Abstract
Although pathogenic bacteria are suspected contributors to colorectal cancer progression, cancer-promoting bacteria and their mode of action remain largely unknown. Here we report that sustained infection with the human intestinal colonizer Pseudomonas aeruginosa synergizes with the Ras1V12 oncogene to induce basal invasion and dissemination of hindgut cells to distant sites. Cross-talk between infection and dissemination requires sustained activation by the bacteria of the Imd–dTab2–dTak1 innate immune pathway, which converges with Ras1V12 signalling on JNK pathway activation, culminating in extracellular matrix degradation. Hindgut, but not midgut, cells are amenable to this cooperative dissemination, which is progressive and genetically and pharmacologically inhibitable. Thus, Drosophila hindgut provides a valuable system for the study of intestinal malignancies.
Keywords: Drosophila , cancer, innate immunity
Introduction
Colorectal cancer represents the third leading cause of cancer mortality in the United States. Intestinal infection has been proposed as a risk factor for colon cancer progression [1, 2], but the aspects of tumourigenesis that are potential targets of infection remain poorly understood; proposed mechanisms include chronic inflammation and production of carcinogenic metabolites by pathogens [3]. Furthermore, in combination with genetic aberrations [4, 5], innate immunity stimulation is likely an important contributor to colorectal cancer progression [6, 7]. However, the nature of the link between infection and tumour progression is poorly understood.
Our previous work on the Drosophila midgut has shown that virulent bacteria induce enterocyte apoptosis. This leads to stem cell-mediated compensatory proliferation that works for the benefit of the host by replenishing dying cells [8]. Oncogenes can divert this process to stimulate tumour formation and growth [8], but the impact of intestinal bacteria on subsequent steps of cancer (that is, cancer cell dissemination and metastasis) remains unclear.
The Drosophila Immune Deficiency (Imd) innate immune pathway is considerably conserved in mammals [9, 10]. It is predominantly activated by Gram-negative bacteria and consists of two branches that are both required for activation of the downstream nuclear factor-κB factor Relish (Rel), while only one of them leads to JNK pathway activation [9]. The two branches might be activated sequentially [11] or synergistically to induce immune responses [9] or act antagonistically [12]. In this study we provide an adult Drosophila model of oncogene-induced cell dissemination, a genetically and pharmacologically tractable system to study epithelial cell invasion and dissemination. We use it to show that prolonged bacterial infection that induces an Imd to JNK innate immune signalling in the Drosophila hindgut facilitates the basal invasion and dissemination of oncogenic hindgut epithelial cells to distant tissues.
Results and Discussion
To explore the role of infection on colorectal cancer progression, we developed an adult Drosophila model by expressing the oncogene Ras1V12 in the adult hindgut enterocytes and their progenitors [13, 14]. We controlled the byn-GAL4 driver with GAL80ts, a temperature-sensitive GAL4 inhibitor, to restrict Ras1V12 activation only in the adult. UAS-gfp was used to monitor Ras1V12-expressing cells over time (Fig 1A).
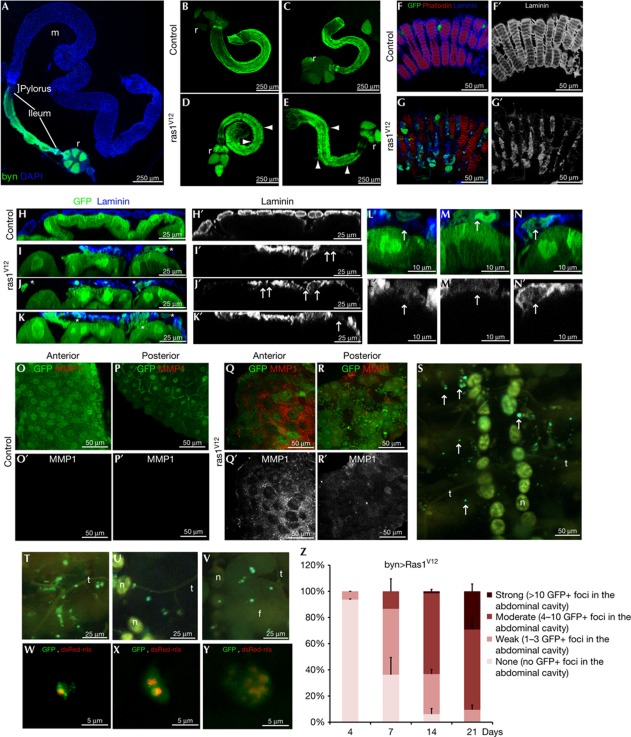
Figure 1.
Ras1V12 expression is sufficient to initiate dissemination of the adult Drosophila hindgut cells. (A) Drosophila intestinal epithelium. Hindgut cells green fluorescent protein (GFP)-marked using byn-GAL4. Blue, nuclei (DAPI); m, midgut, r, rectum. (B–E) Hindguts expressing GFP alone (B,C) or GFP plus Ras1V12 (D,E) 7 days (B,D) or 21 days (C,E) after induction of transgenes. Arrowheads: cellular protrusions throughout ileum. (F,G) Surface views of ileum expressing GFP alone (F) or GFP plus Ras1V12 (G). Blue, laminin (basement membrane). (F′,G′) Laminin channel only of F and G. (H–K′) Cross-sections of control (H,H′) and Ras1V12 (I–K′) hindguts. Ras1V12-expressing hindguts (I–K) show GFP+ cells in the process of invading basally out of the gut epithelium (stars). These regions also have reduced or absent laminin staining (I′–K′, arrows). (L–N) Close-up views of disseminating cells (arrows) from Ras1V12-expressing hindguts. (laminin, blue). (L′,N′) Laminin channel only of L–N. Disseminating cells are present in regions of reduced laminin staining (arrows). (O–R) Matrix metalloprotease 1 (MMP1) expression (red) in control (O,P) and Ras1V12-expressing hindguts (Q,R). (O′–R′) MMP channel only of O–R. (S) Inside view of the abdominal cavity from an animal expressing Ras1V12 in the hindgut showing GFP+ foci (arrows). Stereoscope (T–Y) and confocal (W–Y) live views of GFP+ foci and dsRed nuclei (dsRed-nls). Note: yellow autoflorescence makes nephrocytes (n), fat body (f) and trachea (t) visible. (Z) Quantification of Ras1V12-induced dissemination over time. DAPI, 4,6-diamidino-2-phenylindole; nls, nuclear localization signal.
Seven days after transferring flies to the permissive temperature (29°C) to activate Ras1V12, we observed abundant green fluorescent protein (GFP)-positive hindgut cells delaminating through the basal side of the epithelium (Fig 1B–G). The hindgut showed reduced and non-uniform laminin staining in the gaps where hindgut epithelial cells and cytoplasmic protrusions were mislocalized (Fig 1F–N). Laminin loss can be a result of basement membrane degradation by secreted matrix metalloproteases (MMPs) [4, 15, 16]. Indeed, we found high levels of non-uniform MMP1 expression in Ras1V12 hindgut epithelia that was absent in wild-type control animals (Fig 1O–R).
Following 7 days of Ras1V12 induction, we found that Ras1V12-expressing hindgut cells disseminated away from the hindgut to distant sites within the body. These disseminating cells formed GFP-positive foci—both as individual cells and as cell clusters (Fig 1W–Y)—in the abdominal cavity, including the body wall, trachea, fat body and nephrocytes (Fig 1S–Y). We classified the dissemination phenotypes into four groups according to their quantitative strength: none, weak, moderate and strong based on the number of foci observed in the abdominal cavity (Fig 1Z). Strikingly, dissemination was progressive, increasing in penetrance and severity over time (Fig 1Z). This indicated that migrating cells either do not die or that their dissemination rate is higher than their death rate.
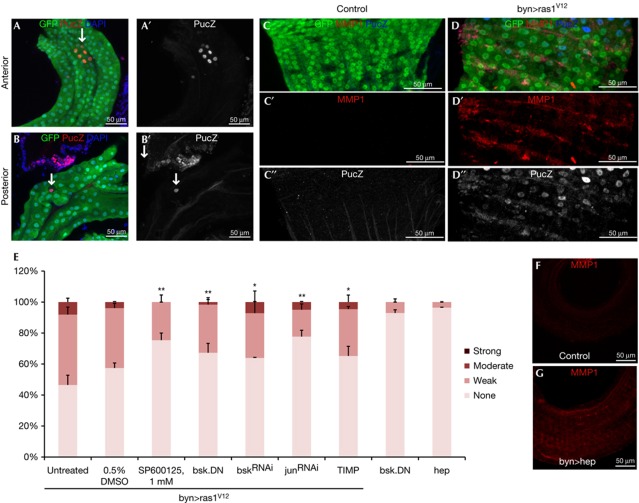
On the basis of the previous observations [16], we assessed the requirement and sufficiency of JNK pathway activation in MMP1 induction and hindgut cell dissemination. The JNK pathway reporter puckered-lacZ (pucZ) [17] was induced in a subset of hindgut cells at 4 days (pucZ+ cells/hindgut =6±3.5 s.d.) and at 7 days (12±2.8 s.d.) after Ras1V12 induction (Fig 2A,B); no pucZ+ cells were present after 2 days of induction. Co-expressing the dominant-negative JNK isoform BskDN or RNA interference (RNAi) for Bsk or Jun [8] or feeding of SP600125, a JNK pathway inhibitor with conserved activity in Drosophila [12, 18], suppressed dissemination of Ras1V12 hindgut cells (Fig 2E). In addition, expression of the JNK kinase Hep sufficed to induce MMP1, facilitating dissemination of hindgut cells specifically in the presence of Ras1V12 (Figs 2E,4B); by contrast, expression of the MMP inhibitor TIMP considerably reduced dissemination (Fig 2E). Of note, delaminating enterocytes did not express detectable pucZ (0%, n=20), and pucZ staining only partially overlapped with MMP1 expression (Fig 2C,D; supplementary Fig S1 online), suggesting that strong JNK pathway activation is either transient or it induces MMP1 non-autonomously to facilitate dissemination of hindgut cells.
Figure 2.
JNK pathway activation contributes to the induction of Ras1V12-mediated dissemination phenotypes. (A,B) PucZ expression in anterior (A) and posterior (B) byn>Ras1V12 hindguts. (C,D) PucZ and matrix metalloprotease 1 (MMP1) expression in wild-type (C) and byn>Ras1V12 (D) hindguts. A′,B′,C″,D″ are PucZ and C′,D′ are MMP1 channel only of A–D. (E) Quantitated dissemination 7 days after induction of the indicated transgenes (*P<0.05, **P<0.01). (F,G) MMP1 of wild-type (F) and byn>hep (G) hindguts. DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein.
Figure 4.
P. aeruginosa infection and Imd activation induce an earlier onset of dissemination phenotypes in the Ras1V12 background. (A) Quantitated dissemination 4 days after induction of Ras1V12 expression in the presence of infection. (B) CF5-induced dissemination in byn>Ras1V12 flies co-expressing the indicated transgenes. (C) Co-expressing Imd, but not relD, with Ras1V12 results in a strong dissemination phenotype 4 days after induction. (D) MMP1 expression (red) in hindguts with indicated genotypes and treatment conditions 4 days after induction. Infection or Imd expression induces MMP1 expression in the presence of Ras1V12 4 days after induction. (E) Early onset of dissemination induced by infection is suppressed by RNAi and mutations of the indicated Imd pathway components. *P<0.05, **P<0.01. DAPI, 4,6-diamidino-2-phenylindole; DMSO, dimethylsulphoxide; GFP, green fluorescent protein; MMP1, matrix metalloprotease 1; RNAi, RNA interference.
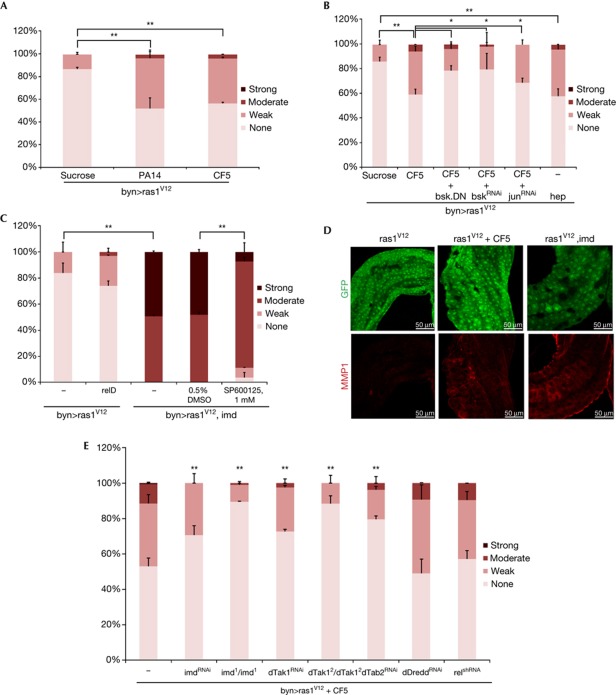
To test whether bacterial infection can synergize with oncogenes to induce dissemination, we challenged flies by feeding them with two strains of P. aeruginosa, a human bacterial pathogen that can also colonize the Drosophila intestine [8, 19]. PA14 strain is lethal to flies starting 5 days of ingestion, while the CF5 strain causes no mortality due to the lack of expression of several main virulence factors [8, 20]. Approximately 300 (±250 s.d.) bacteria from each strain were sustained within the hindgut and both strains induced CecZ, an antimicrobial peptide reporter for Cecropin (supplementary Fig S2 online). To examine the role of PA14 and CF5 in our migratory assay, we induced Ras1V12 for 6 days and then fed flies with these strains for 4 days at 25°C, conditions that avoid fly mortality due to infection (Fig 3A). Importantly, regardless of their virulence, both strains potentiated dissemination to a similar extent (Fig 3B), indicating that sustained immune response rather than virulence is necessary for this potentiation.
Figure 3.
P. aeruginosa intestinal infection potentiates dissemination phenotypes. (A) Outline of the transgene induction and infection strategy. (B) Quantitated dissemination in flies fed on sucrose (suc) only, virulent (PA14) or avirulent (CF5) P. aeruginosa strain. (C,D) pucZ expression in infected (D) and uninfected (C) byn>Ras1V12 hindguts. (C′,D′) pucZ only channels of C,D. (E) Quantitated dissemination in flies co-expressing Ras1V12 and Imd following the induction scheme in A without infection. (F, G) Hindguts co-expressing Ras1V12 and Imd show stronger MMP1 expression (red), particularly in the anterior region (arrows). (F′,G′) MMP1 channels of F,G (arrowheads: migratory cells; *P<0.05, **P<0.01). DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein; MMP1, matrix metalloprotease 1.
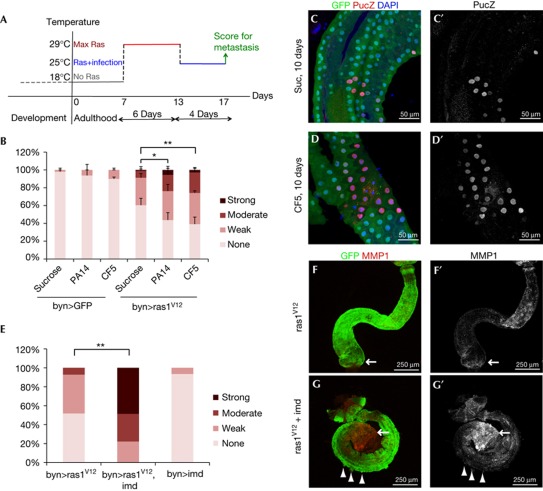
To identify mechanisms by which infection enhanced Ras1V12-dependent dissemination, we further explored JNK activity, a mediator of the immune response. Ras1V12-expressing flies fed with CF5 bacteria showed higher numbers of pucZ+ cells (37±7 s.d.) than those fed with bacteria-free media (14±4.3 s.d., P=0.003; Fig 3C,D), indicating elevated JNK activity; wild-type controls did not show detectable pucZ+ cells. Imd is a central mediator of the Drosophila innate immune response and a potent activator of JNK on bacterial infection in Drosophila [21]. Co-expression of Ras1V12 and Imd in the hindgut (byn>Ras1V12Imd) led to a striking potentiation of dissemination (Fig 3E) coupled with increased MMP1 expression (Fig 3F,G).
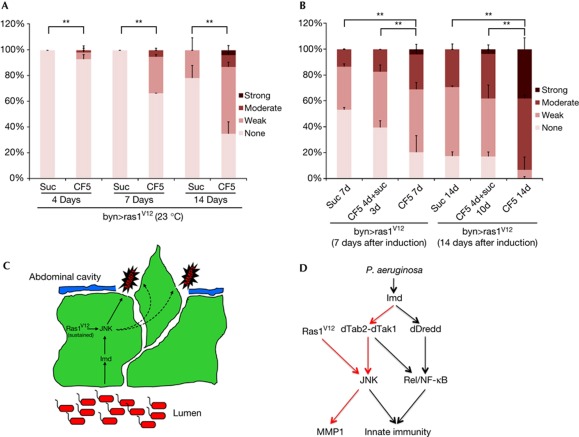
We next tested whether infection can also promote dissemination at 4 days of Ras1V12 induction, a stage at which only 10–15% of uninfected flies show (weak) dissemination (Figs 1Z,4A). Dissemination was suppressed by co-expression of BskDN or RNAi for Bsk or Jun (Fig 4B) and strongly enhanced by Imd co-expression (Fig 4C), indicating that dissemination was dependent on JNK pathway activity. Further, bacterial infection or Imd expression led to detectable MMP1 expression in byn>Ras1V12 adults 4 days after induction (Fig 4D). Reducing activity of the Imd pathway members Imd, dTab2 and dTak1—key regulators of the branch of immune response leading to JNK activation [9]—also reduced the penetrance and severity of dissemination (Fig 4E). In contrast, a distinct branch of the Imd pathway dedicated to nuclear factor-κB/Rel activation, specifically dDredd and Rel [9], was not required for full induction of dissemination (Fig 4E). Also co-expression of an activated form of Rel (relD) for 4 days failed to enhance Ras1V12-induced dissemination (Fig 4C). Our results collectively indicate that the synergistic interaction between infection and Ras1V12 expression is mediated by the interaction of Ras1V12 with the Imd–dTab2–dTak1–JNK axis, but not the Imd–dDredd–Rel innate immunity branch (Fig 5D).
Figure 5.
Requirement for sustained bacterial infection for the induction and enhancement of dissemination. (A) Quantitated dissemination of byn>Ras1V12 flies at 23 °C (semipermissive conditions) fed continuously on sucrose only or infected with CF5 for 4, 7 and 14 days (d). (B) Quantitated dissemination of byn>Ras1V12 flies at days 7 and 14 of Ras1V12 transgene induction at 29 °C. Animals were either fed continuously on sucrose only (‘suc 7d’ and ‘suc 14d’) or infected with CF5 for 4 days followed by 3 or 10 days of bacterial clearance (‘CF5 4d + suc 3d’ or ‘CF5 4d + suc 10d’); or continuously infected for 7 or 14 days (‘CF5 7d’ and ‘CF5 14d’; **: P<0.01). (C) Model illustration depicting the synergistic induction of JNK and MMP1 in the hindgut enterocytes and the destruction of laminin as a mechanism that facilitates cell dissemination. (D) Model illustration delineating the branch of innate immune responses that converge with Ras1V12 signalling (red arrows) and the Imd pathway branch dedicated to the activation of Rel/nuclear factor-κB (black arrows). DAPI, 4,6-diamidino-2-phenylindole; DMSO, dimethylsulphoxide; GFP, green fluorescent protein; MMP1, matrix metalloprotease 1; NF-κB, nuclear factor-κB.
To determine whether P. aeruginosa infection can induce Ras1V12-expressing hindgut cells to disseminate at a stage when uninfected flies are completely dissemination-free, we induced this oncogene at low levels by placing byn>Ras1V12 flies at a semipermissive temperature (23°C). Dissemination was not observed at 7 days, and only weak, low penetrant dissemination was seen at 14 days in uninfected flies (Fig 5A). In contrast, infected flies showed both weak and moderate dissemination as early as 4 days after induction and infection, which became progressively stronger and more penetrant (Fig 5A).
To test if sustained bacterial infection is required for the potentiation of dissemination, we monitored infected animals that were subsequently cleared of P. aeruginosa by feeding them methyl paraben and propionic acid. The enhanced dissemination observed in infected animals was lost within 3 days of clearing the bacteria: no significant differences in dissemination were subsequently observed between previously infected and non-infected flies (Fig 5B). By contrast, continuous infection led to continued enhancement of Ras1V12-mediated dissemination (Fig 5B). We conclude that the process of infection-enhanced dissemination is progressive and requires sustained infection.
The mechanisms by which microbes affect dissemination are not clear. One possibility is that pathogens can damage epithelia, inducing regeneration that might favour tumourigenesis in genetically predisposed hosts [7], as in the Drosophila midgut [8]. However, we observed no dissemination in the midgut model (esg-GAL4>Ras1V12) in the presence or absence of infection and no evidence of hindgut regeneration due to infection (supplementary Fig S3 online). Perhaps hindgut cells, in contrast to midgut cells, are more resistant to stress and apoptosis [22, 23], provoke a stronger immune response (supplementary Fig S2 online) or have a distinct physiology or metabolism [24, 25]. Whatever the mechanism, sustained immune response rather than virulence potentiates Ras1V12-mediated dissemination in the Drosophila hindgut. This dissemination requires potentially transient and non-autonomous JNK pathway activation as well as both autonomous and/or non-autonomous induction of MMP1 (Fig 5C).
Hindgut cells showed features of basal invasion and dissemination rather than shedding of cells due to tissue damage. For example, we observed (i) no evidence of regeneration on infection, (ii) basement membrane degradation, MMP1 expression and basal delamination of cells, (iii) cytoplasmic processes of delaminating cells, (iv) attachment of cell foci in tissues and to each other, and (v) progressive accumulation of foci.
Our Ras1V12-based hindgut cell invasion and dissemination assay likely generates benign-like tumours that might require more mutations to establish secondary tumours and metastasis. Nevertheless, our study emphasizes the potential importance of microbial infections in promoting colon cancer progression, including towards malignancy. Monitoring individuals—especially those genetically predisposed for colon cancer—for microbiota that promote sustained and aberrant innate immune responses might help to reduce their risk for malignant progression.
Methods
Dissemination assay. Anaesthetized females were lined up ventral sides up on microscope slides coated with vaseline and slides were then submerged in 1 × PBS. The abdomens were opened up, intestines and ovaries removed, and the number of GFP+ foci along the abdominal cavity was counted using a Leica MZ 16F dissecting microscope with a GFP filter under a × 10 magnification. Each experiment was performed in duplicate (n=30 in each replicate); error bars indicate standard error. P-values were calculated using Fisher's exact test with a 4 × 2 contingency table to assess the four phenotypic classes between two conditions at a time. Background dissemination level in control uninfected and infected flies were 1% (n=264) and 5–10% (n=60).
Pharmacological treatment. SP600125 (1 mM; Cayman Chemicals) in 0.5% DMSO was prepared by diluting a 200-mM stock (in 100% dimethylsulphoxide, DMSO) in 60°C-heated flyfood using Bloomington's semidefined medium recipe and dispensed into standard Drosophila vials (1 ml/vial). After the flyfood solidified, flies were transferred onto SP600125/DMSO or DMSO only or control flyfood (n=30 flies/vial). Flies were transferred onto fresh compound-treated food every other day until scoring.
Immunohistochemistry. The following primary antibodies were used: rabbit anti-β-gal (1:10,000; Cappel), mouse anti-MMP1 (1:100; DSHB), mouse anti-BRDU (1:10; BD Biosciences) and rabbit anti-laminin (1:500; Abcam). Secondary antibodies conjugated to Alexa Fluor-568, 633 and 555 were diluted to 1:1,000 (Molecular Probes). Standard staining methods were used [13, 14]. Images were collected on a Leica TCS SP2-AOBS confocal microscope. For β-gal cell enumeration, whole hindgut pucZ-positive cells were counted from n=5 or more female hindguts using a × 20 lens. Error represents the standard deviation of the mean. Statistical significance assessment was done using two-tailed Student's t-test assuming equal variance.
Fly strains. byn-GAL4 (hindgut and salivary gland-specific), UAS-GFP and tubGAL80ts [14] were recombined on the III chromosome and crossed with combinations of the following: UAS-Ras1V12 (II), UAS-bskDN (X), UAS-dsRed-nls (II) and UAS-hep (II) obtained from Bloomington Stock Center; UAS-Imd, Imd1 and dTak1/2 [21]; puc-lacZE69 [17]; UAS-dTab2RNAi and UAS-dRelshRNA [26]; UAS-dTak1RNAi (II), UAS-dDreddRNAi (II), UAS-bskRNAi (III), UAS-JunRNAi (II) and UAS-ImdRNAi (II) obtained from the National Institute of genetics in Japan; UAS-relD [27]. esg-GAL4 (midgut progenitor-, malpighian tubule- and salivary gland-specific) was used for the midgut experiments [8].
Fly culture and infection. Fly rearing and collection were done at 18°C (Gal80 repressor, on; Gal4 expression, off). To induce transgenes, flies were transferred at the semipermissive (23°C) or fully permissive (29°C) temperature. Infections with PA14 and CF5 strains added in 4% sucrose media and colony-forming unit measurements of n=6 hindguts were performed as previously described [8]. For bacterial clearance, 0.1% methyl paraben and 0.36% propionic acid were added to sucrose, eliminating bacteria within 24 h.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Bernard Mathey-Prevot for critical comments on the manuscript. E.B. and R.C. were supported by National Cancer Institute grant R01-CA109730.
Author Contributions: E.B. performed and analysed experiments and had main intellectual contributions. C.P. performed experiments and provided comments. L.G.R. provided insightful comments. R.C. co-wrote the paper and provided insightful comments. Y.A. designed, managed and supervised work and performed and analysed experiments and also wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Beebe JL, Koneman EW (1995) Recovery of uncommon bacteria from blood: association with neoplastic disease. Clin Microbiol Rev 8: 336–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmerich S, Scholler M, Duranton B, Gosse F, Galluser M, Klein JP, Raul F (2000) Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis 21: 753–756 [DOI] [PubMed] [Google Scholar]
- Parsonnet J (1995) Bacterial infection as a cause of cancer. Environ Health Perspect 103(Suppl 8): 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39: 305–318 [DOI] [PubMed] [Google Scholar]
- Voulgari A, Pintzas A (2009) Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 1796: 75–90 [DOI] [PubMed] [Google Scholar]
- Park JH, Yoon HE, Kim DJ, Kim SA, Ahn SG, Yoon JH (2011) Toll-like receptor 5 activation promotes dissemination and invasion of salivary gland adenocarcinoma. J Oral Pathol Med 40: 187–193 [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R (2009) Toll-like receptors and cancer. Nat Rev Cancer 9: 57–63 [DOI] [PubMed] [Google Scholar]
- Apidianakis Y, Pitsouli C, Perrimon N, Rahme L (2009) Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci USA 106: 20883–20888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, Aggarwal K, Paquette N, Silverman N (2011) NF-κB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol 349: 25–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25: 697–743 [DOI] [PubMed] [Google Scholar]
- Boutros M, Agaisse H, Perrimon N (2002) Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell 3: 711–722 [DOI] [PubMed] [Google Scholar]
- Kim T et al. (2005) Downregulation of lipopolysaccharide response in Drosophila by negative crosstalk between the AP1 and NF-κB signaling modules. Nat Immunol 6: 211–218 [DOI] [PubMed] [Google Scholar]
- Fox DT, Spradling AC (2009) The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell 5: 290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V (2008) The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454: 651–655 [DOI] [PubMed] [Google Scholar]
- Igaki T, Pagliarini RA, Xu T (2006) Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol 16: 1139–1146 [DOI] [PubMed] [Google Scholar]
- Uhlirova M, Bohmann D (2006) JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J 25: 5294–5304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring JM, Martinez Arias A (1993) puckered, a gene involved in position-specific cell differentiation in the dorsal epidermis of the Drosophila larva. Dev Suppl 251–259 [PubMed] [Google Scholar]
- Bangi E, Garza D, Hild M (2011) In vivo analysis of compound activity and mechanism of action using epistasis in Drosophila. J Chem Biol 4: 55–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley CD, Duan K, Fischer C, Parkins MD, Storey DG, Rabin HR, Surette MG (2008) Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog 4: e1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, Mindrinos MN, Xiao W, Lau GW, Baldini RL, Davis RW, Rahme LG (2005) Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc Natl Acad Sci USA 102: 2573–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P et al. (2001) Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell 1: 503–514 [DOI] [PubMed] [Google Scholar]
- Chen J, Xie C, Tian L, Hong L, Wu X, Han J (2010) Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc Natl Acad Sci USA 107: 20774–20779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenbacher G, Hafen E, Stocker H (2011) MK2-dependent p38b signalling protects Drosophila hindgut enterocytes against JNK-induced apoptosis under chronic stress. PLoS Genet 7: e1002168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami R, Shiotsuki Y (2001) Ultrastructure of the hindgut of Drosophila larvae, with special reference to the domains identified by specific gene expression patterns. J Morphol 248: 144–150 [DOI] [PubMed] [Google Scholar]
- Shanbhag S, Tripathi S (2009) Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J Exp Biol 212: 1731–1744 [DOI] [PubMed] [Google Scholar]
- Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu LP, Villalta C, Booker M, Perkins L, Perrimon N (2008) Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods 5: 49–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ (2009) The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci USA 106: 20853–20858 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.