Abstract
Although treatment of stroke patients with mild hypothermia is a promising therapeutic approach, chemicals inducing prompt and safe reduction of body temperature are an unmet need. We measured the effects of the transient receptor potential vanilloid-1 (TRPV1) agonist rinvanil on thermoregulation and ischemic brain injury in mice. Intraperitoneal or intracerebroventricular injection of rinvanil induces mild hypothermia that is prevented by the receptor antagonist capsazepine. Both intraischemic and postischemic treatments provide permanent neuroprotection in animals subjected to transient middle cerebral artery occlusion (MCAo), an effect lost in mice artificially kept normothermic. Data indicate that TRPV1 receptor agonists are promising candidates for hypothermic treatment of stroke.
Keywords: hypothermia, neuroprotectants, TRPV1 receptor
Introduction
Hypothermia is among the most powerful neuroprotective strategies identified so far (Diller and Zhu, 2009). Intraischemic hypothermia reduces infarct volumes and improves neurologic outcome in different models of brain ischemia. Delayed (2–3 hours) hypothermia also reduces ischemic brain injury provided that the duration of cooling lasts up to several hours. On the clinical side, induction of hypothermia in stroke patients proved safe, although its efficacy is unclear and needs confirmation by additional clinical trials (Yenari and Hemmen, 2010). Uncertainties also exist about depth, time to treatment and duration of hypothermia, as well as efficacious methods of cooling (van der Worp et al, 2010). Because few molecules are able to reduce body temperature (Tb) to an extend consistent with neuroprotective hypothermia (such as neurotensin, 3-iodithyronamine, and hydrogen sulfide), there is enormous interest in identifying drugs able to readily and safely reduce Tb (Yenari and Hemmen, 2010; van der Worp et al, 2010). Interestingly, transient receptor potential vanilloid-1 (TRPV1) activation, besides transducing sensory stimuli, also negatively regulates Tb (Gavva, 2008; Fosgerau et al, 2010). Given that no studies exploited this hypothermic activity for neuroprotection, here we studied the effects of rinvanil, a potent TRPV1 agonist (Appendino et al, 2005), on Tb and ischemic brain injury in mice.
Materials and methods
All the experiments conducted were performed according to the Italian guidelines for animal care (DL 116/92) in application of the European Communities Council Directive (86/609/EEC) and were formally approved by the Animal Care Committee of the Department of Pharmacology of the University of Florence. C57Bl/6 male mice (Harlan, Udine, Italy) were used. Rinvanil (synthesized as reported; Appendino et al, 2005) or capsazepine (Tocris, Minneapolis, MN, USA) was dissolved in dimethyl sulfoxide (DMSO) and injected intraperitoneally at the indicated doses. Tb and skin temperature was measured by means of a rectal or skin probe (Harvard Apparatus, Holliston, MA, USA). Because pilot experiments showed that rectal and temporalis muscle temperature similarly decreased on rinvanil treatment, the rectal probe was routinely used to measure Tb in mice. O2 consumption rate was obtained by means of a closed respirometer (Columbus Instruments, Columbus, OH, USA). Middle cerebral artery occlusion (MCAo) was conducted as reported (Eliasson et al, 1997) and the filament withdrawn after 60 or 90 minutes. Mice (n=8 per group) were killed by decapitation and brains frozen. Indirect infarct determination was performed 48 hours after MCAo as described (Cozzi et al, 2006). Regional cerebral blood flow was measured by Laser-Doppler (PF2B; Perimed, Stockholm, Sweden), using a flexible skull probe. In randomly selected animals, the left femoral artery was cannulated with a PE-10 polyethylene tube for arterial blood pressure measurement and blood gas determination. Arterial blood samples (50 μl) were analyzed for pH, arterial oxygen pressure (PaO2), and partial pressure of carbon dioxide (PaCO2) using a Ciba-Cornig 248 pH/blood gas analyzer (Ciba-Corning Diagnostics, Medfield, MA, USA).
Results
Effects of Rinvanil on Body Temperature Regulation
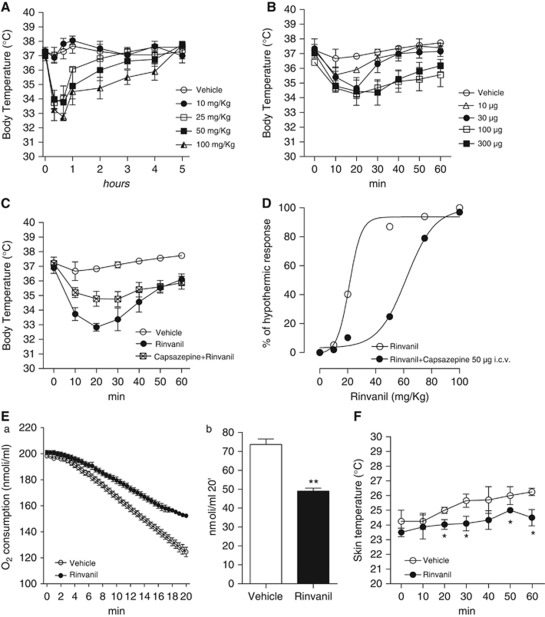
Given the effects of various capsacinoids on thermoregulation (Gavva, 2008), we first investigated whether the TRPV1 receptor agonist rinvanil also affects Tb. At 25 mg/kg, animals reached a Tb of 33.4±0.6°C 30 minutes after the injection (Figure 1A), and returned to control values after 2.2±0.3 hours. Higher doses prompted similar hypothermia after 30 minutes (33.2±3°C at 50 mg/kg and 32.5±5°C at 100 mg/kg) but for longer time lengths (4.4±0.3 and 4.9±0.5 hours at 50 or 100 mg/kg, respectively). Prior work suggests that hypothermia induced by capsaicin analogs is due to activation of peripheral TRPV1 receptor (Gavva, 2008; Fosgerau et al, 2010). However, we found that intracerebroventricular injections of rinvanil also reduced Tb dose dependently (Figure 1B). Notably, hypothermia induced by 25 mg/kg rinvanil intraperitoneally was reduced by a concomitant intracerebroventricular injection of the TRPV1 antagonist capsazepine (50 μg; Figure 1C). The inhibiting effects of capsazepine were surmountable by increasing the dose of rinvanil (Figure 1D), indicating competitive inhibition. To gather information on the mechanisms through which rinvanil alters thermoregulation, we analyzed O2 consumption and skin temperature as indexes of basal metabolism and vascular tone, respectively. We found that rinvanil reduced both O2 consumption and skin temperature (Figures 1E and 1F).
Figure 1.
Effect of rinvanil on thermoregulation. Effect of intraperitoneal (A) or intracerebroventricular (B) injection of different doses of rinvanil on body temperature (Tb) of uninjured mice. (C) The transient receptor potential vanilloid-1 (TRPV1) receptor antagonist capsazepine inhibits the hypothermic effect of rinvanil. (D) The effect of capsazepine is competitive. Effect of rinvanil (25 mg/kg) on O2 consumption during a 20-minute recording (expressed both on time scale (E,a) or as total consumption (E,b)) and skin temperature (F) in mice. Each point/column represents the mean±s.e.m. of three (A–C) or two (E, F) experiments with five animals per group. *P<0.05, **P<0.01 versus Vehicle. Student's t-test.
Effects of Rinvanil on Brain Ischemia-Reperfusion Injury
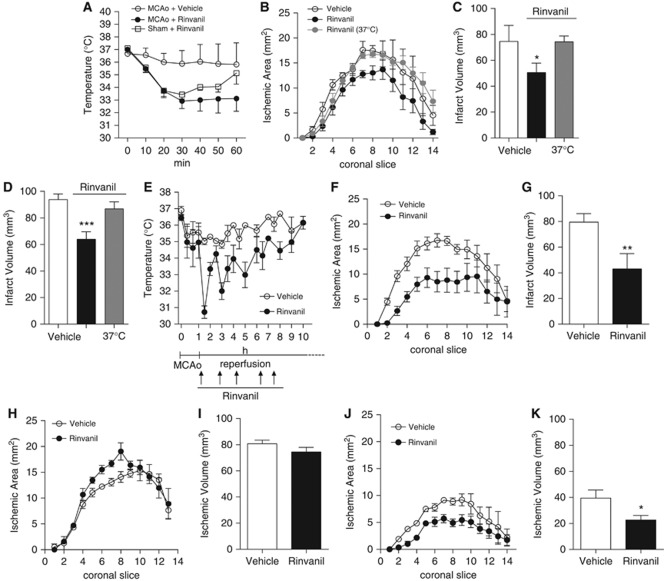
To evaluate whether rinvanil-dependent TRPV1 receptor activation prompts neuroprotective hypothermia during brain ischemia, we assessed compound's effects in mice subjected to transient MCAo. As shown in Figure 2A, hypothermia induced by rinvanil (25 mg/kg) in mice subjected to 1 hour MCAo was similar to that induced in sham-operated mice but lasted longer (6.5±0.8 and 2.5±0.2 hours; respectively. Figure 2A and not shown), indicating that brain ischemia sensitizes animals to TRPV1 receptor-induced hypothermia. However, rinvanil injections did not alter physiological parameters such as blood pressure, PaO2, PaCO2, blood pH, and regional cerebral blood flow during ischemia and reperfusion (not shown).
Figure 2.
Effects of rinvanil on ischemic brain injury in mice. (A) The effect of rinvanil (25 mg/kg intraperitoneally) on body temperature (Tb) of mice subjected to 1 hour middle cerebral artery occlusion (MCAo) is more prolonged than in sham-operated animals. (B, C) Effect of rinvanil (25 mg/kg) on infarct areas and volumes of mice subjected to 1 hour MCAo/24 hours reperfusion. Neuroprotection is lost in animals kept at a Tb of 37°C. (D) Effect of rinvanil (25 mg/kg) on infarct volumes of mice subjected to 1.5 hours MCAo/48 hours reperfusion. Effect of multiple, postischemic injections of rinvanil (25 mg/kg) on Tb (E), infarct areas (F), and volumes (G) of mice subjected to 1 hour MCAo/24 hours reperfusion. (H, I) Effect of 50 mg/kg of rinvanil on infarct areas and volumes of mice subjected to 1 hour MCAo/24 hours reperfusion. Effect of 24 hours hypothermia obtained by multiple (13) injections of rinvanil (25 mg/kg) on infarct areas (J) and volumes (K) of mice subjected to 1 hour MCAo/7 days reperfusion. Each point/column represents the mean±s.e.m. (n=8 per group). *P<0.05, **P<0.01, ***P<0.001 versus Vehicle. Analysis of variance plus Tukey's post hoc test.
In keeping with ischemic neuroprotection by hypothermia, infarct volumes of mice subjected to 1 hour MCAo/24 hours reperfusion were significantly reduced by 25 mg/kg rinvanil (74±12 in DMSO-treated mice and 49±8 mm3 in rinvanil-treated mice; n=8/group, P<0.05) (Figure 2C). To ascertain that neuroprotection was due to hypothermia and not to different central and peripheral effects of TRPV1 receptor activation, we analyzed ischemic neurodegeneration in mice injected with rinvanil and artificially kept at normothermic values. Notably, rinvanil did not reduce ischemic volumes in these animals (Figures 2B and 2C). When the compound was tested in mice subjected to prolonged ischemic insult and reperfusion times (90 minutes MCAo/48 hours reperfusion), reduction of stroke volumes by the TRPV1 receptor agonist was still evident (94±4 in DMSO-treated mice and 76±5 mm3 in rinvanil-treated mice (n=8/group), respectively; P<0.001) and occurred in a hypothermia-dependent manner (Figure 2D). To corroborate the clinical relevance of TRPV1 receptor-induced ischemic neuroprotection, we next analyzed the effect of rinvanil administered in a postischemic paradigm. To this end, in mice subjected to 1 hour MCAo/24 hours reperfusion we injected rinvanil 25 mg/kg in a time window comprised from the time of reperfusion to 8 hours from MCAo (total of five injections) (Figure 2E). Even though injections were conducted when hypothermia recovered to 34°C, we noticed that the hypothermic effects of repetitive injections diminished in amplitude, suggesting TRPV1 receptor desensitization (Figure 2E). Nevertheless, animals subjected to this postischemic treatment paradigm showed reduced infarct areas and volumes compared with those of vehicle-treated mice (79.5±7 in DMSO-treated mice and 43±12 mm3 in rinvanil-treated mice; n=8/group, P<0.05) (Figures 2F and 2G).
It is well appreciated that activation of TRPV1 receptors causes large increases of intracellular Ca2+ concentrations in neurons. Consistently, massive receptor activation prompts neuronal death in vitro and in vivo (Kim et al, 2005; Shirakawa et al, 2008). In principle, these properties might counteract hypothermia-dependent ischemic neuroprotection. To address this issue, we analyzed the effects of a higher dose (50 mg/kg) of rinvanil in mice undergoing 1 hour MCAo/24 hours reperfusion. Notably, ischemic neuroprotection was lost in mice receiving this dose intraperitoneally at time of MCAo (75.8±8 in DMSO-treated mice and 84.5±13 mm3 in rinvanil-treated mice; n=9/group, P<0.05) (Figures 2H and 2I). This finding prompted us to investigate whether neuroprotection afforded by 25 mg/kg of rinvanil was transient or permanent. As shown in Figures 2J and 2K, in the vehicle-treated animals 7 days after MCAo infarct size was smaller than that measured after 24 hours, in keeping with prior work (Yamada et al, 2003). Still, reduction of infarct size by rinvanil was still evident (39.5±8 in DMSO-treated mice and 22±4 mm3 in rinvanil-treated mice; n=7/group, P<0.05).
Discussion
Hypothermia is a robust protectant against experimental ischemic brain injury, even though its clinical relevance needs additional trial to establish efficacy. Pilot studies have clearly indicated that, among the various parameters and variables to be considered, fast cooling of patients is a key for clinical efficacy (Yenari and Hemmen, 2010; van der Worp et al, 2010). In this light, there is ample agreement that drugs able to readily and safely reduce Tb are an unmet need. In the present paper, we show that the potent TRPV1 receptor agonist rinvanil readily induces hypothermia dose dependently acting within the CNS in a capsazepine-sensitive manner. Evidence that the hypothermic effect correlates with ischemic neuroprotection suggests that TRPV1 targeting can be exploited for hypothermic treatment of stroke.
Data are in line with hyperthermia induced by drugs blocking tonic activation of TRPV1 receptors (Gavva, 2008). In this regard, it has been reported that TRPV1-dependent thermoregulation is due to modulation of receptors outside the blood–brain barrier, more precisely those tonically activated in the viscera (Gavva et al, 2007). Our results on the effect of intracerebroventricular injection of rinvanil, however, indicate that central agonism on TRPV1 receptors suffices to induce hypothermia. Of note, although this is in keeping with the complex effects of capsaicin on hypothalamic thermosensitive neurons (Hori, 1984; Hori et al, 1988), the exact localization of TRPV1 receptors within the rodent and human hypothalamus is still debated (Menigoz and Boudes, 2011). Reportedly, peripheral TRPV1 receptors tonically suppress cold defenses by inhibiting thermogenesis and skin vasoconstriction (Gavva, 2008). Although we confirmed that TRPV1 receptor activation reduces O2 consumption (an index of thermogenesis), we found that, rather than vasodilatation, TRPV1 agonism causes skin vasoconstriction. The latter might be a cold defense response due to hypothermia. Alternatively, hypothermia due to central TRPV1 receptor activation might activate autonomic responses partially different from those set into motion by blocking tonically active peripheral TRPV1 receptors. Regardless, the present study qualifies TRPV1 receptor agonists as promising drugs for hypothermic neuroprotection. The finding that reduction of ischemic brain injury also occurs when rinvanil is used in a postischemic treatment paradigm further emphasizes its therapeutic potential. The latter, however, might be reduced because of TRPV1 receptor desensitization after repetitive injections (Figure 2E), or because of the neurotoxic effects originating from excessive activation of TRPV1 receptors and ensuing massive intracellular Ca2+ entrance (Kim et al, 2005; Shirakawa et al, 2008).
In conclusion, in light of the urgent need of hypothermic drugs for clinical trial in stroke patients, our findings suggest that hypothermia due to TRPV1 receptor activation can be exploited for innovative stroke treatments. Also, because of the relevance of hypothermia to cardiac arrest or neonatal hypoxia, the cooling affects of rinvanil-like drugs might be harnessed for additional therapeutic strategies.
The authors declare no conflict of interest.
Footnotes
This study was supported by Fondazione Italiana Sclerosi Multipla, Regione Toscana Progetto Salute 2009, and Ente Cassa di Risparmio di Firenze.
References
- Appendino G, De Petrocellis L, Trevisani M, Minassi A, Daddario N, Moriello AS, Gazzieri D, Ligresti A, Campi B, Fontana G, Pinna C, Geppetti P, Di Marzo V. Development of the first ultra-potent ‘capsaicinoid' agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J Pharmacol Exp Ther. 2005;312:561–570. doi: 10.1124/jpet.104.074864. [DOI] [PubMed] [Google Scholar]
- Cozzi A, Cipriani G, Fossati S, Faraco G, Formentini L, Min W, Cortes U, Wang ZQ, Moroni F, Chiarugi A. Poly(ADP-ribose) accumulation and enhancement of postischemic brain damage in 110-kDa poly(ADP-ribose) glycohydrolase null mice. J Cereb Blood Flow Metab. 2006;26:684–695. doi: 10.1038/sj.jcbfm.9600222. [DOI] [PubMed] [Google Scholar]
- Diller KR, Zhu L. Hypothermia therapy for brain injury. Annu Rev Biomed Eng. 2009;11:135–162. doi: 10.1146/annurev-bioeng-061008-124908. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Fosgerau K, Weber UJ, Gotfredsen JW, Jayatissa M, Buus C, Kristensen NB, Vestergaard M, Teschendorf P, Schneider A, Hansen P, Raunso J, Kober L, Torp-Pedersen C, Videbaek C. Drug-induced mild therapeutic hypothermia obtained by administration of a transient receptor potential vanilloid type 1 agonist. BMC Cardiovasc Disord. 2010;10:51. doi: 10.1186/1471-2261-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavva NR. Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol Sci. 2008;29:550–557. doi: 10.1016/j.tips.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN, Jr, Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ, Louis JC. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T. Capsaicin and central control of thermoregulation. Pharmacol Ther. 1984;26:389–416. doi: 10.1016/0163-7258(84)90041-x. [DOI] [PubMed] [Google Scholar]
- Hori T, Shibata M, Kiyohara T, Nakashima T, Asami A. Responses of anterior hypothalamic-preoptic thermosensitive neurons to locally applied capsaicin. Neuropharmacology. 1988;27:135–142. doi: 10.1016/0028-3908(88)90162-1. [DOI] [PubMed] [Google Scholar]
- Kim SR, Lee DY, Chung ES, Oh UT, Kim SU, Jin BK. Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J Neurosci. 2005;25:662–671. doi: 10.1523/JNEUROSCI.4166-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menigoz A, Boudes M. The expression pattern of TRPV1 in brain. J Neurosci. 2011;31:13025–13027. doi: 10.1523/JNEUROSCI.2589-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa H, Yamaoka T, Sanpei K, Sasaoka H, Nakagawa T, Kaneko S. TRPV1 stimulation triggers apoptotic cell death of rat cortical neurons. Biochem Biophys Res Commun. 2008;377:1211–1215. doi: 10.1016/j.bbrc.2008.10.152. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Macleod MR, Kollmar R. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials. J Cereb Blood Flow Metab. 2010;30:1079–1093. doi: 10.1038/jcbfm.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada I, Kuroiwa T, Endo S, Miyasaka N. Temporal evolution of apparent diffusion coefficient and T2 value following transient focal cerebral ischemia in gerbils. Acta Neurochir Suppl. 2003;86:147–151. doi: 10.1007/978-3-7091-0651-8_31. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go. Stroke. 2010;41:S72–S74. doi: 10.1161/STROKEAHA.110.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]