Abstract
Tissue engineering with adult stem cells is a promising approach for the restoration of focal defects in articular cartilage. For this, progenitor cells would ideally be delivered to (and maintained within) the defect site via a biocompatible material and in combination with soluble factors to promote initial cell differentiation and subsequent tissue maturation in vivo. While growth factor delivery methods are continually being optimized, most offer only a short (days to weeks) delivery profile at high doses. To address this issue, we investigated mesenchymal stem cell (MSC) differentiation and maturation in photocrosslinkable hyaluronic acid (HA) hydrogels with transient exposure to the pro-chondrogenic molecule transforming growth factor-beta3 (TGF-β3), at varying doses (10, 50 and 100 ng/mL) and durations (3, 7, 21 and 63 days). Mechanical, biochemical, and histological outcomes were evaluated through 9 weeks of culture. Results showed that a brief exposure (7 days) to a very high level (100 ng/mL) of TGF-β 3 was sufficient to both induce and maintain cartilage formation in these 3D constructs. Indeed, this short delivery resulted in constructs with mechanical and biochemical properties that exceeded that of continuous exposure to a lower level (10 ng/mL) of TGF-β 3 over the entire 9-week time course. Of important note, the total TGF delivery in these two scenarios was roughly equivalent (200 vs. 180 ng), but the timing of delivery differed markedly. These data support the idea that acute exposure to a high dose of TGF will induce functional and long-term differentiation of stem cell populations, and furthers our efforts to improve cartilage repair in vivo.
Keywords: Hyaluronic Acid, Mesenchymal Stem Cells, Cartilage Tissue Engineering
INTRODUCTION
Articular cartilage is the dense white connective tissue lining the ends of long bones in diarthrodial joints and functions to transfer and distribute loads generated with locomotion (Carney and Muir 1988; Ateshian and Hung 2005). Load transfer occurs through the dense extracellular matrix (ECM), which is primarily composed of type II collagen and proteoglycans (PGs). Matrix mechanical properties are quite high, with an equilibrium modulus on the order of 0.5–1 MPa and a dynamic modulus ranging from 16–40 MPa (Park, Hung et al. 2004; Erickson, van Veen et al. 2011). While the tissue can function remarkably well in a demanding environment over a lifetime of use, focal defects and other trauma can initiate progressive degeneration (Martel-Pelletier 1998; Buckwalter, Martin et al. 2000). As cartilage is avascular, repair processes are limited (Huber, Trattnig et al. 2000), and regenerative strategies, short of total joint replacement, have not yet produced durable and functional repair.
To address this issue, cartilage tissue engineering approaches have been developed with the goal of forming biologic replacement materials with functional mechanical properties (Ateshian and Hung 2005). In addition to cell-based therapies (Knutsen, Engebretsen et al. 2004; Hettrich, Crawford et al. 2008), three dimensional constructs consisting of various hydrogels coupled with chondrocytes have been utilized to generate cell-laden constructs (Buschmann, Gluzband et al. 1995; Mauck, Soltz et al. 2000; Roy, Boskey et al. 2008; Kisiday, Lee et al. 2009). Within this 3D context, a number of culture variables have been explored, including modulation of cell density, growth factor supplementation, oxygen tension and mechanical stimulation (Blunk, Sieminski et al. 2002; Mauck, Wang et al. 2003; Hung, Mauck et al. 2004; Lima, Bian et al. 2007; Meyer, Buckley et al. 2010), to further the biochemical and mechanical maturation of engineered constructs. Indeed, recent reports employing chondrocytes in agarose hydrogels demonstrate functional equivalence between engineered constructs and native tissue, with equilibrium compressive properties approaching 1MPa (Lima, Bian et al. 2007; Byers, Mauck et al. 2008).
While chondrocytes have been instrumental as a cell source for such approaches, their clinical use may be limited due to a scarcity of healthy cells. Mesenchymal stem cells (MSCs) derived from bone marrow have emerged as an attractive alternative cell type (Pittenger and Martin 2004) as they are multipotent and easy to expand, and so are available in a nearly unlimited supply, and in an autologous fashion. Numerous studies have shown that MSCs within hydrogel constructs cultured in a chemically defined media (CM) undergo chondrogenesis when supplemented with transforming growth factor (TGF) family members (Huang, Farrell et al. 2010). We are particularly interested in the translational capacity of hyaluronic acid (HA) hydrogels. HA is a natural constituent of the cartilage extracellular matrix and provides a biologically relevant interface for encapsulated MSCs (Chung and Burdick 2009). Moreover, gel properties are easily tunable (Burdick, Chung et al. 2005; Erickson, Huang et al. 2009), and the gel can be modified to degrade in a controlled fashion (Sahoo, Chung et al. 2008) and to deliver TGF-β3 through co-encapsulated alginate microspheres (Bian, Zhai et al. 2011). Indeed, using methacrylated (and so photo-crosslinkable) HA as a starting point, we have optimized gel formation and functional matrix production by MSCs with variations in gel density (1%, (Erickson, Huang et al. 2009)) and MSC (~60 million cells/mL, (Erickson, Kestle et al. 2011)) concentration, consistently producing cartilage like constructs with compressive properties in the range of native tissue levels (200–300kPa).
In addition to the hydrogel environment, the timing and dose of pro-chondrogenic factors can modulate the growth of MSC-based constructs. In early work with human MSCs in alginate, it was reported that a single high dose of TGF (50ng/mL) resulted in comparable molecular level induction of cartilage genes at day 21 compared to continuous exposure to TGF at much lower levels (10 ng/mL) (Caterson, Nesti et al. 2001). More recently, we showed that transient exposure of MSCs in agarose to 10ng/mL TGF-β3 (for three weeks) induced a stable chondrogenic phenotype, with functional properties at six weeks greater than continual exposure at this same level (Huang, Stein et al. 2009). This suggests that timing, dose, and duration of exposure to chondrogenic factors can influence the long-term growth of engineered constructs formed with MSCs.
Transient exposure presents an interesting paradigm with clinical relevance; in vivo defect filling will require robust maturation of the engineered tissue driven by TGF-β3 delivered from the material itself in a controlled and sustained fashion. As most delivery systems offer the capacity for only a short term, high dose delivery, the purpose of this study was to determine the minimal TGF-β3 dosage and duration of exposure required to promote the most robust chondrogenesis and functional maturation of MSCs in this HA hydrogel system. To this end, MSCs were seeded in 1% HA hydrogels at a high cell density (60 million cells/mL) and cultured under different levels (10, 50, and 100 ng/mL) of TGF-β3 for varying durations (3, 7, 21 and 63 days) in a chemically defined medium. After removal of the growth factor at these defined time points, construct mechanics, biochemical content, and histological features were evaluated through 9 weeks of in vitro culture.
MATERIALS AND METHODS
MSC Isolation and Expansion
MSCs were isolated from femoral and tibial bone marrow from three to five juvenile calves (3 months old, Research 87, Bolyston, MA) and maintained in basal medium consisting of high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Gibco-Invitrogen, Grand Island, NY) and 1% penicillin/streptomycin/fungizone (PSF) (Gibco-Invitrogen, Grand Island, NY). MSCs were expanded in culture through passage three prior to pooling and encapsulation.
Construct Fabrication and Long-term 3D Culture
MSCs were suspended in a chemically defined medium (CM) consisting of high glucose DMEM supplemented with 1% PSF, 0.1 M dexamethasone, 50mg/mL ascorbate 2-phosphate, 40mg/mL L-proline, 100mg/mL sodium pyruvate, 6.25 g/mL insulin, 6.25 g/mL transferrin, 6.25 g/mL selenious acid, 1.25mg/mL bovine serum albumin (BSA), and 5.35 g/mL linoleic acid. The cell solution was centrifuged, and the supernatant was removed. The cells were resuspended in 1% w/v methacrylated hyaluronic acid (HA) solution. Specifics on the synthesis of this photocrosslinkable HA macromer have previously been reported in detail (Burdick, Chung et al. 2005; Erickson, Huang et al. 2009). Briefly, 1 wt% sodium hyaluronate (65kDa HA; Lifecore Biomedical, Chaska, MN) was reacted with methacrylic anhydride (Sigma, St. Louis, MO) at pH 8.0 for 24 hours, followed by dialysis to remove unreacted byproducts, and lyophilization and storage at −20°C. To form cell-seeded constructs, the methacrylated HA was dissolved at 1% w/vol in PBS with 0.05% w/vol photoinitiator (Irgacure I2959, Ciba-Geigy, Tarrytown, NY). MSCs were resuspended at a concentration of 60 million cells/mL, cast into a mold, and exposed to UV using a 365 nm Blak-Ray UV lamp (#UVL-56, San Gabriel, CA) for 10 min at room temperature. The range of the UV was 320–400 nm with a transmission maximum of 70% at 365 nm. Cylindrical cores (Ø4mm × 2.25mm) were removed from the resulting HA sheet with a sterile biopsy punch.
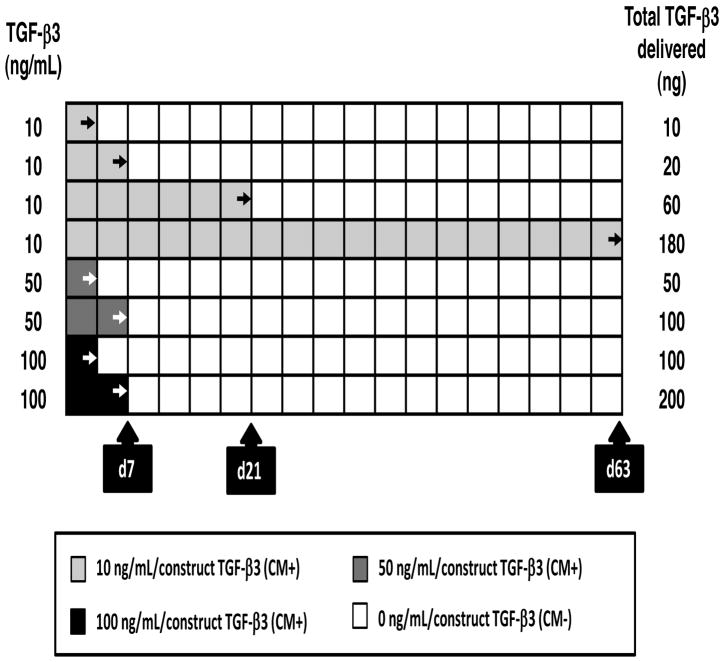
Cored constructs were pooled and randomly placed in a 6-well plate (5~6 constructs/well). The constructs were then cultured in chemically defined media (CM) (1mL per construct) for the duration of the study. TGF-β3 (R&D Systems, Minneapolis, MN) was introduced at several different concentrations and durations (Figure 1). TGF-β3 dose was varied from a standard concentration of 10ng/mL to a high dose of 50ng/mL, or to a very high dose of 100ng/mL. For these higher concentrations, exposure times were limited to 3 or 7 days, with media changed one time over the first week. For the lower dose, exposure was for 3, 7, 21, or 63 days. A total of 8 groups were thus evaluated (10-d3, 10-d7, 10-d21, 10-d63, 50-d3, 50-d7, 100-d3 and 100-7d), where the first number indicates dose, and the second number indicates duration of exposure to TGF-β3. Media was changed twice weekly for the duration of study.
Figure 1.
Schematic of transient exposure to TGF-β3 at varying doses and durations.
Mechanical Analysis
A custom-built mechanical testing device was utilized to examine compressive properties of engineered constructs (Mauck, Soltz et al. 2000). Unconfined compression was applied via a non-porous indenter with constructs hydrated in PBS during testing. Constructs underwent creep loading to 0.02 N for 5 min to ensure even contact between the construct and platens. After equilibration of this creep load, samples were compressed at 0.05%/sec to 10% strain, calculated from the post creep thickness of the construct. Constructs were then allowed to relax for 1000 seconds, and the equilibrium stress noted. In constructs that had matured to a very high level, this relaxation time may not have been sufficient, leading to a slight overestimation of equilibrium properties, but not altering overall conclusions. After stress relexation, a 1% sinusoidal deformation was applied at 1.0 Hz. Equilibrium and dynamic moduli were calculated from stress-strain response and the sample geometry.
Biochemical Analysis
After mechanical testing, the wet weight of constructs was measured followed by digestion in papain (1 mL/construct, 0.56U/mL in 0.1M sodium acetate, 10M cysteine hydrochloric acid, 0.05M ethylenediaminetetraacetic acid, pH 6.0) at 60°C for 16 hours. Glycosaminoglycan (GAG) content was determined using the 1,9-dimethylmethlene blue (DMMB) dye-binding assay and collagen content using the orthohydroxyproline (OHP) assay, with a 1:7.14 OHP:collagen ratio, as previously described (Stegemann and Stalder 1967). DNA content was assessed via the PicoGreen double strand DNA assay (Molecular Probes, Eugene, OR).
Histological Analysis and Cell Viability
Constructs were fixed in 4% paraformaldehyde and embedded in paraffin. Sections (8 m thick) were deparaffinized in a graded series of ethanol and stained with Alcian Blue (pH 1.0) and Picrosirius Red (0,1% w/v in saturated picric acid) for proteoglycans (PG) and collagens, respectively. Viability was assessed on fresh constructs on days 21, 42 and 63 using the LIVE/DEAD staining kit (Molecular Probes, Eugene, OR).
Fourier Transform Infrared Imaging Spectroscopy (FT-IRIS)
Histological sections (8 m) from the day 63 groups were mounted onto Low-e Microscope Slides (1 × 3 inch, Kevely Technologies, Chesterland, OH) and dehydrated completely to avoid spectral interference from water molecules prior to data acquisition. FT-IRIS analysis of construct matrix composition and distribution was carried out with a Spectrum Spotlight 400 imaging system (Perkin-Elmer, Waltham, MA) as in (Kim, Bi et al. 2005; Kim, Foo et al. 2010) with a 25 m2 pixel resolution and a 4 cm−1 spectral resolution. The system consists of an FTIR spectrometer in conjunction with a light microscope and linear array detector (Boskey and Pleshko Camacho 2007). Molecular characteristics were determined in a rectangular region of interest after spatial interpolation to 6.25 μm2. During IR penetration, molecules within the tissue absorb infrared radiation and vibrate at a specific frequency (wavenumber; cm−1) based on the characteristics of the molecular bond (composition) and the number of molecules (intensity) at that location. Collagen molecules were monitored using the integrated area of the amide I absorbance that arises from the C=O stretching vibration, centered at, 1655cm−1. The major absorbances that arise from proteoglycans are found in the amide I region (1640cm−1), as well as in the 1125-920 cm−1 region, attributable to sugar ring vibrations. In native tissue, the Amide I band primarily represents type II collagen while the sugar groups primarily represent proteoglycans. Although proteoglycan absorbance contributes to the amide I absorbance bands, their contributions are generally small compared to that of collagen in native tissue (Camacho, West et al. 2001). However, in this study, because proteoglycan content was significantly higher than collagen content (see results), proteoglycan absorbance did contribute to the amide I peak. Therefore, the proteoglycan contributions to Amide I signal were spatially subtracted to allow for quantification of collagen alone. This was based on the assumption that the integrated absorbance of the PG sugar band was proportional to the PG contribution in the Amide I region for each sample. While this analysis provides for a ‘collagen’ signal from our IR measures, it does not determine the type of collagen present given the nearly identical molecular bonds and spectral characteristics of collagens within the mid infrared region (400–4000 cm−1). Immunostaining (not shown) demonstrated that the majority of this collagen was type II collagen, with little staining for type I or type X collagen observed. Data processing was carried out using the ISys software (version 5.0, Malvern Instruments Ltd., Worcestershire, UK).
Statistical analysis
Statistical analysis was performed using the SYSTAT software (v10.2, SYSTAT software Inc., San Jose, CA). Significance was determined by two-way ANOVA with Tukey’s post hoc test (p<0.05). Data represent the mean ± the standard deviation for one replicate study; a replicate study showed similar trends.
RESULTS
Mechanical properties of MSC-seeded HA constructs
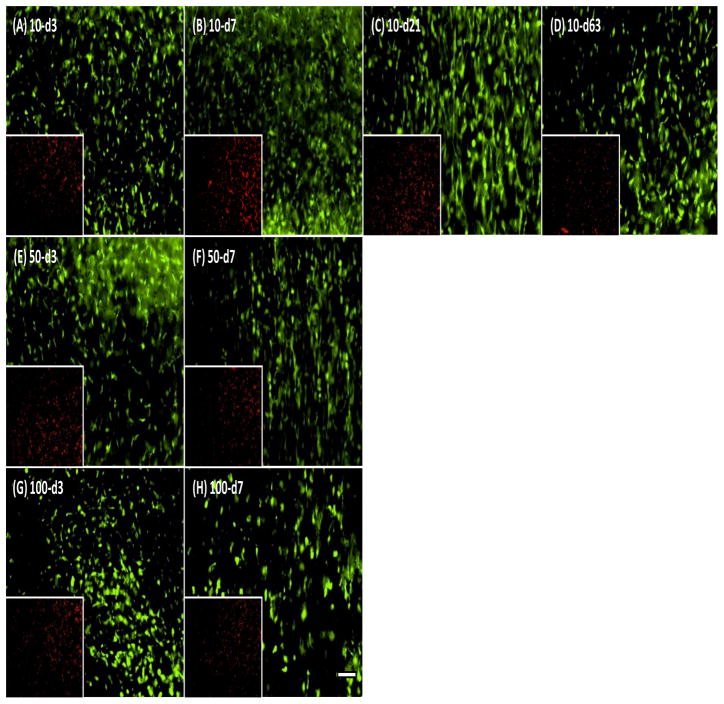
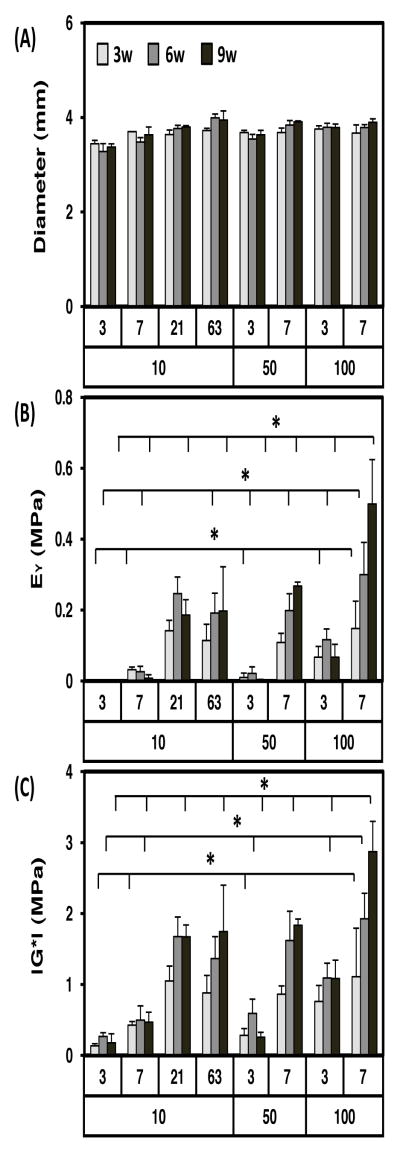
The effect of varying dose and duration of TGF-β3 exposure on the chondrogenesis and functional maturation of MSC-laden HA hydrogels was assessed under free swelling conditions. MSCs were viable and biosynthetically active in HA hydrogels under each condition assessed (Figure 2). Construct diameters were similar between groups and did not change during the culture period (Figure 3A). While constructs were geometrically stable, transient exposure of TGF-β3 altered construct mechanical properties, depending on the dose and duration of exposure. Compared to conditions where TGF-β3 was provided at each media change at 10 ng/mL, constructs exposed to either 10 ng/mL for only the first three weeks, or constructs exposed to higher concentrations (50 and 100 ng/mL) for even shorter periods of time (one week), reached equivalent or higher mechanical properties. For example, the equilibrium (and dynamic) modulus for constructs exposed to TGF-β3 at a high dose (100-d7; two feedings at 100ng/construct) reached ~500 kPa (and ~2.9 MPa) at nine weeks, the highest level achieved in this study (Figure 3). For comparison, acellular HA and day 0 constructs have an equilibrium modulus of ~5 kPa and a dynamic modulus of ~0.1 MPa (Erickson, Huang et al. 2009). Control constructs with continuous delivery of TGF-β3 at the standard concentration (10ng/mL) for the entire culture duration (10-d63) reached only ~200 kPa (and 1.7 MPa) over this same time course. This differential growth occurred despite similar levels of TGF delivery over the culture duration (200ng/construct for 100-d7, 180ng/construct for 10-d63). Groups intermediate to these two extremes (50-d7 and 100-d3) yielded constructs with lower mechanical properties. Equilibrium (and dynamic) modulus for these groups (50-d7 and 100-d3) was 267 kPa (and 1.8 MPa) and 67 kPa (and 1.1 MPa) by 9 weeks, respectively. Groups exposed to 10ng/mL for only 3 or 7 days (10-d3, 10-d7) did not show marked increase in mechanical properties. However, at this low dose, 21 days of exposure (10-d21) was sufficient to match properties in constructs with continual exposure (10-d63).
Figure 2.
Viability in MSCs-seeded HA hydrogel with different exposure conditions on day 63. Live (green; 10X) and dead (red; inset: 2X) staining show comparable cell viability in HA hydrogels with each treatment condition. (A–D) 10 ng/mL TGF-β3 for 3, 7, 21 or 63 days, (E and F) 50 ng/mL for 3 or 7 days, and (G and H) 100 ng/mL for 3 or 7 days. Scale bar indicates 100 m.
Figure 3.
Transient exposure to high levels of TGF-β3 enhances the mechanical properties of MSC-seeded HA constructs. Transient exposure time (day) and concentration of TGF-β3 (ng/mL) over the entire culture period (9 weeks, 9w) shown on x-axis. (A) Construct diameter, (B) equilibrium modulus (EY; MPa), and (C) dynamic modulus (|G*|; MPa) as a function of treatment (n=5/group; *p<0.05 for groups at 3w, 6w, or 9w compared to 100-d7 group at same the time point). For comparison, acellular and day 0 HA constructs have an equilibrium modulus of ~5 kPa and a dynamic modulus of ~0.1 MPa)
Biochemical content of MSC-seeded HA constructs
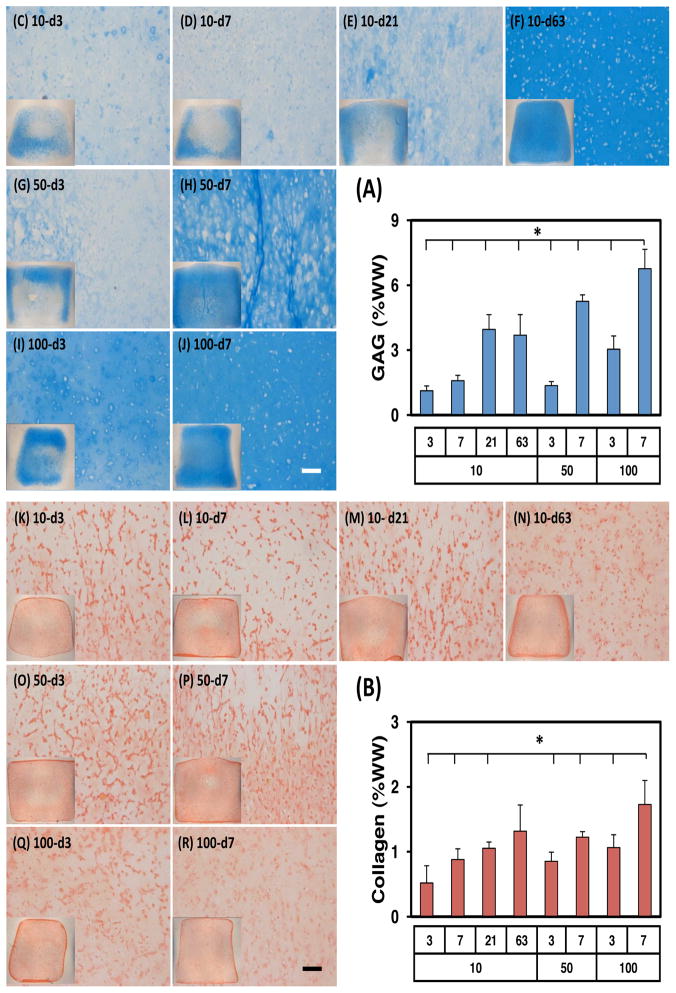
Consistent with the mechanical properties, biochemical content of constructs depended on dose and duration of exposure to TGF (Figure 4A). In control conditions (10-d63, continuous exposure, 10 ng/mL), GAG and collagen content increased with time. By 9 weeks, GAG content in these constructs reached ~3.7%. Interestingly, comparable GAG content was achieved in constructs exposed to this concentration of TGF for only three weeks (10-d21, ~4.0%), while shorter durations at this level resulted in less GAG accumulation (10-d3, 1.1%; 10-d7, 1.6%). With higher doses of TGF, even with short durations of exposure, GAG content of constructs was markedly higher. Indeed, the short-term, high dose TGF-β3 group (100-d7) reached GAG contents higher than all other groups. In these constructs, GAG content was ~6.8% (per wet weight) by 9 weeks, matching or exceeding native tissue levels (Erickson, van Veen et al. 2011). Intermediate groups (i.e., 50-d7 and 100-d3) produced GAG contents comparable to the continuous low concentration group (10-d63). DNA content for most groups decreased slightly with culture duration (not shown). Collagen content followed the same general trend as GAG content, though the difference between groups was less marked. Transient exposure to a high dose of TGF-β3 (i.e., 50-d7, 100-d3 and 100-d7) resulted in comparable levels of collagen content to the control group (10-d63) by week 9 (Figure 4B). The highest collagen content achieved was ~1.7% in the 100-d7 group at 9 weeks.
Figure 4.
Transient exposure to high levels of TGF-β3 enhances biochemical content and matrix distribution of MSC-seeded HA constructs. (A) GAG (%WW), (B) Collagen (%WW), (C–J) Alcian blue, (K–R) Picrosirius red (n=5/group for biochemical analysis; A and B; *p<0.05 for 9w group compared to 100-d7 group at the same time point). Histological images were captured at 9w at a magnification of 10X (inset: 2X). Scale bar indicates 100 m.
Histological analysis of MSC-seeded HA constructs
Histological evaluation generally supported bulk biochemical measures (Figure 4). Low doses of TGF-β3 applied for short periods of time (10-d3, 10-d7) resulted in very little proteoglycan deposition (as evidenced by lack of Alcian Blue staining), while longer periods of exposure (10-d21 and 10-d63) resulted in robust staining (Figure 4C–J) throughout the construct. Interestingly, with a short duration of exposure, PG staining was only apparent at the periphery of the construct. When TGF-β3 was added at higher concentrations for one week (50-d7 and 100-d7), staining patterns were similar to that of continuous exposure in the low dose (10-d63) group. At high doses, with only one exposure (50-d3 and 100-d3), staining was intense only at the construct periphery. Picrosirius red staining of collagen likewise matched bulk biochemical assessment, where collagen staining intensity was not markedly different among groups (Figure 4K–R).
FT-IRIS analysis of MSC-seeded HA constructs
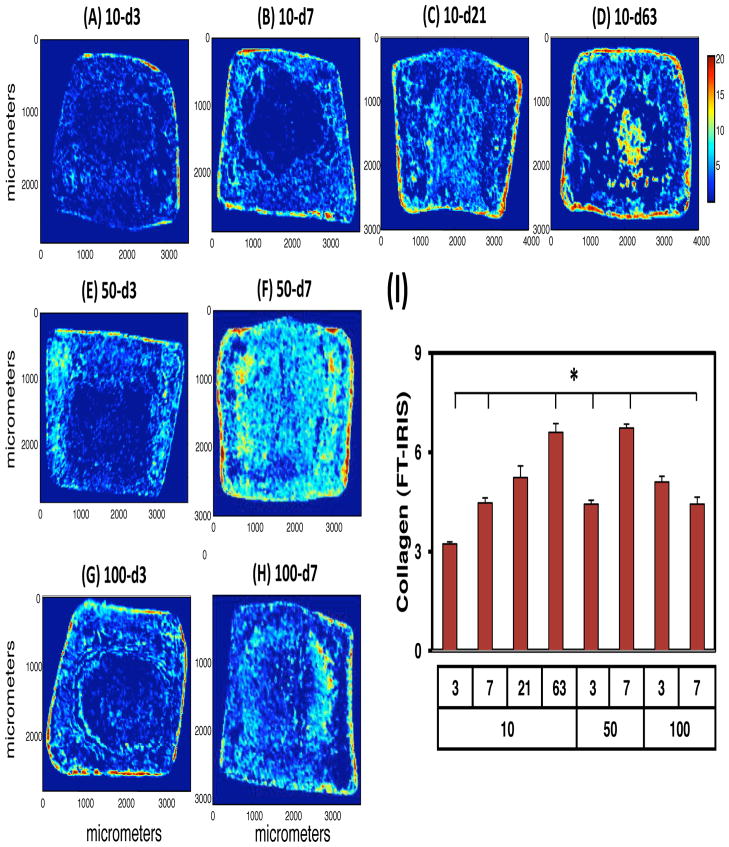
In addition to standard histological assays, FT-IRIS was used to visualize and quantify PG and collagen distribution across the engineered constructs. FT-IRIS allows for the deconvolution of collagen and proteoglycan signal based on specific signatures of these molecules in the IR spectrum. This analysis showed that collagen matrix production was initiated near the outer edge of constructs. With longer durations of exposure, central regions of the construct contained progressively more collagen (Figure 5). Continuous exposure constructs (10-d63) showed abundant collagen distributed throughout the construct. Groups exposed to TGF-β3 at higher concentrations (50-d7, 100-d3 and 100-d7) showed comparable collagen distributions, while those at lower concentrations and for shorter times of exposure (10-d3 and 10-d7) produced less collagen. Summation of the FT-IRIS signal across the construct expanse largely matched patterns of bulk collagen content determined through biochemical analysis.
Figure 5.
FT-IRIS assessment of collagen distribution with transient exposure to TGF-β3. Cross-sectional images showing distribution of collagen within MSC-seeded HA constructs on day 63 (A–H). Collagen intensity was quantified and averaged from 3 serial sections for each group (I). Pixel (25 × 25 m) intensity defined by color bar. *p<0.05 for 9w groups compared to 100-d7 group at the same time point.
DISCUSSION
In this study, we evaluated the impact of TGF-β3 dose and duration of exposure on the functional maturation of MSC-laden HA hydrogels. Our results show that, even with a very short exposure time, a sufficiently high TGF-β3 dose can produce functional cartilage-like materials over a 9 week period. Indeed, these constructs achieved mechanical and biochemical properties greater than those achieved with continuous exposure to TGF-β3 at a lower dose. This ‘release’ response is consistent with that observed previously in both chondrocytes (Lima, Bian et al. 2007; Byers, Mauck et al. 2008) and MSCs (Huang, Stein et al. 2009), though in this study it was apparent after an even shorter exposure period (1 week vs. 3 weeks), though a higher dose was required. Interestingly, chondrogenic differentiation (as evidenced by continued accrual of proteoglycans) progressed over the entire 9 weeks, despite only 1 week of exposure to chondrogenic factors. In the most auspicious conditions identified (100-d7, 100 ng/mL TGF-β3 exposed for 7 days), constructs showed a dramatic increase in mechanical properties and biochemical content, reaching an equilibrium modulus of ~500 kPa and ~6.8% GAG (per wet weight) at 9 weeks. These properties were higher than those in the continual exposure group (~200 kPa and 3.7% GAG), and nearly matched those of native bovine cartilage (Erickson, van Veen et al. 2011). Indeed, these are the highest yet reported in this HA system when seeding with MSCs. This finding confirms that a robust and persistent chondrogenic state can be induced with very short exposure durations to TGF, and has bearing on the clinical translation of this system.
The finding of time and dose varying effects of TGF on progenitor cell behavior should not be surprising, given its developmental relevance. In development, multiple morphogen gradients are established in a temporally and spatially varying manner to direct cellular behavior and tissue differentiation (Wolpert 1969; Gurdon and Bourillot 2001; Ibanes and Izpisua Belmonte 2008; Berezhkovskii, Sample et al. 2011). These soluble factor gradients are established when a local accumulation of differentiated cells initiates production of a given factor. From this origin to cells at a distant location, the morphogen concentration will decrease, depending on simple diffusion and/or consumption/binding of the factor (Gurdon and Bourillot 2001). In some instances, there exist critical concentration thresholds for phenotypic conversion (Raj, Rifkin et al. 2010), allowing for distinct boundaries to form along continuous gradients. The impact of a morphogen gradient can be transient, where either it establishes a pattern of differentiation after which the source cells cease production, or the cells initially regulated by the gradient move away from the point source through other growth mechanisms. An example of such as system is the cartilaginous growth plate in long bones, where multiple gradients of competing factors regulate progenitor cell chondrogenesis and eventual hypertrophy (Farnum, Turgai et al. 1990).
Some of these developmental features are present in our system as well, where the presentation of TGF in the soluble environment rapidly establishes a morphogen gradient, with the highest (and defined) levels located at the construct periphery. Though not directly measured here, overall concentration of TGF in the bathing solution most likely falls between feedings as receptor binding and internalization occurs (Di Guglielmo, Le Roy et al. 2003; Chen 2009; Gunaratne, Benchabane et al. 2011), eventually depleting the source. Depletion via cell utilization have been shown for other media components (such as glucose) in engineered chondrocyte-based cartilage constructs (Heywood, Bader et al. 2006), and may be pronounced in this study where a very high cell concentration was used. While TGF does not bind to the HA hydrogel directly (Bian, Zhai et al. 2011), at later times in culture, it could also be sequestered in the formed ECM, decreasing bioavailability. Ongoing studies designed to directly measure TGF consumption and binding will shed further light on this important issue.
While the ‘source’ concentration may vary with time between feedings, the local concentration is also expected to vary with distance from the boundary. Cells at the periphery are first exposed to the morphogen, and likely bind and sequester it, decreasing its availability to cells in the center. Indeed, under static conditions, MSCs within agarose hydrogels with continuous exposure to TGF at 10 ng/mL deposit matrix inhomogeneously through the depth, with the centers having significantly lower properties and matrix content than the edge regions (Farrell, Comeau et al. 2011). This is apparent in the current study as well, visualized both by variations in staining intensity across the construct expanse (Figure 4) and imaging (Figure 5). With short exposure (three days), increasing the dosage of TGF resulted in increasing penetration of proteoglycan staining. Of note, distinct boundaries were apparent where the staining intensity decreased rapidly from high to low levels, perhaps indicating a threshold concentration necessary to achieve chondrogenesis. This drop off in staining could be attenuated when a second delivery with TGF was applied at the higher doses (e.g., 50-d7 and 100-d7). In these conditions, a contiguous proteoglycan rich matrix was observed through the depth. These findings suggest short term application of TGF at high doses can provide a sufficient supply of the morphogen to reach critical thresholds to initiate chondrogenesis even at the construct center. As an alternative to the direct action of TGF, differentiation itself may have indirectly influenced matrix production through the depth. For example, Heywood et al., showed that oxygen tension and glucose concentration influence chondrocyte matrix production and viability in 3D culture (Heywood, Bader et al. 2006). Moreover, this same group showed that the transition of MSCs from monolayer into a 3D pellet cultured lowered oxygen consumption and altered glucose metabolism (Pattappa, Heywood et al. 2011) While not measured here, local chondrogenesis in HA (due to TGF gradients established during transient exposure) may have shifted the metabolic state of the embedded MSCs, with the subsequent change in consumption by these cells generating more pronounced nutrient gradients (and so gradients in matrix deposition) through the depth of the construct. Future work examining differentiation status and energy utilization at a local (single cell) level will be required to determine how these distinct and regional cellular processes develop in response to transient TGF exposure.
The above findings provide both a cost-effective approach to long-term in vitro culture studies, and also point to the translational potential of this system. In the current study, essentially the same amount of TGF-β3 was delivered (200 ng for the 100-d7 group, 180 ng for the 10-d63 group), with the transient delivery groups reaching mechanical and biochemical properties exceeding the continuous delivery groups (by ~150%), a considerable achievement in terms of construct modulus per nanogram of TGF provided. Even at intermediate doses (i.e., 50-d7, where only 100 nanograms were delivered over the first week), properties were greater than the continuous exposure group (by ~25%). While the study terminated at this point, these findings suggest that for culture durations in excess of 9 weeks, it will be cost-effective to deliver a high dose of TGF in a bolus fashion during the first week.
In terms of translation, our findings suggest that a finite (but large) dose of TGF-β3 need only be delivered for a short period of time to achieve functional chondrogenesis. While material delivery systems are improving, most still only offer the potential for a short term ‘burst’ followed by a much slower sustained release (Lee and Shin 2007; Ionescu, Lee et al. 2010; Meyerrose, Olson et al. 2010). We have recently shown that short term (7 day) delivery of chondrogenic factors can be achieved from co-encapsulated alginate microspheres, both in vitro and in vivo (Bian, Zhai et al. 2011). An important advantage of this system is that TGF can be released at a ‘high’ dose throughout the gel, and so lower overall delivery may be required (i.e., one will not have to create such steep concentration gradients to service cells at the center as the microspheres will establish a near uniform supply throughout the construct expanse). Data from the current study will help define optimal delivery doses for clinical translation of this technology to testing in a large animal cartilage defect model.
While the current findings are exciting, with native tissue properties achieved in our MSC-seeded HA hydrogel system with transient TGF exposure, additional work is required to elucidate the mechanisms governing this response and to fully optimize this system for in vivo application. In particular, it will be important to define the molecular basis of this phenotypic transition with transient exposure. For instance, it will be important to understand how TGF receptor type, density, and internalization and degradation kinetics change, as well as whether autocrine TGF production pathways are initiated, during the transient exposure. It will also be important to monitor hypertrophic markers that could indicate a shift or loss in MSC phenotype after TGF withdrawal. Even chondrocytes can undergo hypertrophy in very long term in vitro culture in the absence of added mineralization factors (Kim, Kraft et al. 2011). Staining of constructs in the present study for type I, II and X collagen showed abundant type II collagen, and very little type I or type X (data not shown). This is consistent with our previous findings in agarose hydrogels (Huang, Stein et al. 2009) and suggests stability of the chondrogenic phenotype. In vivo response may differ significantly, however, and so the effect of transient TGF delivery must also be evaluated in the context of a changing chemical environment, as the construct will be expected mature within the synovial space. Such an environment may provide ‘conflict’ signals that alter the response to transient TGF exposure, or predispose it to hypertrophic conversion. Finally, these studies must be extended to cell types of clinical relevance, namely porcine and human cells from aged patients, so as to enable rapid transition to pre-clinical and clinical testing scenarios. Despite these limitations and unknowns, this study suggests that, when coupled with even simple delivery systems providing controlled release for as little as one week, HA-based MSC-seeded constructs may provide for effective and functional cartilage repair. Such progress would improve the health and mobility of millions of patients worldwide suffering from the ravages of progressive cartilage degeneration and loss of function.
Acknowledgments
This work was supported by the National Institutes of Health (R01 EB008722 and R01 AR056145) and the National Science Foundation (IEE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ateshian GA, Hung CT. Patellofemoral joint biomechanics and tissue engineering. Clin Orthop Relat Res. 2005;436:81–90. doi: 10.1097/01.blo.0000171542.53342.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezhkovskii AM, Sample C, et al. Formation of morphogen gradients: Local accumulation time. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;83(5 Pt 1):051906. doi: 10.1103/PhysRevE.83.051906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Zhai DY, et al. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32(27):6425–34. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blunk T, Sieminski AL, et al. Differential effects of growth factors on tissue-engineered cartilage. Tissue Eng. 2002;8(1):73–84. doi: 10.1089/107632702753503072. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Pleshko Camacho N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28(15):2465–78. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JA, Martin J, et al. Synovial joint degeneration and the syndrome of osteoarthritis. Instr Course Lect. 2000;49:481–9. [PubMed] [Google Scholar]
- Burdick JA, Chung C, et al. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386–91. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, et al. Mechanical compresson modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):745–58. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- Byers BA, Mauck RL, et al. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14(11):1821–34. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho NP, West P, et al. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers. 2001;62(1):1–8. doi: 10.1002/1097-0282(2001)62:1<1::AID-BIP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Carney SL, Muir H. The structure and function of cartilage proteoglycans. Physiol Rev. 1988;68(3):858–910. doi: 10.1152/physrev.1988.68.3.858. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, et al. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57(3):394–403. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chen Y. Endocytic regulation of TGF-beta signaling. Cell Research. 2009;(19):58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. 2009;15(2):243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo G, Le Roy C, et al. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5(5):410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Erickson IE, Huang AH, et al. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogel. Osteoarthitis Cartilage. 2009;17(12):1639–48. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson IE, Kestle SR, et al. High Density MSC-Seeded Hyaluronic Acid Constructs Produce Engineered Cartilage with Near-Native Properties. The 57th Annual Meeting of the Orthopaedic Research Society; Long Beach, CA. January 13–16.2011. [Google Scholar]
- Erickson IE, van Veen SC, et al. Cartilage Matrix Formation by Bovine Mesenchymal Stem Cells in Three-dimensional Culture Is Age-dependent. Clin Orthop Relat Res. 2011 doi: 10.1007/s11999-011-1869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnum CE, Turgai J, et al. Visualization of living terminal hypertrophic chondrocytes of growth plate cartilage in situ by differential interference contrast microscopy and time-lapse cinematography. J Orthop Res. 1990;8(5):750–63. doi: 10.1002/jor.1100080517. [DOI] [PubMed] [Google Scholar]
- Farrell MJ, Comeau E, et al. Depth-Dependent Mechanical Properties of MSC-laden Engineered Cartilage Constructs. The 57th Annual Meeting of the Orthopaedic Research Society; Long Beach, CA. January 13–16.2011. [Google Scholar]
- Gunaratne A, Benchabane H, et al. Regulation of TGF-beta receptor trafficking and signaling by atypical protein kinase C; Cell Signal; 2011. Sep 6, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- Hettrich CM, Crawford D, et al. Cartilage repair: third-generation cell-based technologies-basic science, surgical techniques, clinical outcomes. Sports Med Arthrosc. 2008;16(4):230–5. doi: 10.1097/JSA.0b013e31818cdc98. [DOI] [PubMed] [Google Scholar]
- Heywood HK, Bader DL, et al. Glucose concentration and medium volume influence cell viability and glycosaminoglycan synthesis in chondrocyte-seeded alginate constructs. Tissue Eng. 2006;12(12):3478–96. doi: 10.1089/ten.2006.12.3487. [DOI] [PubMed] [Google Scholar]
- Huang AH, Farrell MJ, et al. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43(1):128–36. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AH, Stein A, et al. Transient exposure to transforming growth factor beta 3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng Part A. 2009;15(11):3461–72. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Trattnig S, et al. Anatomy, biochemistry, and physiology of articular cartilage. </pubmed/11041151>. Invest Radiol. 2000;35(10):573–80. doi: 10.1097/00004424-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Hung CT, Mauck RL, et al. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32(1):35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- Ibanes M, Izpisua Belmonte JC. Theoretical and experimental approaches to understand morphogen gradients. Mol Syst Biol. 2008;4(176):1. doi: 10.1038/msb.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu LC, Lee GC, et al. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials. 2010;31(14):4113–20. doi: 10.1016/j.biomaterials.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Bi X, et al. Fourier transform infrared imaging spectroscopic analysis of tissue engineered cartilage: histologic and biochemical correlations. J Biomed Opt. 2005;10(3):0311051–6. doi: 10.1117/1.1922329. [DOI] [PubMed] [Google Scholar]
- Kim M, Foo LF, et al. Evaluation of early osteochondral defect repair in a rabbit model utilizing fourier transform-infrared imaging spectroscopy, magnetic resonance imaging, and quantitative T2 mapping. Tissue Eng Part C Methods. 2010;16(3):355–64. doi: 10.1089/ten.tec.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kraft J, et al. Characterization of a cartilage-like engineered biomass using a self-aggregating suspension culture model: Molecular composition using FT-IRIS. Journal of Orthopaedic Research. 2011;29(12):1881–7. doi: 10.1002/jor.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiday JD, Lee JH, et al. Catabolic responses of chondrocyte-seeded peptide hydrogel to dynamic compression. Ann Biomed Eng. 2009;37(7):1368–75. doi: 10.1007/s10439-009-9699-9. [DOI] [PubMed] [Google Scholar]
- Knutsen G, Engebretsen L, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A(3):455–64. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):339–59. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Lima EG, Bian L, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthitis Cartilage. 2007;15(9):1025–33. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthitis Cartilage. 1998;6(6):374–6. doi: 10.1053/joca.1998.0140. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Wang CC, et al. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11(12):879–90. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Meyer EG, Buckley CT, et al. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J Biomech. 2010;43(13):2516–23. doi: 10.1016/j.jbiomech.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Meyerrose T, Olson S, et al. Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev. 2010;62(12):1167–74. doi: 10.1016/j.addr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Hung CT, et al. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthitis Cartilage. 2004;12(1):65–73. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Pattappa G, Heywood HK, et al. The Metabolism of human mesenchymal stem cells during proliferation and differentiation. J Cell Physiol. 2011;226(10):2562–70. doi: 10.1002/jcp.22605. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. CircRes. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Raj A, Rifkin SA, et al. Variability in gene expression underlies incomplete penetrance. Nature. 2010;463(7283):913–8. doi: 10.1038/nature08781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, Boskey AL, et al. Non-enzymatic glycation of chondrocyte-seeded collagen gels for cartilage tissue engineering. J Orthop Res. 2008;26(11):1434–9. doi: 10.1002/jor.20662. [DOI] [PubMed] [Google Scholar]
- Sahoo S, Chung C, et al. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules. 2008;9(4):1088–92. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25(1):1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]