicals are known to have a key role in intracellular signaling mechanisms leading either to neuroprotection (through Nrf2 mediated and other mechanisms) or causing cell death through DNA and protein oxidation, the concentration of ferric ion in the cytosol could be a crucial factor in determining the fate of hypoxic cells. In the present study, we observed a decrease in ROS generation on administration of ferric ion chelator, DFO, during hypoxic exposure. Supplementation of NAC, known to improve neuronal survival in hypoxia by improving the synthesis of glutathione (primary antioxidant in the cells) (Jayalakshmi et al, 2005), resulted in decreased ferric ion deposition and reduced generation of ROS during hypoxia, indicating a correlation between the cellular antioxidant status, redox state of iron, and generation of free radicals. The NAC supplementation also maintained Ngb expression close to normoxic levels providing further evidence on the role of cellular antioxidant status on the stability of Ngb. The increased oxidation of Ngb followed by its ubiquitination in hypoxic conditions leading to dissociation of heme and globin along with decrease in ferroportin expression, however, resulted in accumulation of ferric ion in the cytosol. Interestingly, ferroportin expression was independent of protein oxidation mechanisms and remained unaffected on supplementation of NAC to hypoxic animals.
Neuroglobin has been previously shown to interact with ferric cytochrome c indicating its role in docking of ferric cytochrome c and its probable conversion to ferrous cytochrome c (Bonding et al, 2008; Fago et al, 2006). Several studies also implicate ferric cytochrome c to be pro-apoptotic while ferrous cytochrome c is retained within the mitochondria (Fago et al, 2008). Since hypoxic exposure results in disruption in the electron transport chain and is associated with release of cytochrome c to the cytosol, we investigated the possible occurrence of a misbalance in the Ngb–cytochrome c cycle that could lead to release of ferric cytochrome c into the cytosol. Our in-vitro findings showed increased ferric cytochrome c in hypoxic cells, which are supported by our in-vivo findings on decreased Ngb–cytochrome c interaction in hippocampus of hypoxic animals. The increased cytosolic cytochrome c and caspase 3 expression in hippocampus of hypoxic animals was concomitant to Ngb oxidation and degradation. Hence, prolonged exposure to hypoxia could lead to compromised antioxidant status of the cells leading to degradation of Ngb and release of ferric cytochrome c to the cytosol and subsequent activation of caspase 3. Similar increase in caspase 3 and cytosolic cytochrome c has been previously reported in in-vitro and in-vivo hypoxic models (Barhwal et al, 2009b). Since overexpression of Ngb led to increased ferrous cytochrome c, while silencing of Ngb in N2a cells exposed to hypoxia resulted in increased ferric cytochrome c, it is speculative that Ngb could have a key role in maintaining the redox state of cytochrome c and prevent the cell from undergoing apoptosis.
Neuroglobin has been recently implicated in augmenting the survival response and overexpression was found to be neuroprotective during hypoxic-ischemic injury (Sun et al, 2001; Wang et al, 2008). The detailed mechanisms pertaining to Ngb-mediated survival response, however, remain to be explored. Hence, in addition to its role in regulating cellular apoptosis, we also aimed at investigating the possible mechanisms involved in Ngb-mediated neuroprotection. Hypoxia-inducible factor-1α, a master regulator of hypoxic response, is known to have a key role in mediating neuroprotection through upregulation of vascular endothelial growth factor, erythropoietin, and several antiapoptotic proteins (Wang and Semenza, 1993). The stabilization of Hif-1α is dependent on PHD activity, which in turn is regulated by the availability of antioxidants (Epstein et al, 2001). However, Nrf2 is known to be stabilized by oxidative stress and upregulates an array of antioxidant genes including thioredoxin and glutathione synthatase (Li et al, 2007). Based on our present findings on reduced cytosolic cytochrome c on supplementation of NAC during hypoxic exposure, we speculated increased turnover of ferric cytochrome c during hypoxia resulting in increased conversion of ferrous Ngb to ferric Ngb. The ferric Ngb in turn could be converted to ferrous Ngb by utilization of intracellular antioxidants contributing to depletion of the intracellular antioxidant status. As a result of the depleted antioxidant status and altered redox state, the activity of PHD could be inhibited leading to stabilization of Hif-1α. Besides that, oxidative stress due to accumulation of ferric ion in the cytosol would result in stabilization of Nrf2. DNA binding assays performed during the study showed increased nuclear translocation and promoter binding ability of Hif-1α and Nrf2. Similar results on the activation of Hif-1α and Nrf2 has been previously reported in hypoxic-ischemic conditions (Fan et al, 2009; Barhwal et al, 2009a). Interestingly, DNA binding ability of both Hif-1α and Nrf2 was minimum on prolonged exposure, which coincided with decreased Ngb and increased caspase 3 expression in the cytosol. However, silencing of Ngb in N2a cells exposed to hypoxia not only decreased cell viability, but also led to decreased DNA binding of Nrf2 and Hif-1α, suggesting a role for Ngb in hypoxia-mediated survival mechanisms. Further evidence on the role of Ngb in regulating nuclear translocation of Hif-1α and Nrf2 was obtained from real-time expression studies during hypoxic exposure of Ngb-silenced and Ngb overexpressed N2A cells.
Our findings therefore reveal a novel role of Ngb in delaying neuronal apoptosis by augmenting the neuroprotective mechanisms via Hif-1α and Nrf2 regulated genes and preventing release of ferric cytochrome c during hypoxia. Moreover, the redox state of Ngb is detrimental in deciding the fate of the cells during hypoxia. We also show that supplementation of antioxidants could contribute in maintaining Ngb in the ferrous state while depletion of cellular antioxidant status could result in preponderance of ferric state leading to cellular apoptosis. Since oxidative stress and cytochrome c-mediated apoptosis are invariably associated with several neurodegenerative disorders and neuropathological conditions, these findings could establish Ngb as a novel therapeutic target for preventing cell death. The limitation of adequate cellular availability of several known antioxidants, their absorption in the digestive system, and ability to cross the blood–brain barrier also necessitates the development of more effective antioxidant molecules that could adequately maintain Ngb in its ferrous state and prevent neurodegeneration during hypoxia, ischemia and conditions of impaired cerebral circulation.
Acknowledgments
The authors are thankful to the Defence Research and Development Organization (DRDO), Ministry of Defence, Government of India for providing financial grant and support during the study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Barhwal K, Hota SK, Baitharu I, Prasad D, Singh SB, Ilavazhagan G. Isradipine antagonizes hypobaric hypoxia induced CA1 damage and memory impairment: complementary roles of L-type calcium channel and NMDA receptors. Neurobiol Dis. 2009b;34:230–244. doi: 10.1016/j.nbd.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Barhwal K, Hota SK, Jain V, Prasad D, Singh SB, Ilavazhagan G. Acetyl-L-carnitine prevents hypobaric hypoxia-induced spatial memory impairment through extracellular related kinase-mediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience. 2009a;161:501–514. doi: 10.1016/j.neuroscience.2009.02.086. [DOI] [PubMed] [Google Scholar]
- Basu S, Keszler A, Azarova NA, Nwanze N, Perlegas A, Shiva S, Broniowska KA, Hogg N, Kim-Shapiro DB. A novel role for cytochrome c: efficient catalysis of S-nitrosothiol formation. Free Radic Biol Med. 2010;48:255–263. doi: 10.1016/j.freeradbiomed.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BelAiba RS, Djordjevic T, Bonello S, Flugel D, Hess J, Kietzmann T, Gorlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol Chem. 2004;385:249–257. doi: 10.1515/BC.2004.019. [DOI] [PubMed] [Google Scholar]
- Bønding SH, Henty K, Dingley AJ, Brittain T. The binding of cytochrome c to neuroglobin: a docking and surface Plasmon-resonance study. Int J Biol Macromol. 2008;43:295–299. doi: 10.1016/j.ijbiomac.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- Capece L, Marti MA, Bidon-Chanal A, Nadra A, Luque FJ, Estrin DA. High pressure reveals structural determinants for globin hexacoordination: neuroglobin and myoglobin cases. Proteins. 2009;75:885–894. doi: 10.1002/prot.22297. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiueh CC, Krishna G, Tulsi P, Obata T, Lang K, Huang SJ, Murphy DL. Intracranial microdialysis of salicylic acid to detect hydroxyl radical generation through dopamine auto-oxidation in the caudate nucleus: effects of MPP+ Free Radic Biol Med. 1992;13:581–583. doi: 10.1016/0891-5849(92)90151-6. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Fago A, Hundahl C, Dewilde S, Gilany K, Moens L, Weber RE. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin: molecular mechanisms and physiological significance. J Biol Chem. 2004;279:44417–44426. doi: 10.1074/jbc.M407126200. [DOI] [PubMed] [Google Scholar]
- Fago A, Mathews AJ, Brittain T. A role for neuroglobin: resetting the trigger level for apoptosis in neuronal and retinal cells. IUBMB Life. 2008;60:398–401. doi: 10.1002/iub.35. [DOI] [PubMed] [Google Scholar]
- Fago A, Mathews AJ, Moens L, Dewilde S, Brittain T. Reactivity of neuroglobin with the potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett. 2006;580:4884–4888. doi: 10.1016/j.febslet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev. 2009;62:99–108. doi: 10.1016/j.brainresrev.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006;1763:690–699. doi: 10.1016/j.bbamcr.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta- Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Hill JM, Switzer RC. The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 1984;11:595–603. doi: 10.1016/0306-4522(84)90046-0. [DOI] [PubMed] [Google Scholar]
- Hirsilä M, Koivunen P, Günzler V, Kivirikko KI, Myllyharju J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem. 2003;278:30772–30780. doi: 10.1074/jbc.M304982200. [DOI] [PubMed] [Google Scholar]
- Hota SK, Hota BK, Prasad D, Ilavazhagan G, Singh SB. Oxidative stress induced alterations in Sp factors mediate transcriptional regulation of NR1 subunit in hippocampus during hypoxia. Free Radic Biol Med. 2010;49:178–191. doi: 10.1016/j.freeradbiomed.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Jayalakshmi K, Sairam M, Singh SB, Sharma SK, Ilavazhagan G, Banerjee PK. Neuroprotective effect of N-acetyl cysteine on hypoxia induced oxidative stress in primary hippocampal culture. Brain Res. 2005;1046:97–104. doi: 10.1016/j.brainres.2005.03.054. [DOI] [PubMed] [Google Scholar]
- Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci USA. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Calkins MJ, Johnson DA, Johnson JA. Role of Nrf2-dependent ARE-driven antioxidant pathway in neuroprotection. Methods Mol Biol. 2007;399:67–78. doi: 10.1007/978-1-59745-504-6_6. [DOI] [PubMed] [Google Scholar]
- Meguro R, Asano Y, Odagiri S, Li C, Iwatsuki H, Shoumura K. Non heme-iron histochemistry for light and electron microscopy: a historical, theoretical and technical review. Arch Histol Cytol. 2007;70:1–19. doi: 10.1679/aohc.70.1. [DOI] [PubMed] [Google Scholar]
- Myllyla R, Kuutti-Savolainen ER, Kivirikko KI. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Comm. 1978;83:441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- Pasdois P, Parker JC, Griffiths EJ, Halestrap AP. The role of oxidized cytochrome c in regulating mitochondrial reactive oxygen species production and its perturbation in ischemia. Biochem J. 2011;436:493–505. doi: 10.1042/BJ20101957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis. 2010;15:401–411. doi: 10.1007/s10495-009-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss S, Saaler-Reinhardt S, Weich B, Wystub S, Reuss MH, Burmester T, Hankeln T. Expression analysis of neuroglobin mRNA in rodent tissues. Neuroscience. 2002;115:645–656. doi: 10.1016/s0306-4522(02)00536-5. [DOI] [PubMed] [Google Scholar]
- Rybnikova E, Sitnik N, Gluschenko T, Tjulkova E, Samoilov MO. The preconditioning modified neuronal expression of apoptosis-related proteins of Bcl-2 superfamily following severe hypobaric hypoxia in rats. Brain Res. 2006;17:195–202. doi: 10.1016/j.brainres.2006.03.053. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J Lab Clin Med. 1998;131:207–214. doi: 10.1016/s0022-2143(98)90091-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hydroxylation of HIF-1: oxygen sensing at molecular level. Physiology. 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268:513–518. [PubMed] [Google Scholar]
- Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, Lo EH. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wystub S, Laufs T, Schmidt M, Burmester T, Maas U, Saaler-Reinhardt S, Hankeln T, Reuss S. Localization of neuroglobin protein in the mouse brain. Neurosci Lett. 2003;346:114–116. doi: 10.1016/s0304-3940(03)00563-9. [DOI] [PubMed] [Google Scholar]
- Yu Z, Fan X, Lo EH, Wang X. Neuroprotective roles and mechanisms of neuroglobin. Neurol Res. 2009a;31:122–127. doi: 10.1179/174313209X389866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Liu J, Guo S, Xing C, Fan X, Ning M, Yuan JC, Lo EH, Wang X. Neuroglobin overexpression alters hypoxic response gene expression in primary neuron culture following oxygen glucose deprivation. Neuroscience. 2009b;62:396–403. doi: 10.1016/j.neuroscience.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman K, Ryu H, Hall D, O'Donovan K, Lin KI, Miller MP, Marquis JC, Baraban JM, Semenza GL, Ratan RR. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19:9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.