Abstract
It is not uncommon for laboratory animals to be fasted prior to experimentation. Fasting evokes marked reductions in heart rate (HR), blood pressure (BP), heat production, and oxygen consumption (VO2) in rodents. Mice with diet-induced obesity exhibit elevated HR and BP, and lower VO2 and heat production in the fed condition vs. their lean counterparts. It is unknown whether body composition alters the tempo of response to fasting. We tested the hypothesis that cardiovascular and metabolic responses to fasting are delayed in obese vs. lean male C57BL/6J mice. In the fed condition mice that consumed high-fat (HF, 45% fat) chow for 98±5 days had elevated (p<0.05) body fat percentage (DEXA), serum leptin (ELISA), HR and BP (72 h biotelemetry), and lower (p<0.05) heat production and VO2 (72 h metabolic chamber) vs. animals that consumed standard chow (CON, 10% fat; n=16 per group). HR, BP, VO2, heat production, and serum leptin decreased (all p<0.05) in response to a 16 h fast (1600 h to 0800 h) in both groups. Although the overall fold changes in cardiovascular and metabolic parameters were similar in magnitude among animals, fasting-induced reductions in cardiovascular and metabolic variables occurred ~ 4 h and ~ 7 h earlier (p<0.05), respectively, in HF vs. CON mice. These findings indicate that while metabolic and cardiovascular stress evoked by a 16 h fast at 22°C is not different between HF and CON mice, fasting-induced responses occur sooner in obese animals.
Keywords: starvation, blood pressure, heart rate, metabolic rate, animal models
Introduction
It is not uncommon for laboratory animals to be fasted prior to experimentation. While this might be done to minimize risk to the animal during procedures such as endotracheal intubation1, 2, to lessen physiological responses associated with digestion prior to protocols that involve physical exercise3, 4, and/or to maximize agonist (e.g., insulin) induced stimulation of tissue signaling pathways5, fasting represents a substantial challenge to an organism. For example, fasting evokes marked reductions in heart rate (HR) and blood pressure (BP) in rodents.6-9 These reductions result from the organism entering into a state of torpor as it attempts to conserve energy.8, 10-13 Support for this statement is that fasting suppresses oxygen consumption, heat production, locomotion, and body temperature in rodents.8, 10-16 Factors contributing to the severity of these reductions might include the animal species, species strain, gender, ambient and core body temperature, length of fast, and/or composition of the previously consumed diet.
Our laboratory investigates mechanisms responsible for cardiovascular complications (e.g., systemic hypertension and endothelial dysfunction) that exist in mice with diet-induced obesity.5 We recently confirmed previous findings that HR and BP are elevated in obese vs. lean mice during both the light (0600 h – 1759 h) and dark (1800 h - 0559 h) phases of their 24 hour (h) cycle.5, 17 Furthermore, our preliminary data and results from others18 indicate that heat production, oxygen consumption and locomotion are less in obese vs. lean animals. It is unknown whether differences exist between lean and obese mice specifically concerning the severity and timing of cardiovascular and metabolic responses to fasting. Addressing this issue is important to studies of vascular function because BP can significantly impact arterial function19,20, and vascular reactivity is typically determined in vitro using vessels obtained from mice that have completed a 16 h fast (i.e., 1600h – 0800h).5, 17
We hypothesized that fasting-induced reductions in cardiovascular parameters (e.g., HR and BP) and metabolic function (e.g. oxygen consumption and heat production) would be less severe and/or delayed in obese vs. lean mice. Rationale for this hypothesis was that because adipose tissue stores are more abundant in obese vs. lean animals, fasting should pose less of a stress. Biotelemetry and metabolic chamber studies showed that HR, BP, and heat production were higher, and oxygen consumption and locomotion were lower, in obese vs. lean mice in the fed condition. As anticipated, each of these variables decreased in response to fasting. Interestingly, the tempo of these changes was distinct, occurring earlier in the obese model. However, after 16 hours of fasting, cardiovascular and metabolic adaptations had decreased to a similar extent overall. These data indicate that a 16-hour fast is a suitable period after which to study vascular function in models of diet-induced obesity. However, shorter-term periods of fasting could be confounded by differential rates of metabolic and vascular adaptations.
Materials and Methods
Animals and diet
All protocols were approved by the Animal Use and Care Committee at the University of Utah. Ten-week old male C57BL/6J mice were purchased commercially (Jackson Laboratories, Bar Harbor, ME), and housed under controlled temperature (22°C), light (0600-1759h), and dark (1800-0559h) conditions. Animals were allowed ad libitum access to water and standard chow containing (kcal%) 13% fat, 53% carbohydrate, and 34% protein (#8656, Harlan Teklad, Denver, CO; CON, n=32). After a 1-week quarantine period mice continued the CON diet (n=16) or were switched to chow containing (kcal%) 45% fat, 35% carbohydrate, and 20% protein (D12451, Research Diets, Inc; HF, n=16). After 98±5 days on the respective diets, cardiovascular variables were assessed in the fed and fasted condition in one cohort of 7 CON and 7 HF mice, while blood sampling, body composition, metabolic variables, and urine collection procedures were performed in a second cohort of 5 CON and 5 HF mice.
Cardiovascular variables
Mice were anesthetized using 2-5% isoflurane. Using aseptic techniques, the abdomen was opened and a transmitter (model PA-C10, Data Sciences International, St. Paul, MN) was inserted and secured in place to allow ambulation with minimal hindrance. A catheter attached to the transmitter then was advanced subcutaneously and inserted into the right common carotid artery. Thirty second averages of systolic, diastolic, and mean arterial blood pressure (MAP; mmHg) and HR (beats min−1) were recorded every 15-min for 4 consecutive 24-h periods starting ~10 days post surgery.5, 17, 21-23 MAP and HR from the first 3 days were combined to represent fed values from the HF and CON mice. Fed values then were compared to the fourth day that contained the fasting segment from 1600 h to 0800 h.
Blood sampling, body composition, metabolic variables, and urinary catecholamines
Blood was obtained from a tail clip at ~ 0800 h from fed, conscious CON and HF mice to quantify serum leptin (Millipore/Linco, St. Charles, MO) and triglycerides (Sigma-Aldrich, St. Louis, MO). At least 24 h later, body composition (total body mass, fat mass, and lean mass (g)) was assessed in fed CON and HF mice using Dual Energy X-Ray Absorptiometry (DEXA; pDEXA Sabre Bone Densitometer, Norland Medical Systems, Fort Atkinson, WI) on lightly anesthetized mice (0.015-0.02 ml avertin g−1 body mass).5 Three days later VO2 (ml kg−1 h−1), heat production (kcal kg−1 h−1), respiratory exchange ratio (RER), locomotor activity (beam breaks; X+Y+Z planes), food ingestion (g), and water intake (ml) were measured in mice housed individually in metabolic chambers (Oxymax; Columbus Instruments, Columbus, Ohio) for 72 h. This duration consisted of familiarization (24 h) and data collection (24 h) periods wherein mice consumed their respective chow ad libitum, and a 24 h period that contained the fasting segment from 1600 h - 0800 h. Body mass and rectal temperature (model TH-5, Physitemp, Clifton, NJ) were measured prior to chamber entry (~ 0800 h on Day 1), body mass was assessed before the fasting segment (~ 1600 h on Day 2), and both variables again were recorded upon completion of the fasting period (~ 0800 h on Day 3). Mice were returned to their original housing conditions and resumed their respective feeding regimen.
Ten days later mice were housed individually for 72 h in cages designed specifically to collect urine.22, 23 This duration consisted of a familiarization (24 h) and data collection (48 h) period. Urine that accumulated in a vial containing 10 ul of 6 M HCl was recovered at ~ 0800 h on Days 1, 2, and 3, and was stored at −80°C until all samples could be analyzed simultaneously. Urinary epinephrine (ng ul−1) and norepinephrine (ng ul−1) were measured using the 2-CAT ELISA kit (Labor Diagnostika Nord, Nordhorn, Germany). Identical procedures were followed for all collections, except that food was removed at 1600 h on Day 3. When mice were removed from the urine collection chamber at the end of the fasting period (i.e., ~ 0800 h on Day 3), blood samples were obtained from a tail clip to assess leptin and triglycerides. Next, mice were anesthetized deeply (3-5% isoflurane), their chest was opened, heart excised, and gonadal fat pad mass was assessed.
Data analysis and statistics
Comparisons were made: 1) within the CON and HF group (light phase vs. dark phase and FED vs. FAST conditions); 2) between the CON and HF groups at identical time points in the FED and FAST conditions; and 3) between the CON and HF groups concerning the magnitude of the fasting-induced response. To calculate the latter, FED values were subtracted from FAST values at identical time points. This number was divided by the FED value and represents the percent change due to fasting. Comparisons were made using a two-way ANOVA with replication (Microsoft Excel 2007, Redmond, WA). Significance was accepted when p<0.05. Sources of statistical significance were identified using Tukey post hoc analyses (SPSS, Chicago, IL). All values in the Figures (1-7) and Tables (1-2) are presented as mean ± standard error.
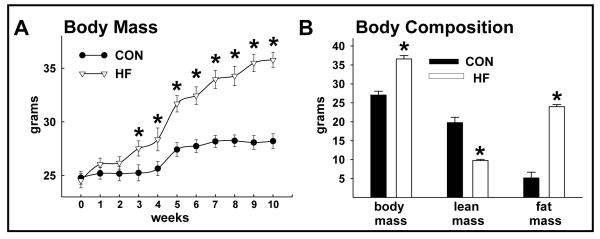
FIGURE 1.
Body mass and composition of random-fed CON and HF mice. Body mass was greater (*p<0.05) in HF vs. CON mice after 3 weeks of feeding (A). Body mass and fat mass were greater and lean mass was less in HF vs. CON mice (all p<0.05; B). Values represent mean ± standard error of the mean (SEM).
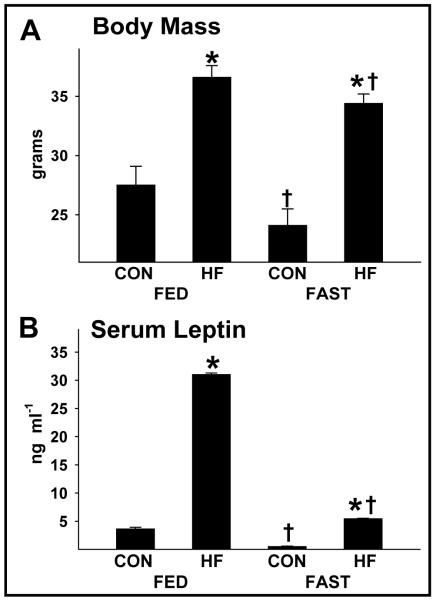
FIGURE 7.
Body mass and serum leptin in CON and HF mice during the FED and FAST conditions. Body mass (A) and serum leptin (B) were elevated (*p<0.05) in HF vs. CON mice during the FED and FAST periods. While fasting reduced (†p<0.05) leptin concentrations similarly between HF and CON mice, the fold reduction in body mass was ~2-fold greater in CON vs. HF mice. Values represent mean ± SEM.
Table 1.
Urinary catecholamines and metabolic averages
| CON | HF | |
|---|---|---|
| Age (days) | 160±5 | 162±6 |
| Gonadal fat (g) | 0.26±0.1 | 1.60±0.2* |
| Urinary epinephrine (ng/d) | ||
| FED | 30±6 | 18±2 |
| FAST | 22±4 | 8±2*† |
| Urinary norepinephrine (ng/ d) | ||
| FED | 240±61 | 211±33 |
| FAST | 178±24 | 82±21*† |
| Heat production during the FED state (kcal/kg h) | ||
| Light phase | 15.0±0.2 | 13.7±0.1* |
| Dark phase | 18.0±0.2‡ | 15.7±0.2*‡ |
| Heat production during the FAST state (kcal/kg h) | ||
| Light phase | 14.7±0.3 | 13.1±0.1*† |
| Dark phase | 15.4±0.3† | 14.1±0.1*†‡ |
| VO2 during the FED state (mL/kg h) | ||
| Light phase | 3099±37 | 2837±18* |
| Dark phase | 3628±45‡ | 3253±29*‡ |
| VO2 during the FAST state (mL/kg h) | ||
| Light phase | 3095±42 | 2814±22* |
| Dark phase | 3174±66† | 2935±28*†‡ |
| RER during the FED state (AU) | ||
| Light phase | 0.900±0.003 | 0.864±0.002* |
| Dark phase | 0.951±0.002‡ | 0.840±0.002*‡ |
| RER during the FAST state (AU) | ||
| Light phase | 0.893±0.004 | 0.831±0.004*† |
| Dark phase | 0.758±0.001†‡ | 0.741±0.001*†‡ |
| Activity during the FED state, beam breaks (X + Y + Z) | ||
| Light phase | 311±30 | 225±24* |
| Dark phase | 1662±119‡ | 1194±83*‡ |
| Activity during the FAST state, beam breaks (X + Y + Z) | ||
| Light phase | 411±48 | 235±29* |
| Dark phase | 1842±122‡ | 931±73*†‡ |
Values are means±SE. CON, mice that consumed standard chow; HF, mice that consumed high-fat chow
P< 0.05 HF versus CON
P< 0.05 FED versus FAST
P< 0.05 light phase versus dark phase
Table 2.
Food and water intake, and urine output
| CON | HF | |
|---|---|---|
| Food consumption (g) | ||
| Light phase | 2.3±0.3 | 1.4±0.1* |
| Dark phase | 4.9±0.3‡ | 1.6±0.1* |
| Total caloric intake (kcal) | ||
| Light phase | 6.7±0.8 | 6.8±0.4 |
| Dark phase | 14±0.8‡ | 7.7±0.6* |
| Calories consumed from fat (kcal) | ||
| Light phase | 0.9±0.1 | 3.0±0.2* |
| Dark phase | 1.9±0.1‡ | 3.4±0.3* |
| Calories consumed from carbohydrate (kcal) | ||
| Light phase | 3.6±0.4 | 2.4±0.1 |
| Dark phase | 7.6±0.4‡ | 2.7±0.2* |
| Calories consumed from protein (kcal) | ||
| Light phase | 2.3±0.3 | 1.4±0.1* |
| Dark phase | 4.9±0.3‡ | 1.5±0.1* |
| Water consumption (mL 24 h−1) | ||
| FED | 4.8±0.3 | 2.5±0.2* |
| FAST | 2.4±0.5† | 1.6±0.2† |
| Urine excretion (mL 24 h−1) | ||
| FED | 1.7±0.3 | 0.84±0.1* |
| FAST | 1.1±0.2† | 0.22±0.02*† |
Values are means±SE. CON, mice that consumed standard chow; HF, mice that consumed high-fat chow
P< 0.05 HF versus CON
P< 0.05 FED versus FAST
P< 0.05 light phase versus dark phase
Results
Animal characteristics
Body mass, gonadal fat pad mass, and total fat mass were greater, while lean mass was less, in HF vs. CON mice (Table 1; Fig. 1A, 1B). Fasting triglycerides (mg dl−1) were higher in HF (39.8±2.4) vs. CON (23.2±2.9) mice. Previously we showed this duration of HF feeding to similarly aged C57Bl6 mice impairs glucose tolerance, evokes hyperinsulinemia, increases free fatty acids, and causes endothelial cell but not vascular smooth muscle cell dysfunction.5, 17
Cardiovascular responses
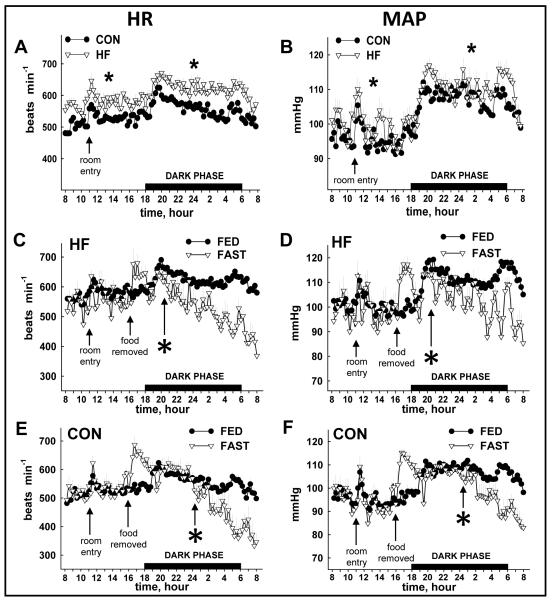
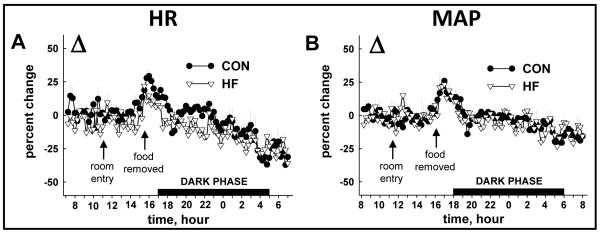
24 h HR and MAP were elevated in HF vs. CON mice during the FED period (Fig. 2A, 2B). While HR and MAP decreased in response to fasting in both groups, the onset was ~ 4 h sooner in HF (Fig.2C, 2D) vs. CON mice (Fig. 2E, 2F). The percent reduction induced by fasting (Δ) was similar between groups for HR (Fig. 3A) and MAP (Fig. 3B), although responses occurred sooner in HF animals.
FIGURE 2.
Heart rate (HR) and mean arterial pressure (MAP) from CON and HF mice during the FED and FAST co nditions. HR (A) and MAP (B) were elevated (*p<0.05) in HF vs. CON mice during the light and dark phase of the FED period. The fasting-induced reduction in HR (C, E) and MAP (D, F) occurred sooner (p<0.05) in HF vs. CON mice. FED values represent mean data from 3 × 24 h periods (i.e., days 1-3); FAST values represent 1 × 24 period (i.e., day 4) that contains a fasting segment from ~1600 to ~0800. Spikes in HR and MAP evoked by room entry for cleaning (~1100) and food removal/cage change (~1600) were not inc luded in the analyses. The “*” indicates the time at which a sustained statistical difference between FED and FAST values was first observed. Values represent mean ± SEM. CON, mice that consumed standard chow; HF, mice that consumed high-fat chow.
FIGURE 3.
Fasting-induced decreases in HR and MAP compared between HF and CON mice. The overall fasting-induced percent change (Δ) in HR (A) and MAP (B) observed from 1600-0800 h was similar between CON and HF mice. Values represent mean ± SEM.
Metabolic responses
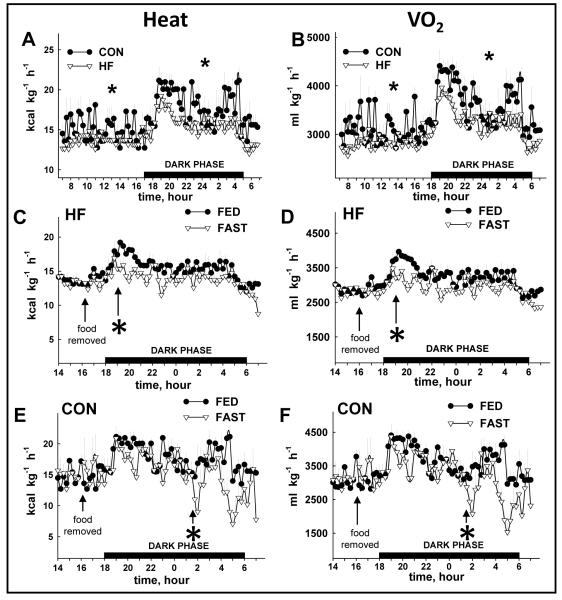
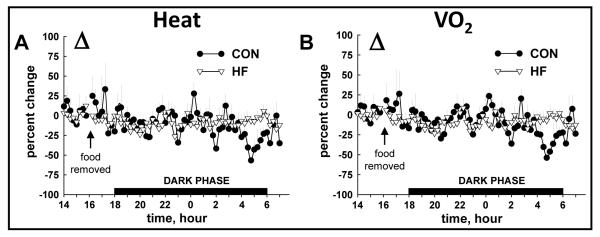
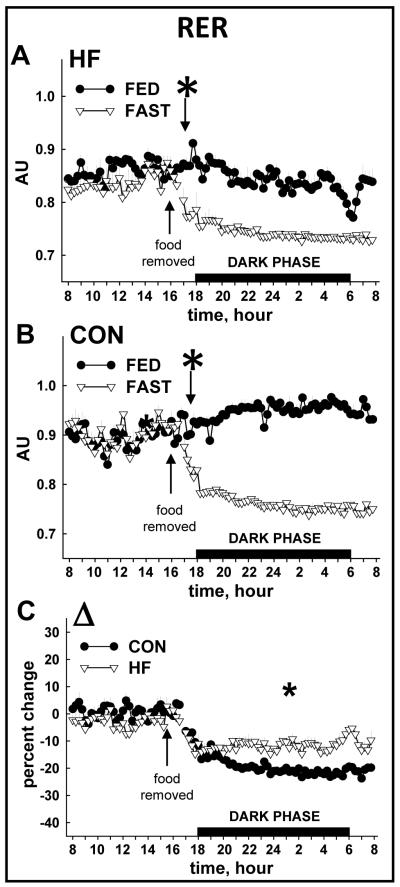
Total 24 h VO2 and heat production were lower in HF vs. CON mice during the FED period (Fig. 4A, 4B; Table 1). While heat production and VO2 decreased in response to fasting in both groups, the onset was ~ 7 h sooner in HF (Fig. 4C, 4D) vs. CON mice (Fig. 4E, 4F). The overall percent reduction induced by a 16 h fast was similar between groups for heat production (Fig. 5A) and VO2 (Fig. 5B). As expected, RER was lower in HF vs. CON mice in both FED and FAST conditions, regardless of diurnal phase, and fasting precipitated decreases of RER in all animals (Table 1; Fig. 6A, 6B). Fasting-induced percent reduction of RER was greater in CON vs. HF mice, which was not surprising considering the lower baseline (i.e., FED) RER in HF vs. CON animals (Fig. 6C). No differences were observed in the tempo of fasting-induced decreases of RER, indicating that the fasting-induced shift of substrate usage occurred similarly in lean and obese animals (Fig. 6A, 6B). Average values for these variables for both groups during the light and dark phases are shown in Table 1.
FIGURE 4.
Heat production (HEAT) and oxygen consumption (VO2) from CON and HF mice during the FED and FAST conditions. Heat production (A) and VO2 (B) were lower (*p<0.05) in HF vs. CON mice during the light and dark phase of the FED period. The fasting-induced reduction in heat production (C, E) and VO2 (D, F) occurred sooner (*p<0.05) in HF vs. CON mice. FED values represent mean data from 2 × 24 h periods (i.e., days 1-2); FAST values represent 1 × 24 period (i.e., day 3) that contains a fasting segment from ~1600 to ~0800. The “*” indicates the time at which a sustained statistical difference between FED and FAST values was first observed. Values represent mean ± SEM. Fasting-induced decreases in heat production (HEAT) and oxygen consumption
FIGURE 5.
( VO2) compared between HF and CON mice. The overall fasting-induced percent change (Δ) in heat production (A) and VO 2 (B) observed from 1600-0800 h was similar between CON and HF mice. Values represent mean ± SEM.
FIGURE 6.
Respiratory exchange ratio (RER) from CON and HF mice during the FED and FAST conditions. The tempo of fasting-induced reduction of RER was not different between HF and CON mice (A, B). Noting the lower baseline (i.e., FED) RER in HF vs. CON mice (A, B), it is not surprising that the overall fasting-induced percent change (Δ) in RER observed from 1600-0800 h on day 3 was greater in CON vs. HF mice. FED values represent mean data from 2 × 24 h periods (i.e., days 1-2); FAST values represent 1 × 24 period (i.e., day 3) that contains a fasting segment from ~1600 to ~0800. The “*” indicates the time at which a sustained statistical difference between FED and FAST values was first observed. Values represent mean ± SEM.
Body temperature, food consumption, body mass, locomotion
Rectal temperatures (°C) during the FED (36.2±0.3 and 36.3±0.4) and at completion of the FAST (36.4±0.3 and 36.5±0.5) period were similar between HF and CON animals, respectively. Fasting-induced reductions in body temperature of ~2-5°C have been observed after 6 h of fasting.12, 14, 24 Therefore, it is curious that we did not observe differences after 16 h of food removal. One explanation is that temperature reductions were minimized/abolished because of increases in skeletal muscle activity secondary to animal restraint that was required to insert the rectal probe.
Food consumption, total caloric intake, and calories consumed from fat, carbohydrate, and protein were similar regardless of the diurnal phase (i.e., light vs. dark) in HF mice. In contrast, all of these variables were greater in the dark vs. light cycle in CON mice (Table 2). While HF mice consumed less food and fewer calories than CON mice during the dark phase, a larger portion of their calories consumed over 24 h was provided from fat, and a smaller portion from carbohydrates and protein (Table 2). No changes in body mass were observed after 2 days in the metabolic chamber (e.g., ad lib feeding; data not shown), but fasting (1600-0800 h on Day 3) reduced body mass by 6.0±0.4% and 12.5±0.6% in HF vs. CON mice, respectively (Fig. 7A). HF mice accumulated more fat and body mass vs. CON animals during their respective 14-week feeding regimens (Fig. 1A, 1B) even though data obtained during the 72 h “snapshot” of metabolic variables at 14-weeks indicated HF mice had less food consumption and caloric intake. While this paradox might partially be explained by decreased metabolic rate and activity in HF vs. CON mice (Table 1), these findings are consistent with previous work done by Lin et al.25 In that study, even though less energy was consumed by fat-fed vs. lean mice at 4-weeks, body mass and adipose tissue mass continued to increase in the former group for an additional 11 weeks. Regarding locomotion, nocturnal (i.e., dark cycle) activity was lower in HF mice upon fasting, whereas fasting did not alter nocturnal activity in lean animals (Table 1).
Water consumption, urine excretion, and urinary catecholamines
In general, HF mice consumed less water and produced less urine per 24 h than CON animals during the FED and FAST period. Water consumption and urine production were less during the FAST vs. FED period for both groups (Table 2). Urinary epinephrine and norepinephrine excretion were similar between HF and CON mice in the FED state. Urinary excretion of both catecholamines was lower in HF vs. CON animals at the end of the FAST period (Table 1).
Leptin
Serum leptin was elevated in HF vs. CON mice in both the FED and FAST conditions (Fig. 7B). Compared to values obtained in the FED condition, fasting-induced reductions in leptin were similar between HF (82.4±0.2%) and CON (86.0±3%) mice.
Discussion
The independent effects of fasting8, 10, 13, 16, 26 and diet-induced obesity17, 27-32 on cardiovascular and metabolic variables in rodents are well known. To our knowledge, specific comparisons of the tempo of fasting-induced reductions in cardiovascular and/or metabolic variables between lean and obese mice have not been made. We hypothesized that fasting would induce a smaller and/or delayed reduction in HR, BP, VO2 and heat production in obese vs. lean mice because the absence of food would pose less of a stress in the former group of animals. Biotelemetry and metabolic chamber studies confirmed that HR and BP were higher, while oxygen consumption, heat production, and locomotion were lower, in obese vs. lean mice in the fed condition. Further, each of these variables decreased in response to a 16 h fast at 22°C. In contrast to our hypothesis, the overall severity of the respective decreases was similar between groups. These data are important for investigators who use murine models of diet-induced obesity because they indicate that the degree of metabolic and cardiovascular adaptations to fasting were similar after 16 h in lean and obese mice.
Cardiovascular and metabolic data were collected at ~ 15-min intervals and averaged hourly. These procedures allowed us to determine the time required for the onset of fasting-induced reductions to become evident, and whether differences existed between groups. Compared to the fed condition, fasting-induced reductions in HR and BP occurred ~ 4 h earlier in obese vs. lean mice. Likewise, VO2 and heat production became statistically lower ~ 7 h earlier in HF vs. CON animals. While the mechanisms responsible for these unexpected findings are unclear, several possible explanations including differences in gender, differential activation of the autonomic nervous system, and altered leptin signaling will be discussed.
First, differences in sex may modulate metabolic responses to fasting. For example, Swoap et al. reported that female mice are more likely to enter a state of fasting-induced torpor than male mice.12 However, gender cannot contribute to our findings because all animals were male. A second possible explanation involves the generalized fasting-induced depression of the sympathetic limb of the autonomic nervous system thought to exist in rodents.13, 33-37 We assessed overall activation of the sympathetic nervous system by measuring urinary epinephrine and norepinephrine following the fed and fasting periods. Fasting-induced reductions of urinary catecholamines occurred in HF but not lean animals. While these data are consistent with the notion that overall sympathetic withdrawal existed to a greater degree in HF vs. CON mice, the exact time when sympathetic activity became lower in HF vs. CON animals cannot be discerned from our data. To accomplish this would have required serial blood sampling throughout the fasting period (rather than urine collection), which is difficult to do in an organism with a small blood volume, i.e., 4% of body weight38 without influencing baroreceptor function. As such, without accurate serial assessment of sympathetic and/or parasympathetic activity, we cannot state with certainty whether differential activation of the two limbs of the autonomic nervous system contributed to earlier reductions in cardiovascular and metabolic variables in obese vs. lean mice.
A third possibility that merits consideration for why reductions in cardiovascular and metabolic variables occurred sooner in HF vs. CON mice is that central and/or peripheral leptin resistance might have developed in the former group of animals. The hormone leptin is secreted in proportion to fat stores. Decreased food intake (e.g., fasting or starvation) results in decreased serum leptin concentration and subsequent decreases in energy expenditure.39 This effect is mediated primarily via leptin signaling in the hypothalamus (i.e., centrally).40 We do not believe central leptin resistance existed in HF vs. CON mice in the present study. Support for this statement is our observation that HF mice, while continuing to gain weight, consumed fewer calories (Fig. 1A, 1B; Table 2). These observations are similar to those reported by Lin et al.25 In that study even though less energy was consumed by fat-fed vs. lean mice after 4 weeks of feeding, body mass and adipose tissue mass continued to increase in the former group for an additional 11 weeks. The authors concluded that hypophagia in the high-fat-fed group likely resulted from an attempt to regulate the rate of adipose tissue accumulation via intact hypothalamic/central leptin signaling. Accelerated decreases in HR and BP in HF vs. CON mice could also be the result of an attempt to regulate energy expenditure via leptin signaling, and thus also suggests intact leptin sensitivity centrally and/or peripherally. However, because neither central nor peripheral leptin signaling was assessed directly in the present study, we cannot state with certainty that the tempo of reductions in VO2, heat production, BP, and HR in obese mice was hastened by factors related to leptin resistance. The ability to make accurate comparisons between lean and obese mice after they have been fasted might be jeopardized if fasting poses a greater/lesser stress on one group vs. another. Our findings indicate that the severity of cardiovascular and metabolic stress evoked by a 16 h fast at 22°C is similar among lean and obese mice. These data indicate that a 16-hour fast is a suitable period after which to study vascular function in models of diet-induced obesity. We also observed that the tempo of fasting-evoked reductions in cardiovascular and metabolic variables is hastened in obese vs. lean animals, although shifts in substrate usage (i.e., from carbohydrate to fat) was not. As such, shorter-term periods of fasting could be confounded by differential rates of metabolic and vascular adaptations, and should be taken into consideration when designing experimental protocols.
Acknowledgments
The assistance of Dr. Robert Cooksey and Deborah Jones (metabolic chambers), and Dr. Andreas Rohrwasser and Elaine Hillas (arterial telemetry) is greatly appreciated. Student support was provided, in part, by the Western States Affiliate of the American Heart Association Summer Student Research Program, the American Physiological Society, and the University of Utah Undergraduate Research Opportunities Program. J.M.T., J.D.S., and D.T.K. designed research; J.M.T., J.D.S., D.T.K., and B.J.K. conducted research; C.S., Z.J., T.Y., E.D.A., and J.D.S. provided essential materials for leptin and catecholamine analyses; J.M.T. and J.D.S. analyzed data and performed statistical analysis, wrote the paper, and had primary responsibility for final content.
Sources of Funding. This work was funded, in part, by the University of Utah College of Health, the University of Utah Research Foundation, American Heart Association Western States Affiliate Grant-In-Aid 06-55222Y, NIH HL 091493-01; and American Diabetes Association Research Grant 7-08-RA-164 (JDS).
Footnotes
Disclosures. None.
REFERENCES
- 1.Symons JD, Gunawardena S, Kappagoda CT, Dhond MR. Volume overload left ventricular hypertrophy: Effects on coronary microvascular reactivity in rabbits. Exp Physiol. 2001;86:725–32. doi: 10.1111/j.1469-445x.2001.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 2.Symons JD, Rendig SV, Fu LW, Longhurst JC. Endothelin-1 limits increases in blood flow to native and collateral dependent myocardium. Am J Physiol. 1997;273:R41–R48. doi: 10.1152/ajpregu.1997.273.1.R41. [DOI] [PubMed] [Google Scholar]
- 3.Symons JD, Firoozmand E, Longhurst JC. Repeated dipyridamole administration enhances collateral-dependent flow and regional function during exercise. A role for adenosine. Circ Res. 1993;73:503–13. doi: 10.1161/01.res.73.3.503. [DOI] [PubMed] [Google Scholar]
- 4.Symons JD, Longhurst JC, Stebbins CL. Response of collateral-dependent myocardium to vasopressin release during prolonged intense exercise. Am J Physiol. 1993;264:H1644–H1652. doi: 10.1152/ajpheart.1993.264.5.H1644. [DOI] [PubMed] [Google Scholar]
- 5.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zang Q-J, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to eNOS in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–94. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duffy PH, Feuers RJ, Hart FW. Effect of chronic caloric restriction on the circadian regulation of physiological and behavioral variables in old male B6C3F1 mice. Chronobiol Int. 1990;7:291–303. doi: 10.1080/07420529009064635. [DOI] [PubMed] [Google Scholar]
- 7.Overton JM, Williams TD, Chambers JB, Rashotte ME. Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol. 2001;280:R1007–R1015. doi: 10.1152/ajpregu.2001.280.4.R1007. [DOI] [PubMed] [Google Scholar]
- 8.Williams TD, Chambers JB, Henderson RP, Rashotte ME, Overton JM. Cardiovascular responses to caloric restriction and thermoneutrality in C57BL/6J mice. Am J Physiol. 2002;282:R1459–R1467. doi: 10.1152/ajpregu.00612.2001. [DOI] [PubMed] [Google Scholar]
- 9.Williams TD, Chambers JB, May OL, Henderson RP, Rashotte ME, Overton JM. Concurrent reductions in blood pressure and metabolic rate during fasting in the unrestrained SHR. Am J Physiol. 2000;278:R255–R262. doi: 10.1152/ajpregu.2000.278.1.R255. [DOI] [PubMed] [Google Scholar]
- 10.Himms-Hagen J. Food restriction increases torpor and improves brown adipose tissue thermogenesis in ob/ob mice. Am J Physiol. 1985;248:E531–E539. doi: 10.1152/ajpendo.1985.248.5.E531. [DOI] [PubMed] [Google Scholar]
- 11.Hudson JW, Scott IM. Daily torpor in the laboratory mouse, mus musculus var. albino. Physiol Zool. 1979;52:205–18. [Google Scholar]
- 12.Swoap SJ, Gutilla MJ. Cardiovascular changes during daily torpor in the laboratory mouse. Am J Physiol. 2009;297:R769–R774. doi: 10.1152/ajpregu.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM. Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol. 2003;30:769–78. doi: 10.1046/j.1440-1681.2003.t01-1-03808.x. [DOI] [PubMed] [Google Scholar]
- 14.Gavrilova O, Leon LR, Marcus-Samuels B, Mason MM, Castle Al, Refetoff S, Vinson C, Reitman ML. Torpor in mice is induced by both leptin-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 1999;96:14623–8. doi: 10.1073/pnas.96.25.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koizumi A, Tsukada M, Wada Y, Masuda H, Weindruch R. Mitotic activity in mice is suppressed by energy restriction-induced torpor. J Nutr. 1992;122:1446–53. doi: 10.1093/jn/122.7.1446. [DOI] [PubMed] [Google Scholar]
- 16.Swoap SJ. Altered leptin signaling is sufficient, but not required, for hypotension associated with caloric restriction. Am J Physiol. 2001;281:H2473–H2479. doi: 10.1152/ajpheart.2001.281.6.H2473. [DOI] [PubMed] [Google Scholar]
- 17.Symons JD, Holland W, Tanner J, Kearns D, Cahoon J, Palionyte M, Duncan B, Losee J, Narra K, Hillas E, Rohrwasser A, Abel ED, Summers S. Inhibiting vascular ceramide synthesis prevents arterial dysfunction and hypertension in mice with diet-induced obesity. FASEB J. 2008;22:743–5. [Google Scholar]
- 18.Makimura H, Mizuno TM, Beasley J, Silverstein JH, Mobbs CV. Adrenalectomy stimulates hypothalamic proopiomelanocortin expression but does not correct diet-induced obesity. BMC Physiol. 2003;3:4. doi: 10.1186/1472-6793-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalili T, Carlstrom J, Kim S, Freeman D, Jin H, Wu TC, Litwin SE, Symons J. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J Cardiovasc Pharmacol. 2006;47:531–41. doi: 10.1097/01.fjc.0000211746.78454.50. [DOI] [PubMed] [Google Scholar]
- 20.Carlstrom J, Symons JD, Wu TC, Bruno RS, Litwin SE, Jalili T. A quercetin supplemented diet does not prevent cardiovascular complications in spontaneously hypertensive rats. J Nutr. 2007;137:628–33. doi: 10.1093/jn/137.3.628. [DOI] [PubMed] [Google Scholar]
- 21.Bek MJ, Wang X, Asico LD, Jones JE, Zheng S, Li X, Eisner GM, Grandy DK, Carey RM, Soares-da-Silva P, Jose PA. Angiotensin II type I receptor-mediated hypertension in D4 dopamine receptor-deficient mice. Hypertension. 2007;47:288–95. doi: 10.1161/01.HYP.0000198427.96225.36. [DOI] [PubMed] [Google Scholar]
- 22.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE, Yang T. Vascular PPARγ controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metabolism. 2008;8:1–10. doi: 10.1016/j.cmet.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang N, Symons JD, Zhang H, Jia Z, Gonzalez FJ, Yang T. Distinct functions of vascular endothelial and smooth muscle PPARgamma in regulation of blood pressure and vascular tone. Toxicol Pathol. 2009;37:21–7. doi: 10.1177/0192623308328545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5:415–25. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24:639–46. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 26.Williams TD, Chambers JB, Gagnon SP, Roberts LM, Henderson RP, Overton JM. Cardiovascular and metabolic responses to fasting and thermoneutrality in Ay mice. Physiol Behav. 2003;78:615–23. doi: 10.1016/s0031-9384(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 27.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang BN, Perlman LV, Epstein FH. Overweight and hypertension: a review. Circulation. 1969;39:403–21. doi: 10.1161/01.cir.39.3.403. [DOI] [PubMed] [Google Scholar]
- 29.Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol. 2004;286:R803–R813. doi: 10.1152/ajpregu.00707.2003. [DOI] [PubMed] [Google Scholar]
- 30.Dustan HP. Mechanisms of hypertension associated with obesity. Intern Med. 1983;98:860–4. doi: 10.7326/0003-4819-98-5-860. [DOI] [PubMed] [Google Scholar]
- 31.Kotsis V, Stabouli S, Bouldin M, Low A, Toumanidis S, Zakopoulos N. Impact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension. 2005;45:602–7. doi: 10.1161/01.HYP.0000158261.86674.8e. [DOI] [PubMed] [Google Scholar]
- 32.Wood JE, Cash JR. Obesity and hypertension: clinical and experimental observations. Ann Intern Med. 1939;13:81–90. [Google Scholar]
- 33.Grassi G, Servalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97:2037–42. doi: 10.1161/01.cir.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda T, Gomi T, Hirawa N, Sakurai J, Yoshikawa N. Improvement of insulin sensitivity contributes to blood pressure reduction after weight loss in hypertensive subjects with obesity. Hypertension. 1996;27:1180–6. doi: 10.1161/01.hyp.27.5.1180. [DOI] [PubMed] [Google Scholar]
- 35.Kushiro T, Kobayashi F, Osada H, Tomiyama H, Satoh K, Otsuka Y, Kurumatani H, Kajiwara N. Role of sympathetic activity in blood pressure reduction with low calorie regimen. Hypertension. 1991;17:965–8. doi: 10.1161/01.hyp.17.6.965. [DOI] [PubMed] [Google Scholar]
- 36.Overton JM, VanNess JM, Casto RM. Food restriction reduces sympathetic support of blood pressure in spontaneously hypertensive rats. J Nutr. 1997;127:655–60. doi: 10.1093/jn/127.4.655. [DOI] [PubMed] [Google Scholar]
- 37.Overton JM, Williams TD, Chambers JB, Rashotte ME. Central leptin infusion attenuates the cardiovascular and metabolic effects of fasting in rats. Hypertension. 2001;37(2):663–9. doi: 10.1161/01.hyp.37.2.663. [DOI] [PubMed] [Google Scholar]
- 38.Hoff J. Methods of blood collection in the mouse. Lab Animal. 2000;29(10):47–53. [Google Scholar]
- 39.Rahmouni K, Haynes WG. Leptin and the cardiovascular system. Recent Prog Horm Res. 2004;59:225–44. doi: 10.1210/rp.59.1.225. [DOI] [PubMed] [Google Scholar]
- 40.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 41.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis HR., Jr Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–90. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP Kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–41. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 43.Mark AL, Correia ML, Rahmouni K, Haynes WG. Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens. 2002;20:1245–50. doi: 10.1097/00004872-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–8. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 45.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–R500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]