Abstract
Our aim was to explore the involvement of the transcriptional suppressor GCF2 in silencing RhoA, disorganization of the cytoskeleton, mislocalization of MRP1, and sensitivity to anti-cancer agents as an upstream gene target in cancer therapy. Increased expression of GCF2 was found in human cisplatin-resistant cells, and overexpression in GCF2-transfected cells results in loss of RhoA expression and disruption of the actin-filamin network. In consequence, the membrane transporter MRP1 was internalized from the cell surface into the cytoplasm, rendering cells sensitive to doxorubicin by more than 10-fold due to increased accumulation of doxorubicin in the cells. The GCF2 transfectants also showed reduced accumulation of cisplatin and increased resistance. siRNA targeted to GCF2 suppressed the expression of GCF2 in cisplatin-resistant cells, re-activated RhoA expression, and restored the fine structure of actin microfilaments. MRP1 was also relocated to the cell surface. siRNA targeted to RhoA increased resistance 3-fold in KB-3-1 and KB-CP.5 cells. These data for the first time demonstrate a novel complex regulatory pathway downstream from GCF2 involving the small GTPase RhoA, actin/filamin dynamics, and membrane protein trafficking. This pathway mediates diverse responses to cytotoxic compounds, and also provides a molecular basis for further investigation into the pleiotropic resistance mechanism at play in cisplatin-resistant cells.
Keywords: chemotherapy, trafficking, cytoskeleton, cisplatin resistance, doxorubicin, cisplatin
1. INTRODUCTION
Cisplatin (cis-Diamminedichloroplatinum II) is a major chemotherapeutic agent used for the treatment of a wide spectrum of solid tumors. However, the ability of cancer cells to become resistant to the drug remains a significant impediment to successful chemotherapy. Intensive efforts have been made to define genes that are involved in acquisition of cisplatin resistance. Recent studies using gene knockout,1 differential display,2 subtractive hybridization,3 cDNA microarrays,4 and microRNA profiling5 have documented that a large number of genes are either up-regulated or down-regulated in cisplatin-resistant cells, including genes that encode transcription factors, DNA damage-repair pathways,6 stress-response proteins, cell cycle checkpoints, apoptosis mediators, and transporters7. Despite this progress, the relationship between genes involved in mediating pleiotropic resistance and miRNA regulation is not understood.
Previous work in the Gottesman laboratory revealed that many membrane transporters, such as the multidrug resistance-associated protein, MRP1, and the folate-binding protein, FBP, are translocated from the cell surface into the cytoplasm, near the trans-Golgi region in human cisplatin-resistant cells.8 However, the cause of this mislocalization is unknown.
Our laboratory has also reported that the same cisplatin-resistant (CP-r) cells were defective in the expression of the small GTPase RhoA, and had disorganized cytoskeletons associated with reduced uptake of drugs that rely on cell-surface transporters for cell entry9. The small guanine triphosphatase (GTPase) protein, RhoA, belongs to the Ras superfamily of proteins. It has been shown to regulate the microtubule dynamics of the actin cytoskeleton via its downstream effector protein Rho-associated, coiled-coil containing protein kinase 1 (ROCK1). Consequently cell morphology, cytokinesis, and proliferation are all controlled by RhoA10–15. The roles of RhoA in regulation of ion channels and tumor invasion and/or metastasis in breast carcinomas have recently been described.16, 17
It was recently reported that GC-binding factor 2 (GCF2)/leucine-rich repeat (in FLII) interacting protein 1 (LRRFIP1) directly interacts with Dishevelled protein to regulate RhoA activity.18 GCF2 is a transcriptional repressor shown to bind to the GC-rich region of promoters. It has been reported that GCF2 negatively regulates expression of several genes, such as epidermal growth factor receptor (EGFR), platelet-derived growth factor (PDGF), excitatory amino-acid transporter 2 (EAAT/SLC1A2) and tumor necrosis factor alpha (TNF-a).19–23
We hypothesized that the disorganization of the actin filaments in CP-r cells might result from down-regulated expression of RhoA, mediated by differences in GCF2 levels in CP-r and sensitive cells. In the present work, we examined the expression of RhoA and GCF2 in CP-r cells. The organization of the cytoskeleton and recycling pathways of the membrane were examined using immunostaining to follow the localization of cell surface transporters including multidrug resistance protein 1 (MRP1) and folate binding protein (FBP). GCF2-transfected cells were generated, and GCF2 and RhoA siRNA tested, to demonstrate the involvement of these genes in the cisplatin resistance phenotype. The transcript levels of 381 drug resistance genes in GCF2-transfected cells were analyzed to identify those genes whose expression was affected by this transcriptional repressor.
2. Materials and Methods
2.1. Cell lines and cell culture
Two populations of CP-r cell lines and their parental cell lines were studied: the human epidermoid carcinoma cell line KB-3-1 and its independent CP-r derivative, KB-CP.5, selected in a single step at 0.5 μg cisplatin/ml.8,24 The KB-CP20 cell line and the human liver carcinoma cell line BEL-7404 and its CP-r derivative 7404-CP20 were selected by stepwise increases to 20 μg of cisplatin/ml of medium, as described previously.9, 25 All the CP-r cells were maintained in the presence of cisplatin, but cisplatin was removed from the medium 3 days prior to preparation of proteins and RNA. All cell lines were grown as monolayer cultures at 37°C in 5% CO2, using Dulbecco’s modified Eagle medium with 4.5 g/l glucose (Invitrogen, Carlsbad, CA), supplemented with L-glutamine, penicillin, streptomycin and 10% fetal bovine serum (BioWhittaker, Walkersville, MD). Cisplatin, cytochalasin D and other chemicals were purchased from Sigma (St. Louis, MO).
2.2. Preparation of RNA and reverse transcription (RT) PCR for GCF2/LRRFIP1
For determination of levels of expression of genes of interest, RNA was isolated from cells using an RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RT-PCR was performed using a GeneAmp kit (Applied Biosystems, Branchburg, NJ) as described by the manufacturer. This was performed twice with similar results. Specific primers for tested genes were: GCF2: 5′-TGA AAG GGA AAA ACA CGC C -3′ (forward), and 5′-TCA TTT TTC ACC TCC ACT TCA C-3′ (reverse). Primers for GAPDH were purchased from Invitrogen for verification of the PCR performance. The quality (purity and integrity) of the RNA samples was assessed using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). The RNA was quantitated using a spectrophotometer (Ultrospec 3100 pro, Amersham Biosciences, Piscataway, NJ).
2.3. Preparation of total RNA and reverse transcription for Taqman Low Density Array (TLDA) analysis
RNA was prepared using an RNEasy kit (Qiagen) and quantitated using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE). The integrity of the RNA samples was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and then stored at −80°C. Synthesis of cDNA from 1 μg total RNA in a 20 μL reaction volume was carried out using the High Capacity cDNA kit with RNAse inhibitor (Applied Biosystems, Foster City, CA) as per the manufacturer’s instructions. The reverse transcription conditions were as follows: 10 minutes at 25°C, 120 minutes at 37°C, 5 seconds at 85°C. Following reverse transcription, cDNA was stored at 4°C.
2.4. Taqman Low Density Array
381 multidrug-resistance-related genes were analyzed using custom-made Taqman Low Density Arrays (Applied Biosystems, Foster City, CA). The ribosomal RNA 18s were arrayed in four replicates, while the 380 other genes were measured in singleton. Genes from the same family were arrayed in various locations and 18s was used for quality control. cDNA was mixed with 2X TaqMan Universal PCR Master Mix (Applied Biosystems), loaded on the TLDA card, and run on an ABI Prism 7900 HT Sequence Detection System (Applied Biosystems) as per the manufacturer’s instructions.
2.5. TLDA data processing
TLDA data were analyzed with RQ Manager Software (Applied Biosystems). Median normalization was performed as follows: the median cycle threshold (CT) value over all genes on each TLDA was subtracted from the CT value for each reaction well on that array such that after normalization, the median CT value on each card was zero.26 For the cluster analysis, any genes that exhibited greater than 50% missing data were filtered out. Hierarchical clustering analysis was then performed to investigate any relationships among the statistically significant differentially expressed genes, and a heatmap was produced to explore the gene expression between the cancer tissue lines. The clustering algorithm used a Euclidean distance metric with complete linkage.
2. 6. Preparation of cell lysates and nuclear proteins
For cell lysates without nuclei, cells were washed with ice-cold PBS three times, then lysed in a lysis buffer [50 mM Tris-HCl, pH 8.0, 140 mM NaCl, 1.5 mM MgCl2, and 0.5% (v/v) Nonidet P-40, and protease inhibitor on ice for 5 min. Samples were then checked under a phase-contrast microscope, showing that more than 99% of the nuclei were released from cells. The cytosol and nuclei fractions were separated by centrifugation at 1000 rpm for 5 min at 4°C. The resulting supernatant was collected as cytosol. The pellets of nuclei were lysed with 5% SDS lysis buffer in 0.1 M Tris, pH 7.4, and sheared 5 times with a syringe needle (25G) to break the DNAs. The proteins were determined by BCA assay (Thermo Scientific, Rockford, IL) and measured at a wavelength of 562 nm using an Ultrospec 3100 pro UV/Visible Spectrophotometer (Amersham Biosciences). All proteins were stored at −80°C until used.
2.7. Immunoblotting and confocal analysis
SDS-PAGE immunoblotting was run as recommended by the manufacturer (Invitrogen). Following electrophoresis, the gels were transblotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH) at 4°C. Immuno-reaction was performed with desired primary antibodies and secondary HRP-conjugated antibodies. Enhanced chemiluminescence reagents were used for developing signals as described by the manufacturer (Pierce Chemical Co., Rockford, IL). The MRP1 antibody was purchased from Alexis Biochem (Farmingdale, NY), and one for RhoA was purchased from Santa Cruz Biotech, (Santa Cruz, CA); those for GCF2, F-phalloidin, b-tubulin, dynamin, filamin, and keratin, were purchased from BD Biosciences (San Diego, CA). HRP-labeled secondary antibodies were purchased from Jackson Immuno-Research Lab (West Grove, PA). For confocal analysis, cells were cultured in a Lab-Tek Chamber Slide (Nalge Nunc International, Naperville, IL), and fixed with 70% ETOH at −20°C for 15 min for visualization of intracellular expression and localization. 2.5% Glutaraldehyde (EM grade, Sigma) in PBS containing 0.1% bovine albumin for fixation, followed by Triton X-100 for permeabilization were used to visualize fluorescence distribution of proteins on the cell surface. The cells were fixed with either ETOH or glutaraldehyde, and then reacted with the primary antibodies, followed by reaction with the secondary antibody labeled with Rhodamine red, or FITC (Jackson Immuno-Research Lab) to show the double-staining images. For determination of accumulation of Alexa Fluor 488-labeled cisplatin (F-CP) (Invitrogen), the fluorescence complex was dissolved in dimethyl-sulfoxide (DMSO) according to the manufacturer, then loaded into cells at a final concentration of 10 U/ml in IMDM without phenol red (Invitrogen) containing 10% FBS for 1 hour of incubation at 37°C. Alexa Fluor 488 (AF488) not conjugated to cisplatin was used as a negative control for the F-CP uptake assay. AF488 was diluted at 1:100 with the medium as mentioned above, and incubated with cells for 1 hour at 37°C. After washing with PBS, cells were fixed with 70% ETOH, and re-probed with the monoclonal antibody directed to GCF2 for 1 hr. Immunofluorescent images of cells were monitored under a laser scanning confocal microscope (Zeiss LSM 510 Laser Scanning Confocal Microscope) at 600 × magnification at excitation at 488 nm and 568 nm to collect green and red fluorescence, respectively. Each immunofluorescent image represents an average of at least 10 confocal microscopic fields examined. Each immunoblotting figure represents a triplicate determination
2.8. Transfection of GCF2/LRRFIP1 and siRNA
We obtained the full-length cDNA for the gene encoding GCF2 in the pCMV-XL5 vector (OriGene, Rockville, MD). The GCF2 cDNA was released from the vector by restriction enzymes, and re-inserted into a mammalian expression vector, pcDNA5.1, with a selective marker for neomycin (Invitrogen), as described by the manufacturer. Gene transfection was done with Lipofectin (Invitrogen). Stable transfected clones were isolated after selection with 0.8 mg/ml of G418. The pre-designed SMART pool siRNA for GCF2 (AGAGGAAAUGCUCGAGAAA; AAAAUGAAAUCGUGGCGAA; GGUCAUGGGUGCACCAGAU; AGGGCAUAGUUUAGAGAAA), and the Target-Plus reagents for siRNA transfection were purchased from Dharmacon Thermo Scientific (Lafayette, CO). The transfection was done as described by the manufacturer. Briefly, cells were seeded in a 6-well Cell Culture Cluster (Costar #3516, Corning, NY) for protein assays, or seeded in a Lab-Tek Chamber Slide (Nalge Nunc International) for confocal examination as described above, and the protocols provided by the manufacturer were followed.
2.9. Transfection of anti-RhoA siRNA
Transfection was performed with RNAiMax (Invitrogen, Carlsbad, CA) (5 μL/mL). The pre-designed GeneSolution siRNAs (Hs_RHOA_7 CAAGCTAGACGTGGGAAGAAA, Hs_RHOA_8 TACCTTATAGTTACTGTGTAA, Hs_RHOA_6 TTCGGAATGATGAGCACACAA, Hs_RHOA_1 TACCCAGATACCGATGTTATA) (Qiagen, Valencia, CA), and AllStars Negative Control (mock) (Qiagen) were plated with cells on day 1 at 20nM. The transfection was done as described by the manufacturer.
2.10. Analysis of drug sensitivity after anti-RhoA siRNA using the MTT assay
Cell survival was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) viability assay. Cells were transfected with siRNA as described above and seeded at a density of 5,000 cells per well in 96-well plates and incubated at 37°C in humidified 5% CO2 for 24 h. Serially diluted cisplatin was added to give the intended final concentrations. Cells were then incubated an additional 72 h, and the MTT assay was performed according to the manufacturer’s instructions (Molecular Probes, Eugene, OR). Absorbance values were determined at 570 nm on a Spectra Max 250 spectrophotometer (Molecular Devices, Sunnyvale, CA). All MTT assays were performed three times in triplicate. The 50% inhibitory concentration (IC50) values were defined as the drug concentrations required to reduce cell viability to 50% of the untreated control well and are reported here as such. Resistance ratio (RR) values were reported as a measure of relative drug sensitivity and were defined for each drug as the IC50 of the anti-RhoA treated lines divided by the IC50 of the mock treated lines.
2.11. Assays of cellular sensitivity to chemotherapeutic agents
Cell sensitivities to doxorubicin, cisplatin, and other chemicals were tested by two methods. For clonogenic assays, cells were seeded at 4 × 102 in 3 ml of medium per 35 mm dish, and then colonies were stained with methylene blue after 9–10 days of incubation, and counted. For short term killing assays, cells were seeded at 5 × 103 in 0.1 ml of medium in a 96-well plate, and then quantitated by a colorimetric assay after 3 days’ incubation, using a Cell Counting Kit-8 at an absorbance of 450 nm, as described by the manufacturer (Dojindo, Gaithersburg, MD). Drugs at a desired concentration were introduced into each well or dish prior to cell seeding. Control cells were transfected with insert-free vector only. The values are means of triplicate determinations.
3. Results
3.1. Overexpression of GCF2 in cisplatin-resistant cells
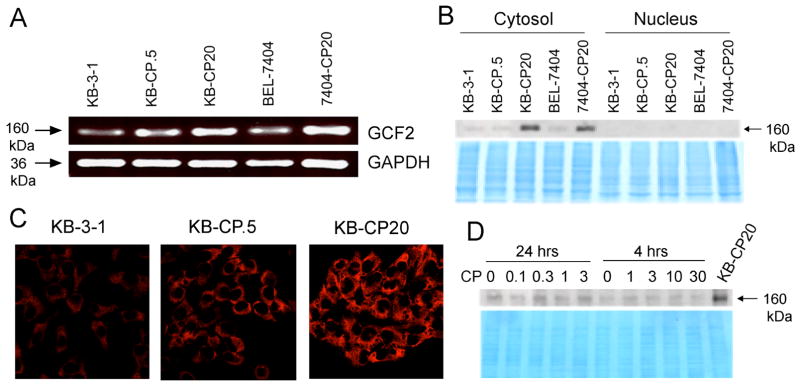
We first wanted to determine if expression of the transcription factor GCF2 was indeed increased in our CP-r cells. Total RNA from five cell lines, including CP-r cells from both human epidermal cancer (KB-3-1) and hepatoma (BEL-7404) were prepared to determine GCF2 expression levels by RT-PCR. GAPDH, a housekeeping gene, served as a control. Figure 1A shows increased expression of the GCF2 gene (upper panel) in the CP-r cell lines KB-CP.5, KB-CP20, and 7404-CP20, compared to their cisplatin-sensitive parental cell lines KB-3-1 and BEL-7404. KB-CP.5, an early stage CP-r cell line that was selected directly with 0.5 mg/mL of cisplatin, showed elevated expression of GCF2, but less than the expression level of the most CP-r cell line, KB-CP20, suggesting that elevated expression of GCF2 is associated with the level of resistance to cisplatin. Expression of GAPDH, used as a loading control, was similar in all of the 5 cell lines that were examined. GCF2 has been reported to be located in both the nuclear and cytosolic compartments within cells, depending on the cell line and the agents used for treatment.21 The proteins of the 5 cell lines mentioned above were prepared and separated into two fractions: nuclear extracts and whole cell lysates without nuclei (labeled cytosol). Figure 1B is an immunoblot comparing expression levels of GCF2 in the nucleus and cytosol among the 5 cell lines. Strong bands can be seen in the cytosol fraction in both KB-CP20 and 7404-CP20, whereas there was no signal in the nuclear extracts among the 5 cell lines that were tested. So it is clear that the localization of the transcription factor GCF2 is mainly in the cytoplasm, not in the nucleus in our cell lines. The lower part of the panel shows a Coomassie blue-stained gel, confirming equivalent loading for all cytosolic and nuclear fractions. This localization pattern was further confirmed by confocal microscopy. Immunofluorescence of GCF2 was detected only in the cytoplasm and was not visible in the nucleus. It is possible that there might be a nucleo-cytoplasmic shuttling pathway with GCF2 in the nucleus being too low to be detected, or that cytoplasmic localization may be responsible for the effect on RhoA. It should be noted that the basal level of GCF2 mRNA was easily detectable in the CP-s cells, as seen in the RT-PCR analysis in Figure 1A, but the protein was almost undetectable by immunoblots (Figure 1B). This could result from increased post-transcriptional regulation leading to higher protein levels, especially in the highly resistant CP-r cells (Figure 1B).
FIGURE 1.
Overexpression of GCF2 in cisplatin-resistant cells. (A) RT-PCR analysis shows overexpression of GCF2 in cisplatin-resistant cell lines, KB-CP.5, KB-CP20 and 7404-CP20, compared with their parental cell lines: KB-3-1 and BEL-7404. (B) Immunoblot showing increased expression of GCF2 in the cytosol of KB-CP20 and 7404-CP20 cells. There was no signal in the nuclear extracts, as shown at the right. (C) Confocal fluorescence images reveal increasing expression of GCF2 in the cytosol of KB-CP.5 and KB-CP20. (D) Immunoblot showing no significant acute effect of cisplatin on expression of GCF2 after exposure to cisplatin at different concentrations of the drug for 24 and 4 hrs. KB-CP20 served as a positive control. The lower panel is a Coomassie blue-stained gel, serving as a loading control.
It was found that the fluorescence intensities of GCF2 were slightly increased in KB-CP.5 cells, and much more in the cells that were the most resistant to cisplatin (KB-CP20). To determine if there were any acute effect of cisplatin on GCF2 expression, KB-3-1 cells were treated with cisplatin at different concentrations (0, 0.1, 0.3, 1, or 3 mg/mL) for 24 hrs, or a short exposure (0, 1, 3, or 10 mg/mL) for 4 hrs. Immunoblotting results indicated that the expression of GCF2 was not affected by cisplatin (Figure 1D).
3.2. Disruption of cytoskeleton results in mislocalization of membrane proteins
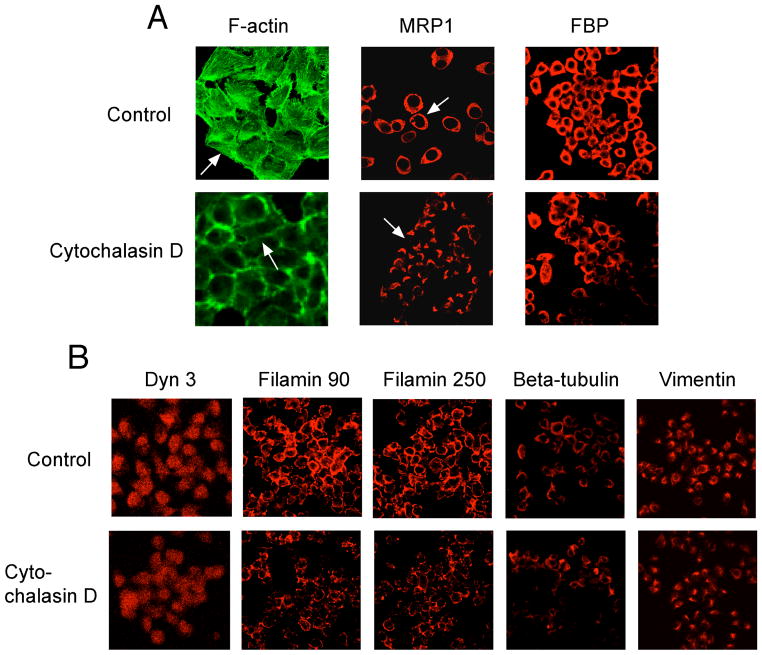
Cytochalasin D, a known cytoskeleton inhibitor,27 was applied to see if disruption of the actin network would result in an altered distribution of the membrane proteins MRP1 and FBP. Cisplatin-sensitive KB-3-1 cells were treated with cytochalasin D at 30 ng/mL for 6 hrs. Figure 2 shows the effects of cytochalasin D on membrane proteins and the cytoskeleton. The most striking features are disruption of actin filaments and mislocalization of the membrane transport, MRP1. The fine organization of F-actin was lost on the cell surface in the cytochalasin D-treated cells, in comparison with the untreated cells (control), as shown by arrows. In cytochalasin D-treated cells, MRP1 appeared as cap-like structures in the cytoplasm, not on the cell surface. In contrast, in control cells MRP1 appears as a ring-like shape on the cell surface, as indicated by arrows. Trafficking of another membrane protein, FBP, was partially affected by the cytochalasin D. The structure and distribution of filamins 90 and 250 were also significantly altered by the cytochalasin D, in comparison with their counterparts. β-tubulin was partially inhibited. Dynamin 3 and vimentin were only slightly affected. These results confirm that disruption of the actin cytoskeleton is sufficient to cause mislocalization of the plasma membrane transporter, MRP1, and disturbs organization of other cytoskeletal elements. These observations that drug transporters are mislocalized from the cell surface also support our previous observations that the cytochalasins lower cisplatin accumulation in cells.28
FIGURE 2.
Confocal fluorescence visualization of the effects of cytochalasin D on expression, distribution, and localization of the cytoskeletons in KB-3-1 cells after exposure to the compound at 30 ng/ml for 6 hrs. F-actin, MRP1, FBP, Dyn 3, filamin 90, filamin 250, beta-tubulin, and vimentin are labeled at the top, The arrows point out changes in structures and distribution in cells that were treated with cytochalasin D.
3.3. Silencing RhoA by GCF2 results in mislocalization of MRP1
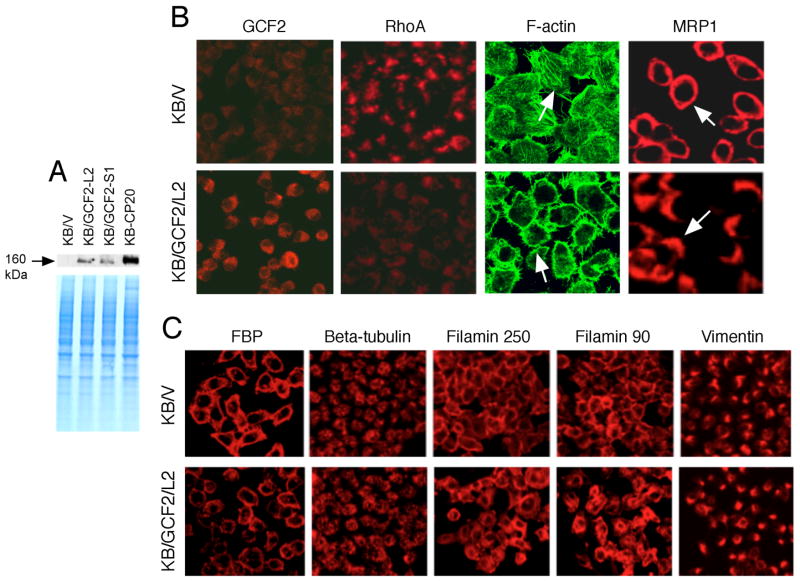
We previously reported that expression of RhoA was remarkably reduced in CP-r cells.9 Was this related to a negative regulator of RhoA, the repressive factor GCF2? We excised the full-length cDNA of GCF2 from the pCMV6-XL5 vector (OriGene), and inserted it into a mammalian expression vector, pcDNA5.1, with the selective marker neo (Invitrogen), and named this vector hGCF2/pcDNA. Transfection of this expression vector into the parental cell line KB-3-1 was performed, and cells were selected with G418 at 0.8 mg/mL to obtain GCF2-positive cells. Two clones, KB/GCF2/L2 and KB/GCF2/S1, were isolated. KB/V was a negative control transfected with the pcDNA5.1 vector without an insert, and selected with the same concentration of G418, serving as a control. Figure 3A is an immunoblot showing that the mAb reacted with human GCF2 transfected into KB-3-1 cells. Increased expression of GCF2 was seen in the two transfected clones (KB/GCF2/L2 and KB/GCF2/S1), though moderate in comparison to the highly CP-r KB-CP20 cells. Figure 3B shows confocal immunofluorescence microscopic images comparing the KB/V control (upper panels) and KB/GCF2/L2 (lower panels) to demonstrate the differences in expression and distribution of GCF2, RhoA, F-actin, and MRP1. GCF2 was overexpressed in the KB/GCF2/L2 clone, and the small GTPase RhoA was reduced to an undetectable level in the same clone. As expected, F-actin, a major component of microfilaments known to be regulated by the small GTPase RhoA, was reduced in expression, and disrupted in organization. No fine filaments were observed in the KB/GCF2/L2 cells with GCF2 overexpression as indicated by arrows. MRP1 was relocated from the cell surface to the interior of the cell. MRP1 shows a ring-like distribution in the control KB/V cells, but in the GCF2-transfected KB/GCF2/L2 cells, MRP1 is relocalized to an intracellular region near the TGN, appearing as cap-like structures or clusters as shown by arrows. These results indicate that overexpression of GCF2 affects the expression and distribution of multiple proteins, including RhoA, F-actin, and the membrane protein MRP1 in CP-r cells.
FIGURE 3.
Overexpression of GCF2 in GCF2-transfected cells. (A) Immunoblot showing increased expression of GCF2 in two clones of GCF2-transfected cells, KB/GCF2/L2 and KB/GCF2/S1, compared with KB/V cells that were transfected with vector only. The cell line most resistant to cisplatin, KB-CP20, served as a positive control. The lower panel is a Coomassie blue-stained gel, serving as a loading control. (B) Confocal immunofluorescence analysis of expression, distribution, and localization of GCF2, RhoA, F-actin, and MRP1. KB/V was transfected with vector only, serving as controls; KB/GCF2/L2 is a clone of GCF2-transfected cells. (C) Confocal images reveal differences between KB/V and KB/GCF2/L2 cells in expression, distribution, and organization of FBP, β-tubulin, filamin 250, filamin 90, and vimentin, in KB/V and KB/GCF2/L2, as described above.
To determine if changes in expression and distribution of actin and MRP1 result from the down-regulation of RhoA by GCF2, we checked the phenotypes of other proteins such as the membrane protein folate-binding protein (FBP), and other cytoskeleton proteins: β-tubulin, filamin 250, filamin 90, and vimentin. As shown in Figure 2C, in the GCF2-transfected cells, the fluorescence intensities of FBP were reduced, with slightly altered membrane distribution. β-tubulin and vimentin showed little change, but the intermediate filaments, filamin 90, and 250 appear to be more densely distributed along the cell membrane in comparison with the control KB/V cells, revealing that some of the membrane proteins and cytoskeletal components are regulated by the transcription factor GCF2 and the small GTPase RhoA, but some of them, such as FBP and β-tubulin are only partially affected, and vimentin is little changed.
3.4. Overexpression of GCF2 mediates sensitization to doxorubicin
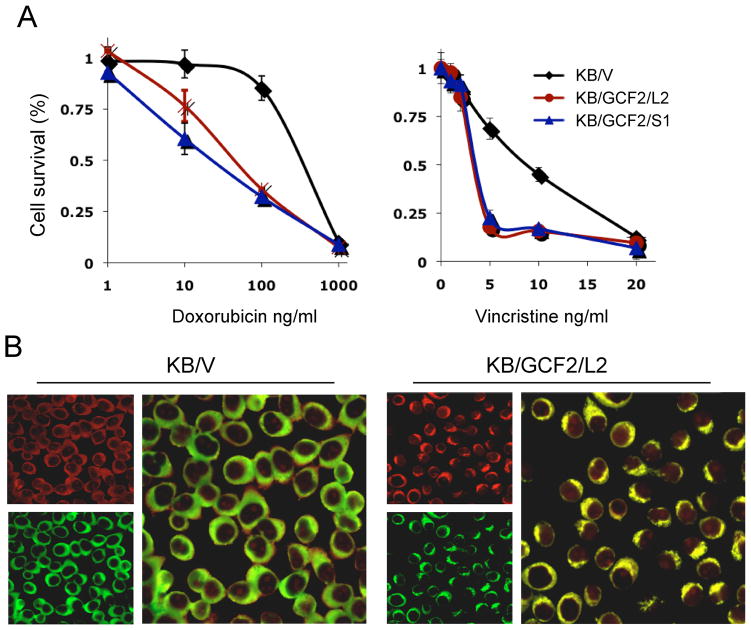
To find out whether upregulation of GCF2 and mislocalization of the membrane transporter MRP1 affected toxicity of MRP1-related substrates, doxorubicin and vincristine were tested. As shown in Fig. 4A, the two GCF2-transfected clones, KB/GCF2/L2 and KB/GCF2/S1 were found to be more sensitive to doxorubicin than their control KB/V cells by 8- and 14-fold, respectively. Sensitivity to vincristine was also increased by about 3-fold in these two clones as compared to control cells. As doxorubicin is a compound that can be detected by confocol fluororesence microscopy, we found that the intensity of doxorubicin was significantly increased in the KB/GCF2/L2 cells compared to the control KB/V cells after exposure to doxorubicin for 2 hours. Fig. 4B shows double-stained images in which MRP1 (green) was located at the cell surface in KB/V cells, whereas it was internalized in the cytoplasm in the KB/GCF2/L2 cells. Interestingly, doxorubicin (red) was co-localized with MRP1, appearing yellowish at the cell surface in the control cells, but seen at the intracellular region and nucleus with increased intensity in KB/GCF2/L2 cells. These results indicate that overexpression of GCF2 in the wild-type cells results in MRP1 internalization and sensitization to its substrates, due to decreased efflux and increased accumulation of the drug.
FIGURE 4.
Upregulation of GCF2 affects toxicity of MRP1-related substrates (A) Overexpression of GCF2 mediates cell sensitization to doxorubicin and vincristine, as determined by CCK assays. (B) Confocal images show increased accumulation of doxorubicin (red) in GCF2-transfected cells once the MRP1 (green) is internalized from the cell surface to the intracellular region.
3.5. Overexpression of GCF2 mediates resistance to cisplatin
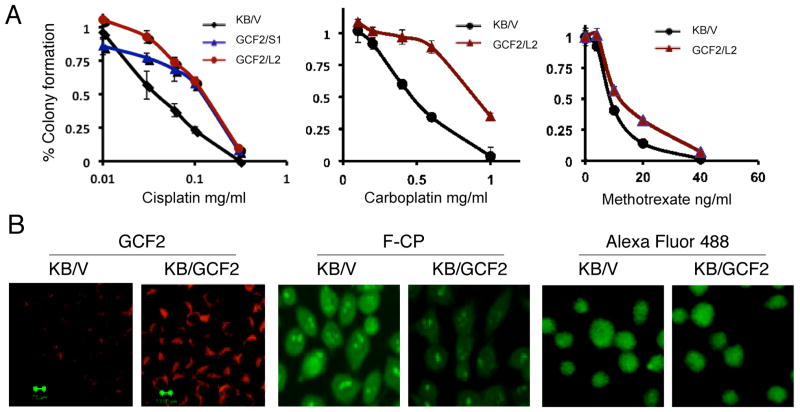
A question concerning the function of GCF2 was whether its overexpression is causative in, or a consequence of, the development of resistance to cisplatin and other anti-cancer drugs. As seen in Figure 3A, GCF2 was overexpressed in the GCF2-transfected KB/GCF2/S1 and KB/GCF2/L2 clones. Clonogenic assays were performed to measure the resistance of the GCF2-positive clones to cisplatin and other anti-cancer drugs. Figure 5A shows that the GCF2-positive clones KB/GCF2/S1 and KB/GCF2/L2 were about 3-fold more resistant to cisplatin than the control KB/V cells (left panel). Cross-resistance to another platinum-based compound, carboplatin, was also found (middle panel). The KB/GCF2/L2 clone was 2-fold more resistant than the control KB/V cells, and slightly cross-resistant to methotrexate (right panel).
FIGURE 5.
Overexpression of GCF2 mediates drug resistance and decreases F-CP accumulation. (A) Clonogenic assays show resistance levels of the GCF2-transfectants to cisplatin, carboplatin, and methotrexate. (B) Confocal images demonstrating: left panels, increased GCF2 expression in KB/GCF2 cells but not in KB-V control cells; middle panels, decreased accumulation of Alexa Fluor 488-labeled cisplatin (F-CP) in KB/GCF2 cells; right panels, control fluorophore Alexa Fluor 488 dye not linked to cisplatin.
An uptake assay using fluorescent Alexa Fluor 488-labeled cisplatin was performed to trace intensities and distributions of a platinum compound in cells. Figure 5B shows that the intensities of GCF2 (red) were markedly increased in the KB/GCF2/L2 cells (left panels), whereas the intensities of the Alexa-488-cisplatin (F-CP; green) were significantly reduced in KB/GCF2-L2 (right panels). As expected, the control KB/V cells showed strong fluorescence of Alexa Fluor 488-cisplatin (green) in the cytoplasm, as well as in the nucleus and nucleoli. In contrast to the decreased accumulation of F-CP in KB/GCF2/L2 cells, both KB/V and KB/GCF2/L2 cells show an even distribution of the fluorophore (AF488) not bound to cisplatin in cellular compartments, with no clear concentration in the nucleus or nucleoli (right panels). These results suggest a correlation between increased expression of GCF2 with reduced accumulation of cisplatin and resistance to cisplatin.
3.6. Changes in MDR-linked gene expression profile determined by TLDA
Analysis of the expression profile of 381 multidrug-resistance-linked genes29 was performed on two GCF2-transfected clones, KB/GCF2/L2, and KB/GCF2/S1, and the control KB/V cells, to help ascertain whether suppression of RhoA expression was the only reason why GCF-2 transfected cells were CP-r. Each cell line was profiled in singleton. Genes whose expression was reduced or increased more than 2-fold in both types of GCF2-transfected cells are listed in Table 1. The gene most reduced in expression in the GCF2-transfected cells was SLC2A5 (29-fold lower in KB/GCF2/L2, and 5-fold lower in KB/GCF2/S1). GJA1 was the gene with the most increased expression in both clones in which GCF2 is overexpressed (approximately 118- to 124-fold more than the mock vector-transfected cells). Interestingly, some ABC transporters, such as ABCA3, ABCC2 and ABCC8, as well as SLC7A2 and SLC7A11 were up-regulated in both GCF2-transfected cell lines. These might be involved in drug resistance or other functions.30 Suppression of TNF, TRAF1, and TGFA was detected in the GCF2 clones by approximately 4- to 9-fold, indicating that GCF2 is an upstream repressor of these genes. Thus, GCF2 likely has multiple effects, which could contribute to drug resistance after transfection into KB-3-1 cells.
Table 1.
TLDA (TaqMan Low Density Array) analysis of expression of two GCF2-transfected cell lines*
| Gene | KB/GCF2-L2 (Fold change) | KB/GCF2-S1 (Fold change) |
|---|---|---|
| Down-regulated | ||
| SLC2A5 | −29.6 | −5.4 |
| IL6 | −5.3 | −2.6 |
| TRAF1 | −4.9 | −3.8 |
| TNF | −4.5 | −9.4 |
| TXNIP | −2.9 | −3.0 |
| MVP | −2.7 | −2.5 |
| MT1X | −2.6 | −3.6 |
| BIRC3 | −2.5 | −2.5 |
| TAP1 | −2.4 | −1.9 |
| CLDN1 | −2.3 | −2.6 |
| CIAPIN1 | −2.3 | −2.9 |
| MMP9 | −2.2 | −3.9 |
| CCL2 | −2.2 | −2.1 |
| SLCO1B3 | −1.9 | −3.3 |
| Up-regulated | ||
| SLC7A11 | 1.9 | 2.2 |
| ABCA3 | 1.9 | 2.2 |
| AKR1C1** | 1.9 | 3.7 |
| BCL2 | 2.0 | 2.0 |
| BAD | 2.2 | 2.2 |
| GGT1 | 3.0 | 2.9 |
| SRC | 3.0 | 3.1 |
| ABCC2 | 3.1 | 2.0 |
| SLC7A2 | 4.2 | 4.8 |
| ABCC8 | 7.7 | 4.0 |
| GJA1 | 118.6 | 124.5 |
Values represent fold change for gene expression in the GCF2-transfected cell line compared with its untransfected counterpart used as a control.
This assay also detects AKR1C2.
3.7. Silencing GCF2 and reactivating RhoA by siGCF2
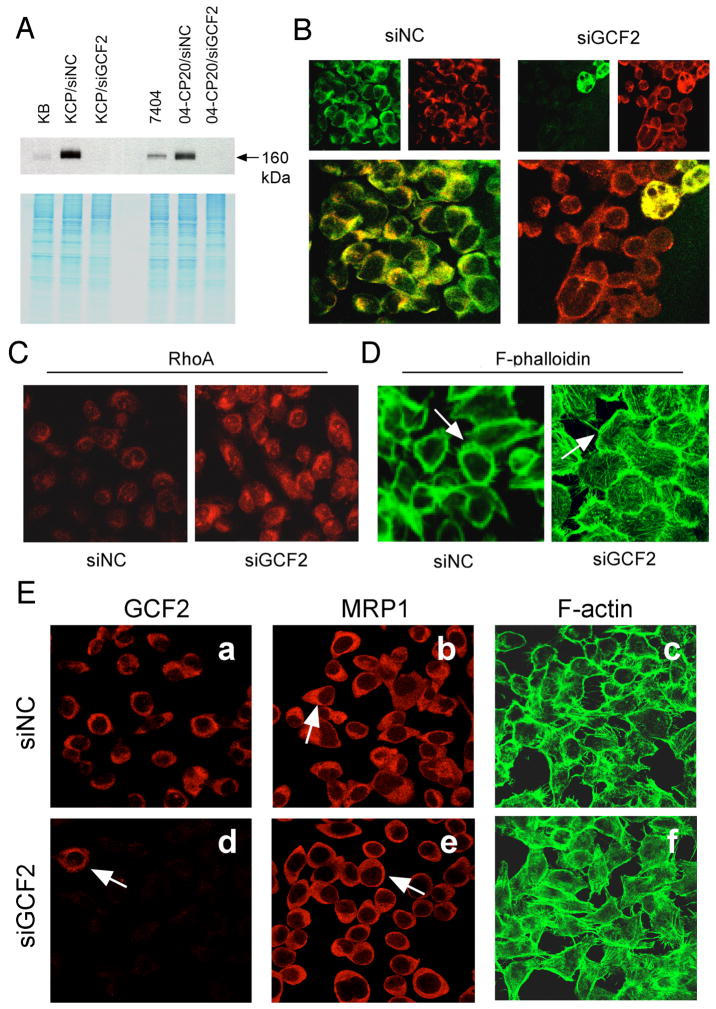
Small interfering RNA (siRNA) was used to reduce expression of GCF2 in human CP-r cells. The KB-CP20 and 7404-CP20 cells were transfected with an siRNA pool specific to GCF2 (termed siGCF2). The negative control (termed siNC) was designed, modified, and microarray-confirmed by ON-TARGETplus (Dharmacon) to have minimal targeting of known human genes. Figure 6A shows that the expression of GCF2 was knocked-down to undetectable levels in both KB-CP20 and 7404-CP20 cells after transfection with siGCF2 for 7 days, labeled as KB-CP20/siGCF2 and 7404-CP20/siGCF2, respectively. Both cell lines transfected with the control siNC showed strong signals. The parental cell lines, KB-3-1 and BEL-7404, showed weak intensities as compared to their CP-r derivatives. The lower part of the panel is a Coomassie blue-stained gel, serving as a loading control.
FIGURE 6.
siRNA of GCF2 reverses phenotypes of CP-r cells. (A) Immunoblot revealing suppression of GCF2 expression in siGCF2-transfected cells, labeled as KB-CP20/siGCF2, and 04-CP20/siGCF2 cells, in comparison to their negative control siNC, labeled as KB-CP20/siNC, and 7404-CP20/siNC, respectively. (B-D): KB-CP20 cells, (B) Double-stained confocal images (larger, lower panels) show that siGCF2 silenced the expression of GCF2 (stained with green, upper left), and MRP1 (stained with red, upper right) re-appeared at the cell surface as seen at the right, and two giant multinucleated cells at the top right show a mixed pattern of double-fluorescence, and seemed to not be affected by the siGCF2. siNC represents siRNA of a negative control (mock-transfected cells), seen at the left. (C) Expression of RhoA was recovered in the siGCF2-transfected cells (on the right, labeled as siGCF2) after GCF2 was knocked-down by the specific siRNA of GCF2, in comparison to the mock cells seen on the left, labeled siNC. (D) F-phalloidin stained immunofluorescence images indicate fine fibers were re-formed on the cell surface of siGCF2-transfected cells compared to siNC. (E) Human liver carcinoma cisplatin-resistant 7404-CP20 cells were transfected with siGCF2, labeled as siGCF2, or with siRNA as a negative control, labeled as siNC. In the GCF2 panels, an arrow points to the single cell in this field still expressing GCF2, while all other cells show no signal, as expression of GCF2 was inhibited by the siRNA of GCF2. In the MRP1 panels, an arrow points to cap-like staining of MRP1 in siNC-transfected cells (upper panel), and an arrow indicates MRP1 was re-located to the cell surface (lower panel). In the F-actin panels, the fine filaments were visualized at the cell surface in siGCF2-transfected cells (lower panel) compared to siNC mock-transfected cells in which the cells appear as hollow circles (upper panel).
Using indirect double immuno-fluorescence staining, Figure 6B shows confocal images of the KB-CP20 cells that were transfected with siGCF2, or siNC as labeled above the two panels. The GCF2 expression (green) was silenced in most siGCF2-transfected KB-CP20 cells, except two multinucleated cells at the top right corner of the field seemed not to be inhibited by the siGCF2, showing bright green fluorescence, indicated by an arrow. In the majority of the cells in which GCF2 was extinguished, MRP1 (red) re-localized from the intracellular cytoplasm to the periphery of the cells, with a red ring-shaped distribution. The bottom of the panel shows images with double staining with GCF2 (green), and MRP1 (red). The two multinucleated cells, indicated by an arrow, exhibit yellowish fluorescence, indicating overlapping of the two proteins GCF2 and MRP1, distributed in the same intracellular area. The cells transfected with negative control siNC as labeled at the top, show well-expressed GCF2 (green), and cap-like MRP1 (red) in the cytoplasm. In Figure 6C, RhoA is seen to be clearly expressed in the siGCF2-transfected cells (right), as compared to the mock siNC-transfected cells (Figure 6C, left). The fibers of F-actin also re-formed in the siGCF2-transfected cells, seen as fine filaments on the cell surface at the periphery of the cells (Figure 6D, right), whereas the siNC-transfected mock cells showed disorganized microfilaments (Figure 6D, left). Thus, reducing expression of GCF2 in the CP-r cells reverses most of the changes seen when KB-3-1 cells are transfected with GCF2.
The 7404-CP20 cells shared very similar features, as shown in Figure 6E. 7404-CP20 cells were transfected with siNC or siGCF2 for the same period of time as mentioned above. GCF2 was clearly silenced in most of the siGCF2-transfected cells (Figure 6E-d); only one cell in this field was not affected, indicated by an arrow. MRP1 re-localized from the cytoplasm to the cell surface in siGCF2-transfected cells as shown by an arrow (Figure 6E-e), compared to the siNC-transfected cells with cap-like structures in the cytoplasm as indicated by an arrow (Figure 6E-b). The fine F-actin network re-appeared in siGCF2-transfected cells (Figure 6E-f), whereas the si-NC-transfected mock control cells appear as hollow circles, with fewer fibers on the cell surface (Figure 6E-c). These results further verify that the negative transcription factor GCF2 plays an important role in mislocalization of the multidrug-associated protein MRP1 in at least two cell lines by silencing the small GTPase RhoA, interrupting microfilament dynamics.
In order to find out whether the resistant phenotype in CP-r cells was reversed by siRNA targeted to GCF2, both KB-CP20 and 7404-CP20 cells were transfected with siGCF2, or siNC as a negative control, and tested for levels of resistance to cisplatin by a CCK8 three-day assay. As seen in Fig. S1, the sensitivities to cisplatin were changed, but only slightly, in both siGCF2-transfected KB-CP20 and 7404-CP20 cells, compared to their siNC-transfected cells. The lower level, single-step selected CP-r KB-CP.5 cells were more sensitized to cisplatin after transfection with siGCF2 than siGCF2-transfected KB-CP20 cells, while there was little difference between siNC- and siGCF2-transfected parental cisplatin-sensitive (CP-s) KB-3-1 cells and BEL-7404 cells. These results indicate that resistance to cisplatin is less detectable by siGCF2 in highly CP-r cells, but could be partially reversed in the lower-level CP-r cells.
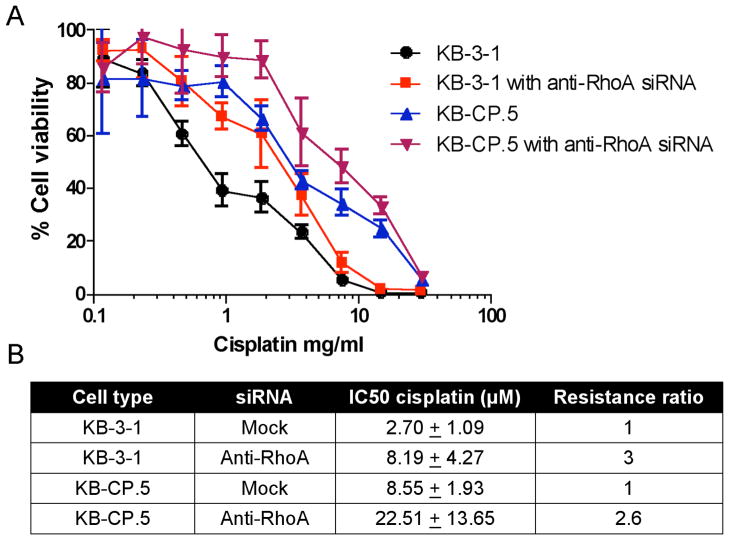
3.8. Silencing RhoA increases resistance
In order to confirm that reduction of RhoA contributes to cisplatin resistance, siRNAs directed against RhoA were transfected into KB-3-1, KB-CP.5, and KB-CP20 cell lines as described in Materials and Methods. As shown in Figure 7, knockdown of RhoA ≥80% resulted in a 3-fold increase in resistance in sensitive parental KB-3-1 cells and a 2.6-fold increase in lower level, single-step selected cisplatin resistant KB-CP.5 cells. Silencing of RhoA in KB-CP20 did not further modulate resistance to cisplatin (data not shown) as these cells are already highly resistant to cisplatin and an IC50 cannot be obtained. Experiments resulting in RhoA being silenced less than 80% had no effect on cisplatin resistance (data not shown). IC50s (Figure 7B) show that treatment of parental KB-3-1 cells with anti-RhoA siRNA increases resistance to an IC50 similar to that of KB-CP.5 cells, which were obtained following single-step selection to cisplatin. These results indicate that silencing RhoA recapitulates the effect of resistance in KB-3-1 cells and suggests that the silencing of RhoA is an important contributor to modulating resistance.
FIGURE 7.
siRNA against RhoA confers cisplatin resistance to KB-3-1 and KB-CP.5 cells. (A) Knockdown of RhoA using siRNA (20 nM) and RNAiMax (Invitrogen, Carlsbad, CA) (5 μL/mL), cells were challenged at 24 hours with cisplatin. After 72 hours, cell viability was measured using MTT assays. Absorbance was measured at 570 (MTT) and 690 (background). (B) IC50s show an increase in resistance when RhoA is knocked down.
4. Discussion
Until now, an explanation for the mislocalization of the membrane proteins MRP1 and FBP occurring in human CP-r cells8 has been elusive. We hypothesized that there might be a link between this phenomenon and the reduced expression of small GTPases and disorganization of the cytoskeleton.9 RhoA plays important roles in both organization of the actin network and membrane trafficking.31–33 However, whether RhoA is directly related to MRP1 mislocalization in CP-r cells, and whether GCF2 is related to cellular response to cancer chemotherapeutic agents was not known. In this paper, we show that an increase in GCF2 levels lower RhoA levels and can account for the protein mislocalization in our cisplatin-selected cells, and for altered sensitivity to doxorubicin and vincristine, cisplatin, and carboplatin. Downregulation of RhoA alone also results in cisplatin resistance.
Mechanisms of cisplatin resistance are multifactorial, as a number of genes are either upregulated or down-regulated during development of resistance to the drug.30 We previously reported that a pleiotropic defect occurs in CP-r cells isolated in single steps, including reduced expression of several small GTPases, disorganization of the cytoskeleton, and mislocalization of the membrane proteins MRP1 and FBP. Here we show that increased GCF2 expression is associated with increased sensitivity to doxorubicin, and resistance to cisplatin.
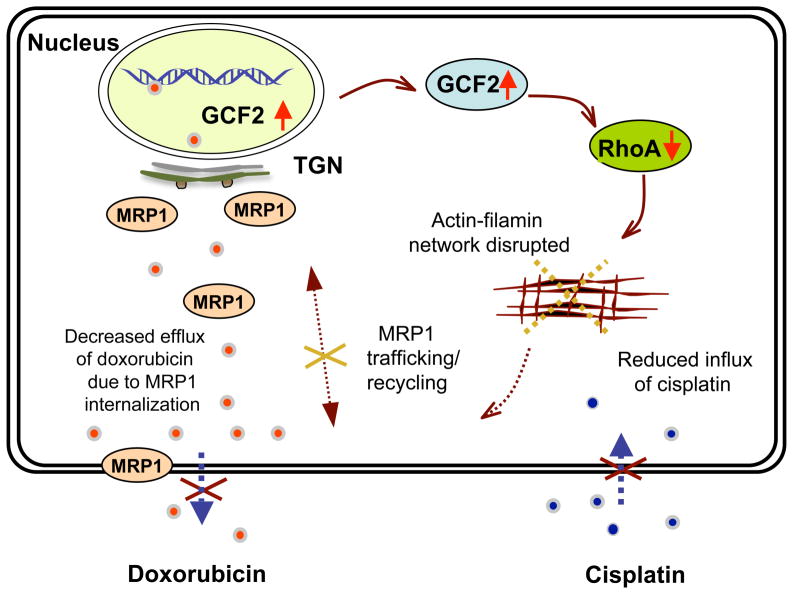
How does GCF2 control increased sensitivity to doxorubicin, and resistance to cisplatin? Figure 8 summarizes our proposed pathway. In this study, we demonstrated that the decreased expression of RhoA identified previously in our CP-r cells is a result of increased expression of the transcription repressor GCF2. The GTPase RhoA directly activates Rho-associated, coiled-coil containing protein kinase 1 (ROCK1), whose downstream targets regulate the actin cytoskeleton. A loss of RhoA expression leads to disruption of actin-myosin filament assembly.34 One result of destabilization of the actin cytoskeleton by RhoA is disruption of endocytic and exocytic processes.35 The net result is that transporters ordinarily trafficked to the cell surface are mislocalized to intracellular compartments, consistent with previous reports from our lab that transporters such as MRP1/2 and FBP are not effectively delivered to the cell surface in CP-r cells.36
FIGURE 8.
Schematic illustration of the proposed GCF2 pathway. This schematic shows a mechanism by which cisplatin induces upregulation of GCF2, then silences rhoA expression, interrupting assembly/organization of the actin/filamin network, resulting in internalization of the membrane protein MRP1, resulting in decreased efflux and increased accumulation of doxorubicin in the cytoplasm and nucleus. The decreased influx of cisplatin in the cytoplasm and nucleus is postulated to be due to altered localization of putative proteins needed for cisplatin uptake as well as reduced endocytosis.
The mislocalization of membrane-bound proteins, including transporters, orchestrated by GCF2 in our CP-r cells is demonstrated here by two separate phenotypes. Firstly, the low-affinity uptake transporters that partly mediate cisplatin uptake into the cell are not present at the surface of CP-r cells, reducing drug uptake and therefore efficacy.7 This is supported by the fact that GCF2-transfected cells are cross-resistant to other drugs also requiring facilitated uptake (carboplatin, methotrexate). Secondly, efflux transporters such as MRP1 that normally extrude lipophilic molecules from cells are not present at the surface, and are therefore unable to extrude cytotoxins from cells. This is supported by the fact that GCF2-transfected cells are hypersensitized to the MRP1 substrates doxorubicin and vincristine.
The folate binding protein, FBP, found translocated from the membrane into the cytoplasm in CP-r cells, was only partially affected in the GCF2-transfected cells, indicating that RhoA has a greater effect on MRP1 recycling, and a less significant effect on FBP. Filamins stabilize the actin network, and play essential roles in integrating cellular architecture and interaction of signaling functions.37, 38 The results obtained from this work suggest that the intermediate filament proteins filamin 90 and 250 may also be regulated by the small GTPase RhoA, and contribute to the dysfunctional recycling of the membrane protein MRP1. The expression and distribution of dynamin 3 and vimentin were not changed in GCF2-transfectants and as such it is likely that these two cytoskeletal proteins belong to a different regulatory pathway. One important conclusion from this analysis is that GCF2 alone does not explain the entire pleiotropic phenotype seen in CP-r cells. If a single pathway is disrupted in these single-step mutants, then there must be another pleiotropic regulator proximal to GCF2 in the pathway accounting for the full phenotype.
We observed localization of GCF2 in the cytoplasm, but not in the nucleus in our CP-r cells. This is similar to the observations by Ohtsuka et al. that endogenous GCF2 was primarily present in the cytoplasm of Hela cells.18 There are a number of reports of nucleo-cytoplasmic shuttling, translocation, or redistribution of transcription factors.39–42 One example is the forkhead family transcription factor, Foxc, which is found in the cytoplasm rather than in the nucleus. Increased cytoplasmic expression of Foxc2 activates epithelial-mesenchymal transition (EMT) and correlates with epithelial differentiation and tumor metastasis. Tedesco et al.43 also reported that STRA8 (stimulated by retinoic acid 8) shuttles between nucleus and cytoplasm and possesses transcriptional activity. HuR, a ubiquitously expressed member of the Hu protein family that binds and stabilizes AU-rich element (ARE)-containing mRNAs, is known to shuttle between the nucleus and the cytoplasm via several export pathways under heat-shock stress 40.
Resistance to cisplatin and cross-resistance to other metals and unrelated compounds is one of the major characteristics of CP-r cells. In this work, we also show that GCF2-transfected cells were about 3-fold more resistant than the parental cells, indicating that overexpression of the GCF2 gene mediates resistance, via silencing of RhoA and/or other genes. Cross-resistance to carboplatin was significant, but the transfected cells were only slightly resistant to methotrexate, as methotrexate is an anti-folate compound, and GCF2 overexpression did not have a significant effect on distribution of the methotrexate uptake transporter FBP.
Resistance to cisplatin is commonly associated with reduced accumulation of the compound. In this work, GCF2-transfected cells showing 3-fold more resistance to cisplatin also exhibited a significant reduction of cisplatin accumulation assayed with an Alexa Fluor labeled platinum complex. Our results demonstrate a significant reduction of F-CP in the cytoplasm and nucleus of the overexpressed GCF2 cells (KB/GCF2/L2) in comparison to their control mock-transfected cells (KB/V).
To verify if mislocalization of the membrane protein MRP1 in these cells was due to elevated expression of GCF2, we applied siRNA against GCF2/LRRFIP1 in two cell lines highly resistant to cisplatin, KB-CP20 and 7404-CP20. Once GCF2 was silenced, the expression of RhoA was restored. The F-actin network was also restored, and the membrane protein MRP1 reappeared at the cell surface. Recovered RhoA expression and a restored actin network and membrane protein recycling also coincided with some decrease in resistance to cisplatin in siGCF2-transfected KB-CP.5, KB-CP20, and 7404-CP20 (Figure 7). siRNA directed against RhoA resulted in a 3-fold increase in resistance to cisplatin in KB-3-1 cells and an IC50 similar to that seen in KB-CP.5 cells, indicating that GCF2 negative regulation of RhoA is an important factor in the cellular ability to resist killing by cisplatin.
It has been largely accepted that cisplatin resistance is multifactorial, facilitated in part by the fact that platinum (Pt) binds to DNA randomly, and forms numerous Pt-DNA adducts and lesions, resulting in global changes in gene expression and structural mutation in genes after long-term increases that occur during cisplatin selection. The KB-CP20 cells, selected in multiple steps, were more resistant to cisplatin by ~200 fold, showing dozens of genes that were altered compared to wild-type cells. It would be reasonable to expect that one gene could confer ~3-fold more resistance in wild-type cells, which would be easy to detect, but it would be far less likely that siRNA knockdown of a single gene of the many that cause resistance could affect the resistance of highly resistant KB-CP20 cells to a level that would be easy to detect. Therefore, GCF2 may play a role in conferring cisplatin resistance in association with other genes that have been reported or as yet are undiscovered.
Gene expression profiling using TLDA on GCF2-transfected clones confirmed that TNF (tumor necrosis factor) and TRAF1 (TNF receptor-associated factor 1) are downstream targets of GCF2,22 and provided new insights into several other targets of GCF2. Among the genes suppressed by GCF2 is SLC2A5, a member of the solute carrier family, identified as a facilitated glucose/fructose transporter.44 The gene most highly up-regulated by GCF2 was GJA1, a gap junction protein also known as connexin 43 or CX43,45 with more than 100-fold increased expression in both GCF2-transfected clones, GCF2/L2, and GCF2/S1. Whether these genes regulated by GCF2 affect cisplatin resistance remains to be determined.
The detailed analysis of the complex regulatory changes that occur in a single step in cisplatin-selected cells suggests that we are exposing the existence of a pre-determined pathway that protects cells against environmental toxins. Whether this is a hard-wired, primordial response to enhance cell survival after toxic insult, or represents adaptation of a pathway that subserves a developmental program remains to be determined.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Supporting Information: Supplemental Figure 1, showing the sensitivity of cisplatin-resistant cells after GCF2 transfection. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Niedner H, Christen R, Lin X, Kondo A, Howell SB. Identification of genes that mediate sensitivity to cisplatin. Mol Pharmacol. 2001;60:1153–1160. [PubMed] [Google Scholar]
- 2.Francia G, Man S, Teicher B, Grasso L, Kerbel RS. Gene expression analysis of tumor spheroids reveals a role for suppressed DNA mismatch repair in multicellular resistance to alkylating agents. Mol Cell Biol. 2004;24:6837–6849. doi: 10.1128/MCB.24.15.6837-6849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yasui K, Mihara S, Zhao C, Okamoto H, Saito-Ohara F, Tomida A, Funato T, Yokomizo A, Naito S, Imoto I, Tsuruo T, Inazawa J. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403–1410. doi: 10.1158/0008-5472.can-3263-2. [DOI] [PubMed] [Google Scholar]
- 4.Roberts D, Schick J, Conway S, Biade S, Laub PB, Stevenson JP, Hamilton TC, O’Dwyer PJ, Johnson SW. Identification of genes associated with platinum drug sensitivity and resistance in human ovarian cancer cells. Br J Cancer. 2005;92:1149–1158. doi: 10.1038/sj.bjc.6602447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 6.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 7.Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- 8.Liang XJ, Shen DW, Garfield S, Gottesman MM. Mislocalization of membrane proteins associated with multidrug resistance in cisplatin-resistant cancer cell lines. Cancer Res. 2003;63:5909–5916. [PubMed] [Google Scholar]
- 9.Shen DW, Su A, Liang XJ, Pai-Panandiker A, Gottesman MM. Reduced expression of small GTPases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells. Br J Cancer. 2004;91:270–276. doi: 10.1038/sj.bjc.6601956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Longo FM, Zhou H, Massa SM, Chen YH. Signaling through Rho GTPase pathway as viable drug target. Curr Med Chem. 2009;16:1355–1365. doi: 10.2174/092986709787846569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol. 2009;11:71–77. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navenot JM, Fujii N, Peiper SC. Activation of Rho and Rho-associated kinase by GPR54 and KiSS1 metastasis suppressor gene product induces changes of cell morphology and contributes to apoptosis. Mol Pharmacol. 2009;75:1300–1306. doi: 10.1124/mol.109.055095. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida T, Clark MF, Stern PH. The small GTPase RhoA is crucial for MC3T3-E1 osteoblastic cell survival. J Cell Biochem. 2009;106:896–902. doi: 10.1002/jcb.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998;18:6325–6339. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin LM. Rock ‘n’ Rho: regulation of ion channels. Am J Physiol Heart Circ Physiol. 2009;296:H908–H909. doi: 10.1152/ajpheart.00185.2009. [DOI] [PubMed] [Google Scholar]
- 17.Wu D, Asiedu M, Wei Q. Myosin-interacting guanine exchange factor (MyoGEF) regulates the invasion activity of MDA-MB-231 breast cancer cells through activation of RhoA and RhoC. Oncogene. 2009;28:2219–2230. doi: 10.1038/onc.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohtsuka H, Oikawa M, Ariake K, Rikiyama T, Motoi F, Katayose Y, Unno M, Johnson AC. GC-binding factor 2 interacts with dishevelled and regulates Wnt signaling pathways in human carcinoma cell lines. Int J Cancer. 2011;129:1599–1610. doi: 10.1002/ijc.25837. [DOI] [PubMed] [Google Scholar]
- 19.Kageyama R, Pastan I. Molecular cloning and characterization of a human DNA binding factor that represses transcription. Cell. 1989;59:815–825. doi: 10.1016/0092-8674(89)90605-3. [DOI] [PubMed] [Google Scholar]
- 20.Khachigian LM, Santiago FS, Rafty LA, Chan OL, Delbridge GJ, Bobik A, Collins T, Johnson AC. GC factor 2 represses platelet-derived growth factor A-chain gene transcription and is itself induced by arterial injury. Circ Res. 1999;84:1258–1267. doi: 10.1161/01.res.84.11.1258. [DOI] [PubMed] [Google Scholar]
- 21.Rikiyama T, Curtis J, Oikawa M, Zimonjic DB, Popescu N, Murphy BA, Wilson MA, Johnson AC. GCF2: expression and molecular analysis of repression. Biochim Biophys Acta. 2003;1629:15–25. doi: 10.1016/s0167-4781(03)00156-8. [DOI] [PubMed] [Google Scholar]
- 22.Suriano AR, Sanford AN, Kim N, Oh M, Kennedy S, Henderson MJ, Dietzmann K, Sullivan KE. GCF2/LRRFIP1 represses tumor necrosis factor alpha expression. Mol Cell Biol. 2005;25:9073–9081. doi: 10.1128/MCB.25.20.9073-9081.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed AL, Yamazaki H, Kaufman JD, Rubinstein Y, Murphy B, Johnson AC. Molecular cloning and characterization of a transcription regulator with homology to GC-binding factor. J Biol Chem. 1998;273:21594–21602. doi: 10.1074/jbc.273.34.21594. [DOI] [PubMed] [Google Scholar]
- 24.Shen D, Pastan I, Gottesman MM. Cross-resistance to methotrexate and metals in human cisplatin-resistant cell lines results from a pleiotropic defect in accumulation of these compounds associated with reduced plasma membrane binding proteins. Cancer Res. 1998;58:268–275. [PubMed] [Google Scholar]
- 25.Shen DW, Liang XJ, Gawinowicz MA, Gottesman MM. Identification of cytoskeletal [14C]carboplatin-binding proteins reveals reduced expression and disorganization of actin and filamin in cisplatin-resistant cell lines. Mol Pharmacol. 2004;66:789–793. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- 26.Orina JN, Calcagno AM, Wu CP, Varma S, Shih J, Lin M, Eichler G, Weinstein JN, Pommier Y, Ambudkar SV, Gottesman MM, Gillet JP. Evaluation of current methods used to analyze the expression profiles of ATP-binding cassette transporters yields an improved drug-discovery database. Mol Cancer Ther. 2009;8:2057–2066. doi: 10.1158/1535-7163.MCT-09-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.May JA, Ratan H, Glenn JR, Losche W, Spangenberg P, Heptinstall S. GPIIb-IIIa antagonists cause rapid disaggregation of platelets pre-treated with cytochalasin D. Evidence that the stability of platelet aggregates depends on normal cytoskeletal assembly. Platelets. 1998;9:227–232. doi: 10.1080/09537109876744. [DOI] [PubMed] [Google Scholar]
- 28.Liang XJ, Yin JJ, Taylor B, Winkovitch SM, Garfield SH, Shen DW, Gottesman MM, Aszalos A. Disruption of microfilaments by cytochalasin B decreases accumulation of cisplatin in human epidermal carcinoma and liver carcinoma cell lines. Cancer Chemother Pharmacol. 2008;62:977–984. doi: 10.1007/s00280-008-0687-9. [DOI] [PubMed] [Google Scholar]
- 29.Calcagno AM, Salcido CD, Gillet JP, Wu CP, Fostel JM, Mumau MD, Gottesman MM, Varticovski L, Ambudkar SV. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Natl Cancer Inst. 2010;102:1637–1652. doi: 10.1093/jnci/djq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 31.Matas OB, Fritz S, Luna A, Egea G. Membrane trafficking at the ER/Golgi interface: functional implications of RhoA and Rac1. Eur J Cell Biol. 2005;84:699–707. doi: 10.1016/j.ejcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Millman EE, Zhang H, Zhang H, Godines V, Bean AJ, Knoll BJ, Moore RH. Rapid recycling of beta-adrenergic receptors is dependent on the actin cytoskeleton and myosin Vb. Traffic. 2008;9:1958–1971. doi: 10.1111/j.1600-0854.2008.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 35.Ridley AJ. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–310. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- 36.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin Resistance: A Cellular Self-Defense Mechanism Resulting from Multiple Epigenetic and Genetic Changes. Pharmacol Rev. 2012 doi: 10.1124/pr.111.005637. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. Filamins as integrators of cell mechanics signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 38.Thelin WR, Chen Y, Gentzsch M, Kreda SM, Sallee JL, Scarlett CO, Borchers CH, Jacobson K, Stutts MJ, Milgram SL. Direct interaction with filamins modulates the stability and plasma membrane expression of CFTR. J Clin Invest. 2007;117:364–374. doi: 10.1172/JCI30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hader C, Marlier A, Cantley L. Mesenchymal-epithelial transition in epithelial response to injury: the role of Foxc2. Oncogene. 2009;29:1031–1040. doi: 10.1038/onc.2009.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasegawa H, Kakuguchi W, Kuroshima T, Kitamura T, Tanaka S, Kitagawa Y, Totsuka Y, Shindoh M, Higashino F. HuR is exported to the cytoplasm in oral cancer cells in a different manner from that of normal cells. Br J Cancer. 2009;100:1943–8. doi: 10.1038/sj.bjc.6605084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurooka H, Yokota Y. Nucleo-cytoplasmic shuttling of Id2, a negative regulator of basic helix-loop-helix transcription factors. J Biol Chem. 2005;280:4313–4320. doi: 10.1074/jbc.M412614200. [DOI] [PubMed] [Google Scholar]
- 42.Verhagen J, Donnelly M, Elliott G. Characterization of a novel transferable CRM-1-independent nuclear export signal in a herpesvirus tegument protein that shuttles between the nucleus and cytoplasm. J Virol. 2006;80:10021–10035. doi: 10.1128/JVI.01322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedesco M, La Sala G, Barbagallo F, De Felici M, Farini D. STRA8 shuttles between nucleus and cytoplasm and displays transcriptional activity. J Biol Chem. 2009;284:35781–35793. doi: 10.1074/jbc.M109.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C, Wu X, Yu Y, Amlal H, Seidler U, Zuo J, Soleimani M. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem. 2009;284:5056–5066. doi: 10.1074/jbc.M808128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laird DW. Closing the gap on autosomal dominant connexin-26 and connexin-43 mutants linked to human disease. J Biol Chem. 2008;283:2997–3001. doi: 10.1074/jbc.R700041200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.