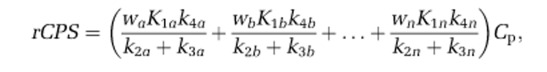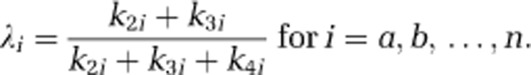Abstract
A spectral analysis approach was used to estimate kinetic parameters of the L-[1-11C]leucine positron emission tomography (PET) method and regional rates of cerebral protein synthesis (rCPS) on a voxel-by-voxel basis. Spectral analysis applies to both heterogeneous and homogeneous tissues; it does not require prior assumptions concerning number of tissue compartments. Parameters estimated with spectral analysis can be strongly affected by noise, but numerical filters improve estimation performance. Spectral analysis with iterative filter (SAIF) was originally developed to improve estimation of leucine kinetic parameters and rCPS in region-of-interest (ROI) data analyses. In the present study, we optimized SAIF for application at the voxel level. In measured L-[1-11C]leucine PET data, voxel-level SAIF parameter estimates averaged over all voxels within a ROI (mean voxel-SAIF) generally agreed well with corresponding estimates derived by applying the originally developed SAIF to ROI time-activity curves (ROI-SAIF). Region-of-interest-SAIF and mean voxel-SAIF estimates of rCPS were highly correlated. Simulations showed that mean voxel-SAIF rCPS estimates were less biased and less variable than ROI-SAIF estimates in the whole brain and cortex; biases were similar in white matter. We conclude that estimation of rCPS with SAIF is improved when the method is applied at voxel level than in ROI analysis.
Keywords: [11C]leucine, cerebral protein synthesis, parametric imaging, positron emission tomography, spectral analysis
Introduction
Functional quantification with positron emission tomography (PET) is generally based on kinetic modeling approaches that relate a particular biological process of interest to measurements of activity in blood and tissue following administration of a radiolabeled tracer. Kinetic models used in PET are necessarily simplified representations of tissue processes, and one of the simplifying assumptions frequently made is that tissue regions are kinetically homogeneous, that is, rates of blood flow, delivery and efflux of tracer to/from tissue, metabolism, and incorporation into labeled products do not vary in the tissue region. In the brain, these assumptions are difficult to meet. At spatial resolutions approximately an order of magnitude higher than PET, such as achieved in autoradiographic studies, one clearly sees heterogeneity of rates of blood flow, glucose metabolism, and protein synthesis across the brain (Schmidt and Smith, 2005). Not only are rates of these processes different in gray and white matter, but within gray matter structures themselves, for example in cortical layers, rates of these processes can vary considerably. At the relatively lower spatial resolution of PET scanning, therefore, activities measured in the brain can be expected to originate from kinetically heterogeneous mixtures of tissue. Application of kinetic models designed for homogeneous tissues to heterogeous tissues leads to errors in estimated rates of cerebral blood flow and glucose metabolism, as well as to errors in estimates of receptor binding parameters (Schmidt and Turkheimer, 2002).
Protein synthesis in the nervous system is a fundamental process essential for adaptive responses such as long-term memory formation. With L-[1-11C]leucine and PET, it is possible to fully quantify the measurements of regional rate of cerebral protein synthesis (rCPS) in human subjects (Schmidt et al, 2005; Smith et al, 2005). Quantification of rCPS with the L-[1-11C]leucine PET method was initially carried out at the region-of-interest (ROI) level and was based on a kinetic model that assumes tissue ROIs are kinetically homogeneous (Schmidt et al, 2005); effects of tissue heterogeneity were not taken into account.
We recently reported two approaches to address effects of tissue heterogeneity on rCPS estimated in [11C]leucine PET studies: reducing the size of tissue regions by using a voxel-by-voxel analysis, while retaining the tissue homogeneity assumption (Tomasi et al, 2009) and, conversely, by keeping data analysis at the ROI level, but employing a spectral analytic approach that applies to heterogeneous as well as homogeneous tissues (Veronese et al, 2010a). Use of the voxelwise analysis reduced, but did not entirely eliminate, effects of tissue heterogeneity visible in model fits of measured tissue time-activity curves (TACs) (Tomasi et al, 2009). The spectral analytic approach, spectral analysis with iterative filter (SAIF), detected heterogeneity in all ROIs examined (Veronese et al, 2010a). Not all parameters are identifiable in heterogeneous tissue, however, without use of parameter constraints. Specifically, determination of rCPS requires an estimate of the fraction of unlabeled leucine in the precursor pool for protein synthesis that is derived from arterial plasma. This quantity is defined as λ. In a homogeneous tissue, λ can be calculated directly from kinetic model or SAIF parameters, whereas in a heterogeneous tissue values of λ in different tissue subregions are not individually identifiable. As a first approximation, we introduced the constraint that values of λ in all tissue subregions are equal, and on that basis λ and rCPS in heterogeneous tissue were estimated. Under this constraint, differences between rCPS estimated with SAIF at the ROI level and rCPS estimated by homogenous voxelwise analysis were small (mean relative difference ∼2%), but rCPS determined with SAIF had a tendency to be somewhat lower in the whole brain and cortical regions, and higher in some subcortical and white matter regions, than rCPS determined by voxelwise analysis (Veronese et al, 2010a). The equality constraint on λ was understood to be an imperfect approximation, as parametric maps of λ show some spatial variation (Tomasi et al, 2009), but sensitivity analysis suggested that the use of the constraint should have only a small effect on calculated rCPS (Veronese et al, 2010a).
In the current study, we investigated the possibility of adapting SAIF to estimate parameters of the [11C]leucine kinetic model on a voxel-by-voxel basis. Our aim was to develop a method to deal with heterogeneity at the voxel level, the degree of which may be dependent on the resolution of the PET scanner. Reducing the size of the tissue volume examined may also reduce effects arising from the equality constraint on the λs in all subregions of heterogeneous tissues. Due to vastly different signal-to-noise ratios in voxel and ROI data, SAIF developed for ROI analysis could not be directly used for voxelwise estimation. In the present study, the SAIF method was optimized for high levels of noise typical of voxelwise data, and simulations were performed to assess precision and accuracy of parameter estimates. We also investigated the capacity of SAIF to correctly identify the number of kinetic components in each voxel. The method was then used to reanalyze data from previously acquired L-[1-11C]leucine PET studies in six awake healthy young men.
Materials and methods
Spectral Analysis Iterative Filter
The spectral analysis method is based on a single input–single output model used to identify kinetic components of tissue tracer activity (Cunningham and Jones, 1993; Turkheimer et al, 1994). The system output, concentration of radioactivity in tissue, Ctiss*(t), is described by the convolution of the input function, that is, plasma concentration of parent tracer, Cp*(t), with an exponential transfer function that has real-valued, nonpositive exponents. That is,
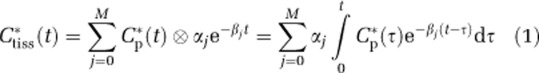 |
where αj and βj are assumed to be real-valued and nonnegative. Not all compartmental models satisfy these assumptions, but they are met by the kinetic models used with many PET tracers (Schmidt, 1999) including L-[1-11C]leucine (Veronese et al, 2010a). In spectral analysis, the maximum number of terms to be included in the model and the possible values of βj are preset, and the αj are estimated from measured blood and tissue TACs (Cunningham and Jones, 1993). The estimated spectrum of kinetic components, together with knowledge of the tracer's biochemical and/or physiological properties, is then related to the process of interest. The number of compartments in the kinetic model of the tracer is also obtained (Bertoldo et al, 1998).
From equation (1) one observes that ‘high-frequency' components (βj very large) equilibrate rapidly with and become proportional to Cp*(t), whereas ‘low-frequency' components (βj≅0) are proportional to ∫Cp*(t)dt and account for trapping of tracer. Components with intermediate values of βj reflect tissue compartments that exchange material directly or indirectly with plasma; these are referred to as equilibrating components. Noise in the data greatly influences the accuracy with which very low- and high-frequency components can be detected, and the problem is exacerbated when the method is applied where the signal-to-noise ratio is low. In these cases, application of numerical filters becomes essential. Spectral analysis iterative filter, previously developed for estimating rCPS from region-of-interest data (Veronese et al, 2010a), was investigated in the present study for use with voxelwise estimation. Spectral analysis with iterative filter uses a passband filter [βL,βU] to separate trapping and blood components from equilibrating components. The passband lower limit βL represents the smallest exponent β for a component that can be distinguished from trapping of tracer, and the upper limit βU is the largest exponent of a component that can be distinguished from blood in the brain. The passband limits are determined based on prior information on expected tracer kinetics, data sampling schedule, and noise in the data. As in the previous study, we utilized 100 logarithmically distributed values βj between 0.0038 and 1.3 min−1 in the estimations (Veronese et al, 2010a). Spectral analysis with iterative filter conserves the main characteristics of spectral analysis—it does not require any prior assumptions concerning number of compartments in the system, and it can be applied to heterogeneous as well as homogeneous tissues. The added benefit of SAIF is that it improves estimates of both α0 and 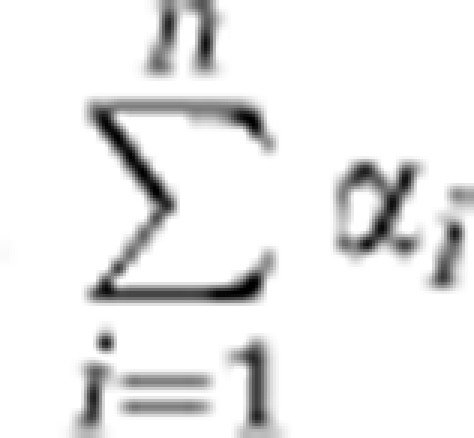 whose accurate estimation is essential for quantification of rCPS (Veronese et al, 2010a).
whose accurate estimation is essential for quantification of rCPS (Veronese et al, 2010a).
Leucine Kinetic Model
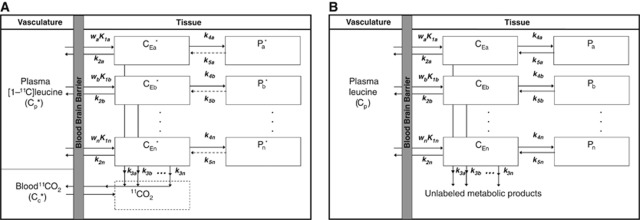
The model for L-[1-11C]leucine that explicitly allows for tissue heterogeneity is shown in Figure 1A. The model assumes that each tissue is composed of n subregions (n⩾1), and the kinetics of L-[1-11C]leucine in each subregion are described by the homogeneous tissue model given in Schmidt et al (2005). Activity in the tissue as a whole is a convex combination of activity in its subregions. The model used to describe labeled leucine holds also for unlabeled leucine (Figure 1B), except that unlabeled leucine and protein are in steady state. Assuming no isotope effect, rate constants for unlabeled and labeled leucine are identical. The steady-state breakdown of unlabeled protein is greater than zero.
Figure 1.
(A) Heterogeneous tissue model for L-[1-11C]leucine. The heterogeneous tissue is composed of n kinetically homogeneous subregions. The constants wa, wb, …, wn represent relative tissue weights of the subregions, where wa+wb+…+wn=1. In each subregion i, K1i and k2i are the rate constants for transport of leucine from plasma to tissue and back, respectively. k3i is the rate constant for the first two steps in leucine catabolism, transamination, and decarboxylation, which yield 11CO2. k4i and k5i are the rate constants for leucine incorporation into protein and for the release of free leucine from proteolysis, respectively. Because of the long half-life of protein in the brain (Lajtha et al, 1976), it is assumed that there is no significant breakdown of labeled product (Pi*) during the experimental interval, that is, k5iPi*∼0. Under the assumptions of negligible fixation of 11CO2 during the experimental period (Siesjo and Thompson, 1965) and rapid equilibration of 11CO2 between the brain and blood (Buxton et al, 1987), the model for each subregion reduces to two-tissue compartments (free L-[1-11C]leucine, CEi*, and L-[1-11C]leucine incorporated into tissue protein, Pi*) plus the 11CO2 compartment in which the concentration is assumed known. In each subregion, the kinetics of leucine are described by the homogeneous tissue model given in Schmidt et al (2005), which assumes that the concentrations of amino acids, rates of blood flow, rates of transport and metabolism of amino acids, and rates of leucine incorporation into protein do not vary within the subregion. Total tracer activity 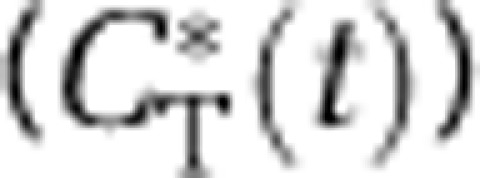 in the volume is given by
in the volume is given by
where Vb is the fraction of the volume occupied by blood; Cb* is the total 11C concentration in whole blood; VD is the brain:blood equilibrium distribution volume of 11CO2; and Cc* is the 11CO2 concentration in whole blood. VD is fixed at 0.41 (Smith et al, 2005; Brooks et al, 1984). (B) Heterogeneous tissue model for unlabeled leucine. The model used to describe labeled leucine (A) also holds for unlabeled leucine. Assuming no isotope effect, rate constants for labeled and unlabeled leucine are identical. The model for unlabeled leucine in heterogeneous tissue is composed of n homogeneous subregions; in each homogeneous tissue the unlabeled leucine (CEi) and protein (Pi) are assumed to be in steady state, and the steady-state breakdown unlabeled protein (k5iPi) is greater than zero. Therefore, the weighted average of regional rate of cerebral protein synthesis (rCPS) is
where Cp is the concentration of unlabeled leucine in arterial plasma and the rate constants are defined above (Schmidt et al, 2005; Veronese et al, 2010a). The fraction of unlabeled leucine in the precursor pool for protein synthesis in subregion i that is derived from arterial plasma, λi, can be expressed as
and the weighted average influx rate constant for the mixed tissue, K1, as
When total activity in a brain region or voxel following injection of L-[1-11C]leucine is written in terms of the spectral analysis parameters (symbols are defined as follows: CT*, total 11C concentration in volume (region or voxel); Vb, fraction of volume occupied by blood; Cp*, concentration of L-[1-11C]leucine in arterial plasma; Cb*, total 11C concentration in whole blood; VD, brain:blood equilibrium distribution volume of 11CO2 (fixed at 0.41); Cc*, 11CO2 concentration in whole blood; K1i rate constant in ith subregion for transport of leucine from plasma to tissue; k2i, rate constant in ith subregion for transport of leucine from tissue to plasma; k3i, rate constant in ith subregion for the first two steps in leucine catabolism, transamination, and decarboxylation, which yield 11CO2; k4i, rate constant in ith subregion for leucine incorporation into protein; wi, relative tissue weight of ith subregion; n, number of homogeneous tissue subregions), we obtain (Veronese et al, 2010a)
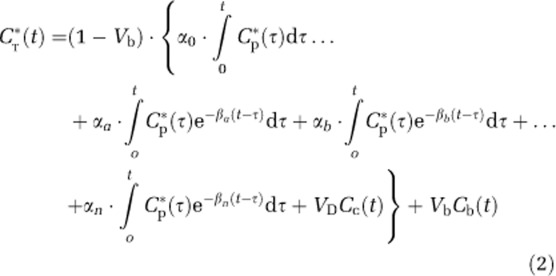 |
where
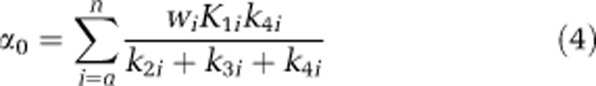 |
Spectral analysis with iterative filter is used to estimate n and the spectral analysis parameters α0, αa, αb, …, αn, βa, βb, …, βn, and Vb. From these parameters, the leucine parameters of interest can be determined. The latter include the weighted average influx rate constant for the mixed tissue, 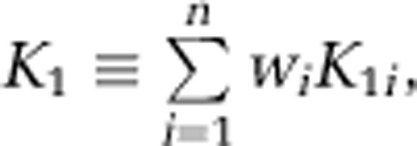 which can be estimated as
which can be estimated as
 |
The fraction of unlabeled leucine in the precursor pool for protein synthesis in tissue subregion i that is derived from arterial plasma, λi≡(k2i+k3i)/(k2i+k3i+k4i) is identified for each subregion under the constraint that λa=λb=…=λn=λ, as
 |
Finally, rCPS in the mixed tissue can be computed as
 |
where Cp is the arterial plasma concentration of unlabeled leucine.
Simulation Studies
Simulation 1: Spectral analysis with iterative filter passband: Due to the large discrepancy in signal-to-noise ratio between voxel and ROI TACs, the equilibrating component passband optimized for ROI analysis may not be optimal for voxelwise quantification. To define the passband interval bounds [βL,βU], we followed a simulation strategy similar to that used for application of SAIF at the ROI level (Veronese et al, 2010a). The procedure consisted of simulating noisy voxel-TACs that were subsequently analyzed by SAIF with different passband intervals. The optimal passband was chosen as the one that provided the best trade-off between precision and accuracy of the estimates of rCPS and other parameters of interest.
Noise-free voxel-TACs were simulated according to a heterogeneous kinetic model, with two equilibrating components and one trapping component. Model parameters were generated by the random sampling of normal distributions as follow: Vb=0.064±0.009 (unitless), α0=0.012±0.002 (mL g−1 min−1), α1=0.0096±0.0004 (mL g−1 min−1), α2=0.0197±0.0015 (mL g−1 min−1), β0=0 (min−1), β1=0.032±0.004 (min−1), and β2=0.15±0.03 (min−1). These values are the intersubject mean values±s.d. of spectra estimated with ROI-SAIF in whole brain (Veronese et al, 2010a). One thousand noisy TACs were simulated by adding to the noise-free simulated TACs Gaussian noise with zero-mean and variance consistent with the level of noise in voxel data, as estimated in Tomasi et al (2009). Different passband intervals (βL=0.005 to 0.06 min−1; βU=0.1 to 0.6 min−1) were tested. To select the best interval, performance indices percent bias (Bias%) and percent root mean square error (RMSE%) were computed as
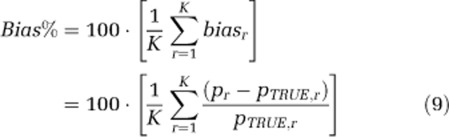 |
and
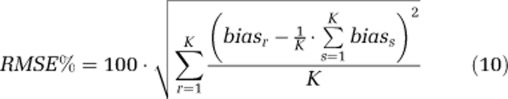 |
where pr represents the rth SAIF parameter estimate, pTRUE,r indicates the true parameter value for the rth simulated TAC, biasr indicates bias for the rth simulated TAC, and K indicates total number of simulated TACs. Any noise realization that resulted in a negative entry in the simulated TAC was discarded and replaced with a new realization.
Simulation 2: performance of voxelwise spectral analysis with iterative filter: Once the choice of passband was made, a new simulation was performed to quantify the effect of noise on estimated parameters of interest. In these simulations, a bootstrap approach was used as an alternative to random noise generation with a Gaussian model.
To simulate data as close as possible to that measured in a previously acquired PET study, we proceeded as follows:
Using the optimal passband interval obtained in Simulation 1, data measured in a representative slice of an individual brain volume, masked to include brain voxels only, were analyzed voxel-by-voxel with SAIF. The set of parameters obtained became the reference, or ‘true,' parameter values used in the simulations. Outliers and algorithm failures produced at this step were excluded from the simulation.
- Noise-free TACs were generated by convolving the original measured arterial input function of the subject with the sum of exponential terms defined by the reference parameters from step 1. In addition, for each voxel, normalized residuals Ri(t) were computed as:
where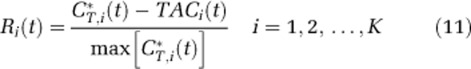
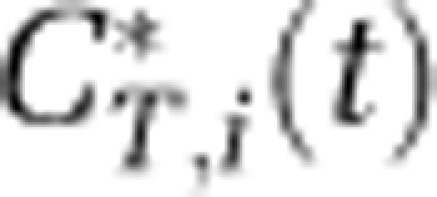 represents total concentration of 11C in the field of view of the PET camera for the ith voxel, TACi(t) indicates the noise-free TAC for the ith voxel, and K is the number of voxels in the chosen slice.
represents total concentration of 11C in the field of view of the PET camera for the ith voxel, TACi(t) indicates the noise-free TAC for the ith voxel, and K is the number of voxels in the chosen slice. - Voxel noise was simulated using a bootstrap approach (Efron, 1979) based on random resampling of normalized residuals. With this approach, noise for the i-th voxel (Ni(t)) was simulated as
where Rj(t) is the resampled normalized residual, originally associated with the jth voxel, chosen by random sampling with replacement from among all brain voxels of the slice.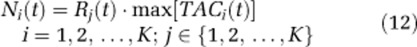
The procedure was repeated 50 times, generating 50 different bootstrap-simulated slices. Note that the bootstrap procedure used here differs from the usual approach of normalizing each residual vector by its standard deviation in order to assure that all resampled residuals have equal variance. In this approach, the required rescaling would be by the standard deviation in the target voxel residual. In the current study, however, we do not have good estimates of the variance in each individual voxel due to the inherent high noise levels of voxel data. Instead, we empirically chose normalization and rescaling to the maxima of the TACs, as these scale factors have the added advantage of preventing negative values in the simulated noisy TACs. The sample variances of the original and bootstrapped residuals were compared to check the effect of the chosen scale factors.
All 50 bootstrap-simulated slices were analyzed with the voxelwise SAIF approach. Spectral analysis with iterative filter applied at the voxel level with the passband interval optimized for voxel analyses will be referred to as voxel-SAIF. We looked at three regions: the whole slice and two ROIs placed on the slice (frontal cortex and corona radiata). As in Simulation 1, to assess the quality of voxel-SAIF results, for each region and each parameter of interest, Bias% and RMSE% were computed. The number of voxels with estimated parameter values considered to be outside the normal physiological range, that is, those with K1>1 mL g−1 min−1, Vb>1, or rCPS>10 nmol g−1 min−1, was recorded (all SAIF parameter estimates are already restricted to be nonnegative by algorithm design), and outlier voxels were excluded from bias and RMSE statistics. For comparison, we also averaged voxel-TACs in each bootstrap-simulated slice over the three regions to obtain ROI TACs, which were then analyzed with SAIF using the passband interval optimized for ROI data (ROI-SAIF) (Veronese et al, 2010a).
We also investigated the capacity of voxel-SAIF to correctly identify the number of kinetic components in each voxel by comparing the number of tissue equilibrating components in the reference data with the number detected in bootstrap-simulated slices.
Positron Emission Tomography Studies
Data from previously reported studies (Bishu et al, 2008, 2009) of six healthy awake male subjects were reanalyzed in the current study. Subject inclusion criteria and the PET study procedure are described in detail in Bishu et al (2008). Briefly, all subjects underwent 90-minute dynamic L-[1-11C]leucine scan on a ECAT High-Resolution Research Tomograph, with a spatial resolution of ∼2.6 mm full width at half maximum (Wienhard et al, 2002). Images were reconstructed and motion-corrected (Carson et al, 2003) as 42 frames of data (16 × 15, 4 × 30, 4 × 60, 4 × 150, 14 × 300 seconds); voxel size was 1.21 × 1.21 × 1.23 mm3. Arterial blood was sampled over the course of the study and concentrations of unlabeled and labeled leucine in arterial plasma and total 11C and 11CO2 activities in whole blood were measured (Bishu et al, 2008). All subjects underwent a T1-weighted brain MRI (magnetic resonance imaging) for the ROI placement. The MRI was registered to the mean of the 30 to 60 minutes PET leucine data, and ROI masks were resliced on PET space (Tomasi et al, 2009).
The whole brain and 12 regions were evaluated in all subjects. Estimates of Vb, K1, λ, and rCPS were determined in each ROI and in each voxel by using ROI-SAIF and voxel-SAIF, respectively. Weights were inversely proportional to the variance of decay-corrected measured activity in the region, which was modeled assuming Poisson statistics as
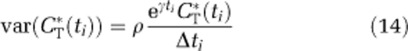 |
(Wu and Carson, 2002), where γ is the decay constant for 11C, Δti is length of Frame i, and ρ is a proportionality coefficient reflecting the noise level in the data estimated a posteriori (Veronese et al, 2010a). For voxel-SAIF, weights were based on whole brain activity; in ROI-SAIF, weights were based on activity in the region itself. Tracer arrival delay was calculated as in Veronese et al (2010a).
For all subjects, ROI-SAIF estimates were compared with the mean of voxel-SAIF estimates in all voxels of the region. To assess agreement between methods, we calculated the slope and intercept of the fitted regression line with ROI-SAIF estimates and mean voxel-SAIF estimates in each region and each subject as independent and dependent variables, respectively. Pearson's R2 values were added as correlation measures.
We also visually examined the spatial distribution of voxels found to be kinetically heterogeneous. The ‘heterogeneity map' was constructed by fusing the MR image with a map of the spatial distribution of voxels with two or more SAIF-estimated equilibrating components.
Results
Simulation 1: Spectral Analysis with Iterative Filter Passband
In simulations performed to find an appropriate filter passband, rCPS bias ranged from 3% to 25%, depending on the cutoff frequencies βL and βU. Root mean square error ranged from 15% to 35% for rCPS estimates, and from 8% to 38% for the other parameters of interest, that is, K1, λ, and Vb. Due to the high level of noise typical of voxel-TACs, these values were much higher than those obtained with ROI-SAIF (bias in rCPS: 0.03% to 7% RMSE 2.4% to 21.5% (Veronese et al, 2010a)). For voxel-SAIF, we chose 0.02 min−1 and 0.2 min−1 for βL and βU, respectively, as a good compromise between precision and accuracy of rCPS (bias 3% RMSE 18%) and λ (bias 2% RMSE 8%). With this passband interval, K1 and Vb displayed, respectively, 8% and 6% bias, and 14% and 28% RMSE. This choice for the voxel-SAIF passband interval differs from that made for ROI-SAIF (βL=0.03 min−1; βU=0.3 min−1) (Veronese et al, 2010a); when the optimal ROI passband was applied to voxelwise analysis bias exceeded 5% for all parameters of interest.
In the following text, voxel-SAIF refers to SAIF applied at voxel level with its optimal passband of [0.02, 0.2] min−1, and ROI-SAIF refers to SAIF applied at the ROI level with its optimal passband interval of [0.03, 0.3] min−1.
Simulation 2: Performance of Voxel-Spectral Analysis with Iterative Filter
Less than 1% of brain voxels in the slice were excluded from the bootstrap simulations due to outlier parameter estimates or failure of the voxel-SAIF algorithm to converge. Table 1 provides results of the bootstrap simulations. Mean values and s.d. of voxel-SAIF bootstrap estimates were in good agreement with parameter mean values and s.d. of the simulated reference values. Bias% and RMSE% of voxel-SAIF estimates for Vb, K1, λ, and rCPS determined in 50 bootstrap simulations for three ROIs are also provided. Bias (<2%) and RMSE (<8%) were both low in estimates of λ. Estimates of rCPS had limited biases (3.9% to 6.0%) and variability consistent with the high level of noise typical of voxel-TACs (RMSE 17.3% to 19.7%). Blood volume, Vb, was also estimated with good accuracy (maxBias% 6.2%) but not high precision (maxRMSE% 21.5%), particularly in corona radiata. Similar results were obtained for K1 (maxBias% 9.2% maxRMSE% 12.7%). Normalizing and rescaling led to a small reduction (mean: 5% range: 0% to 13%) in the standard deviation of the resampled residual values compared to those of the reference data set (see Supplementary Figure).
Table 1. Performance of SAIF algorithm in bootstrap simulationa.
| Regionb | Vb (unitless) | K1 (mL g−1 min−1) | λ (unitless) | rCPS (nmol g−1 min−1) |
|---|---|---|---|---|
| Whole slice (11,081 voxels) | ||||
| Simulated reference valuesc | 0.068±0.050 | 0.048±0.017 | 0.68±0.14 | 1.68±0.92 |
| Voxel-SAIF estimatesd | 0.066±0.049 | 0.051±0.019 | 0.68±0.14 | 1.73±0.89 |
| Voxel-SAIF Bias%e | 0.1% | 7.2% | 0.9% | 4.7% |
| Voxel-SAIF RMSE%f | 17.2% | 12.0% | 7.4% | 19.7% |
| Regional voxel-SAIF estimatesg | 0.066±0.0001 | 0.051±0.0001 | 0.68±0.001 | 1.73±0.003 |
| ROI-SAIF estimatesh | 0.068±0.0002 | 0.049±0.0002 | 0.69±0.002 | 1.54±0.013 |
| Frontal cortex (1,166 voxels) | ||||
| Simulated reference valuesc | 0.076±0.043 | 0.055±0.023 | 0.67±0.24 | 2.00±1.00 |
| Voxel-SAIF estimatesd | 0.074±0.042 | 0.059±0.025 | 0.67±0.24 | 2.04±1.00 |
| Voxel-SAIF Bias%e | −2.4% | 6.5% | 0.9% | 3.9% |
| Voxel-SAIF RMSE%f | 12.5% | 10.9% | 7.1% | 17.3% |
| Regional voxel-SAIF estimatesg | 0.074±0.0002 | 0.059±0.0002 | 0.67±0.001 | 2.04±0.007 |
| ROI-SAIF estimatesh | 0.075±0.0004 | 0.055±0.0004 | 0.69±0.004 | 1.78±0.025 |
| Corona radiata (1,837 voxels) | ||||
| Simulated reference valuesc | 0.036±0.020 | 0.030±0.011 | 0.71±0.25 | 0.91±0.54 |
| Voxel-SAIF estimatesd | 0.038±0.022 | 0.033±0.013 | 0.71±0.25 | 0.96±0.47 |
| Voxel-SAIF Bias%e | 6.2% | 9.2% | 1.6% | 6.0% |
| Voxel-SAIF RMSE%f | 21.5% | 12.7% | 6.4% | 19.2% |
| Regional voxel-SAIF estimatesg | 0.038±0.0003 | 0.033±0.0001 | 0.71±0.001 | 0.96±0.005 |
| ROI-SAIF estimatesh | 0.041±0.0005 | 0.032±0.0004 | 0.71±0.008 | 0.94±0.028 |
rCPS, regional rate of cerebral protein synthesis; RMSE, root mean square error; ROI, region of interest; SAIF, spectral analysis with iterative filter; Vb, fraction of volume occupied by blood.
Number of bootstrap realizations=50.
Regions were limited to particular slice used for the bootstrap simulation.
Values are mean±intervoxel s.d. of the simulated voxel parameters within the region.
Values are mean±intervoxel s.d. of the estimated SAIF-voxel parameters from bootstrap simulations.
Values are mean values of SAIF-voxel parameter bias% calculated over the bootstrap simulations.
Values are mean values of SIAF-voxel parameter RMSE% calculated over the bootstrap simulations.
Values are mean±s.d. of the mean SAIF-voxel parameter estimates within the region, calculated over the bootstrap simulations.
Values are mean±s.d. of the ROI-SAIF parameter estimates calculated over the bootstrap simulations.
Table 1 also shows comparisons between voxel-SAIF estimates for the parameters, averaged over each ROI, and the corresponding parameters estimated with ROI-SAIF. Estimates of λ, Vb, and K1 show good agreement between voxel and ROI methods. Regional rate of cerebral protein synthesis, which has a small positive bias (⩽6%) when estimated on a voxel-by-voxel basis, is underestimated with ROI-SAIF in the whole slice and frontal cortex by 8% and 11%, respectively. Variability of estimates of λ and rCPS among the 50 bootstrap simulations is lower for the mean of the voxel-SAIF estimates than those determined with ROI-SAIF.
Spectral Analysis with Iterative Filter Application to Measured Positron Emission Tomography Data
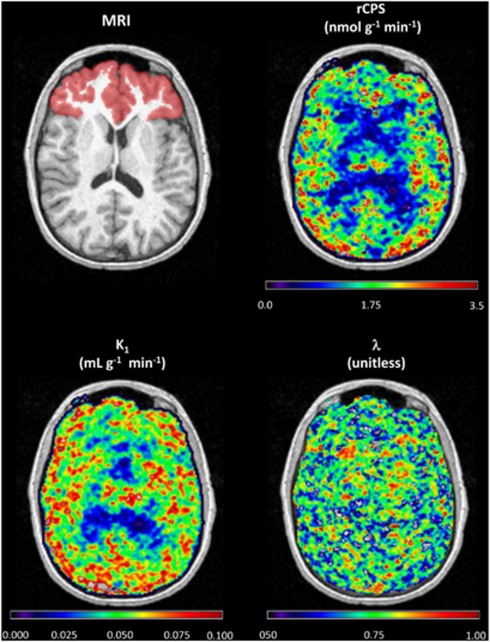
In measured PET data, the percentage of voxels in a given ROI in which voxel-SAIF either failed to converge or produced nonphysiological estimates, averaged over all subjects, ranged from 0.7% (Putamen) to 2.9% (Amygdala), depending on the brain area. The mean fraction of outlier voxels over all brain regions was 1.3%, and ∼0.2% of voxels were excluded for failure to converge. The percentage of voxels included in the analysis was >95% for all subjects and regions. In whole brain 0.5% of voxels and in white matter 0.8% of voxels (mean over all subjects) had rates of protein synthesis not detectably different from zero. Figure 2 shows parametric maps for rCPS, λ, and K1 in a representative transaxial slice at level of frontal cortex in one subject. The subject's MRI is also shown. In this slice, rCPS was 1.73±0.96 nmolg−1 min−1 (mean±s.d. over all brain voxels), 5th to 95th percentile range 0.04 to 3.01 nmolg−1 min−1. Regional rate of cerebral protein synthesis shows the well-known pattern with areas of white matter tending to have lower rCPS than gray matter. Voxel-SAIF estimates of λ were 0.68±0.11 (mean±s.d.), corresponding to intraslice variability of ∼16%. Although noisy, the λ image shows a tendency of higher values in white than gray matter areas. The 5th to 95th percentile range of K1 estimates was 0.023 to 0.064 mLg−1 min−1; there is a clear spatial distribution of higher and lower values concentrated in gray and white matter, respectively.
Figure 2.
Magnetic resonance imaging (MRI) and parametric maps of regional rate of cerebral protein synthesis (rCPS), λ, and K1 obtained with voxel-spectral analysis with iterative filter (SAIF). Images are of a representative transaxial slice for one subject. Red area in the MRI image represents the frontal cortex region for the particular slice. A Gaussian filter (full width at half maximum (FWHM) 1.7 mm) was employed to smooth each of the parametric images in three dimensions before visualization.
Comparison of Voxel-Spectral Analysis with Iterative Filter and ROI-Spectral Analysis with Iterative Filter in Measured Positron Emission Tomography Data
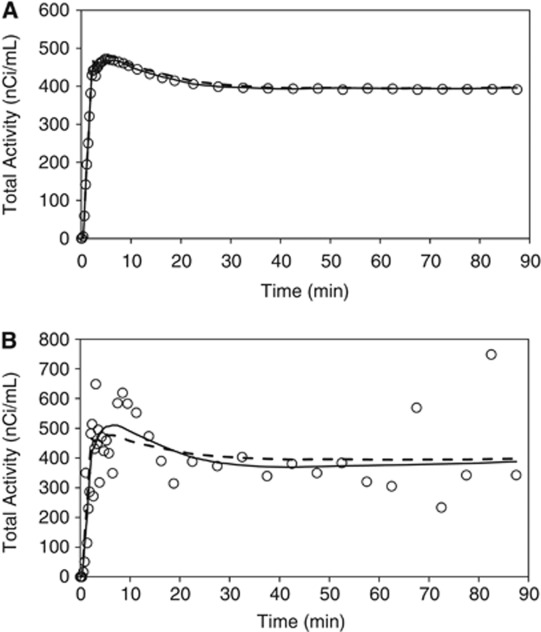
Spectral analysis with iterative filter fits of ROI and voxel-TACs are shown in Figure 3. Overall, good agreement is seen when the mean of voxel-SAIF fits is compared with the ROI-SAIF fit of ROI TAC (Figure 3A). There are, however, small discrepancies: the mean voxel-SAIF fit slightly overestimates the measured activity between ∼2 and 30 minutes, whereas the ROI-SAIF fit has a slight underestimation ∼2 to 8 minutes and a very slight overestimation from ∼15 to 25 minutes. After ∼30 minutes, fits of the two methods are indistinguishable. The measured single voxel-TAC shown in Figure 3B illustrates the very high noise level present in voxel data and the variability between individually fit voxel-TACs (solid line) and the mean of all voxel-TACs in the region (dashed line).
Figure 3.
(A) Kinetic model fit of a region-of-interest (ROI) time-activity curve (TAC). Open circles (○) indicate activity measured in the whole brain region of one subject. Solid line represents activity estimated by fitting the ROI TAC with spectral analysis with iterative filter (SAIF). Dashed line represents mean of the fitted voxel-TACs within the ROI. (B) Kinetic model fit of a single voxel-TAC in the frontal cortex. Open circles (○) indicate activity measured in a single brain voxel. Solid line represents activity estimated by fitting the voxel-TAC with SAIF. Dashed line represents mean of the fitted voxel-TACs within the ROI.
Table 2 compares regional mean voxel-SAIF estimates with estimates determined with ROI-SAIF in measured PET data. Mean values and standard deviations for six subjects are reported for 12 ROIs and whole brain. There was excellent agreement between methods in estimates of Vb, K1, and λ; mean relative differences were 1%±4%, −1%±4%, and −2%±2% (mean±s.d. among regions) for Vb, K1, and λ, respectively (Table 2). Except in corona radiata, estimates of rCPS were somewhat higher with voxel-SAIF compared with ROI-SAIF; the mean percent difference between methods ranged from 2% (putamen) to 16% (occipital cortex), with average over all regions ∼7.7%. Differences between voxel- and ROI-SAIF estimates of rCPS tended to be larger in cortical than in subcortical or white matter regions.
Table 2. Regional estimates of Vb, K1, λ, and rCPSa.
|
Voxel-SAIFb | |||||
|---|---|---|---|---|---|
| Region | Region size (number of voxels) | Vb | K1 (mL g−1 min−1) | λ | rCPS (nmol g−1 min−1) |
| Whole brain | 808,068±88,180 | 0.073±0.010 | 0.043±0.007 | 0.73±0.02 | 1.77±0.07 |
| Cerebellum | 75,640±6,080 | 0.094±0.013 | 0.053±0.009 | 0.72±0.02 | 2.21±0.10 |
| Cortical | |||||
| Frontal cortex | 85,452±17,015 | 0.073±0.013 | 0.047±0.008 | 0.71±0.03 | 2.02±0.06 |
| Temporal cortex | 42,125±6,437 | 0.075±0.011 | 0.044±0.007 | 0.71±0.02 | 1.90±0.09 |
| Occipital cortex | 26,971±10,079 | 0.090±0.017 | 0.055±0.008 | 0.72±0.03 | 2.30±0.14 |
| Parietal cortex | 13,127±3,241 | 0.079±0.014 | 0.048±0.007 | 0.71±0.02 | 2.10±0.14 |
| Subcortical | |||||
| Thalamus | 12,757±1,862 | 0.073±0.012 | 0.043±0.008 | 0.74±0.01 | 1.59±0.10 |
| Caudate | 6,038±784 | 0.050±0.009 | 0.035±0.007 | 0.76±0.02 | 1.12±0.11 |
| Putamen | 6,488±794 | 0.061±0.011 | 0.047±0.010 | 0.78±0.01 | 1.37±0.06 |
| Hippocampus | 3,709±1,080 | 0.088±0.014 | 0.034±0.006 | 0.69±0.03 | 1.68±0.19 |
| Amygdala | 3,522±516 | 0.069±0.012 | 0.031±0.004 | 0.68±0.03 | 1.60±0.12 |
| White matter | |||||
| Corona radiata | 32,655±3,350 | 0.035±0.007 | 0.027±0.005 | 0.76±0.03 | 0.90±0.06 |
| Cerebellar peduncles | 9,554±1,227 | 0.054±0.010 | 0.036±0.007 | 0.75±0.02 | 1.25±0.10 |
|
ROI-SAIFc | ||||
|---|---|---|---|---|
| Region | Vb | K1 (mL g−1 min−1) | λ | rCPS (nmol g−1 min−1) |
| Whole brain | 0.074±0.011 | 0.045±0.007 | 0.75±0.03 | 1.58±0.10 |
| Cerebellum | 0.095±0.012 | 0.056±0.010 | 0.75±0.02 | 2.00±0.18 |
| Cortical | ||||
| Frontal cortex | 0.070±0.013 | 0.048±0.009 | 0.74±0.03 | 1.76±0.05 |
| Temporal cortex | 0.076±0.012 | 0.043±0.007 | 0.72±0.02 | 1.75±0.12 |
| Occipital cortex | 0.085±0.016 | 0.057±0.008 | 0.75±0.03 | 1.99±0.12 |
| Parietal cortex | 0.074±0.014 | 0.050±0.007 | 0.74±0.03 | 1.87±0.25 |
| Subcortical | ||||
| Thalamus | 0.070±0.012 | 0.044±0.008 | 0.77±0.02 | 1.39±0.05 |
| Caudate | 0.051±0.009 | 0.033±0.007 | 0.77±0.03 | 1.07±0.07 |
| Putamen | 0.059±0.011 | 0.046±0.008 | 0.78±0.02 | 1.34±0.07 |
| Hippocampus | 0.089±0.014 | 0.032±0.006 | 0.68±0.03 | 1.59±0.24 |
| Amygdala | 0.073±0.012 | 0.030±0.004 | 0.68±0.04 | 1.51±0.12 |
| White matter | ||||
| Corona radiata | 0.037±0.007 | 0.027±0.006 | 0.75±0.03 | 0.93±0.06 |
| Cerebellar peduncles | 0.052±0.009 | 0.039±0.007 | 0.78±0.03 | 1.16±0.18 |
|
Mean relative differenced
and correlatione | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region | Vb |
K1 |
λ |
rCPS |
||||
| mrd | R2 | mrd | R2 | mrd | R2 | mrd | R2 | |
| Whole brain | 0% | 0.99 | −4% | 0.99 | −3% | 0.81 | 12% | 0.51 |
| Cerebellum | −1% | 0.99 | −5% | 1.00 | −4% | 0.89 | 10% | 0.81 |
| Cortical | ||||||||
| Frontal cortex | 4% | 0.99 | −1% | 1.00 | −4% | 0.88 | 15% | 0.73 |
| Temporal cortex | −1% | 0.99 | 1% | 0.99 | −2% | 0.68 | 9% | 0.54 |
| Occipital cortex | 5% | 1.00 | −4% | 0.99 | −5% | 0.97 | 16% | 0.76 |
| Parietal cortex | 6% | 0.99 | −4% | 0.99 | −4% | 0.60 | 12% | 0.76 |
| Subcortical | ||||||||
| Thalamus | 4% | 1.00 | −4% | 0.99 | −4% | 0.85 | 14% | 0.58 |
| Caudate | −1% | 0.96 | 5% | 0.98 | 0% | 0.50 | 5% | 0.79 |
| Putamen | 3% | 0.99 | 2% | 0.99 | 0% | 0.62 | 2% | 0.89 |
| Hippocampus | −2% | 0.99 | 6% | 0.99 | 2% | 0.65 | 6% | 0.90 |
| Amygdala | −5% | 0.99 | 4% | 0.92 | 1% | 0.56 | 6% | 0.82 |
| White matter | ||||||||
| Corona radiata | −6% | 0.98 | 0% | 0.98 | 1% | 0.89 | −2% | 0.56 |
| Cerebellar peduncles | 4% | 0.98 | −9% | 0.99 | −4% | 0.87 | 8% | 0.85 |
rCPS, regional rate of cerebral protein synthesis; ROI, region of interest; SAIF, spectral analysis with iterative filter; TAC, time-activity curve; Vb, fraction of volume occupied by blood.
Values are mean±s.d. for six subjects.
Voxel-SAIF estimates based on voxel TACs and optimal voxel-SAIF filter; weights based on the whole brain TAC; parameters averaged over the volume of interest.
ROI-SAIF estimates based on the ROI TACs and optimal ROI-SAIF filter; weights based on the ROI TAC.
For each region, mean relative difference (mrd) based on the percent relative difference between voxel-SAIF and ROI-SAIF estimates, averaged among subjects.
Regional estimate correlation, between voxel-SAIF and ROI-SAIF, indicated by Pearsons's coefficient (R2).
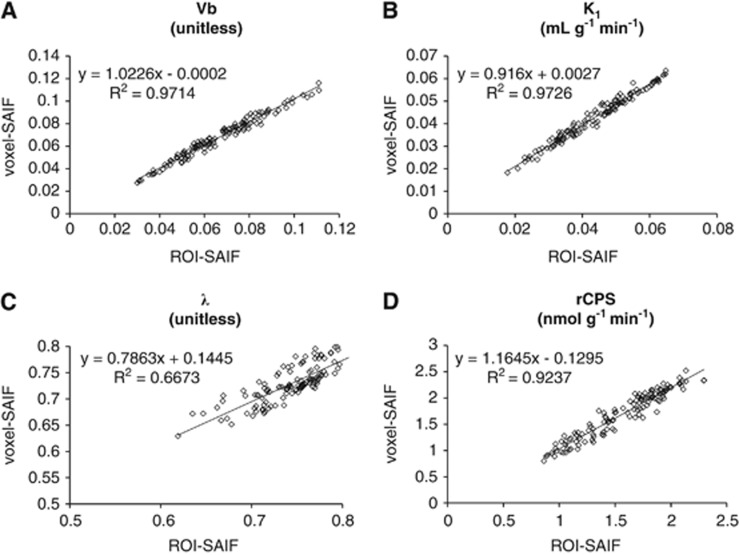
Figure 4 provides scatter plots that compare ROI-SAIF estimates of Vb, K1, λ, and rCPS with voxel-SAIF estimates averaged over the same ROI. All ROIs and all subjects are included. In each plot, the equation of the estimated regression line and Pearson's correlation coefficient are reported. Vb, K1, and rCPS show close correlation between voxel-SAIF and ROI-SAIF estimates (R2>0.92). Estimates of λ are less tightly correlated (R2=0.67).
Figure 4.
Scatter plots of regional rate of cerebral protein synthesis (rCPS) (A), λ (B), K1 (C), and Vb (D). Parameter values estimated using region-of-interest spectral analysis with iterative filter (ROI-SAIF) are plotted on the x axis and the average (over all voxels in the ROI) of the parameters estimated using voxel-SAIF are on the y axis. All subjects and ROIs are included. In each scatter plot, the slope and intercept of the fitted regression line and Pearson's R2 are reported.
Heterogeneity Analysis
From analysis of the simulated reference and bootstrap-estimated number of equilibrating components, we evaluated the capacity of voxel-SAIF to correctly determine the number of compartments in the tissue voxel. In 72.3% of voxels, the number of equilibrating components estimated with voxel-SAIF agreed with simulated reference values. In the remaining 27.7%, voxel-SAIF detected a lower (11.4%) or higher (16.3%) number of equilibrating components compared with the reference number. A trapping component was correctly detected in 100% of simulated voxel-TACs.
This behavior is similar to ROI-SAIF performance in simulation, where the accuracy rate for correct determination of number of equilibrating components was 73.1%. In the ROI data, however, ROI-SAIF detected a higher number of equilibrating components in 26% of cases and a lower number in only 1% of regions. As in the voxelwise analysis, a trapping component was correctly detected in all simulated ROIs.
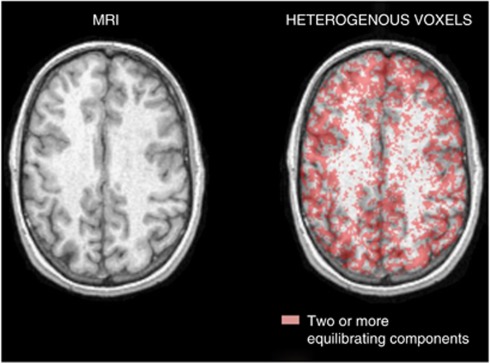
In measured data, differences in the number of detected tissue components were found between the voxelwise and ROI-level analyses, as might be expected. In ROI-SAIF one trapping component, one blood component, and at least two equilibrating components were detected in all regions and subjects. With voxel-SAIF, one trapping and one blood component were found in 100% of successfully estimated voxels, but only 45% of them included two or more equilibrating components. The finding of more than one equilibrating component suggests kinetic heterogeneity of the tissue in these voxels. In the remaining voxels, only one equilibrating component was detected, indicating the homogenous tissue model was appropriate to describe 55% of brain voxel-TACs.
Figure 5 illustrates the spatial distribution of voxels in which two equilibrating components were detected in a representative transaxial slice at the cortical level. From the distribution it appears that the concentration of kinetically inhomogenous voxels is higher at the border between gray and white matter than within the gray or white matter itself. A similar pattern was found in all subjects and brain slices.
Figure 5.
Magnetic resonance imaging (MRI) and spatial distribution of heterogeneous voxels. T1-weighted MR image (left) and the same image fused with the spatial distribution of voxels estimated to be heterogeneous (right). The red area corresponds to those voxels in which voxel-spectral analysis with iterative filter (SAIF) detects two or more equilibrating components.
Discussion
Before this study, voxelwise estimation of kinetic model parameters of L-[1-11C]leucine could only be performed by a method that assumes kinetic homogeneity of tissue within the voxel (Tomasi et al, 2009). Observed differences between measured and fitted TACs suggested that effects of tissue heterogeneity had not been entirely eliminated by performing analyses at the voxel rather than the ROI level (Tomasi et al, 2009). The SAIF method (Veronese et al, 2010a) was developed to specifically allow for tissue heterogeneity in the kinetic model for leucine used for rCPS quantification. In the present study, we explored use of SAIF for quantification of rCPS from L-[1-11C]leucine data at the voxel level to understand the possible presence of kinetic heterogeneity at the voxel level and its impact on rCPS estimates.
Setting the passband filter limits and assessing performance of SAIF at the voxel level was carried out in two simulations that used different noise generation methods. To be consistent with the strategy defined in Veronese et al (2010a) for selection of the passband, we used a Monte Carlo approach, a standard method for noise generation in simulation, widely used and easy to implement. Due to the high noise levels, however, the Monte Carlo approach led to simulated TACs in some voxels with negative activity values. To avoid negative activities in simulated TACs, we utilized bootstrapping of residuals for noise generation in the simulation to assess performance of voxel-SAIF. Negative entries in simulated TACs were avoided by normalizing reference residuals and rescaling the resampled residuals by the maxima of the associated TACs; this choice of scale factors had only a modest effect on noise variance. Results of the two types of simulation were consistent: performance indices for rCPS and other parameters were comparable. Of note, in addition to the assumption of equal residual variances discussed previously, the bootstrap approach assumes that residuals have zero-mean and are statistically independent. The zero-mean assumption was easy to verify (mean of voxel residuals: −0.002 nCi/mL). The statistical independence assumption is known to be incompletely met for voxel data: the process of PET data reconstruction leads to spatial correlation between nearby voxels, although correlation drops off rapidly as distance between voxels increases (Pajevic et al, 1998). Accounting for spatial correlation is a difficult statistical problem beyond the scope of the present study. Methods that perform kinetic modeling in a transformed space, for example, in the wavelet domain, can account for spatial correlation in the data, but apply only to models in which the estimation operator is linear in the data, such as a Patlak plot or Logan analysis (Turkheimer et al, 2000). Further investigation is needed to determine the performance of these methods with voxel-SAIF analysis. We utilized here the routinely used approach that treats each voxel-TAC as independent from the others.
One remarkable outcome of the analysis was the good performance of voxel-SAIF in estimation of leucine kinetic parameters and rCPS. When simulated reference values were compared with bootstrap-estimated parameters, voxel-SAIF demonstrated low bias and good precision. Moreover, voxel-SAIF produced a low number of outliers. Essential to the performance of voxel-SAIF was selection of an appropriate passband whose role is to reduce the influence of noise on parameter estimates. The optimal voxel-SAIF passband filter differed somewhat from that used for ROI-SAIF. In particular, high-frequency noise present in voxel data but attenuated in the ROI data reduces the accuracy with which high-frequency components can be estimated. The upper bound for the voxel-SAIF passband filter was more selective compared to the filter for ROI-SAIF (0.2 min−1 instead of 0.3 min−1); this reduces the impact of high-frequency noise. At the other end of the spectrum, the less restrictive lower bound of the voxel-SAIF passband (0.02 min−1 for voxel-SAIF; 0.03 min−1 for ROI-SAIF) has the effect of allowing greater kinetic variability in low-frequency components among individual brain voxels.
In measured data, when means of voxel-SAIF estimates within a region were compared with ROI-SAIF estimates, there was generally good agreement between analysis methods. Unlike fixed compartmental model analyses, spectral analysis allows the tissue ROI to be represented as a linear combination of any number of tissue compartments. Because the tissue ROI could be represented as a linear combination of the compartments of each voxel comprising the ROI, we could expect good agreement between the ROI-SAIF estimates and mean voxel-SAIF estimates of linear parameters of the model. Indeed, the linear parameter Vb and the parameter K1, which is the sum of the linear coefficients αi, exhibit the best agreement (1%±4% and 1%±4%, mean±s.d. of the difference between ROI-SAIF and mean voxel-SAIF estimates of Vb and K1, respectively) and highest correlation between methods (0.97 for both Vb and K1).
In contrast to linear parameters, λ and rCPS are nonlinear combinations of parameters, and therefore less agreement between ROI-SAIF and mean voxel-SAIF estimates is to be expected. Estimates of λ were 2%±2% higher with ROI-SAIF and correlation between estimates provided by the two methods was 0.67. Correlation between rCPS estimated with the two methods was very high (R2=0.92), but estimates were 8%±6% lower with ROI-SAIF. Determination of λ from the SAIF-estimated coefficients αi is based on the assumption that all λ in the subregions of a heterogeneous tissue are equal; this constraint is likely to have a greater impact when data are analyzed at the ROI level, where a uniform value of λ for the entire region is assumed, than when data are analyzed at the voxel level and λ can vary among voxels within the region. The constraint contributes to differences between ROI-SAIF and voxel-SAIF estimates of λ and, by extension (equation), estimates of rCPS.
Spectral analysis can also be used to provide information on number of compartments in the kinetic model of the tracer in tissue. For the tracer L-[1-11C]leucine, each equilibrating compartment corresponds to the precursor pool of L-[1-11C]leucine in a kinetically homogeneous tissue that exchanges with the plasma. Multiple exchangeable compartments suggest that the tissue is kinetically heterogeneous. When applied to measured PET data, voxel-SAIF found multiple equilibrating tissue compartments in ∼45% of the brain voxels. In the remaining voxels one equilibrating compartment, that is, a homogeneous tissue model, was found to adequately describe the data. Spectral analysis with iterative filter accuracy in determining the correct number of compartments was >70% in the remaining voxels SAIF had a tendency to overestimate the number of tissue equilibrating components, at the ROI as well as at the voxel level. This is typical of spectral analysis methods—since they are not constrained by a fixed model, they tend to add extra components into the model in order to provide a better fit of the data, and this behavior is particularly evident at high noise levels. The inclusion of extra compartments, however, does not have a significant impact on voxel-SAIF estimates, as evidenced by the good performance statistics found in the bootstrap simulation data.
Analysis of the spatial maps of heterogeneous voxels, in which voxel-SAIF selected the heterogeneous kinetic model to describe the voxel-TACs, suggests the presence of a distribution pattern: in our scans heterogeneous voxels appear to be concentrated at the borders between gray and white matter. This finding was somewhat surprising; we had expected more gray matter voxels to be considered heterogeneous due to inherent gray matter heterogeneity, for example, differences in rCPS among the cortical layers, and partial volume effects arising from the limited spatial resolution of the scanner. The partial volume effects would be greater, and therefore more voxels may be considered as heterogeneous, in lower-resolution scans. The present results do, however, confirm that use of a homogenous kinetic model to describe voxel-TACs is not appropriate for all voxels in brain, even on a high-resolution scanner.
Use of the iterative filter implemented in SAIF improved the robustness of the method to noise; voxel-SAIF provided good estimates of leucine kinetic parameters and rCPS, even at high noise levels. Region-of-interest-based- and mean voxel-based-rCPS estimates were highly correlated, but ROI-based estimates were higher. Simulations showed that mean voxel-level rCPS estimates had lower bias than ROI-based estimates in whole brain and cortex, had comparable bias in white matter, and had lower variability in all regions. We conclude that estimation of rCPS with SAIF is improved when the method is applied at the voxel level. Voxel-SAIF may also provide useful information about the spatial distribution of voxel heterogeneity.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research was supported in part by the Intramural Research Program, National Institute of Mental Health. Portions of this work were presented in preliminary form at The Eighth International Symposium on Functional Neuroreceptor Mapping of the Living Brain (NRM2010) (Veronese et al, 2010b).
Supplementary Material
References
- Bertoldo A, Vicini P, Sambuceti G, Lammertsma AA, Parodi O, Cobelli C. Evaluation of compartmental and spectral analysis models of [18F]FDG kinetics for heart and brain studies with PET. IEEE Trans Biomed Eng. 1998;45:1429–1448. doi: 10.1109/10.730437. [DOI] [PubMed] [Google Scholar]
- Bishu S, Schmidt KC, Burlin T, Channing M, Conant S, Huang T, Liu Z-H, Qin M, Vuong B, Unterman A, Xia Z, Zametkin A, Herscovitch P, Smith CB. Regional rates of cerebral protein synthesis measured with L-[1-11C]leucine and PET in conscious, young adult men: normal values, variability, and reproducibility. J Cereb Blood Flow Metab. 2008;28:1502–1513. doi: 10.1038/jcbfm.2008.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishu S, Schmidt KC, Burlin T, Channing M, Horowitz L, Huang T, Liu Z-H, Qin M, Vuong B, Unterman A, Xia Z, Zametkin A, Herscovitch P, Quezado Z, Smith CB. Propofol anaesthesia does not alter regional rates of cerebral protein synthesis measured with L-[1-11C]leucine and PET in healthy male subjects. J Cereb Blood Flow Metab. 2009;29:1035–1047. doi: 10.1038/jcbfm.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Lammertsma AA, Beaney RP, Leenders KL, Buckingham PD, Marshall J, Jones T. Measurement of regional cerebral pH in human subjects using continuous inhalation of 11CO2 and positron emission tomography. J Cereb Blood Flow Metab. 1984;4:458–465. doi: 10.1038/jcbfm.1984.65. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Alpert NM, Babikian V, Weise S, Correia JA, Ackerman RH. Evaluation of the 11CO2 positron emission tomographic method for measuring brain pH. I. pH changes measured in states of altered PCO2. J Cereb Blood Flow Metab. 1987;7:709–719. doi: 10.1038/jcbfm.1987.125. [DOI] [PubMed] [Google Scholar]
- Carson R, Barker W, Liow J-S, Johnson C. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. IEEE Trans Nucl Sci. 2003;5:3281–3285. [Google Scholar]
- Cunningham VJ, Jones T. Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab. 1993;13:15–23. doi: 10.1038/jcbfm.1993.5. [DOI] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: another look at the jackknife. Ann Statist. 1979;7:1–26. [Google Scholar]
- Lajtha A, Latzkovits L, Toth J. Comparison of turnover rates of proteins of the brain, liver and kidney in mouse in vivo following long term labeling. Biochim Biophys Acta. 1976;425:511–520. doi: 10.1016/0005-2787(76)90015-0. [DOI] [PubMed] [Google Scholar]
- Pajevic S, Daube-Witherspoon ME, Bacharach SL, Carson RE. Noise characteristics of 3-D and 2-D PET images. IEEE Trans Med Imag. 1998;17:9–23. doi: 10.1109/42.668691. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Thompson WO. The rate of incorporation of gaseous 14CO2 into brain tissue constituents. Experientia. 1965;20:98–99. doi: 10.1007/BF02151265. [DOI] [PubMed] [Google Scholar]
- Schmidt KC. Which linear compartmental systems can be analyzed by spectral analysis of PET output data summed over all compartments. J Cereb Blood Flow Metab. 1999;19:560–569. doi: 10.1097/00004647-199905000-00010. [DOI] [PubMed] [Google Scholar]
- Schmidt KC, Cook MP, Qin M, Kang J, Burlin TV, Smith CB. Measurement of regional rates of cerebral protein synthesis with L-[1-11C]leucine and PET with correction for recycling of tissue amino acids: I. Kinetic modeling approach. J Cereb Blood Flow Metab. 2005;25:617–628. doi: 10.1038/sj.jcbfm.9600067. [DOI] [PubMed] [Google Scholar]
- Schmidt KC, Smith CB. Resolution, sensitivity and precision with autoradiography and small animal positron emission tomography: implications for functional brain imaging in animal research. Nucl Med Biol. 2005;32:719–725. doi: 10.1016/j.nucmedbio.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Schmidt KC, Turkheimer FE. Kinetic modeling in positron emission tomography. Q J Nucl Med. 2002;46:70–85. [PubMed] [Google Scholar]
- Smith CB, Schmidt KC, Qin M, Burlin TV, Cook MP, Kang J, Saunders RC, Bacher JD, Carson RE, Channing MA, Eckelman WC, Herscovitch P, Laverman P, Vuong BK. Measurement of regional rates of cerebral protein synthesis with L-[1-11C]leucine and PET with correction for recycling of tissue amino acids: II. Validation in rhesus monkeys. J Cereb Blood Flow Metab. 2005;25:629–640. doi: 10.1038/sj.jcbfm.9600066. [DOI] [PubMed] [Google Scholar]
- Tomasi G, Bertoldo A, Bishu S, Unterman A, Smith CB, Schmidt K. Voxel-based estimation of kinetic model parameters of the L-[1-11C]leucine PET method for determination of regional rates of cerebral protein synthesis: validation and comparison with region-of-interest-based methods. J Cereb Blood Flow Metab. 2009;29:1317–1331. doi: 10.1038/jcbfm.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer F, Moresco RM, Lucignani G, Sokoloff L, Fazio F, Schmidt K. The use of spectral analysis to determine regional cerebral glucose utilization with positron emission tomography and [18F]fluorodeoxyglucose: theory, implementation, and optimization procedures. J Cereb Blood Flow Metab. 1994;14:406–422. doi: 10.1038/jcbfm.1994.52. [DOI] [PubMed] [Google Scholar]
- Turkheimer FE, Banati RB, Visvikis D, Aston JAD, Gunn RN, Cunningham VJ. Modeling dynamic PET-SPECT studies in the wavelet domain. J Cereb Blood Flow Metab. 2000;20:879–893. doi: 10.1097/00004647-200005000-00015. [DOI] [PubMed] [Google Scholar]
- Veronese M, Bertoldo A, Bishu S, Unterman A, Tomasi G, Smith CB, Schmidt KC. A spectral analysis approach for determination of regional rates of cerebral protein synthesis with the L-[1-(11)C]leucine PET method. J Cereb Blood Flow Metab. 2010a;30:1460–1476. doi: 10.1038/jcbfm.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese M, Bertoldo A, Schmidt KC.2010b. A spectral analysis approach for voxelwise determination of regional rates of cerebral protein synthesis with the L-[1-(11)C]leucine PET method, Neuroimage, Volume 52, Supplement 1, Eighth International Symposium on Functional Neuroreceptor Mapping of the Living Brain, 22–24 June 2010, Glasgow, UK
- Wienhard K, Schmand M, Casey ME, Baker K, Bao J, Eriksson L, Jones WF, Knoess C, Lenox M, Lercher M, Luk P, Michel C, Reed JH, Richerzhagen N, Treffert J, Vollmar S, Young JW, Heiss WD, Nutt R. The ECAT HRRT: performance and first clinical application of the new high resolution research tomograph. IEEE Trans Nucl Sci. 2002;49:104–110. [Google Scholar]
- Wu Y, Carson R. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab. 2002;22:1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.