Abstract
[18F]-FEPPA binds to the 18-kDa translocator protein (TSPO) and is used in positron emission tomography (PET) to detect microglial activation. However, quantitative interpretations of the PET signal with new generation TSPO PET radioligands are confounded by large interindividual variability in binding affinity. This presents as a trimodal distribution, reflecting high-affinity binders (HABs), low-affinity binder (LAB), and mixed-affinity binders (MABs). Here, we show that one polymorphism (rs6971) located in exon 4 of the TSPO gene, which results in a nonconservative amino-acid substitution from alanine to threonine (Ala147Thr) in the TSPO protein, predicts [18F]-FEPPA total distribution volume in human brains. In addition, [18F]-FEPPA exhibits clearly different features in the shape of the time activity curves between genetic groups. Testing for the rs6971 polymorphism may allow quantitative interpretation of TSPO PET studies with new generation of TSPO PET radioligands.
Keywords: genetics, inflammation, microglia, mitochondria, positron emission tomography
Introduction
Microglia express a protein in their mitochondria called the translocator protein (TSPO) 18 kDa or TSPO (Braestrup et al, 1977), which was previously known as the peripheral benzodiazepine receptor (PBR) (Anholt et al, 1986). Activated microglia thus represent an important marker of neuroinflammation (for a recent review see Venneti et al, 2009). This has been confirmed in a large number of studies that have shown that levels of TSPO and/or microglia are greatly increased in examples of inflammation (as reviewed by Chen and Guilarte, 2008). Positron emission tomography (PET) imaging of TSPO can be used to quantify TSPO in-vivo using appropriate radiotracers that bind selectively and specifically to TSPO. The first and most widely used radiotracer is [11C]-PK11195 (Camsonne et al, 1984); however, this radiotracer has recognized limitations including high nonspecific binding, low brain penetration, high plasma protein binding, and a difficult synthesis. The deficiencies of [11C]-PK11195 coupled with the recognized importance of TSPO imaging have fueled considerable efforts to develop radiotracers with greater sensitivity to detect TSPO binding (James et al, 2006; Okubo et al, 2004). While [11C]-PBR28 (Fujita et al, 2008) and [11C]-DPA713 (Boutin et al, 2007) circumvent many of the deficiencies of [11C]-PK11195, both are radiolabeled with the short-lived carbon-11, rendering unsuitable for wide-spread dissemination. The advantages of fluorine-18-labeled radiotracer are several; improved targetry means that [18F]-fluoride can now be produced by low/medium energy cyclotrons in large quantities, the imaging quality of this radionuclide are superior to carbon-11, and most importantly the longer half-life (t1/2≈ 109 minutes), allows shipment to imaging sites distant from the site of production, which is particularly useful for clinical applications. Only two [18F]-labeled TSPO radiotracers have been evaluated so far in humans. [18F]-FEDAA1106 displays good brain penetration, but this compound is exceedingly lipophilic and its binding kinetics are very slow (Fujimura et al, 2006). Very recently, [18F]-PBR06 has been studied in human (Fujimura et al, 2009). [18F]-PBR06 has many favorable properties, including appropriate kinetics, good brain penetration, and ease of preparation. Unfortunately, initial reports suggest that it produces a brain-penetrant radiolabeled metabolite that confounds quantification of TSPO binding (Fujimura et al, 2009). Recently, our group reported the radiosynthesis and initial evaluation of [18F]-FEPPA, an F-18 radiolabeled analog PBR28 (Wilson et al, 2008), and we have validated its quantification using the unconstrained two-tissue compartment model with 120 minutes of scan data (Rusjan et al, 2011).
Second generation of TSPO tracers present, in humans, three affinity patterns that are related to two distinct binding sites of high and low affinity for the ligands. High-affinity binders and low-affinity binders (HABs and LABs) express a single binding site for TSPO with either high or low affinity, respectively, whereas mixed-affinity binders (MABs) express approximately equal numbers of the high- and low-affinity binding sites (Owen et al, 2010, 2011). Recently, Owen et al (2012) showed that one polymorphism (rs6971) located in exon 4 of the TSPO gene, which results in a nonconservative amino-acid substitution at position 147 from alanine to threonine (Ala147Thr) in the fifth transmembrane domain of the TSPO protein, predicts binding affinity phenotype in human platelets. However, no data regarding brain binding affinity was provided. In this work, we therefore present the results of the impact of the rs6971 polymorphism on in-vivo brain time activity curves (TACs) and total distribution volume (VT) in humans using [18F]-FEPPA.
Materials and methods
Radiochemistry
Details of [18F]-FEPPA synthesis has been described elsewhere (Wilson et al, 2008). It is reliably and quickly labeled with [18F] by nucleophilic displacement of a tosylate leaving group in a fast one-step reaction, yielding a sterile, pyrogen-free product after purification and formulation.
Human Subjects
Nineteen healthy individuals participated in this study. Twelve of this cohort have been presented in a recent quantification study (Rusjan et al, 2011). One extra healthy subject who was later found to be an LAB is included. However, given that a different protocol was carried out for a whole body biodistribution and dosimetry study (to be presented elsewhere) brain uptake data are not presented for this subject. All subjects provided written informed consent after all procedures were fully explained, and were approved by the Center for Addiction and Mental Health Ethics Review Board.
Positron Emission Tomography Image Acquisition and Analysis for High-Affinity Binders and Mixed-Affinity Binders
A dose of 173±13 MBq (4.69±0.35 mCi) of intravenous [18F]-FEPPA was administered as a bolus for the PET scans (mass 0.8±0.8 μg, range: 0.12 to 3.22). An automatic blood sampling system (ABSS, Model #PBS-101 from Veenstra Instruments, Joure, The Netherlands) was used to measure arterial blood radioactivity continuously at a rate of 2.5 mL/min for the first 22.5 minutes. Manual blood samples were obtained at 2.5, 7, 12, 15, 30, 45, 60, 90, and 120 minutes. These samples were used to determine the temporal evolution of the ratio of radioactivity in whole blood to radioactivity in plasma, and the unmetabolized radioligand in plasma needed to create the input function for the kinetic analysis (more details in Rusjan et al, 2011).
The scan duration was 125 minutes following the injection of [18F]-FEPPA. The images were reconstructed into 34 time frames. Frames were acquired as followed: 1 frame of variable length, 5 × 30, 1 × 45, 2 × 60, 1 × 90, 1 × 120, 1 × 210, and 22 × 300 seconds. The PET images were obtained using 3D HRRT brain tomography (CPS/Siemens, Knoxville, TN, USA), which measures radioactivity in 207 slices with an interslice distance of 1.22 mm. All PET images of MABs and HABs were corrected for attenuation using a single photon point source, 137Cs (T50=30.2 years, Eγ=662 keV) and were reconstructed by filtered back projection algorithm, with a HANN filter at Nyquist cutoff frequency.
Region of Interest-Based Analysis
For the anatomical delineation of region of interest (ROIs), a brain magnetic resonance image was acquired for each subject. 2D axial proton density magnetic resonance images were acquired with a General Electric (Milwaukee, WI, USA) Signa 1.5 T magnetic resonance image scanner (slice thickness=2 mm, repetition time >5,300 milliseconds, echo time=13 milliseconds, flip angle=90 degree, number of excitations=2, acquisiton matrix=256 × 256, and field of view=22 cm). Regions of interest were automatically generated using the in-house software, ROMI (Rusjan et al, 2006). Briefly, ROMI (CAMH, Toronto, Ontario, Canada) fits a standard template of ROIs to an individual high-resolution proton density magnetic resonance image scan based on the probability of gray matter, white matter, and cerebrospinal fluid. The individual magnetic resonance image with ROIs properly superimposed are then coregistered to the summed [18F]-FEPPA PET image using a mutual information algorithm to generate the TAC from each ROI. Time activity curves were used to estimate the VT using a two-tissue compartment model, which has been shown previously as the optimum outcome for [18F]-FEPPA quantification (Rusjan et al, 2011).
DNA Extraction and Polymorphism Genotyping
Genomic DNA was obtained from peripheral leukocytes, using high salt extraction methods (Lahiri and Nurnberger, 1991). The polymorphism rs6971 was genotyped variously using a TaqMan assay on demand C_2512465_20 (Applied Biosystems, Foster City, CA, USA). The allele T147 was linked to Vic and the allele A147 was linked to FAM. Polymerase chain reaction reactions were performed in a 96-well microtiter-plate on a GeneAmp PCR System 9700 (Applied Biosystems). After PCR amplification, end point plate read and allele calling was performed using an ABI 7900 HT (Applied Biosystems) and the corresponding SDS software (v2.2.2, Applied Biosystems).
Statistical Analysis
The [18F]-FEPPA binding (VT) to human brains was analyzed using repeated measures analysis of variance with ROI as a repeated measure and MAB or HAB as predictor. Independent analysis of variances were carried out for each ROI as follow-up tests. All analyses are two tailed with the conventional α=0.05.
Results
Twenty healthy individuals were recruited (mean±s.d. age, 51±16 years; range: 24 to 78 years; 9 males and 11 females) who participated in this study. All subjects were free of current medical illness based on history, physical examination, electrocardiogram, urinalysis (including drug screening), and blood tests (complete blood count and serum chemistry). All 20 subjects were free of present or past psychiatric illness, as determined by a psychiatrist (R.M.) and interviews using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). No significant differences were found in amount injected, specific activity, and mass injected across genetic groups (HABs, MABs, LABs; Table 1).
Table 1. PET parameters and demographic data.
| HAB (n=13) | MAB (n=6) | LAB (n=1) | Statistics (df=2,17) | P | |
|---|---|---|---|---|---|
| Injected parameters (s.d.) | |||||
| Amount injected (MBq) | 173.22 (14.80) | 175.07 (8.72) | 174.27 | F=0.04 | 0.96 |
| Specific activity (GBq/μmol) | 190.85 (144.93) | 144.11 (163.63) | 215.3 | F=0.23 | 0.797 |
| Mass injected (μg) | 0.77 (0.86) | 1.02 (0.86) | 0.3 | F=0.37 | 0.693 |
| Age (s.d.) | 48.00 (14.33) | 59.17 (19.16) | 30 | F=1.88 | 0.183 |
| Gender | |||||
| Male | 7 | 1 | 1 | ||
| Female | 6 | 5 | 0 | χ2=3.58 | 0.167 |
HAB, high-affinity binder; LAB, low-affinity binder; MAB, mixed-affinity binder; PET, positron emission tomography.
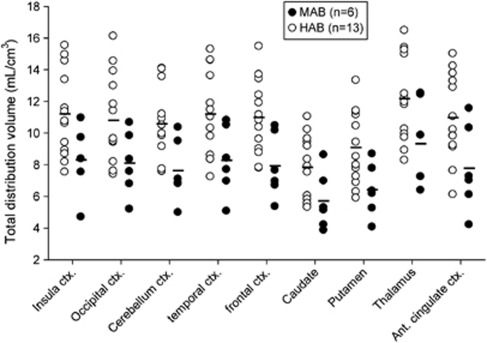
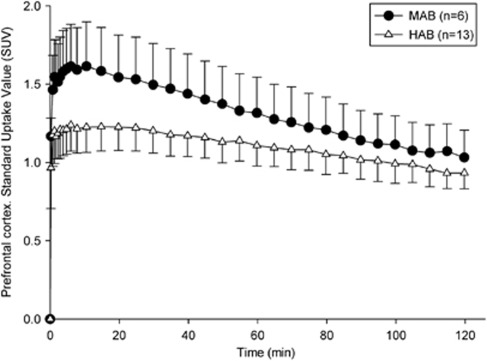
Based on the rs6971 polymorphism, our population showed 13 HABs, 6 MABs, and 1 LAB, which reflected on binding phenotype as observed in VT and TACs (Table 2; Figures 1 and 2). The LAB brain uptake is not presented given that this subject followed a different protocol, but the very different whole body biodistribution will be presented in a follow-up study. This is the first report of a LAB with [18F]-FEPPA. Total distribution volume for MABs and HABs are shown in Figure 1. The LAB is not presented due to lack of arterial data for this subject. There was a significant difference between ROIs VTs (F=24.82, P<0.001), and genetic group (F=6.37, P=0.02), with no significant interaction (F=0.42, P=0.79). VTs show a reduction of 27% on average (range 23% and 29% depending of the ROI) in MABs compared with HABs, showing a significant difference in all ROIs ranging from F=4.2 P=0.057 for the occipital cortex to F=7.41 P=0.014 for the frontal cortex (Figure 1). Regional average TACs between MABs and HABs genotype showed moderate differences (Figure 2) in all the ROIs. Mixed-affinity binders presented a higher peak (≈1.6 SUV) than HABs (≈1.25 SUV) and a faster washout. While the TACs' peaks of the MABs were localized between 3 and 7 minutes depending on the ROI, the TACs for the HABs were flatter and therefore the exact timing of the peak difficult to determine accurately. At 2 hours, the TACs of the MABs decreased in average 32% across ROIs with respect to the peak value, and only 23% in the case of the HABs.
Table 2. Distribution of rs6971 genotypes against ligand-binding classification.
|
TSPO genotype |
Binding phenotype (subject, n) |
|||
|---|---|---|---|---|
| DNA (polymorphism rs6971) | Protein (position 147) | HAB | MAB | LAB |
| C/C | Ala/Ala | 13 | ||
| C/T | Ala/Thr | 6 | ||
| T/T | Thr/Thr | 1 | ||
Ala, alanine; HAB, high-affinity binder; LAB, low-affinity binder; MAB, mixed-affinity binder; Thr, threonine; TSPO, translocator protein.
Genotypes correspond to carriage of the 147 amino acid as follows: CC=Ala147/Ala147; CT=Ala147/Thr147; TT=Thr147/Thr147.
Figure 1.
Comparison of total distribution volume (VT) for high-affinity binders (HABs) and mixed-affinity binders (MABs) showing a significant difference between HABs and MABs (P<0.02) for all regions.
Figure 2.
Comparison of averages time activity curves (TACs) in the prefrontal cortex for high-affinity binders (HABs) and mixed-affinity binders (MABs). The error bars represent the standard errors.
Discussion
Here, we show that the rs6971 polymorphism predicts [18F]-FEPPA VTs in human brains. This finding is highly significant for the interpretation of PET studies using [18F]-FEPPA. These data are consistent with a recent study showing complete agreement between platelets binding of PBR28 and the rs6971 genetic polymorphism (Owen et al, 2012). Although there are no data confirming that other new generation of TSPO radioligands bind at the same site(s) on TSPO, Owen et al (2011) have previously demonstrated that binding class (HAB, MAB, LAB) shows consistency across radioligands; in other words, all tissue samples classified as HABs with PBR28 are also classified as HABs with the other new generation of TSPO radioligands.
In the absence of an available TSPO radioligand, which binds with equivalent affinity in all subjects and has a high signal-to-noise ratio, genotyping the TSPO rs6971 polymorphism will enable confident, quantitative comparisons of [18F]-FEPPA PET data between groups of patients. This can be achieved either by screening out certain subjects to ensure all study participants are from the same binding class, or by including all subjects but correcting PET data based on their genetic binding class. Our results have the same implications for PET studies using [11C]-PBR28, [18F]-PBR06, [11C]-DAA1106, [11C]-DPA713, [18F]-PBR111, and [11C]-AC-5216.
In our study, we did not test the relationship between genetic variation, platelet binding, and brain binding. While this would be an important step following previous work (Owen et al, 2012), our data directly show a robust relationship between rs6971 gene and in-vivo brain binding in humans. Furthermore, we show clear differences between binding profile (VT) and TACs between groups, highlighting the importance of genetic profiling subjects before statistical analysis.
The prevalence of major (Ala147) and minor (Thr147) allele varies in different populations with significant variation between Caucasians and Africans; in the Hapmap database the prevalence of the Thr147 (low-binding allele) is 30% in Caucasians, 25% in Africans, 2% in Han Chinese, and 4% in Japanese (http://hapmap.ncbi.nlm.nih.gov/). In our small predominantly white Caucasian sample, we observed 65% of HABs, 30% MABs, and 5% LABs, which is consistent with the recently reported study by Owen et al (2012).
While the biological impact of this polymorphism and consequent different binding affinity of the new generation of TSPO radioligands is currently unknown, it is interesting to note that complete knockout of the TSPO gene in mice leads to a lethal outcome (Papadopoulos et al, 1997). While anecdotal, the fact that this genetic variability (i.e., the presence of LABs) has been observed in both healthy and disease states (i.e., multiple sclerosis (Owen et al, 2011)) suggests that it may not be specific to a particular neurological condition; this needs to be addressed in future studies. Previous literature suggests an association between the rs6971 polymorphism and variations in pregnenolone production and plasma levels of low-density lipoprotein cholesterol (Costa et al, 2009a), and anxiety in depressive individuals (Costa et al, 2009b). Future studies should be carried out in different patient populations to understand the impact of this polymorphism in humans.
In summary, we demonstrate that TSPO Ala147Thr polymorphism predicts [18F]-FEPPA VT in human brains and report the first LAB as imaged with [18F]-FEPPA. Testing for this polymorphism will allow quantitative interpretation of TSPO PET studies with this and other radioligands.
Acknowledgments
The authors thank Armando Garcia, Winston Stableford, and Ming Wong for their excellent technical assistance; Laura Miler for help in image analysis; and Tamara Arenovich for statistical guidance.
The authors declare no conflict of interest.
Footnotes
This study was supported by Canada Foundation for Innovation, and the Ontario Ministry of Research and Innovation, and partially by the Scottish Grant Charitable Foundation. Dr Mizrahi was supported by the New Investigator Award from CIHR and the Ontario Mental Health Foundation New Investigator Fellowship.
References
- Anholt RR, Pedersen PL, De Souza EB, Snyder SH. The peripheral-type benzodiazepine receptor. Localization to the mitochondrial outer membrane. J Biol Chem. 1986;261:576–583. [PubMed] [Google Scholar]
- Boutin H, Chauveau F, Thominiaux C, Gregoire MC, James ML, Trebossen R, Hantraye P, Dolle F, Tavitian B, Kassiou M. [11C]-DPA-713: a novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J Nucl Med. 2007;48:573–581. doi: 10.2967/jnumed.106.036764. [DOI] [PubMed] [Google Scholar]
- Braestrup C, Albrechtsen R, Squires RF. High densities of benzodiazepine receptors in human cortical areas. Nature. 1977;269:702–704. doi: 10.1038/269702a0. [DOI] [PubMed] [Google Scholar]
- Camsonne R, Crouzel C, Comar D, Mazière M, Prenant C, Sastre J, Moulin M, Syrota A. Synthesis of N-(11C)methyl, N-(methyl-1-propyl), (chloro-2-phenyl)-1-isoquinoleine carboxamide-3 (PK-11195): a new ligand for peripheral benzodiazepine receptors. J Labelled Comp Radiopharm. 1984;21:985–991. [Google Scholar]
- Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118:1–17. doi: 10.1016/j.pharmthera.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Pini S, Gabelloni P, Da Pozzo E, Abelli M, Lari L, Preve M, Lucacchini A, Cassano GB, Martini C. The spontaneous Ala147Thr amino acid substitution within the translocator protein influences pregnenolone production in lymphomonocytes of healthy individuals. Endocrinology. 2009a;150:5438–5445. doi: 10.1210/en.2009-0752. [DOI] [PubMed] [Google Scholar]
- Costa B, Pini S, Martini C, Abelli M, Gabelloni P, Landi S, Muti M, Gesi C, Lari L, Cardini A, Galderisi S, Mucci A, Lucacchini A, Cassano GB. Ala147Thr substitution in translocator protein is associated with adult separation anxiety in patients with depression. Psychiatr Genet. 2009b;19:110–111. doi: 10.1097/YPG.0b013e32832080f6. [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Ikoma Y, Yasuno F, Suhara T, Ota M, Matsumoto R, Nozaki S, Takano A, Kosaka J, Zhang MR, Nakao R, Suzuki K, Kato N, Ito H. Quantitative analyses of 18F-FEDAA1106 binding to peripheral benzodiazepine receptors in living human brain. J Nucl Med. 2006;47:43–50. [PubMed] [Google Scholar]
- Fujimura Y, Zoghbi SS, Simeon FG, Taku A, Pike VW, Innis RB, Fujita M. Quantification of translocator protein (18 kDa) in the human brain with PET and a novel radioligand, 18F-PBR06. J Nucl Med. 2009;50:1047–1053. doi: 10.2967/jnumed.108.060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, Hong J, Pike VW, Innis RB. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. NeuroImage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ML, Selleri S, Kassiou M. Development of ligands for the peripheral benzodiazepine receptor. Curr Med Chem. 2006;13:1991–2001. doi: 10.2174/092986706777584979. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Yoshikawa R, Chaki S, Okuyama S, Nakazato A. Design, synthesis, and structure-activity relationships of novel tetracyclic compounds as peripheral benzodiazepine receptor ligands. Bioorg Med Chem. 2004;12:3569–3580. doi: 10.1016/j.bmc.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, Innis RB, Pike VW, Reynolds R, Matthews PM, Parker CA. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52:24–32. doi: 10.2967/jnumed.110.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Howell OW, Tang SP, Wells LA, Bennacef I, Bergstrom M, Gunn RN, Rabiner EA, Wilkins MR, Reynolds R, Matthews PM, Parker CA. Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation. J Cereb Blood Flow Metab. 2010;30:1608–1618. doi: 10.1038/jcbfm.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, Stjean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997;272:32129–32135. doi: 10.1074/jbc.272.51.32129. [DOI] [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, Tetsuya S, Houle S, Kapur S. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S, Mizrahi R. Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab. 2011;31:1807–1816. doi: 10.1038/jcbfm.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Wiley C, Kofler J. Imaging microglial activation during neuroinflammation and Alzheimer's disease. J Neuroimmune Pharmacol. 2009;4:227–243. doi: 10.1007/s11481-008-9142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Parkes J, McCormick P, Stephenson KA, Houle S, Vasdev N. Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl Med Biol. 2008;35:305–314. doi: 10.1016/j.nucmedbio.2007.12.009. [DOI] [PubMed] [Google Scholar]