Abstract
Minocycline has been proposed as a way to blunt neurovascular injury from matrix metalloproteinases (MMPs) during stroke. However, recent clinical trials suggest that high levels of minocycline may have deleterious side-effects. Here, we showed that very high minocycline concentrations damage endothelial cells via calpain/caspase pathways. To alleviate this potential cytotoxicity, we encapsulated minocycline in liposomes. Low concentrations of minocycline could not reduce tumor necrosis factor α (TNFα)-induced MMP-9 release from endothelial cells. But low concentrations of minocycline-loaded liposomes significantly reduced TNFα-induced MMP-9 release. This study provides proof-of-concept that liposomes may be used to deliver lower levels of minocycline for targeting MMPs in cerebral endothelium.
Keywords: brain endothelial cell, calpain, caspase, cell death, liposome, minocycline
Introduction
Many studies have implicated matrix metalloproteinases (MMPs) as neurovascular proteases that contribute to blood–brain barrier injury, edema, hemorrhage, and brain cell death after cerebral ischemia (Rosell and Lo, 2008). Thus, finding ways to block MMPs may provide a potential therapeutic approach for stroke.
Recently, minocycline has been proposed as a way to dampen the damaging effects of MMPs in stroke (Fagan et al, 2011). In animal models of cerebral ischemia, minocycline downregulated MMPs and decreased edema and hemorrhagic conversion associated with thrombolysis (Murata et al, 2008). Based in part on these experimental findings, clinical trials have been initiated to test minocycline in human stroke (Fagan et al, 2010; Switzer et al, 2011). So far, the results have been promising, and ‘biomarker' levels of MMPs in patient plasma show reductions in concordance with minocycline treatment (Switzer et al, 2011).
However, with any neuroprotectant or neurovascular target, there may always be biphasic properties involved. For example, MMPs cause neurovascular injury during acute stroke but may mediate neurovascular remodeling during stroke recovery (Rosell and Lo, 2008). For minocycline itself, there may also be biphasic caveats worth considering. Beneficial effects include MMP inhibition, amelioration of neuroinflammation, and prevention of cell death (Fagan et al, 2011). But some data suggest that minocycline may not always be benign. Under some experimental conditions, higher doses of minocycline may exacerbate neurotoxicity (Matsukawa et al, 2009; Tsuji et al, 2004; Yang et al, 2003). A recent clinical trial in amyotropic lateral sclerosis suggested that minocycline may worsen outcomes (Gordon et al, 2007).
In this study, we asked whether minocycline can have damaging effects in brain endothelium, and whether a previously established liposome technology (Asahi et al, 2003) can be used to lower the concentrations of minocycline required for targeting MMPs in cerebral endothelium.
Materials and methods
Reagents
RPMI1640, trypsin-EDTA, L-glutamine, fetal bovine serum, sodium pyruvate, minimum essential media (MEM) nonessential amino acids, MEM vitamins, and antibiotics for cell culture were from Invitrogen (Carlsbad, CA, USA). NuSerum was from BD Biosciences (San Jose, CA, USA). Cytotoxicity Detection Kit lactate dehydrogenase (LDH) was from Roche Diagnostics (Mannheim, Germany). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was from Sigma-Aldrich (St Louis, MO, USA). Caspase inhibitors (z-VAD-fmk and z-DEVD-fmk) and tumor necrosis factor α (TNFα) were from R&D (Minneapolis, MN, USA). Calpain inhibitor III was from Calbiochem (San Diego, CA, USA). Rabbit anti-cleaved caspase-3, cleaved caspase-7, and cleaved poly(ADP-ribose) polymerase (PARP) primary antibodies were from Cell Signaling (Danvers, MA, USA). Spectrin-α primary antibody was from Santa Cruz (Santa Cruz, CA, USA). In all, 4% to 20% Tris-Glycine gel, nitrocellulose membrane, 10% Zymogram (Gelatin) gel, Zymogram Renaturing buffer, and developing buffer were from Invitrogen.
Human Brain Endothelial Cell Culture
A human brain microvascular endothelial cell line was cultured in RPMI1640 supplemented with 10% fetal bovine serum, 10% NuSerum, 1 mM sodium pyruvate, MEM nonessential amino acids, MEM vitamins, and 100 units/mL penicillin/streptomycin. This cell line has been previously confirmed to express brain endothelial phenotypes (Supplementary Figure 1). To further confirm this, CD31, von Willebrand and VE-cadherin expression were documented via western blotting (Supplementary Figure 1). Cells were incubated with minocycline (3.75, 7.5, 15, and 30 μg/mL) for 24 hours. For inhibitor experiments, a pretreatment protocol (1 and 5 μmol/L of calpain inhibitor III, 40 μmol/L of z-VAD-fmk, and 40 μmol/L of z-DEVD-fmk) was used for 1 hour before addition of 30 μg/mL minocycline for 24 hours. Cytotoxicity was evaluated by standard LDH and MTT assays (repeated at least three times in triplicate). To detect the activation of caspase-3, -7, and calpain, the cells were incubated with 30 μg/mL of minocycline for 4, 8, 16, and 24 hours. Western blotting of cell lysates was used to detect activation of caspase-3, caspase-7 and calpain, and PARP cleavage. Experiments were also replicated in a second human brain endothelial cell line (CSC Cell System, Kirkland, WA, USA) (Supplementary Figure 2).
Minocycline–Liposome Formulation
Previously published techniques were used to encapsulate minocycline in liposomes (Hu et al, 2009). Briefly, a lipid film composed of dipalmitoyl phosphatidylcholine, cholesterol, and PEG2000-PE (molar ratio 1.85:1:0.15) was prepared in a round-bottom flask by removing chloroform from lipid solution. The film was further dried for 4 hours under high vacuum and then rehydrated in 120 mM sodium acetate, 120 mM CaCl2 pH 7.9 up to a final liposome concentration of 10 mg/mL. The hydrated lipids were extruded through 200 nm polycarbonate membrane. Unencapsulated calcium chloride and sodium acetate were removed by dialysis against sodium chloride for 1 hour at RT. To 8 mL liposomal formulation, 80 mg minocycline hydrochloride was added. After 15 minutes of incubation at 50 °C, the liposomes were dialyzed overnight against PBS pH 7.5 at 4 °C.
All formulations were characterized by size, size distribution using dynamic light scattering on a N4 Coulter (Beckman-Coulter, Brea, CA, USA) and ζ potential using the ζ potential analyzer Zeta-Plus (Brookhaven Instrument Corporation, Holtsville, NY, USA). A portion (10 μL) of each liposome suspension was diluted up to 2 mL in PBS buffer and then analyzed for the size distribution; for the ζ potential, each sample was diluted in 1 mM KCl (5 μL/1.5 mL). To determine the concentration of minocycline, liposomal samples were diluted with methanol (1/10) and absorbance was measured at 350 nm in water.
Matrix Metalloproteinase Assays
Matrix metalloproteinase release from endothelial cells was induced by exposure to TNFα, and this bioassay was used for comparing the efficacy of ‘naked' minocycline versus liposome-encapsulated minocycline for targeting MMPs. The MMP-2 and MMP-9 in cell culture media was measured by gelatin zymogram following standard procedures.
Statistical Analysis
Data were expressed as mean±s.d. Three to five separate experiments were performed. Data were analyzed using t-tests between two groups or analysis of variance with Tukey post hoc tests between multiple groups (SPSS version 11.5, Chicago, IL, USA). Statistical significance was set at P<0.05.
Results
Cytotoxic Effect of Minocycline on Human Brain Microvascular Endothelial Cells
Cytotoxicity of minocycline was evaluated using a standard LDH release assay. Exposure to minocycline (3.75 to 30 μg/mL) for 24 hours induced a dose-dependent cell death in cerebral endothelial cells (Figure 1A). To further confirm these LDH cytotoxicity findings, we also measured cell viability using an MTT technique. Endothelial cell viability was similarly reduced after exposure to minocycline for 24 hours (Figure 1B). Similar effects of minocycline cytotoxicity were obtained in a second human cerebral endothelial cell line (Supplementary Figure 2).
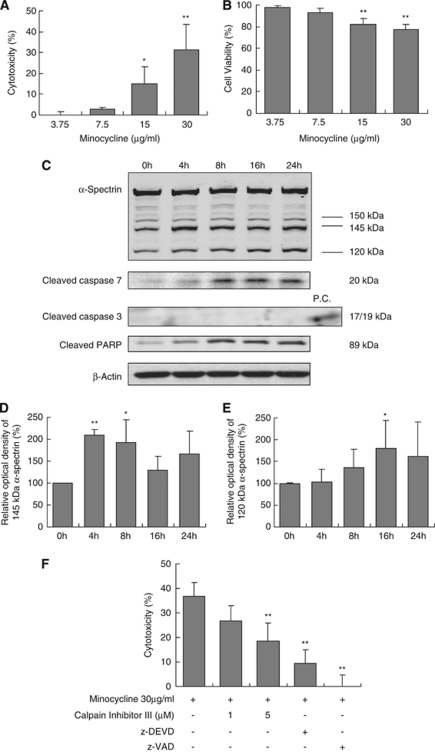
Figure 1.
Cytotoxic effect of minocycline on human brain microvascular endothelial cells and involved signaling pathways. Human brain microvascular endothelial cells were treated with different concentrations of minocycline for 24 hours or with 30 μg/mL minocycline for indicated times, then cytotoxicity and involved pathways were detected. (A) Exposure to minocycline for 24 hours induced a dose-dependent cytotoxic response (LDH assay). *P<0.05, **P<0.01 compared with the control group. (B) Cell viability reduced after exposure to 15 and 30 μg/mL of minocycline for 24 hours (MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays). **P<0.01 compared with the control group. (C) Minocycline induced a time-dependent calpain activation and cleavage of caspase-7 and PARP. Representative blots of 145 and 120 kDa breakdown product of α-spectrin, cleaved caspase-7 (20 kDa), and cleaved PARP (89 kDa) were shown. (D, E) Quantitative analyses of the levels of 145 kDa (D) or 120 kDa (E) breakdown product of α-spectrin (n=5). *P<0.05, **P<0.01 compared with the control group. (F) Pretreatment with calpain inhibitor III or 40 μM of caspase inhibitors (z-VAD-fmk and z-DEVD-fmk) significantly reduced minocycline-induced cell death. Cell death was detected by LDH assay. n=3 independent experiments performed in triplicate. **P<0.01 compared with minocycline alone. LDH, lactate, dehydrogenase; PARP, poly(ADP-ribose) polymerase.
Minocycline-Induced Cell Death Involves Calpain and Caspase Activation
Mechanisms of cell death in cerebral endothelial cells are known to typically involve activation of calpain and caspases (Pober et al, 2009). Hence, we asked whether the cytotoxicity triggered by high concentrations of minocycline also involved similar pathways. Cerebral endothelial cells were incubated with 30 μg/mL minocycline, and cleavage products of α-spectrin were assessed by western blots over 24 hours. A calpain-specific 145 kDa fragment of α-spectrin was rapidly increased within 4 to 8 hours (Figures 1C and 1D), and a caspase-specific 120 kDa fragment of α-spectrin was progressively elevated by 16 to 24 hours (Figures 1C and 1E). Western blots showed that PARP was cleaved, suggesting the involvement of caspase-3 or capase-7 (Figure 1C). Although we were unable to detect any cleaved/activated caspase-3, the 20-kDa cleaved/activated band of caspase-7 was readily observed (Figure 1C). Similar profiles of minocycline-induced activation of calpain and caspase were obtained in a second human cerebral endothelial cells line (Supplementary Figure 2). Finally, consistent with these proteolytic markers of α-spectrin degradation, treatment with either a calpain inhibitor or various caspase inhibitors (Figure 1F) significantly reduced minocycline-induced endothelial cell death.
Effect of Minocycline on the Production of Matrix Metalloproteinases
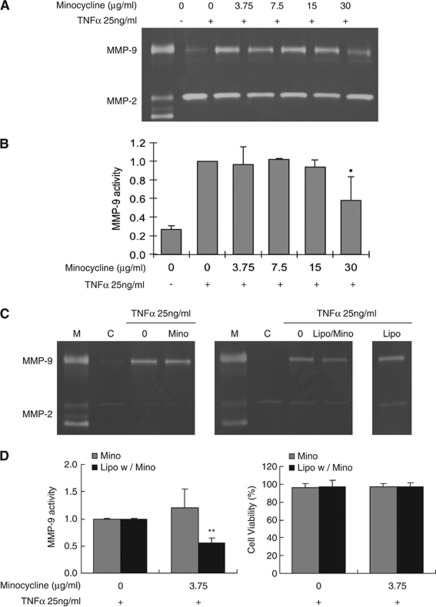
Minocycline may be a potent way to block MMPs in cerebral endothelium. But our data suggest that high concentrations of minocycline may induce calpain and caspase-mediated cytotoxicity. Previously, we had proposed liposomes as a method for delivering drugs to cerebral endothelium (Asahi et al, 2003). Can we use this liposome technology to reduce concentrations of minocycline to avoid cytotoxicity while retaining its ability to target MMPs? Tumor necrosis factor α potently evokes a release of MMP-9 from cerebral endothelial cells (Figure 2A), and as expected, high concentrations of minocycline can reduce MMP-9 levels in this bioassay (Figure 2B). In contrast, low concentration (3.75 μg/mL) of minocycline was unable to block TNFα-induced MMP-9 (Figures 2C and 2D). However, when these low concentrations of minocycline were encapsulated with liposomes, MMP-9 release was significantly reduced (Figures 2C and 2D). Empty liposomes had no effect on TNFα-induced MMP-9 release (Figure 2C). Importantly, these low levels of minocycline were not toxic to cerebral endothelial cells, regardless of whether they were ‘naked' or encapsulated in liposomes (Figure 2D).
Figure 2.
Gelatin zymograms of matrix metalloproteinase (MMP) responses. (A) Representative zymograms of MMPs. Only high concentration (30 μg/mL) of minocycline significantly decreased tumor necrosis factor α (TNFα)-induced MMP-9 release. (B) Bar graph of quantified densitometry of MMP-9 activity. *P<0.05 compared with TNFα group. (C) Representative zymograms of MMPs showing that 3.75 μg/mL of minocycline did not decrease TNFα-induced MMP-9 release (left panel). In contrast, the same dose of minocycline-loaded liposomes significantly reduced TNFα-induced MMP-9 release (middle panel). There is no effect of empty nonminocycline-loaded liposome (right panel). Because pro versus active bands of MMP-9 were not always distinguishable, zymogram densitometry included both pro and active forms. Furthermore, MMP-2 levels were variable so these responses could not be reliably quantified. (D) Bar graph of quantified densitometry of MMP-9 levels. There were no changes in cell viability. **P<0.01 compared with TNFα group.
Discussion
Minocycline is a tetracycline derivative, and has been used safely and effectively as an antibiotic in adult humans for decades. Recently, minocycline has been proposed as a way to target MMPs in stroke (Fagan et al, 2011). In experimental animal models, minocycline markedly reduced infarct volume and improved neurological outcomes (Murata et al, 2008). In the first reported clinical trial in humans, minocycline therapy appeared to be safe and feasible, and importantly, seemed to effectively lower MMP-9 biomarker levels in plasma (Switzer et al, 2011).
However, some literature suggests that high concentrations of minocycline may not be completely benign. In experimental models of neurodegeneration, minocycline may exacerbate neurotoxicity (Yang et al, 2003). Minocycline seemed to worsen outcomes in a neonatal mouse model of hypoxia-ischemia (Tsuji et al, 2004). And a recent clinical trial in amytrophic lateral sclerosis suggested that with long-term high-dose treatments, some patients may have worsened outcomes (Gordon et al, 2007). Hence, we embarked on this small proof-of-concept study to assess the effects of minocycline in cerebral endothelium. In our cell culture model system, high concentrations of minocycline appeared to trigger calpain and caspase-mediated cytotxicity in human cerebral endothelial cells. By encapsulating minocycline in liposomes, we were able to reduce the concentrations of minocycline required to block MMPs while eliminating the potential cytotoxicity that may be associated with higher doses (Supplementary Figure 3). Liposomes are artificial phospholipid vesicles that can be designed for effective encapsulation of the therapeutic agent (usually a small molecule drug), and have proven to be useful in stabilizing drugs and improving their pharmacological properties. There are several advantages in using drug delivery systems: to protect a drug against the inactivating action of the biological microenvironment, to protect nonpathological tissues against the nonspecific toxic action of a drug, and to favorably change and control drug pharmacokinetics. In this study, liposomes are not ‘protective' per se. Instead, liposomes may enhance the cellular delivery of minocycline into the endothelium, thus allowing one to use lower noncytotoxic levels of minocycline to target neurovascular MMPs.
However, there are four important caveats with this initial proof-of-concept study. First, it is difficult to truly reconcile minocycline concentrations used in our cell culture models with what happens in vivo. We found that 30 μg/mL was toxic in endothelial cultures. This may be consistent with a previous study demonstrating a biphasic effect of minocycline in corneal endothelial cultures—10 to 40 μM minocycline prevented apoptosis but 75 to 100 μM (equivalent to 37.5 to 50 μg/mL) minocycline promoted apoptosis (Kernt et al, 2010). But how do these levels compare with in vivo situations? In the human stroke clinical trial, intravenous mincocycline at doses between 3 and 10 mg/kg daily for 3 days yielded average serum concentrations around 5.03 to 23.65 μg/mL (Fagan et al, 2010). A more careful analysis of minocycline effects on endothelial function in vivo may be very useful. A second caveat involves the mechanisms of endothelial cell death. Our data implicate both calpain and caspase but the precise molecular sequence of these pathways are not fully known. Nevertheless, calpain and caspases represent common mechanisms of neuronal death in many central nervous system disorders including stroke (Chan, 2004; Ray and Banik, 2003). Hence, combination therapies utilizing calpain or caspase inhibitors plus minocycline are options that should be further explored. Third, our experiments focus on cerebral endothelial cells because vascular MMPs represent a critical target for stroke therapy. However, whether liposome-encapsulated minocycline may also have important effects in other brain cell types such as neurons and glia remain to be determined. Finally, inflammation is a very complicated reaction to injury or disease in the brain. Tumor necrosis factor α treatment in endothelial cell cultures is a very limited model that attempts to mimic some of these inflammatory responses. Nevertheless, TNFα elevation is a robust phenomenon in stroke, and TNFα treatment is a reproducible and stable way to induce MMP-9 release in endothelial cells. This in vitro model allows us to test the effect of minocycline as MMP-9 inhibitor in this study. Future studies to validate these findings using in vivo stroke models are required.
Minocycline may represent an important repurposed drug candidate for stroke therapy. Our proof-of-concept study suggests that liposome technology may provide a way to deliver lower levels of minocycline for targeting MMPs in cerebral endothelium.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported in part by NIH Grants R01-NS56458, R37-NS37074, and P01-NS55104.
Supplementary Material
References
- Asahi M, Rammohan R, Sumii T, Wang X, Pauw RJ, Weissig V, Torchilin VP, Lo EH. Antiactin-targeted immunoliposomes ameliorate tissue plasminogen activator-induced hemorrhage after focal embolic stroke. J Cereb Blood Flow Metab. 2003;23:895–899. doi: 10.1097/01.WCB.0000072570.46552.DF. [DOI] [PubMed] [Google Scholar]
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Cronic LE, Hess DC. Minocycline development for acute ischemic stroke. Transl Stroke Res. 2011;2:202–208. doi: 10.1007/s12975-011-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC. Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke. 2010;41:2283–2287. doi: 10.1161/STROKEAHA.110.582601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, Neville HE, Boylan K, Mozaffar T, Belsh JM, Ravits J, Bedlack RS, Graves MC, McCluskey LF, Barohn RJ, Tandan R. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Hu W, Metselaar J, Ben LH, Cravens PD, Singh MP, Frohman EM, Eagar TN, Racke MK, Kieseier BC, Stuve O. PEG minocycline-liposomes ameliorate CNS autoimmune disease. PLoS One. 2009;4:e4151. doi: 10.1371/journal.pone.0004151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernt M, Hirneiss C, Neubauer AS, Kampik A. Minocycline is cytoprotective in human corneal endothelial cells and induces anti-apoptotic B-cell CLL/lymphoma 2 (Bcl-2) and X-linked inhibitor of apoptosis (XIAP) Br J Ophthalmol. 2010;94:940–946. doi: 10.1136/bjo.2009.165092. [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, Kaneko Y, Ojika K, Hess DC, Borlongan CV. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 2009;10:126. doi: 10.1186/1471-2202-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Switzer JA, Hess DC, Ergul A, Waller JL, Machado LS, Portik-Dobos V, Pettigrew LC, Clark WM, Fagan SC. Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke. 2011;42:2633–2635. doi: 10.1161/STROKEAHA.111.618215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Wilson MA, Lange MS, Johnston MV. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp Neurol. 2004;189:58–65. doi: 10.1016/j.expneurol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Yang L, Sugama S, Chirichigno JW, Gregorio J, Lorenzl S, Shin DH, Browne SE, Shimizu Y, Joh TH, Beal MF, Albers DS. Minocycline enhances MPTP toxicity to dopaminergic neurons. J Neurosci Res. 2003;74:278–285. doi: 10.1002/jnr.10709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.