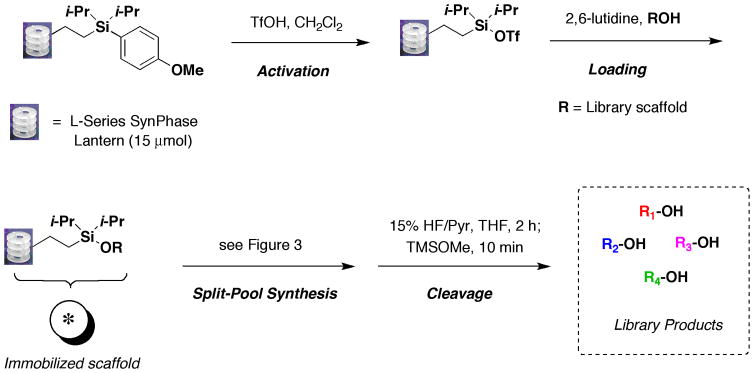
Abstract
Silicon-functionalized SynPhase Lanterns are useful for the combinatorial synthesis of small-molecule libraries. Lanterns bearing an alkyl tethered diisopropylarylsilane are first activated with triflic acid to afford the corresponding diisopropylsilyl triflate, which is then reacted with a library scaffold bearing a free alcohol. Once the scaffold has been loaded onto the solid phase, a variety of transformations can be run, including amine cappings, cross-coupling reactions and amide bond formation. These reactions can yield a variety of products when run sequentially using split-pool synthesis strategies. Upon completion of the solid-phase transformations, the small-molecules are released from the Lanterns using HF/pyridine. Using the techniques described within, libraries can be made ranging from a few compounds to >10,000 members in a highly efficient manner.
INTRODUCTION
Combinatorial chemistry is a powerful tool in the synthesis of small-molecule libraries for the development of biological probes and novel therapeutics (Dolle et al., 2009; Crooks and Charles, 2000). The use of combinatorial strategies, such as diversity-oriented synthesis (DOS) (Nielsen and Schreiber, 2008), can afford highly diverse and structurally complex small-molecules with great synthetic efficiency. Combinatorial libraries can be synthesized in either solution- or solid-phase formats, both of which have their merits. Solution-phase parallel synthesis has the benefit of being compatible with a broad spectrum of reactions and allows for easy reaction monitoring. Meanwhile, solid-phase synthesis has the advantage of employing simple ‘split-pool’ techniques, allowing for rapid generation of large compound libraries without need for automation.
Assuming a successful synthesis, products of solid-phase synthesis are generally of sufficient purity for biological testing, while libraries produced by solution-phase synthesis often require purification, typically by HPLC. This need for purification can be reduced or avoided through the judicious selection of solid-phase scavengers and reagents (Ley and Baxendale, 2002; Ley et al., 2000; Weinbrenner and Tzschucke, 2006).
Advances in solid-phase combinatorial synthesis have provided a variety of methods for compound immobilization, including different options for solid supports and a wide array of linkers (Scott, 2009). SynPhase Lanterns are a practical alternative to conventional resins for library synthesis due to favorable reaction kinetics, easy handling and simple washing (www.mimotopes.com). Figure 1 shows a schematic of the composition of SynPhase Lanterns, as well as the different sizes offered by Mimotopes. SynPhase Lanterns are cylindrical in shape, containing a rigid polypropylene base coated with either a polystyrene (PS) or polyamide (PA) surface. The PS-Lanterns are most suitable for general organic synthesis in non-polar solvents while PA-Lanterns are useful for conducting reactions in hydrophilic or aqueous conditions. Many different linkers are available, such as the Rink Amide (Verdié et al., 2008; Brucoli et al., 2009) and Backbone Amide (Zajdel et al. 2009) linkers. Herein, we focus on the L-series silicon-functionalized SynPhase PS-Lanterns (Ryba et al., 2009), due to the compatibility of this linker with a wide array of transformations and proven robustness for library synthesis (Marcaurelle et al., 2009; Taylor et al., 2004; Tallarico et al., 2001).
Figure 1.
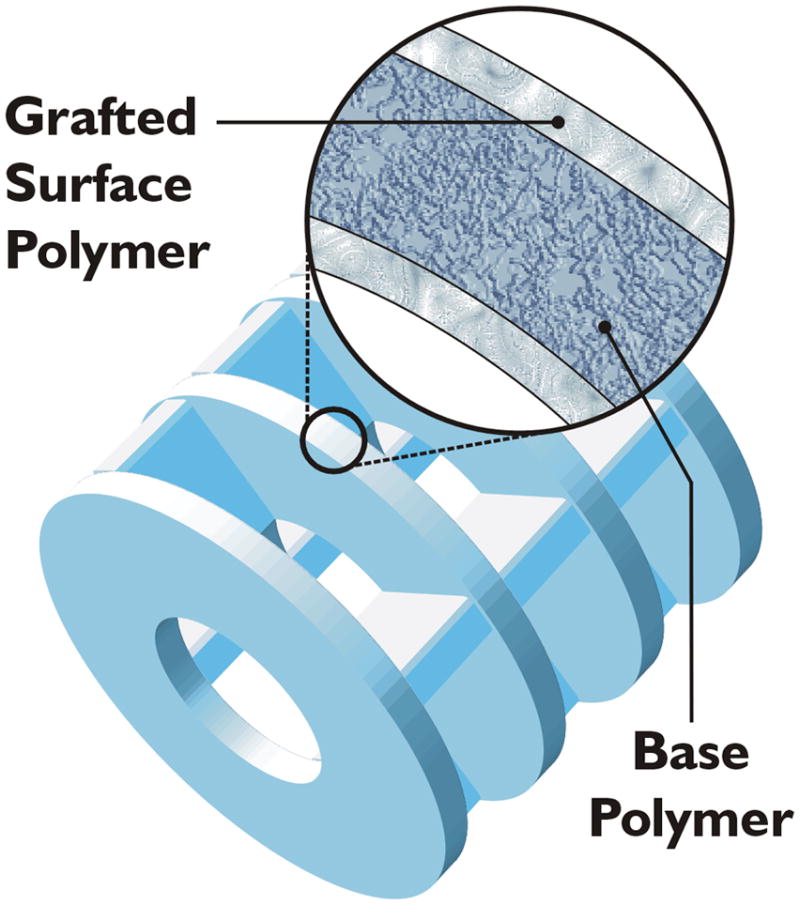
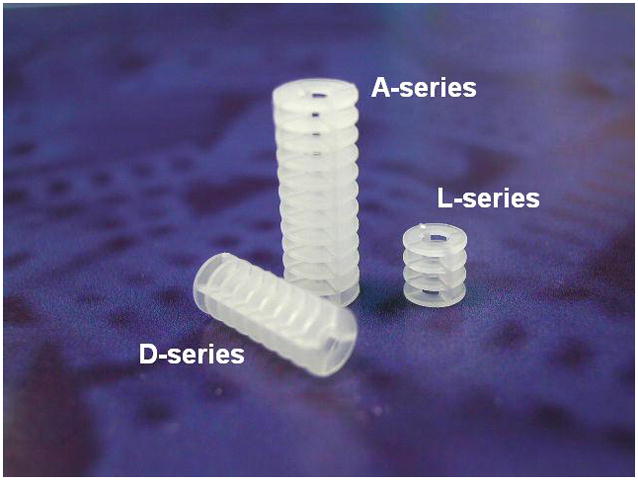
(a) Composition of SynPhase Lanterns: an unreactive base polymer and an outer polymer graft consisting of polystyrene or polyamide. (b) Lanterns are available in three sizes to accommodate different loading needs. A-series: 75 μmol/Lantern. D-series: 35 μmol/Lantern. L-series: 15 μmol/Lantern. Figure 1 was reproduced with permission from Mimotopes.
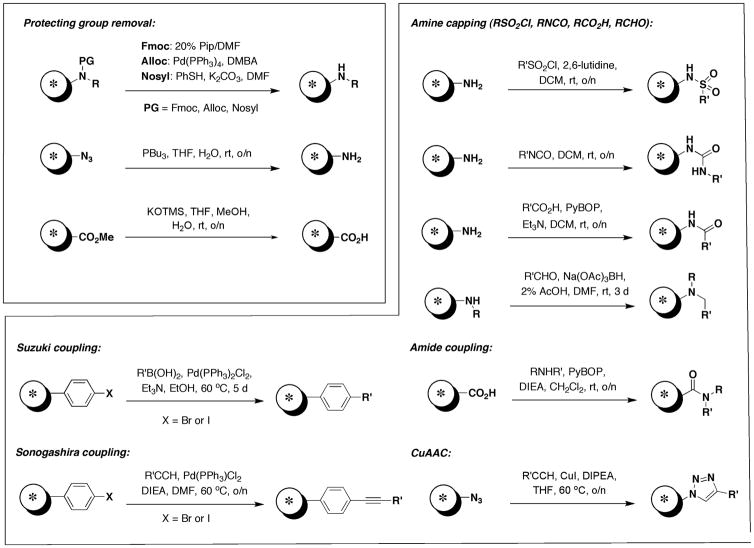
As depicted in Figure 2, the following protocol is divided into 3 sections: (1) activation of Lanterns and loading of the library scaffold, (2) solid-phase transformations of the immobilized scaffold via split-pool synthesis and (3) cleavage of the small molecule from the Lantern. Activation of the Lantern is achieved via treatment with TfOH to form the intermediate diisopropyl silyl triflate. This reactive intermediate is then immediately treated with the library scaffold bearing an alcohol in the presence of excess 2,6-lutidine to form the silyl ether. A variety of solid-phase transformations can then be carried out depending on the functional groups present on the library scaffold. Representative reactions compatible with the silyl ether linker are shown in Figure 3 and detailed procedures are provided in Basic Protocol 2. A number of nitrogen protecting groups are compatible with the silicon linker including Fmoc, Alloc and Nosyl. Capping of the resulting amines can be achieved with sulfonyl chlorides, isocyanates, acids and aldehydes to afford the corresponding sulfonamides, ureas, amides and tertiary amines respectively. An azide can also serve as a masked amine, as reduction can be performed with PBu3 in aqueous THF. Alternately azides can be converted to triazoles via a Huisgen 1,3-dipolar cycloaddition with alkynes. Esters can be hydrolyzed under mild conditions (KOTMS) to provide an acid for coupling with amines. Lastly, aryl halides can undergo cross-coupling reactions such as Suzuki and Sonogashira reactions with boronic acid and alkynes respectively. Cleavage of library products (Figure 2) from the Lantern can be achieved via treatment with HF/pyridine in THF. Quenching of the reaction with TMSOMe provides volatile by-products TMSF and MeOH, which can be removed by evaporation.
Figure 2.
Use of silicon-functionalized Lanterns for library synthesis: activation, loading, split-pool synthesis and cleavage.
Figure 3.
Representative solid-phase transformations useful for split-pool library synthesis on silicon- functionalized Lanterns
STRATEGIC PLANNING
As mentioned above, solid-phase synthesis is a proven method for developing collections of small molecules, whether the objective is a small focused library for medicinal chemistry purposes or a large discovery library for initial screening efforts. Regardless of the type of library being synthesized, it is important to be mindful of the physicochemical properties (e.g. molecular weight, logP, polar surface area) of the library products at the outset of the synthesis (Blake, 2004). In order to prioritize library synthesis, cheminformatics methods such as principal component analysis (PCA) (Feher and Schmidt, 2003), multi-fusion similarity (MFS) maps (Medina-Franco et al., 2007), ChemGPS (Oprea and Gottfries, 2001) and principal moments of intertia (PMI) plots (Sauer and Schwarz, 2003) can be utilized to analyze chemical diversity.
Having designed a set of small-molecules for synthesis, another critical element is the tracking of compounds during the library production. There are several options for tagging Lanterns depending on the library design. If the library size is large (>100 compounds), radiofrequency (RF) stems can be used for their ease in sorting and identification of the immobilized small molecule. The RF Transtems (Figure 4a) fit conveniently onto the Lanterns and a Transort RF reader (Figure 4c) can be used to sort the library at each synthesis step. RF-directed sorting requires the enumeration of the library and encoding of compound identities onto the Transtems. Several tools are available for library enumeration, including Pipeline Pilot, Chemaxon, Cambridgesoft and Daylight. Enumeration and encoding for smaller libraries can be avoided by tracking members with colored spindles and cogs (Figure 4b). Record keeping is essential when using this method of identifying compounds, especially for sorting Lanterns, to ensure the intended compounds are synthesized.
Figure 4.
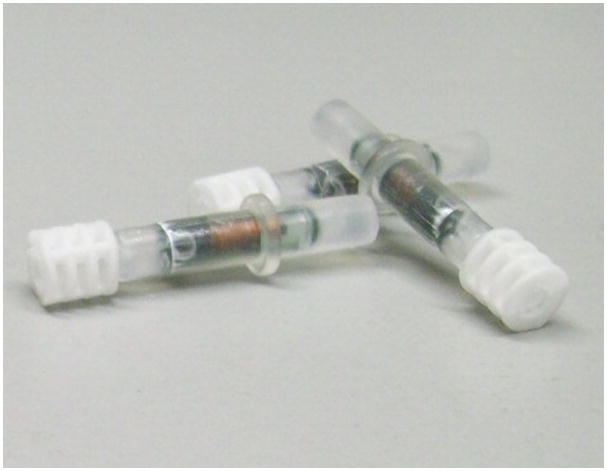
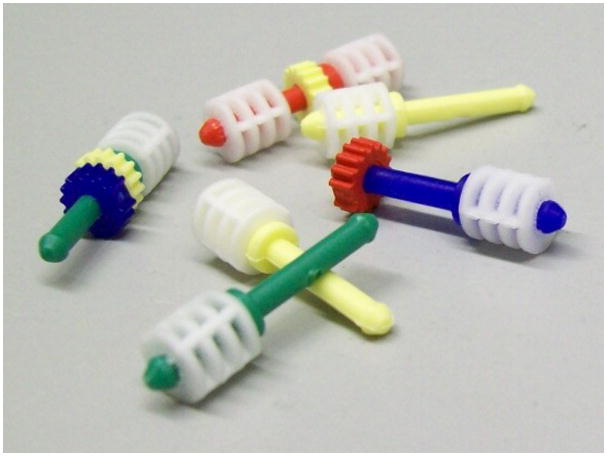
L-Series SynPhase Lanterns equipped with (a) radio frequency (RF) tags and (b) color-coded spindles and cogs. (c) Work station for RF-directed sorting.
To ensure a library of high purity is synthesized, quality control (QC) Lanterns should be included at each synthesis step for LCMS (or NMR) analysis. Colored RF Transtems are commercially available for this purpose to allow easy identification from a reaction flask. For smaller libraries the color-coded cogs and spindles can be used for this purpose. If yield determination is required than a full Lantern should be cleaved, otherwise Lanterns can be cut into quarters for cleavage and analysis to preserve material. When cleaving full Lanterns, the inclusion of multiple QC copies is recommended in case re-subjection of the reaction is necessary. In general it is a good idea to determine yield at each step of the synthesis to ensure premature cleavage from the Lantern is not occurring under the reaction conditions. The number of QC Lanterns per reaction flask will depend on the size of the library and the number of combinatorial steps. It is recommended to sample a variety of compounds per reaction as the success of the reaction may depend on the building block used in the previous step.
BASIC PROTOCOL 1 LOADING OF LIBRARY SCAFFOLD
Once a library has been designed and Lanterns have been suitably tagged and encoded ( if necessary), the solid-phase library production can begin. The following protocol describes the loading of a library scaffold bearing a free alcohol onto a silicon-functionalized Lantern. After the loading has been completed and before subsequent reactions are run, QC analysis is carried out to determine the success of the loading. Generally, this is done via cleavage and recovery of the scaffold (see Basic Protocol 3), but Fmoc quantitation (Gude et al., 2002) can also be used, if applicable.
Materials
L-series alkyl tethered diisopropylarylsilane Lanterns (Mimotopes, MIL10431000, see also Ryba et al., 2009)
Transtems (stems with enclosed RF transponder, Mimotopes MIT10260010)
Standard color tagging kit (colored cogs and spindles, Mimotopes MIT10430001)
3% trifluoromethanesulfonic acid solution in dichloromethane (TfOH in DCM, see Reagents and Solutions)
2,6-lutidine (anhydrous)
Library scaffold containing primary or secondary alcohol (co-evaporated from benzene or toluene)
Dichloromethane (DCM, anhydrous)
Dichloromethane (DCM, HPLC grade for washings)
N,N-dimethylformamide (DMF, HPLC grade)
Tetrahydrofuran (THF, containing BHT as inhibitor)
Water (H2O)
Isopropanol (i-PrOH)
Oven-dried reaction vessel with screw top (e.g., ChemGlass CG-1880-42, Mimotopes MIA10140006 )
Rubber septa (Sigma-Aldrich, Z512222 or similar)
Incubator shaker (New Brunswick Scientific, model M1353-0004 or similar for large libraries)
Washing apparatus (ceramic Buchner funnel and waste container)
Lyophilizer or high-vacuum manifold
SynPhase work station (cleavage tray, Lantern tray, stem ejector, SynPhase press and stem tray, Mimotopes MIA10910001)
Transort RF reader and software (Mimotopes, MIT10520001)
NOTE: Transtems can be attached to Lanterns using the SynPhase press (see SynPhase workstation) to enable RF sorting with the transort reader and software.
CAUTION: TfOH is highly corrosive and personal contact can result in injury. Extreme caution should be exercised.
Activate Lanterns with TfOH
-
1
To an oven-dried shaker vessel containing the Lanterns equipped with RF or color-coded tags, add a 3% TfOH solution in DCM under a flow of nitrogen. Add enough TfOH solution to cover all Lanterns. Cap vessel and shake at room temperature for 10 minutes.
In general, for large-scale reactions (>100 Lanterns) the TfOH solution can be poured directly into the reaction vessel under a flow of nitrogen. For smaller reactions, a syringe can be used for transferring this reagent to a flask equipped with a rubber septa.
The Lanterns should turn an orange or deep red color upon addition of TfOH.
Carry out loading
-
2
After 10 minutes, exchange the cap for a rubber septa and remove the TfOH solution with a syringe (or canula) while maintaining an inert atmosphere inside the flask.
-
3
Add anhydrous 2,6-lutidine (12.0 eq with respect to Si). Purge with nitrogen and shake Lanterns for several minutes.
The Lanterns should turn white. Add additional 2,6-lutidine as needed.
-
4
Add the library scaffold (1.2 eq) in anhydrous DCM via syringe or canula and purge with nitrogen. Exchange rubber septa for screw cap, and transfer reaction vessel to incubator shaker. Shake at room temperature overnight.
The scaffold should be co-evaporated with either benzene or toluene (benzene is recommended) prior to the loading to remove any residual water and then placed on high-vacuum overnight.
DCM should be used conservatively during the loading to maintain as high of a concentration of the scaffold as possible, while still covering all the Lanterns. A slight excess of solvent should be used to account for swelling of Lanterns.
For scaffolds with low reactivity or limited solubility in DCM, longer reaction times (up to 4 days) can be used to facilitate loading. In general higher loading levels are achieved with primary alcohols than secondary alcohols.
Wash and dry Lanterns
-
5
After shaking overnight, remove the reaction mixture and set aside. Wash Lanterns with DCM.
The reaction mixture is kept until the analysis for the loading has been completed to ensure loading was successful. If the loading was unsuccessful, the scaffold can be salvaged from this mixture via acidic aqueous wash to remove excess 2,6-lutidine and purified by silica gel purification. For washing Lanterns, the washing solvent and Lanterns are filtered through a ceramic Buchner funnel, with the wash going into a waste container and the Lanterns returned to the reaction flask. Alternatively, a drilled cap with filter holes (Mimotopes, MIA10070012) can be used to drain the solvent from the reaction flask.
-
6
Wash the Lanterns using the following solvents for 30 min each (~0.8 mL/Lantern): DMF (x2), THF/H2O (3:1), THF/IPA (3:1), THF and DCM.
THF containing BHT as an inhibitor should be used to avoid possible oxidation of amines.
-
7
Place the washed Lanterns on a lyophilizer or high-vacuum overnight before proceeding to the next reaction.
Determine loading level
-
8
Follow cleavage protocol (see Basic Protocol 3) with 3 Lanterns for loading analysis.
At least 3 Lanterns should be used to measure consistency of loading across all Lanterns. Expected loading amounts are typically ~15 μmol/Lantern. QC analysis ensures there was no decomposition during the loading step. Where applicable Fmoc-quantitation (Gude et al., 2002) can also be used to measure success of loading.
-
9
Sort the Lanterns using Transort RF reader and software for upcoming reactions, if necessary.
If color-coded stems and cogs were used rather than RF tags, refer back to your master sheet to sort for the upcoming reactions.
BASIC PROTOCOL 2 SOLID-PHASE TRANFORMATIONS
Once the loading of a library scaffold has been achieved, a split-pool combinatorial approach is applied for the introduction of appendage diversity. Below we outline a series of solid-phase transformations that we have found to be compatible with silicon-functionalized Lanterns. The protocol includes conditions for the removal of a variety of protecting groups (e.g., Fmoc, Alloc, Nosyl), amine capping, amide coupling, azide-alkyne cycloaddition and cross coupling reactions. Once a solid-phase transformation has been carried out, LCMS analysis can be utilized to determine the success of the reaction. If a reaction is deemed incomplete, the Lanterns can simply be re-subjected to the reaction conditions until the desired results are observed. Between each synthetic step Lanterns can be sorted using either the color-coded spindles/cogs or RF tags (using the Transort reader). The next reaction can then be run, and the QC process repeated. This process is continued until the synthesis is complete. The following set of procedures are not intended for sequential use, but rather implemented as desired to fit the design of the library. The success of each reaction may be dependent on the library scaffold used, therefore, some optimization may be required.
Materials
THF (anhydrous containing BHT inhibitor)
Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4)
1,3-dimethylbarbituric acid (DMBA)
DMF (anhydrous)
0.1M sodium cyanide in a 1:1 THF/H2O solution
20% by volume piperidine in DMF
Potassium trimethylsilanolate (KOTMS)
Methanol (MeOH)
H2O
Tributylphosphine (PBu3)
Sulfonyl chlorides
2,6-lutidine (anhydrous)
DCM (anhydrous)
Isocyanates
Carboxylic acids
(Benzotriazol-1-yloxy)tripyrrolidinophosphonium hexafluorophosphate (PyBOP)
Triethylamine (NEt3, anhydrous)
Aldehydes
Sodium triacetoxyborohydride (Na(OAc)3BH)
2% acetic acid in DMF
Boronic acids
Bis(triphenylphosphine)palladium(II) dichloride (Pd(PPh3)2Cl2)
Ethanol (EtOH, anhydrous)
Alkynes
N,N-diisopropylethylamine (DIPEA)
Copper (I) iodide (CuI)
Amines
Oven-dried reaction vessel with screw top (e.g., ChemGlass CG-1880-42, Mimotopes MIA10140006)
Incubator shaker (New Brunswick Scientific, model M1353-0004 or similar for large libraries)
CAUTION: Some washes require the use of a solution of NaCN to remove residual metals (e.g., Pd, Cu). Exercise great caution when performing these washes as NaCN is highly toxic.
Alloc removal
-
To a reaction vessel containing Lanterns, add THF (0.8 mL/Lantern), followed by Pd(PPh3)4 (1 eq) and 1,3-dimethylbarbituric acid (30 eq). Seal the vessel and shake at room temperature overnight.
In general we have found 1,3-dimethylbarbituric acid to be a superior π-allyl scavenger as compared to other reagents (e.g., phenysilane, morpholine) for preventing N-allylation.
Once the shaking has been completed, remove the reaction mixture and wash the Lanterns with DMF followed by a solution of NaCN (0.1 M in 1:1 THF/H2O) (3x).
Continue washing Lanterns by following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete or N-allyation is observed repeat Step 1.
Fmoc removal
To a reaction vessel containing Lanterns add a solution of 20% piperidine in DMF (0.8 mL/Lantern). Seal the vessel and shake for 30 minutes.
Remove the piperidine solution and wash Lanterns following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete, repeat Step 1.
Nosyl removal
To a reaction vessel containing Lanterns add DMF (0.8 mL/Lantern) followed by thiophenol (20 eq) and potassium carbonate (30 eq). Seal the vessel and shake at room temperature overnight.
Remove the reaction mixture and wash Lanterns following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete, repeat Step 1.
Ester hydrolysis
To a reaction vessel containing Lanterns add THF/MeOH/H2O (5:2:2) (0.8 mL/Lantern) followed by KOTMS (10 eq). Seal the vessel and shake at room temperature overnight.
Remove the reaction mixture and wash Lanterns following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete, repeat Step 1.
Azide Reduction
To a reaction vessel containing Lanterns add 9:1 THF/H2O (0.8 mL/Lantern) followed by PBu3 (10 eq). Seal the vessel and shake at room temperature overnight.
Remove the reaction mixture then add THF/H2O (3:1). Seal the vessel and shake at room temperature for 6 h.
Wash the Lanterns following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete repeat Steps 1 and 2.
Amine Capping – Sulfonyl Chlorides
To a reaction vessel containing Lanterns add DCM (0.8 mL/Lantern) followed by 2,6-lutidine (25 eq) and a sulfonyl chloride (20 eq). Seal the vessel and shake at room temperature overnight.
Remove the reaction mixture and wash Lanterns with DCM. Continue to wash Lanterns by following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete repeat Step 1.
Amine Capping – Isocyanates
To a reaction vessel containing Lanterns add DCM (0.8 mL/Lantern) followed by an isocyanate (15 eq). Seal the vessel and shake at room temperature overnight.
Remove the reaction mixture and wash Lanterns with DCM. Continue to wash Lanterns by following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete, repeat step 1.
Amine Capping – Carboxylic Acids
To a reaction vessel containing Lanterns add DCM (800 uL/Lantern), followed by Et3N (30 eq), a carboxylic acid (20 eq) and PyBOP (20 eq). Seal the vessel and shake at room temperature overnight.
-
Remove the reaction mixture and wash with DCM. Continue to wash Lanterns by following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete repeat Step 1.
Occasionally, using the above protocol with a primary amine, bis-capping can result in imide formation. Treatment with a 3:1 THF/pyrrolidine solution at room temperature overnight typically will provide the desired amide.
Amine Capping – Aldehydes
To a reaction vessel containing Lanterns add DMF containing 2% AcOH (0.8 mL/Lantern) followed by an aldehyde (20 eq). Seal the vessel and shake at room temperature for 1 hour.
Remove cap and add Na(OAc)3BH (22 eq). Seal the vessel and shake at room temperature for 3 days.
-
Remove the reaction mixture and wash Lanterns following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete repeat Steps 1 and 2 as needed.
The amine capping with aldehydes is ideally suited for secondary amines (or the di-alkylation of primary amines), as it is difficult to selectively mono-alkylate primary amines under this protocol.
Cross Coupling – Boronic Acids
To a reaction vessel containing Lanterns add EtOH (0.8 mL/Lantern), followed by a boronic acid (20 eq), Et3N (40 eq) and Pd(PPh3)2Cl2 (1 eq). Purge vessel with nitrogen, cap and shake at 60 °C for 5 days.
Remove the reaction mixture and wash Lanterns with DMF several times. Then wash Lanterns with a solution of NaCN (0.1M in 1:1 THF/H2O solution) (3x).
Continue to wash Lanterns by following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete repeat Steps 1 and 2.
Cross Coupling – Alkynes
To a reaction vessel containing Lanterns add DMF (0.8 mL/Lantern), followed by an alkyne (20 eq), DIPEA (30 eq), CuI and Pd(PPh3)2Cl2 (2.0 eq). Purge vessel with nitrogen, cap and shake at 60 °C overnight.
Remove the reaction mixture and wash Lanterns with DMF several times. Then wash Lanterns with a solution of NaCN (0.1M in 1:1 THF/H2O) (3x). Continue washing Lanterns by following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete repeat Step 1.
Cu-catalyzed azide/alkyne cycloaddition (CuAAC)
To a reaction vessel containing Lanterns add THF (0.8 mL/Lantern) followed by an alkyne (30 eq), CuI (10 eq) and DIPEA (30 eq). Seal the vessel and shake at 60 °C for 24 hours.
Remove the reaction mixture and wash Lanterns with DMF several times. Then wash Lanterns with a solution of NaCN (0.1M in 1:1 THF/H2O) (3x).
Continue washing Lanterns by following Step 6 in Basic Protocol 1. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete repeat Steps 1 and 2.
Amidation – PyBOP Coupling
To a reaction vessel containing Lanterns add a 3:1 DCM/DMF solution (0.8 mL/Lantern), followed by the amine (20 eq), DIPEA (10 eq) and PyBOP (10 eq). Seal the vessel and shake at room temperature overnight.
Remove the reaction mixture and follow Step 6 in Basic Protocol 1 for washing Lanterns. Follow Basic Protocol 3 for QC analysis. If reaction is incomplete repeat Step 1.
BASIC PROTOCOL 3 CLEAVAGE OF FUNCTIONALIZED SCAFFOLD FROM SOLID SUPPORT
Once the desired solid-phase transformations have been performed the product is removed from the Lantern via treatment with HF/pyridine. Cleavage may be carried out during the course of a library synthesis to determine yield after loading (see Basic Protocol 1) or to monitor the success of solid-phase reactions (see Basic Protocol 2). Here, we introduce a standardized cleavage protocol for use with silicon-functionalized Lanterns which can be carried out in a deep 96-well plate or Eppendorf tube. When processing multiple samples at once it is preferred to use a 96-well plate as this allows for the use of a multi-channel pipette. The following protocol describes cleavage of up to 96 Lanterns on a single 96-well plate using a multichannel pipette. If working on a large scale library production, the use of an automated liquid handler is preferred.
Materials
Cleavage solution (15% HF/pyridine in THF containing BHT as an inhibitor)
Methoxytrimethylsilane (99%, Aldrich, catalog # 253006-250g)
Methanol
Dichloroethane (DCE)
Dimethylsulfoxide (DMSO)
SynPhase work station (cleavage tray, Lantern tray, stem ejector, SynPhase press and stem tray, Mimotopes MIA10910001)
SynPhase stem recycler module for L- and D-series Lanterns (Mimotopes, catalog # MIA10880001)
Labeled deep 96-well plate (Seahorse Biosciences, catalog # XPO128)
Spreadsheet software
Multi-channel pipette (1000 μL)
Polypropylene reservoir
Labeled tube rack with pre-weighed 2D barcoded glass mini-tubes (Tradewinds 1.2 mL hi-recovery mini-tube, catalog # 063227-0301 or similar)
Centrifugal evaporator (e.g. Genevac HT12 or HT24, SP Industries)
Automated weighing station (e.g. FlexiWeigh, Mettler Toledo) 2D barcode reader (e.g. VisionMatePlus, Thermo Fisher Scientific)
LC/MS vials
CAUTION: Use proper personal protection equipment when handling HF/Pyridine (safety glasses, nitrile gloves and lab coat). Labware that comes into contact with HF/Pyridine should be quenched with methoxy- or ethoxytrimethylsilane. Prepare solution of 25% methoxytrimethylsilane in THF and keep it nearby at all times.
NOTE: Remove any identifiers (Transtems or colored spindles) prior to cleavage. For removing Transtems the use of a Stem Recycler Module is required.
Cleave Lanterns with HF/Pyridine
-
1
Put one Lantern per well in a deep 96-well plate. Note the position of each Lantern in spreadsheet software.
For testing at an intermediate stage of the synthesis it is not necessary to cleave a whole Lantern if yield determination is not required. Lanterns can be cut into quarters using a razor blade. The amount of cleavage solution and TMSOMe should be reduced accordingly. For ¼Lantern 200 μL of HF/Pyridine solution is sufficient.
After extensive testing of different deep 96-well plates, Seahorse Biosciences cleavage plates were selected for use as they released the least amount of detectable plasticizers.
-
2
Using multi-channel pipette add 350 μL of 15% HF/pyridine in THF (cleavage solution) to each well containing Lantern. Lantern should be fully submerged in cleavage solution.
When using the multi-channel pipette, the cleavage solution should be transferred from a polypropylene reservoir due to the reactive nature of HF/pyridine and THF.
-
3
Cover cleavage plate with another 96 well plate and let sit for 3 hours at room temperature.
-
4
Quench reaction with two volumes of methoxytrimethylsilane (700 μL).
Addition of methoxytrimethylsilane should be slow as the reaction is exothermic.
Transfer cleavage mixture to glass tubes
-
5
After 10–15 minutes use a multi-channel pipette to mix the contents of the wells and transfer quenched solution from each well into its own pre-weighed 2D barcoded glass tube (or other appropriate glass tube).
Glass tubes should be used to avoid release of plasticizers into compound solution.
-
6
Add 200 μL of MeOH to each well containing Lanterns.
-
7
Using a multi-channel pipette mix the content of the wells to wash the Lanterns and transfer the wash into respective tube.
-
8
Dry contents of the tubes using centrifugal evaporator.
To ensure proper drying conditions, program the evaporator to remove most of the THF first (~2 hours at 23 mbar, use REMP feature to prevent solvent bumping), then apply full vacuum for at least 12 hours to remove pyridine.
Determine yield and purity
-
9
Weigh tubes using automated weighing station.
-
10
Calculate yield for each compound by subtracting tare tube weight from filled tube weight. Determine loading level if required.
-
11
Dissolve product in DCE/MeOH to approximate 10 mg/mL concentration.
-
12
Remove a 5 μL aliquot, and add it to 45 μL of DMSO. Mix well and analyze by LCMS.
Analysis can be done in LC/MS vial or from a 96- or 384-well microplate depending on number of samples.
At times the formation of a TMS adduct can be observed by LCMS (M+72). This impurity can be reduced by resuspending the compound in MeOH, soaking for 4 hours at room temperature and drying in a centrifugal evaporator.
Reagents and Solutions
3% TfOH Solution in DCM
Remove the Sure-Seal of a 100-mL Sure-Seal dicholomethane (do not discard) and pour 5 g of triflic acid (from a 5-g ampule) directly into the dichloromethane bottle. Replace the Sure-Seal cap, along with the screw cap and shake well. Use the solution soon after preparing.
HF/Pyridine Cleavage Solution
To prepare 100 mL of cleavage solution, add 15 mL of 30% hydrofluoric acid in pyridine (Aldrich, cat # 184225-100g) to 85 mL of anhydrous stabilized THF containing BHT as an inhibitor (Aldrich cat # 401757-1L). Use a polypropylene bottle to store the solution. The cleavage solution can be made in advance and stored in a freezer for future use.
COMMENTARY
Background Information
SynPhase PS-Lanterns produced by Mimotopes are solid-supports containing a rigid polypropylene base coated with a polystyrene (PS) mobile surface. The unique “Lantern” shape allows for the attachment of snap-fitting tags for encoding purposes, and allows for the free flow of reactants and rapid drainage of washing solvents. PS-Lanterns can be functionalized with a variety of different linkers allowing for flexibility in loading strategies. For example, amines can be immobilized via reductive alkylation using Backbone Amide (BAL) Lanterns while carboxylic acids and phenols can be loaded through Hydroxymethylphenoxy (HMP) Lanterns. Meanwhile, primary and secondary alcohols can be loaded using a variety of linkers (Nam et al., 2003) including silicon-functionalized Lanterns (Ryba et al., 2009) now commercially available from Mimotopes. We chose to focus on this later technology due to the compatibility and robustness of the linker for library synthesis applications (Marcaurelle et al., 2009; Taylor et al., 2004; Tallarico et al., 2001). Primary and secondary alcohols can also be used as a handle for affinity chromatography, small-molecule microarrays and the attachment of biasing elements during subsequent follow-up in biological assays (Wang et al., 2008; Radner et al., 2006; Wong et al., 2003).
The mobile surface of the silicon-functionalized Lantern contains a p-methoxyphenyl (PMP) protected diisopropylsilyl linker. Upon activation with triflic acid, the PMP group is removed and the subsequent silyl triflate is formed. Due to the highly reactive nature of silyl triflates, inert reaction conditions are required to prevent hydrolysis of the activated Lanterns, resulting in unsatisfactory loading. Under these inert conditions, in the presence of base, a scaffold containing a free alcohol can be easily immobilized onto the Lantern.
The silicon-functionalized linker can withstand a variety of reaction conditions, but does have certain limitations. Although the linker is stable to a wide range of basic conditions, including reductions and metal-mediated reactions, the use of strongly acidic conditions as well as sources of fluoride should be avoided as this can result in premature cleavage of compound from the Lantern. In addition, while a library scaffold can be recovered if the loading is unsuccessful, the Lanterns cannot be re-used or re-subjected to the loading reaction. Thus, great care should be taken during the loading step.
Critical Parameters
The success of a library synthesis depends largely on the extent to which solid-phase feasibility studies are carried out prior to production. For example, loading levels may vary from one scaffold to another, thus it is highly recommended that loading be tested on a minimum of 5 Lanterns prior to library synthesis. While, the procedures provided in Protocol 2 serve as a good starting point for solid-phase diversification, some optimization may be required as the success of each reaction may be scaffold dependent. It is recommended that a representative set of building blocks with varying reactivity (e.g., alkyl, aryl, electron-withdrawing, electron-donating, hindered) be tested under the suggested reaction conditions prior to library production. The cross coupling reactions in particular may require the screening of multiple building blocks. Varying the recommended reaction conditions (e.g, temperature, solvent, Pd source) may also be necessary. In general scouting reaction conditions in solution phase is not necessary, or even worthwhile, as the conditions often do not translate to the solid-phase.
It is important to consider the sequence of steps to be employed in a library synthesis, particularly with respect to protecting groups. When the reaction sequence is not obvious multiple options may be pursued in parallel during feasibility studies. In general Fmoc is quite labile and should be removed early in the library synthesis, while Alloc is compatible with most solid-phase transformations with the exception of cross-coupling procedures. In order to prevent premature cleavage of products from the Lantern, protecting groups that require the use of harsh acid or base for removal should be avoided.
Other critical parameters for a successful library production include the use of reagents and scaffolds of high purity to ensure clean reactions, and ultimately, clean products directly from the library production. In general it is recommended that 3–5% percent of the library be sampled for QC to ensure good results overall. The synthesis of a pilot library (100–500 compounds) can also be beneficial prior to engaging in a large-scale (>1000 compounds) library production.
Troubleshooting
Poor Loading
The easiest problem to diagnose in the low loading of a library scaffold is poor solubility. If a scaffold has low solubility in DCM the amount of solvent employed in the loading can be increased and reaction time can be extended to up to 5 days to try improving loading levels. Sonication of the scaffold in DCM prior to addition to Lanterns may also be beneficial. If results are still sub-optimal then changes to the library scaffold may be required to improve solubility. For example a simple change in protecting groups may facilitate dissolution in DCM. Heating the loading reaction generally does not lead to improved results, and co-solvents such as THF, DMF or MeOH are incompatible with the loading reaction.
An unsuccessful loading reaction can also be due to moisture. Although difficult to detect, trace amounts of water in the triflic acid, 2,6-lutidine or DCM can lead to poor loading results. The TfOH solution should always be prepared fresh prior to use and the 2,6-lutidine obtained from an unopened Sure Seal bottle (or freshly-distilled). The Lanterns should also be washed with anhydrous DCM and dried under high vacuum prior to use and the library scaffold co-evaporated from benzene or toluene. The use of an oven- or flame-dried reaction vessel is also recommended.
If the library scaffold is highly soluble and great care has been taken to exclude moisture from the loading reaction and loading levels are still low (<10 μmol), the loading site may be inaccessible. In general, higher loading levels are obtained with primary alcohols than secondary alcohols, however reasonable loading levels (>10 μmol) can be achieved for secondary alcohols if the loading site is not sterically hindered. Phenols can also be utilized as a handle for loading but the aryl silyl ether linkage is slightly more sensitive to basic conditions. If modifications to the loading site are not feasible (or desirable) then the use of an alternate linker strategy may be needed.
Low conversion
Depending on the type of reaction or building block employed, low conversion may be observed that is not overcome by simply repeating the recommended reaction conditions. This typically can be remedied by employing harsher reaction conditions, assuming the required conditions are compatible with the silyl linker. For example, when capping an amine with a sterically hindered isocyanate, heating the reaction in toluene may be required to drive the reaction to completion. Meanwhile, capping an amine with a sulfonyl chloride that is sluggish can often be overcome by switching from 2,6-lutidine to pyridine. Reaction optimization will vary from substrate to substrate and feasibility testing can prevent this from happening during the actual library production.
Low purity
Reagent quality can have the greatest impact on the purity of final products. It is recommended that good records be maintained during a library production in case undesirable results are obtained for a particular building block (e.g., supplier information). In some cases impurities may be traced back to the reagent. In other instances a ‘combinatorial effect’ may be observed where certain building block combinations yield products of low purity. For the most part this problem cannot be avoided even with thorough solid-phase feasibility studies.
A common impurity, which may be observed upon HF cleavage, is a TMS adduct (M+72) resulting from quenching of the reaction mixture with TMSOMe. Fortunately, this impurity can be reduced by treatment with MeOH at room temperature.
On rare occasions unexpected rearrangements may occur under the HF/pyridine cleavage conditions. In such instances the use of buffered HF/pyridine (e.g., 15% HF/Pyridine in 1:1 THF/pyridine) may prevent or minimize undesired rearrangement. If the product yield is lower than expected using buffered conditions then the cleavage can be repeated. For the cleavage of highly acid-sensitive products the use of TBAF-may be preferred. In this case HPLC purification of the final products will be needed to remove excess TBAF.
For products containing tertiary amines there is a risk of N-oxide formation if unstabilized THF is used during HF-cleavage or washing steps. This problem is easily detectible by LCMS (M+16). For this reason THF containing BHT as an inhibitor should be used for all washing and cleavage steps. The use of DCM/MeOH (or DCE/MeOH) is recommended for all volatile transfers post-cleavage as the continued use of stabilized THF leads to an increase in BHT impurities.
Low yield
If a low yield of final product is obtained after treatment with HF/pyridine, a second round of cleavage should be carried out to ensure all compound has been removed from the Lantern. Assuming initial loading levels were satisfactory, if additional product is not recovered after a second cleavage event, this may indicate premature release of compound from the Lantern.
Anticipated Results
The loading step (Basic Protocol 1) typically yields ~15 μmol of core/Lantern for L-seried Lanterns. During the subsequent steps, this number should remain constant and the final product obtained in similar amounts. The solid-phase manipulations are usually clean, but the conversions may not always be complete. Re-subjecting the Lanterns to the reaction conditions can be expected at some point during the library production. Driving the reaction to completion is always recommended unless the formation of byproducts begins to appear and counter the progress of increasing the conversion. Purity levels can vary based on the choice of reactions, building blocks and number of steps. Feasibility studies and optimization of reactions should be carried out to ensure a majority of the library is above the desired purity threshold. Typically, with the reactions described in Protocol 2 and simple building blocks, you can expect >85% of the compounds to be >80% purity for a 4–6-step sequence.
Time Considerations
The amount of time needed to produce a library of small molecules using silicon-functionalized Lanterns depends on several factors and can range from a few weeks to several months. This time frame does not include time required for solid-phase feasibility studies, which depends highly on the scaffold and associated chemistry. Very little time, relative to the whole, is spent on the library setup. The main factors in contributing to the length of time are size of the library, number of steps and the difficulty of the reaction sequence. The size of the library does not increase the reaction time but does add more time during the sorting and cleavage steps, as well as LCMS analysis. A very difficult reaction can result in poor conversion and the need for repeating a reaction step. Some reactions require longer reaction times (such as the Suzuki reaction), which may add to the overall production time line. For a medium- to large-sized library, typically one week should be set aside for each step in the library synthesis to account for reaction set up, reaction time (assuming overnight reaction), washing, drying, QC and sorting. For example, the solid-phase synthesis of 1000-membered library involving 6 synthesis steps, including loading, protecting group removal, amine capping, ester hydrolysis, amide coupling and cleavage, would take approximately 6 weeks assuming no resubjections are required.
Acknowledgments
This work was supported in part by CMLD funding from the NIH (Broad Institute CMLD; P50 GM069721). The authors would like to thank Dr. Lakshmi Akella for helpful reading of this Protocol.
Footnotes
Internet Resources
http://www.mimotopes.com/knowledgeBase.asp?cid=26,34
An introduction to SynPhase Lanterns is provided on the Mimotopes website, as well as a variety of protocols for Lanterns with alternate linkers. Figure 1 was reproduced with permission from Mimotopes.
Literature Cited
- Blake JF. Integrating cheminformatic analysis in combinatorial chemistry. Curr Opin Chem Bio. 2004;8:407–411. doi: 10.1016/j.cbpa.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Brucoli F, Howard PW, Thurston DE. Efficient solid-phase synthesis of a library of distamycin analogs containing novel biaryl motifs on SynPhase Lanterns. J Comb Chem. 2009;11:576–586. doi: 10.1021/cc900009r. [DOI] [PubMed] [Google Scholar]
- Crooks SL, Charles LJ. Overview of combinatorial chemistry. Current Protocols in Pharmacology. 2000;9:1–16. doi: 10.1002/0471141755.ph0903s10. [DOI] [PubMed] [Google Scholar]
- Dolle RE, Le Bourdonnec B, Goodman AJ, Morales GA, Thomas CJ, Zhang W. Comprehensive survey of chemical libraries for drug discovery and chemical biology: 2008. J Comb Chem. 2009;11:739–790. doi: 10.1021/cc9000828. [DOI] [PubMed] [Google Scholar]
- Feher M, Schmidt JM. Property distributions: Differences between drugs, natural products, and molecules from combinatorial chemistry. J Chem Inf Comput Sci. 2003;43:218–227. doi: 10.1021/ci0200467. [DOI] [PubMed] [Google Scholar]
- Gude M, Ryf J, White PD. An accurate method for the quantitation of Fmoc-derivatized solid phase supports. Lett Pept Sci. 2002;9:203–206. [Google Scholar]
- Ley SV, Baxendale IR. New tools and concepts for modern organic synthesis. Nat Rev Drug Discovery. 2002;1:573–586. doi: 10.1038/nrd871. [DOI] [PubMed] [Google Scholar]
- Ley SV, Baxendale IR, Bream RN, Jackson PS, Leach AG, Longbottom DA, Nesi M, Scott JS, Storer RI, Taylor SJ. Multi-step organic synthesis using solid-supported reagents and scavengers: a new paradigm in chemical library generation. J Chem Soc, Perkin Trans. 2000;1:3815–4195. [Google Scholar]
- Marcaurelle LA, Johannes C, Yohannes D, Tillotson BP, Mann D. Diversity-oriented synthesis of a cytisine-inspired pyridone library leading to the discovery of novel inhibitors of Bcl-2. Bioorg Med Chem Lett. 2009;19:2500–2503. doi: 10.1016/j.bmcl.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Medina-Franco JL, Maggiora GM, Giulianotti MA, Pinilla C, Houghten RA. A similarity-based data-fusion approach to the visual characterization and comparison of compound databases. Chem Biol Drug Des. 2007;70:393–412. doi: 10.1111/j.1747-0285.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Nam NH, Sardari S, Parang K. Reactions of solid-supported reagents and solid supports with alcohols and phenols through their hydroxyl functional group. J Comb Chem. 2003;5:479–546. doi: 10.1021/cc020106l. [DOI] [PubMed] [Google Scholar]
- Nielsen TE, Schreiber SL. Towards the optimal screening collection: a synthesis strategy. Angew Chem Int Ed. 2008;47:48–56. doi: 10.1002/anie.200703073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprea TI, Gottfries J. Chemography: the art of navigating in chemical space. J Comb Chem. 2001;3:157–166. doi: 10.1021/cc0000388. [DOI] [PubMed] [Google Scholar]
- Radner JE, McPherson OM, Mazitschek R, Barnes-Seeman D, Shen JP, Dhaliwal J, Stevenson KE, Duffner JL, Park SB, Neuberg DS, Nghiem P, Schreiber SL, Koehler AN. A Robust Small-molecule Microarray Platform for Screening Cell Lysates. Chem Biol. 2006;13:493–504. doi: 10.1016/j.chembiol.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Ryba TD, Depew KM, Marcaurelle LA. Large scale preparation of silicon-functionalized SynPhase polystyrene Lanterns for solid-phase synthesis. J Comb Chem. 2009;11:110–116. doi: 10.1021/cc8000986. [DOI] [PubMed] [Google Scholar]
- Sauer WHB, Schwarz MK. Molecular shape diversity of combinatorial libraries: A prerequisite for broad bioactivity. J Chem Inf Comput Sci. 2003;43:987–1003. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
- Scott PJH. Linker strategies in solid-phase organic synthesis. Wiley; Chichester, U.K: 2009. [Google Scholar]
- Tallarico JA, Depew KM, Pelish HE, Westwood NJ, Lindsley CW, Shair MD, Schreiber SL, Foley MA. An alkylsilyl-tethered, high-capacity solid support amenable to diversity-oriented synthesis for one-bead, one-stock solution chemical genetics. J Comb Chem. 2001;3:312–318. doi: 10.1021/cc000107i. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Taylor AM, Schreiber SL. Synthetic strategy toward skeletal diversity via solid-supported, otherwise unstable reactive intermediates. Angew Chem Int Ed. 2004;43:1681–1685. doi: 10.1002/anie.200353466. [DOI] [PubMed] [Google Scholar]
- Verdié P, Subra G, Averland-Petit MC, Amblard M, Martinez J. Solid-phase synthesis of 4-methylcarboxy-1,4-benzodiazepine-2,5-diones. J Comb Chem. 2008;10:869–874. doi: 10.1021/cc800085d. [DOI] [PubMed] [Google Scholar]
- Wang X, Imber BS, Schreiber SL. Small-molecule reagents for cellular pull-down experiments. Bioconjugate Chem. 2008;19:585–587. doi: 10.1021/bc700297j. [DOI] [PubMed] [Google Scholar]
- Weinbrenner S, Tzschucke CC. Purification principles in high-speed solution-phase synthesis. In: Bannwarth W, Hinzen B, editors. Methods and Principles in Medicinal Chemistry, Vol 26: Combinatorial Chemistry. Wiley-VCH Verlag GmbH & Co KGaA; Weinheim: 2006. pp. 1–31. [Google Scholar]
- Wong JC, Hong R, Schreiber SL. Structural biasing elements for in-cell histone deacetylase paralog selectivity. J Am Chem Soc. 2003;125:5586–5587. doi: 10.1021/ja0341440. [DOI] [PubMed] [Google Scholar]
- Zajdel P, Subra G, Verdie P, Gabzdyl E, Bojarski AJ, Duszyñska B, Martinez J, Pawłowski M. Sulfonamides with the N-alkyl-N′-dialkylguanidine moiety as 5-HT7 receptor ligands. Bioorg Med Chem Lett. 2009;19:4827–4831. doi: 10.1016/j.bmcl.2009.06.038. [DOI] [PubMed] [Google Scholar]