Abstract
PTEN hamartoma tumor syndrome (PHTS) presents in a spectrum that encompasses the epononymous disorders, Cowden’s and Bannayan-Riley-Ruvalcaba. Herein, we delineate the distinctive histopathology of a predominantly intramuscular lesion in PHTS, often called “arteriovenous malformation” because of certain imaging and histopathologic features. Cases were identified by review of lesions resected from patients with PHTS registered in our Vascular Anomalies Center and of unusual intramuscular vascular anomalies in our pathology database from 1985 to 2008. Thirty-four patients with this lesion were identified: 20 had a clinical diagnosis of, or were suspected to have, PHTS (genetically confirmed in 16). In 4 patients without clinical manifestations of PHTS, 2 had PTEN mutations, 1 did not, and in another the mutation was intronic. In the remaining 10, there was insufficient clinical information to fully assess whether they had manifestations of PHTS. Lesions manifested by 15 years of age, normally with pain and swelling, most often located in the lower extremity. The major mass was usually intramuscular, but often there were fascial and subcutaneous components and not infrequently a cutaneous vascular stain. MRI generally showed an infiltrative soft tissue lesion involving muscle, fascia and subcutis with frequent enlarged, serpiginous vessels and a prominent adipocytic component. Some lesions involved contiguous muscles and 20% were multifocal. Resected specimens ranged in size from 3–25 cm; in one patient, amputation was necessary.
Histopathologically, these unencapsulated masses, oftentimes with a nodular appearance at scanning magnification, consisted of: 1) variable admixture of mature adipocytic and dense and/or myxoid fibrous tissues (50–90% of surface area); 2) vascular component (10–50% of surface area) with: a) clusters of venous channels, some with excessively and irregularly muscularized complex walls and lumens, and others with thin walls resembling pulmonary alveoli, b) tortuous, thick-walled arteries with concentric muscular hyperplasia and relatively small lumens, c) numerous small vessels (arteries, veins and indeterminate channels), and d) occasional arteriovenous communications; 3) lymphoid follicles (50%); 4) foci of bone (20%); 5) hypertrophic nerves with “onion bulb” proliferation of periaxonal spindled cells (9%).
We designate this disorganized overgrowth of essentially mesenchymal elements, PTEN hamartoma of soft tissue (PHOST). It differs from other vascular and connective tissue lesions that occur in patients with PHTS. PHOST is histopathologically distinctive and its identification should prompt a thorough investigation for PHTS.
INTRODUCTION
For nearly a decade we have observed in young individuals an unusual, benign, mainly intramuscular, soft tissue lesion with a characteristic histopathology. It is composed of an admixture of connective tissue elements, primarily fat, fibrous tissue and abnormal blood vessels. With increasing awareness of the clinical manifestations of germline PTEN mutations, it became apparent that these patients had Bannayan-Riley-Ruvalcaba (BRRS) or Cowden’s syndromes (CS).(27, 53) Herein, we describe the clinical, imaging and distinctive histopathologic features of this lesion which we have termed PTEN hamartoma of soft tissue (PHOST).
MATERIALS AND METHODS
The cases were selected from a search of the departmental files of Children’s Hospital Boston from 1985 to 2005 for specimens previously classified as unusual arteriovenous malformations, intramuscular angiolipomas or vascular hamartomas, and from a review of soft tissue lesions removed from patients with Bannayan-Riley-Ruvalcaba (BRRS) and Cowden’s (CS) syndromes registered in our Vascular Anomalies Center.
The medical records, imaging, pathology reports, and slides were reviewed. Diagnoses of BRRS or CS were made following clinical and/or genetic evaluations according to published criteria.(26) (52) More than half of these patients were tested for PTEN mutations, insertions and deletions, but not for germline epigenetic promoter methylation of KILLIN or succinate dehydrogenase mutations, which have been found in a subset of PTEN negative CS patients.(4, 42)
Routine hematoxylin and eosin stained sections were available in all cases and Miller elastic tissue and Masson trichome were performed in 11 and 10 cases respectively.
As a pilot study, routine immunohistochemical staining for the lymphatic endothelial marker D2-40 was performed on 5 specimens using a streptavidin-biotin-based alkaline phosphatase detection kit (Signet Laboratories, Dedham, MA). Immunostaining for PTEN expression, using a murine monoclonal anti-PTEN antibody (Clone 6H2.1; Cascade Biosciences, Winchester, MA) was performed on paraffin embedded sections of 5 lesions according to a previously described protocol(32) using normal proliferative endometrium as control and compared with 5 cases each of venous, lymphatic and arteriovenous malformations. Two “lipomas” (see next paragraph) were immunostained with an HMGA2 antibody (HMGA2-P1-KLH) (Biocheck, Foster City, CA). Staining was performed in an automatic closed immunostainer (Ventana XT, Tucson, AZ).
Since specimen photographs were available from only a few specimens, a “representative” slide from a specimen in every patient was selected for semiquantitative microscopic assessment of the relative amount of the vascular, adipocytic, fibrous, and myxoid stromal components present.
The histopathology of vascular lesions other than PHOST was also reviewed in the final selected cohort of patients. Five patients with PHOST also had lipomas diagnosed clinically or after resection; their histopathology was not available for review. The histopathology of 2 “lipomas” is presented from a patient recently diagnosed with BRRS.
RESULTS
Clinical Findings
Thirty-four patients with PTEN hamartoma of soft tissue (PHOST), the distinct lesion described herein, were identified (Table 1). The lesions manifested by 15 years of age, usually with pain and swelling. The vast majority occurred in the lower extremity (mostly thigh and calf), followed by the upper extremity (mostly forearm and hand), trunk and head and neck (Figure 1). The bulk of the lesion was usually intramuscular. Fascia, subcutis and dermis were also frequently involved, sometimes more extensively than the muscle. Fifteen patients underwent either biopsy or resection at Children’s Hospital Boston and 19 at other institutions. The series included 22 females and 12 males with age at initial resection ranging from 3 to 42 years (mean 12 years). Four PHOSTs were noted at birth. Before surgery, 8 patients had undergone sclerotherapy, 5 therapeutic embolization, and 7 have received 1 or more anti-angiogenic agents. Twenty patients had a clinical diagnosis of, or were suspected of having, PTEN hamartoma tumor syndrome (PHTS); genetic confirmation was obtained in 16, 2 were negative, and two were not tested. Four other patients who did not manifest the common conditions associated with PHTS were tested for PTEN mutations; 2 had mutations, 1 had an intronic polymorphism and 1 was negative. In the remaining 10 patients, there was insufficient information to determine whether other manifestations of PHTS were present (the majority were consultations without in-house clinical examination of the patients).
Table 1.
Clinical, Pathologic and Genetic Data
| Age at biopsy/Sex | Family History | Clinical History | Other | PTEN mutation status | Location | Size (cm) | Syndrome | |
|---|---|---|---|---|---|---|---|---|
| Patients with clinical manifestations of PTEN hamartoma tumor syndrome | ||||||||
| 1 | 4y/F | Mother negative for PTEN mutation, renal cancer | Macrocephaly, thyroglossal duct cyst | Right ovarian mixed germ cell cell tumor, left ovarian “cavernous hemangioma”, soft tissue tumors occiput & abdomen | PTEN− | Left thigh & calf | 24x10x9 (excision) | BRR/Cowden’s |
| 2 | 5y/F | Breast cancer,brain cancer, liver cancer, renal cancer | Macrocephaly | Unknown | PTEN− | Right knee | 3x2x1(excision) | BRR |
| 3 | 5y/F | Unknown | Macrocephaly, global | Nodule in thyroid (thyroiditis) | R233X, Exon 7 | Left thigh | >20 (imaging) | BRR |
| 4 | 7y/M | Father and brother positive for PTEN mutation | Macrocephaly, multicystic thyroid, penile pigmentation | Squamous papilloma esophagus, colonic ganglioneuromatous polyps | PTEN+ | Left leg | 10.5x5x2.5 (excision); 7x4.5x2.5 (excision) | BRR |
| 5 | 8y/M | Parents negative for PTEN mutations | Penile pigmentation, intracranial developmental venous anomaly | Thyroid carcinoma, rectal juvenile polyps, cutaneous storiform collagenoma, plantar fibromatosis | c.499_505del7, Exon 6 | Right pelvis, thigh and calf | 22x9x3.5; 12x7x3; 15x11x5; 4.5 (debulking) | BRR |
| 6 | 8y/M | Unknown | Macrocepahly, genital freckles | Unknown | PTEN+ | Left foot | <2 (excision) | BRR |
| 7 | 8y/M | AVMs, Hashimoto | Macrocephaly, penile pigmentation, intracranial developmental venous | Lipoma groin, “hemangioma” of ankle | A34D, Exon 2 | Left hand | 4x3x1(imaging) | BRR |
| 8 | 9y/M | Parents negative for PTEN mutation | Macrocephaly, hypothyroidism, hearing loss, epidermal nevus right leg | Lipomatous masses in trunk and arms, mesentery, kidney. Splenic, hepatic, and left neck masses of uncertain nature | R335X germline, Exon 8; R130X somatic (in PHOST) | Entire right lower extremity and right pelvis | Entire right lower extremity and right pelvis (imaging, amputation) | BRR (Proteus-like) |
| 9 | 10y/F | Negative for cancer | Macrocephaly, hemithyroidectomy for | Vascular abnormality of right humeral cortex | Unknown | Right leg | 2.8x1.3x0.5 (excision) | BRR |
| 10 | 11y/F | Macrocephaly, lipomas, hyperthyroidism, borderline mental retardation, PTEN | Macrocephaly, cafe au lait spots, Graves disease, seizures, | lipomas, left chest “AVM” and fast- flow lesion left foot | IVS3+1G-A, Intron 3 | Left thigh and calf | 14x5.3x5.2 (excision) | BRR |
| 11 | 11y/F | Thyroid cancer, breast cancer, lung cancer | Intracranial developmental venous anomaly | Breast tubular adenomas | PTEN+ | Left chest wall, left periclavicular region, right forearm | 3x2.6x2.2 (excision);1.2 (excision); 3.5 (excision) | BRR/Cowden’s |
| 12 | 12y/F | Unknown | Macrocephaly | Unknown | Unknown | Left gastrocnemius | 2.3x1.5x1.2 (excision) | Unknown |
| 13 | 13y/F | See #13 | Unknown | Colonic juvenile polyposis | PTEN+ | Bilateral thigh | 10x7 (imaging) | Cowden’s |
| 14 | 13y/F | Prostate cancer, pancreatic cancer, thyroid disease, father negative for PTEN mutation | Macrocephaly, goiter | Papillary thyroid carcinoma, breast tumors, colonic juvenile polyposis and ganglioneuromatosis, cutaneous papillomas, lipomas and cherry angiomas | IVS6-1, G-C | Left leg | 8x3x2 (imaging) | Cowden’s |
| 15 | 16y/M | Macrocephaly | Macrocephaly, Chiari I | Lipoma ankle | R130X, Exon 5 | Right thoracic paraspinal | 14x10x3 (excision) | BRR |
| 16 | 16y/F | See #13 | Colonic juvenile polyposis | PTEN+ | Left thigh | >6 (excision) | Cowden’s | |
| 17 | 17y/F | Macrocephaly | Macrocephaly, learning disability, prominent incisors, long fingers, cutaneous | Lipoma, ovarian cystadenofibroma, ovarian juvenile granulosa cell tumor, | L108P, Exon 5 | Right knee | 4.9x3x1.6 (excision) | BRR/Cowden’s |
| 18 | 18y/F | Unknown | Hyperthyroidism | Soft tumors removed from left breast, thumb, malleolus | PTEN+ | Right face, left knee | 9x7x1.2 (excision);unknown | BRR |
| 19 | 42y/M | Cowden’s; father of patients | Unknown | Lipomas, colonic juvenile polyposis | PTEN+ | Right upper arm | 13x5.4x4 (excision) | Cowden’s |
| 20 | 53y/F | Prostate cancer, early breast cancer, vascular anomaly | Multinodular goiter, intracranial developmental venous anomalies | Tricholemmomas face, bilateral breast carcinoma (BRCA2+), ovarian carcinoma | Promoter VUS 903 G/A | Left face and masseter, lip | 4.5x4.5x2.5(excision); 2.7x2.3x1.3 (excision) | Cowden’s |
| Patients with genetic testing but no clinical manifestations of PTEN hamartoma tumor syndrome | ||||||||
| 21 | 9y/F | None | None | None | PTEN− | Right thigh | 6.7x4.8x2.4 (excision); 10.5x6x3.2 | None |
| 22 | 12y/F | Glaucoma | Unknown | Retinoblastoma | PTEN+ | Right forearm and hand | 8.5x5x2 (excision); 9.4 (excision) | Unknown |
| 23 | 14y/M | AVM | Head circumference 90%ile | Unknown | VUS Intron | Right flank | 25x3.8x2 (excision) | BRR |
| 24 | 19y/M | Unknown | Unknown | Dermatofibroma | IVS7+1G>T, Intron 7 | Left face and neck, Left elbow | 5x3x1.5 (excision); 5x3x1.5 (excision) | BRR |
| Patients with incomplete clinical data and no genetic testing | ||||||||
| 25 | 7y/F | Unknown | Unknown | Unknown | Unknown | Left thigh | 6x4x3 (imaging) | Unknown |
| 26 | 9y/M | Unknown | Unknown | Unknown | Unknown | Right thigh | 22x6.5x3.4 (excision) | Unknown |
| 27 | 9y/F | Unknown | Unknown | Unknown | Unknown | Right forearm, back, chest | >10(forearm;clinical) | Unknown |
| 28 | 11y/M | Unknown | Unknown | Unknown | Unknown | Right thigh | 2.5x2.5x1 (excision) | Unknown |
| 29 | 11y/M | Unknown | Unknown | Unknown | Unknown | Right thigh (2 masses) | 18x18x3.5 (excision); 13x7x2.6 | Unknown |
| 30 | 12y/F | Unknown | Unknown | Unknown | Unknown | Right forearm, hand | 9x5x1 (excision) | Unknown |
| 31 | 12y/F | Unknown | Unknown | Unknown | Unknown | Left foot | 9.5x5.5x4 (excision) | Unknown |
| 32 | 16y/M | Unknown | Unknown | Unknown | Unknown | Right hand and forearm | 2.5 (excision); 4 (excision) | Unknown |
| 33 | 18y/F | Unknown | Unknown | Unknown | Unknown | Left forearm | 6.5x2x1 (excision); 5.3x1.8x0.8 | Unknown |
| 34 | 19y/F | Unknown | Asthma, obesity | Unknown | Unknown | Left buttock | 4.8 (excision) | Unknown |
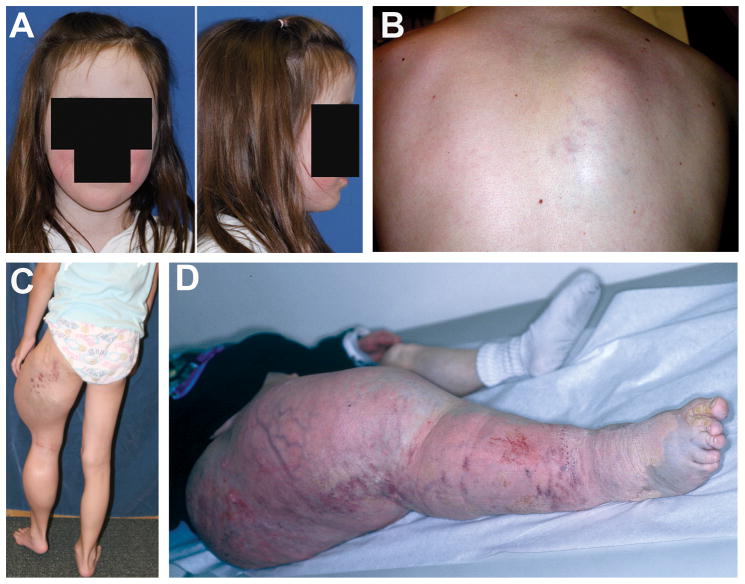
Figure 1.
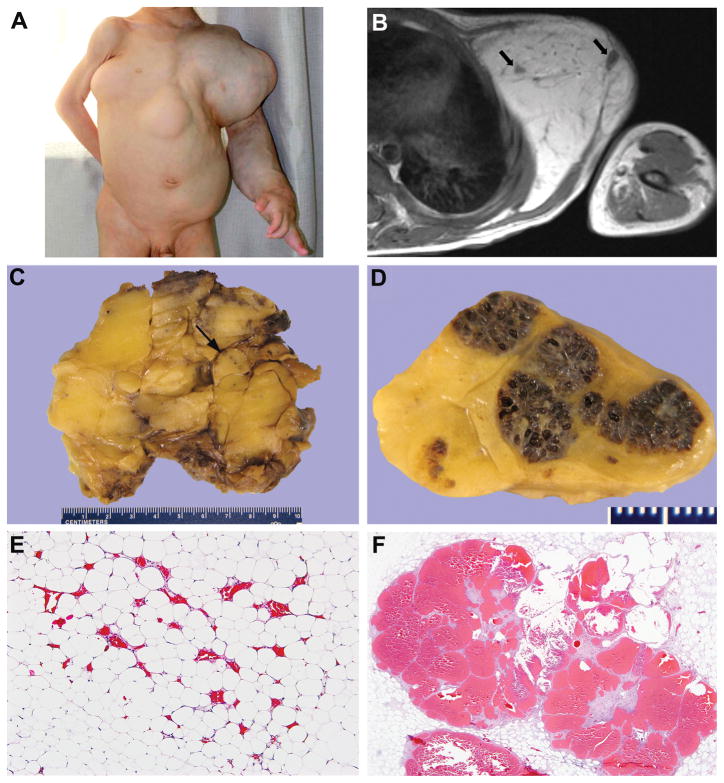
Clinical features. A. Six year old girl with BRRS: note macrocephaly with square cranium and broad, high forehead. B. Adolescent with BRRS and PHOST in right posterior paraspinal musculature. Note blotchy cutaneous stain. C. Boy with BRRS and large, primarily intramuscular PHOSTs of left thigh and calf. Note extensive cutaneous stain and prominent veins in thigh. D. Boy with germline inactivation of both PTEN alleles and massive overgrowth of right lower extremity and pelvis. Note cutaneous stain, prominent veins, and epidermal nevus over dorsum of foot and 1st and 2nd toes.
Imaging Findings
Radiographic imaging was available in 31 patients. MRI evaluations documented T1-contrast-enhancing and T2-hyperintense, irregularly-shaped vascular lesions (Figure 2A,B). Most PHOSTs were primarily intramuscular with a dilated, serpiginous vascular component. Angiography showed small arteriovenous fistulae with a draining varix (disproportionate venous dilatation) (Figure 2C). A variable adipocytic component, frequently bulky, was noted in many. Lesions often extended through tissues planes sometimes disrupting underlying cortical bone. Some PHOSTs involved contiguous or non-contiguous muscles and at least 18% were multifocal. In some instances, it was not possible to determine if the lesions were contiguous or multifocal. A developmental venous anomaly was found in 3 of 6 patients who had an MRI of the brain.
Figure 2.
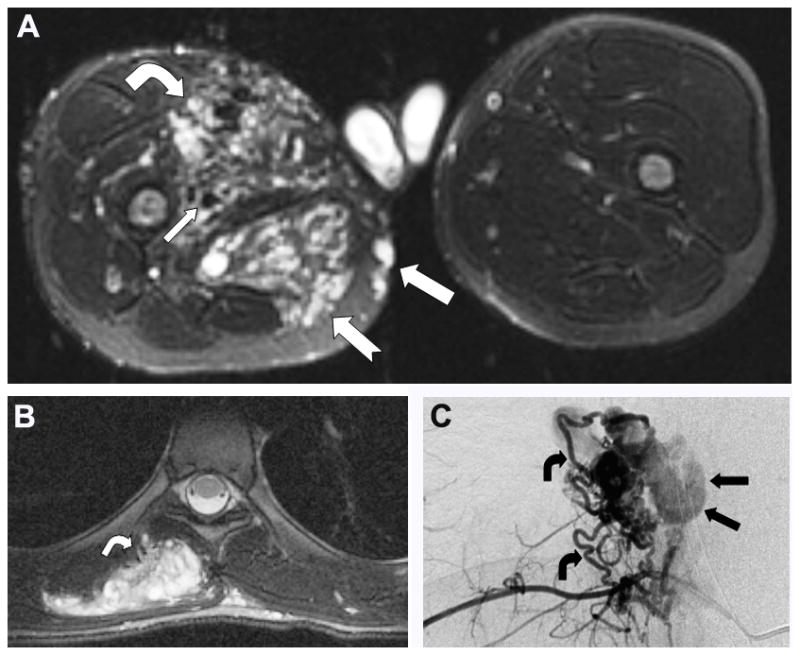
Imaging features. A. Extensive PHOST of right thigh in boy with BRRS. Infiltrative heterogeneous lesion involving vastus medialis muscle (bent arrow) with increased arterial supply (small arrow). Less hypervascular lesion in semimembranosus and gracilis muscles (notched arrow). Note venous nodules in subcutaneous layer of medial thigh (arrow). (Axial fat saturated T2-weighted MR image). B. PHOST in patient depicted in 1B showing heterogenous high signal intensity and hypervascularity evident by enlarged arterial feeders at periphery of lesion (arrow). (Axial T2-WI MR image with fat saturation) C. Selective right 8th intercostal arteriogram in lesion depicted in B demonstrates hypervascular mass with multiple tortuous feeding arteries (bent arrows) and intratumoral shunting into dilated veins (straight arrows).
Operative Findings
At operation, the lesions were hard and multilobulated. They did not have a distinct capsule but could be separated from adjacent structures with blunt dissection. They often involved the muscles and subcutaneous tissue simultaneously and typically surrounded the neurovascular structures. In the subcutis, they appeared as vascular clusters, usually having 1 or 2 nutrient arteries and a large draining vein. The clusters were connected by fibrous tissue and surrounded by abnormal darker fat.
Pathologic Findings
Gross
A total of 48 PHOSTs from the 34 patients were reviewed. Excluding needle and incisional biopsies, the 34 specimens ranged in size from 1.2 to 25 cm (mean, 9.1 cm; median, 6.6 cm); 1 specimen was an amputated lower extremity. PHOSTs were unencapsulated and usually poorly demarcated from the surrounding skeletal muscle and subcutaneous fat. They consisted of a variable admixture of fat, fibrous tissue, vascular clusters and large veins; in some, the adipocytic component was prominent (Figure 3). The patient with intractable pain, mass effect, and cardiac overload who required amputation had marked enlargement of the lower extremity with diffuse involvement of all tissue planes including skin, subcutaneous tissue, skeletal muscle and bone(59) (Figures 1D and 3D).
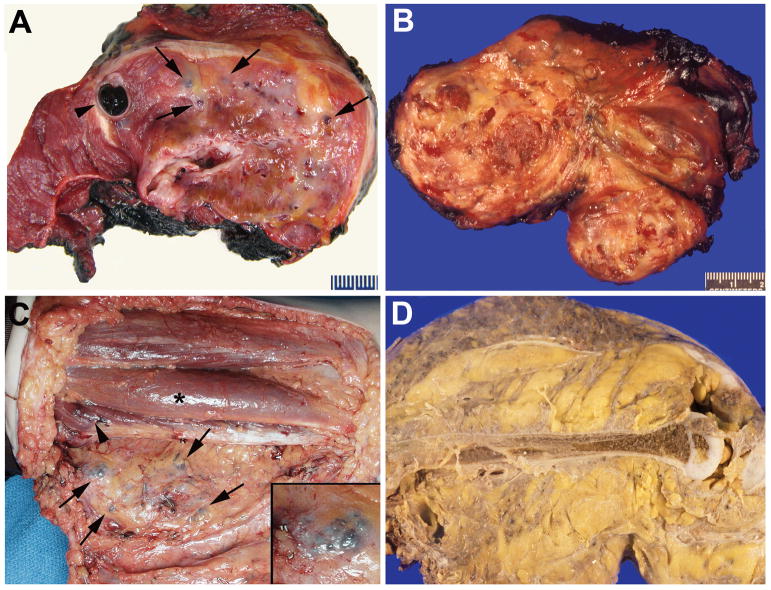
Figure 3.
Macroscopic appearance. A. Intramuscular PHOST with minimal amount of fat (yellow), fibrous tissue (white-gray), vascular nodules (arrows), and massive draining channels, one containing clotted blood (arrowheads). B. Intramuscular PHOST with abundant adipose tissue and vascular nodules (patient in 1B). C. Reflected flap of posterior thigh exposing vascular nodules (arrows) and fatty overgrowth in subcutis. One nodule at higher magnification in inset. Adjacent muscles involved by visible (arrowhead) and non-visible PHOST under bulging surface (asterisk) (patient in 2A). D. Amputation specimen showing PHOST diffusely involving all tissue planes by fibroadipose tissue and blood vessels (patient in 1D).
Histopathology
The lesions were composed of a variable admixture of adipose tissue, dense and/or myxoid fibrous tissue and abnormal vessels involving skeletal muscle, fascia, subcutaneous fat, occasionally dermis and rarely bone (Figure 4). The tissues appeared mature without cytologic atypia. Mitoses were uncommon and there was no necrosis. The vascular component occupied 10–50% (average 30%) of the lesion and the nonvascular component 50–90% (average 70%). Adipose tissue was the most prevalent nonvascular component, ranging from 1–65% (average 35%), followed by dense fibrous tissue 5–65% (average 30%), and myxoid stroma 0–30% (average 5%). When the subcutis was involved, it was usually not possible to distinguish the adipocytic component from normal subcutaneous fat.
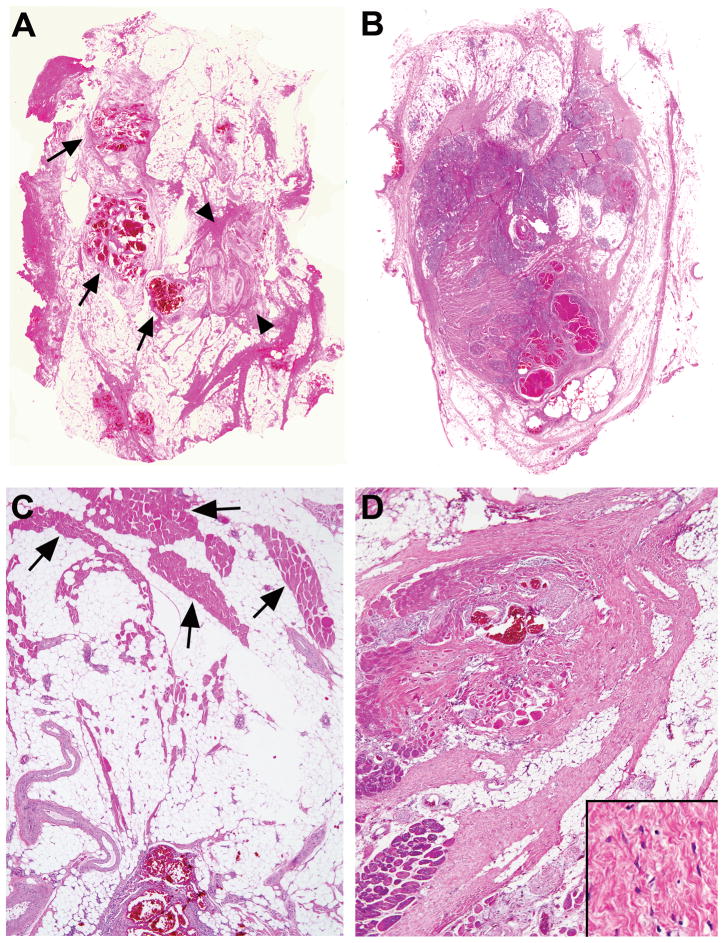
Figure 4.
Typical architecture at scanning magnification. A. Dominant adipocytic component containing clusters of thin-walled veins (arrows), and thick-walled tortuous arteries (arrowheads). B. Equally abundant fibrous and adipocytic components with clusters of abnormal venous structures and myxoid nodules with small vessels. C. Adipose tissue separating bundles of skeletal muscle (arrows). D. Fibrous component dense, broad, and band-like, intersecting adipose tissue and skeletal muscle and surrounding abnormal veins. Inset showing paucicellular fibrous component at high magnification.
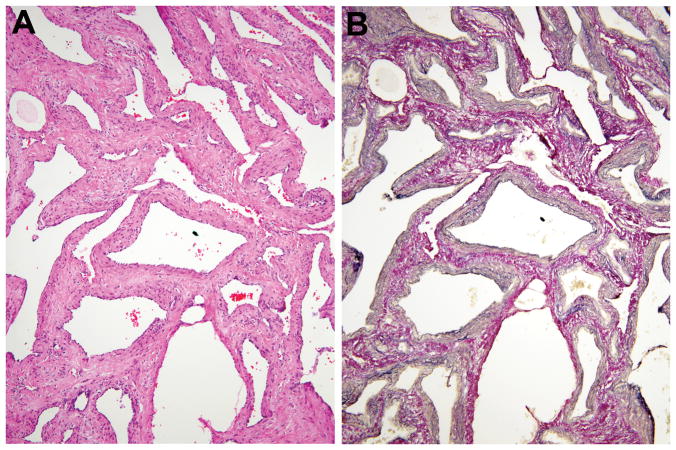
At scanning magnification, the venous blood-filled nodules were usually the most noticeable (Figure 5A). These venous structures varied from clusters of back-to-back thin-walled dilated channels with absent smooth muscle mimicking pulmonary alveoli (Figures 5B,C) to those with complex asymmetric walls having a thin to markedly thick or nodular smooth muscle coat (Figures 5D,E).
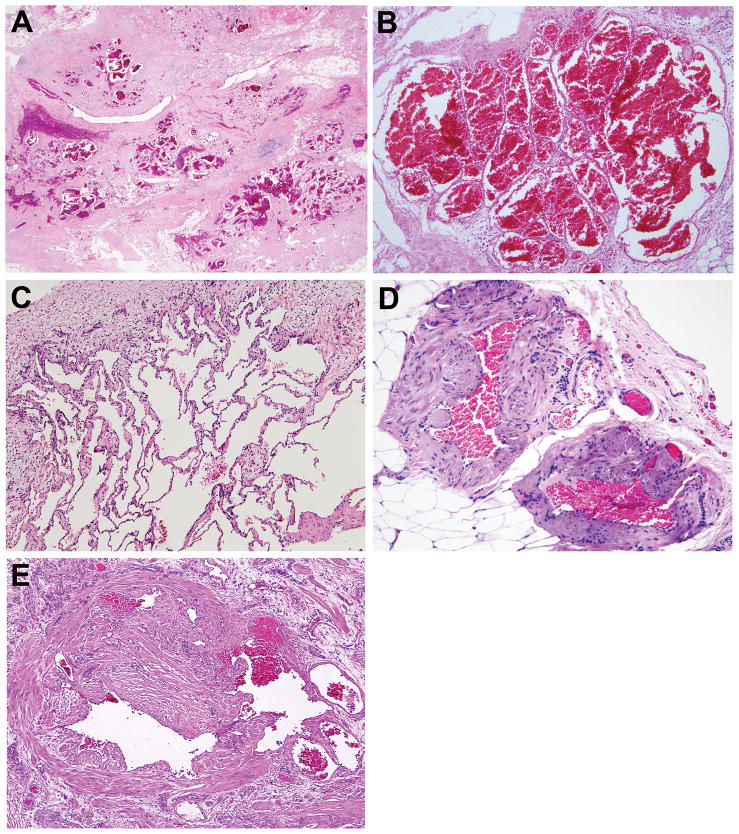
Figure 5.
Venous component. A, B, C. Conspicuous well-circumscribed clusters of blood-filled or empty, back-to-back, thin-walled channels. Superficial resemblance to pulmonary alveoli when empty. D, E. Some abnormal veins have uneven and striking excess of smooth muscle and highly irregular and complex lumens.
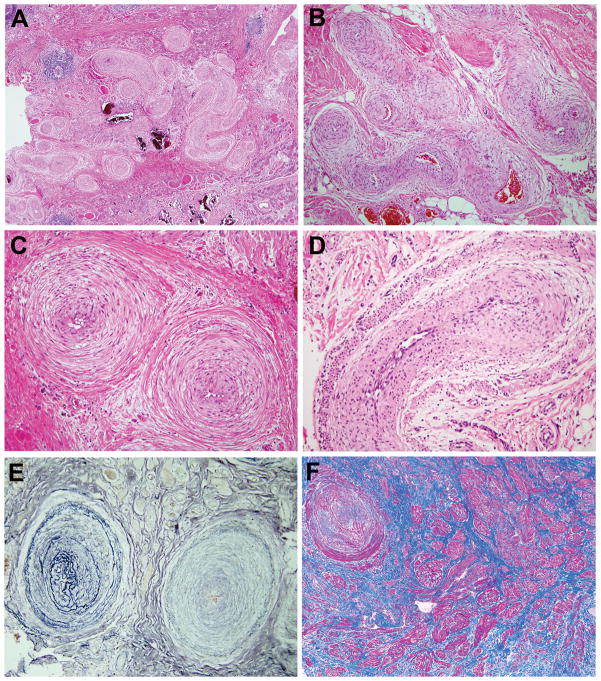
Although the arterial component was less obvious than the venous, its morphology was characteristic. It consisted of medium-sized coiled, tortuous or curved arteries with marked concentric intimal and/or medial smooth muscle hyperplasia, frequently arranged in clusters (Figures 6A–D). Usually, the lumens were markedly narrowed. The arterial elastic constituent was often altered with increased or diminished lamellation (Figure 6E). Occasionally, haphazardly arranged fascicles of smooth muscle without obvious connection to blood vessels were seen (Figure 6F).
Figure 6.
Arterial component. A, B, C. Aggregate of coiled thick-walled arteries with tiny lumens. Concentric smooth muscle hyperplasia often involving intima, media and adventitia. D. Artery with two smooth muscle layers, inner one being intimal. E. Replication and abnormal conformation of elastic laminae (Miller elastic stain). F. Smooth muscle fascicles (stained red) with no apparent vascular connection (Masson trichrome stain).
Small vessels, either dispersed or clustered, were always present. Their morphology was reminiscent of arteries or veins, but was often indeterminate. The indeterminate vessels generally had relatively thick walls with a variable amount of smooth muscle and collagen (Figure 7A,B). Nodules of myxoid stroma containing arteries with numerous “sprouts” were characteristic (Figure 7C).
Figure 7.
Small vessel component. A. Cluster of indeterminate vessels with prominent smooth muscle reminiscent of arteries. B. Small vessels reminiscent of veins dispersed within adipose tissue. C. Small vessels within nodules of myxoid stroma.
In some PHOSTs there were foci of closely apposed large arteries, veins and channels with indeterminate morphology. These latter vessels had walls with muscular and elastic components that transitioned from those of artery-to-vein and presumably were direct communications between the two (Figures 8A,B). There was usually a vicinal component of small vessels (vide supra), as well as large tortuous venous or thin-walled indeterminate channels with minimal or absent elastic fibers or smooth muscle. Lymphatics were inconspicuous in the intramuscular component except in 2 lesions in which there were several clusters of abnormally dilated and thin-walled channels. In some cutaneous specimens, superficial dermal lymphatics were dilated, likely a consequence of obstruction.
Figure 8.
Arteriovenous shunts. A. Arteries transitioning to veins. B. Attenuation and focal loss of internal elastic lamina (stained black) (Miller elastic stain of a contiguous section of A).
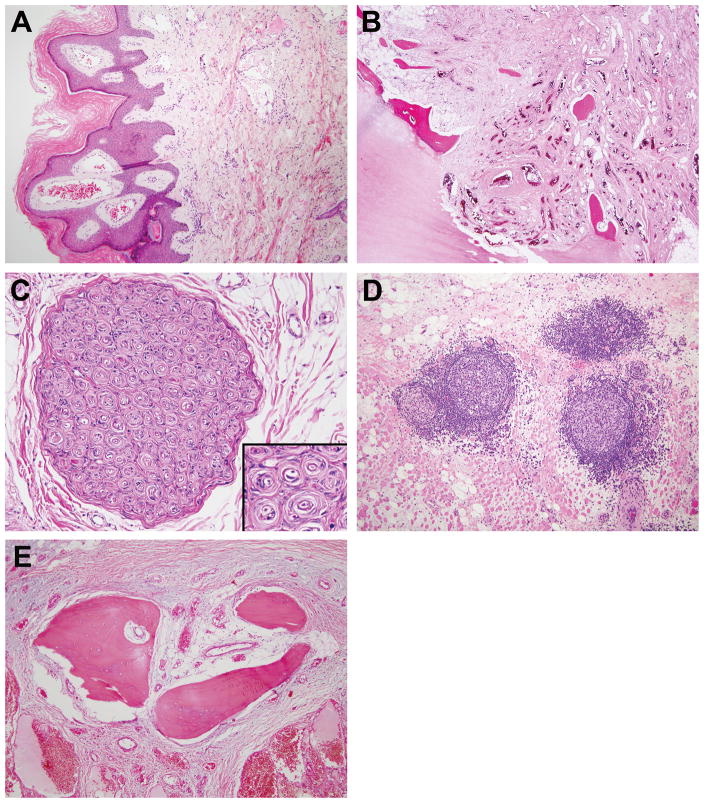
Specimens with a cutaneous capillary malformation-like stain showed dermal and subdermal thin-walled dilated channels, often in clusters and sometimes accompanied by verruciform epidermal hyperplasia (Figure 9A). Only 1 patient had significant intraosseous involvement (amputation specimen), characterized by arteries, veins, and indeterminate vessels within a fibrous stroma (Figure 9B). Nerves sometimes were enveloped by the dense fibrous component of PHOST and on occasion, enlarged arteries and veins were present around and within the fascicles of the nerves themselves. Perineurial hyperplasia was sometimes seen. Three PHOSTs had abnormal nerves with periaxonal spindled cell proliferation resulting in “onion bulb” formation reminiscent of hypertrophic neuropathy or intraneural perineurioma (Figure 9C); however, immunohistochemical stains were not available to distinguish between Schwann and perineurial cells. Lymphoid aggregates and/or lymphoid follicles (often with germinal centers), as well as plasma cells, were observed in all specimens (Figure 9D). Foci of metaplastic bone, usually small, were present in 7 specimens (Figure 9E) and psammomatous calcifications, with or without multinucleated giant cells, were seen in 2.
Figure 9.
Additional features. A. Cutaneous verruciform epidermal hyperplasia and thin-walled venous channels in papillary and reticular dermis. B. Small vessel component within bone showing medullary and cortical disruption and trabecular dissolution (patient in 1D). C. Proliferation of periaxonal spindled cells in nerve imparting onion bulb appearance reminiscent of hypertrophic neuropathy or intraneural perineurioma. D. Lymphoid aggregates and follicles within adipose tissue and skeletal muscle. E. Island of lamellar and woven bone.
Immunohistochemistry
D2-40 immunostaining of PHOSTs showed only occasional lymphatic channels. PTEN immunohistochemistry showed strong nuclear and cytoplasmic expression in all lesional and non-lesional cells. No clear distinction was apparent between these PHOSTs and controls (venous, arteriovenous, and lymphatic vascular malformations).
Adipocytic and other vascular lesions
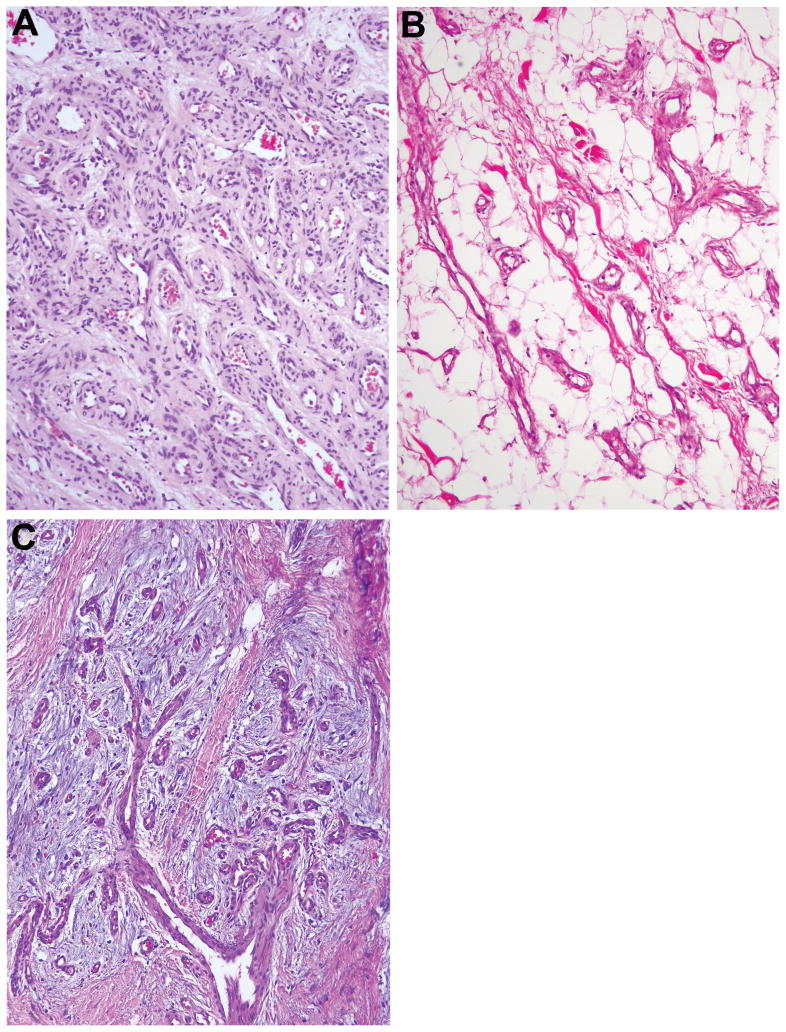
Adipocytic lesions in a boy with BRRS are depicted in Figure 10A–F. Two resected lesions were composed of adipose tissue and coarse fibrous septa, some containing interstitial mucin. Adipocytes varied in size and occasionally had nuclear and cytoplasmic vacuoles. The left axillary mass also had small foci of dilated abnormal venous channels dispersed among the adipocytes (Figure 10E) or clustered in grossly visible nodules (Figure 10C,D,F). The adipocytes and stromal cells in these 2 lesions were immunonegative for HMGA2.
Figure 10.
A. “Adipocytic” bilateral axillary and abdominal wall masses in boy with BRRS. Left forearm and hand with adipocytic overgrowth, high flow lesion, and prominent veins (by imaging). B. Left axillary mass well-defined and hyperintense containing small venous clusters (arrows). (Axial T1-weighted MR image). C. Cross section of left axillary mass showing large adipocytic lobules and occasional abnormal venous clusters (arrow). The resected substernal subcutaneous mass consisted of adipose tissue only. D. Magnified view of left axillary mass showing well-circumscribed nodules of abnormal veins with cribriform appearance (part of lesion not shown in C). E. Focus of small dilated veins among fat cells. F. Discrete area with back-to-back venous channels with thin walls.
Two patients with PHOST had other vascular anomalies removed: one had a cranial dural arteriovenous fistula and another had a small cutaneous lymphatic malformation.
DISCUSSION
PTEN hamartoma tumor syndrome (PHTS) comprises a spectrum of uncommon autosomal dominant disorders associated with germline mutations in the PTEN tumor suppressor gene on 10q23.3. The well-characterized disorders in this spectrum are Bannayan-Riley-Ruvalcaba syndrome (BRRS) and Cowden’s syndrome (CS).(26) Mutational analysis has documented germline PTEN alterations in approximately 70% of BRRS and 80% of CS patients.(34–36, 60) There is also clinical overlap between BRRS and CS with occasional individuals having characteristics of both.10 Identical mutations can occur in both disorders and individuals with BRRS or CS features have been identified in the same family.(26) In some PTEN mutation-negative individuals categorized as CS and CS-like, promoter mutations, promoter methylation affecting expression of the adjacent gene, KILLIN, or mutations encoding mitochondrial succinate dehydrogenase B or D subunits have been identified.(4, 42, 60)
BRRS (OMIM 153480) most commonly manifests as macrocephaly, developmental delay, penile lentigines, and benign lesions of primarily mesodermal origin, such as enteric ganglioneuromatous polyps, lipomas, and variously designated and not well-characterized vascular lesions. CS (OMIM 158350) is associated with multiple mucocutaneous, soft tissue and visceral hamartomas/tumors, usually manifesting by late adolescence.(52) Mucocutaneous lesions are pathognomonic, and include multiple trichilemmomas, palmar/plantar keratoses, and facial and oral papillomatoses.(26) Multiple “hamartomas”, involving all germ cell layers can occur and include gastrointestinal polyps, mammary fibrocystic disease, subcutaneous lipomatous lesions, and cerebellar dysplastic gangliocytomas (Lhermitte-Duclos disease).(26) Benign tumors include breast papillomas, thyroid adenomas, uterine leiomyomas, and subcutaneous lipomas. Individuals with BRRS and CS carry an increased risk for malignancy, particularly of breast, thyroid, endometrium and kidney.(26) Expanding the genotype-phenotype spectrum of PHTS, individuals having inactivated germline mutations of both PTEN alleles and variant phenotypes, have been reported.(6, 8) This is the situation of 1 patient in our study who was incorrectly reported as Proteus-like syndrome.(59)
PHTS is associated with “vascular” lesions primarily in soft tissue and skin, but also in bone, viscera, and central nervous system. Various designations have been used to describe them including hemangioma,(17, 19, 22, 25, 30, 37, 49) cavernous hemangioma,(10) hemangioma/arteriovenous malformation,(56) arteriovenous malformation,(2, 6, 13, 14, 22, 30, 40, 46, 51, 55, 60) vascular malformation,(14) fibrovascular hamartoma,(44) angiokeratoma,(10) lymphangioma,(2) lymphatic malformation,(6) lymphangiokeratoma,(13) lymphangioma/lymphangiomyoma/lymphangioleiomyoma,(31) admixed hemangioma/angiolipoma,(31) lipolymphangiohemangiomas,(2) hamartoma with lipomatous hemangiomatous and lymphangiomatous components,(10) and complex vascular malformation with lipomatous cavernous and capillary hemangiomatous components.(37) However, it is difficult to fully assess their characteristics since the majority lack histopathologic documentation and the few cases with illustrations contain only 1 or 2 photomicrographs.(2, 19, 31, 49, 59)
We characterize a distinctive soft tissue lesion highly associated with the PHTS and termed it PTEN hamartoma of soft tissue (PHOST). More than one half of the patients were clinically diagnosed or suspected to have either BRRS or CS, and a germline PTEN mutation was present in 85% of them. In most of the remaining patients, clinical information was insufficient to assess for all possible manifestations of these disorders. In 2007, we reported the clinical and imaging features of 27 patients with confirmed PTEN mutation; vascular anomalies, predominately fast-flow, were identified in 56% of them (86% intramuscular).(53)
PHOST is an overgrowth of mingled soft tissue elements, primarily adipose, dense and myxoid fibrous tissues and abnormal blood vessels of various types. While one or more of these elements are found in other vascular anomalies, it is their combination and unusual morphology that makes PHOST distinct. A lymphoid component, foci of metaplastic bone, and hypertrophic nerves with “onion bulb” formation (the latter 2 present only in some specimens), are also helpful in its recognition.
While we have termed PHOST a “hamartoma”, disproportionate growth relative to the patient might not be included in the most stringent definition of hamartoma. In the absence of clonality studies it remains speculative if PHOST should be considered a hamartoma or a benign neoplasm.
The histopathological complexity of PHOST is reflected by the various diagnosis rendered at other institutions of cases submitted for consultation, which were: intramuscular or fibrous “hemangioma” (n=10), “angiomatosis” (n=4), arteriovenous malformation (n=3) and non-definitive (n=2). The differential diagnosis of PHOST includes arteriovenous, venous, and other vascular anomalies.(18) In arteriovenous malformation, the abnormal arteries, veins and indeterminate vessels are usually intermixed with less nodular segregation, the arteries are not as coiled or tortuous, their lumens are not as narrowed, muscular hyperplasia is generally confined to the media and the characteristic nodular venous clusters are lacking. Extensive involvement of a limb by PHOST may raise the possibility of Parkes Weber syndrome. In this fast-flow disorder, there is congenital skeletal and muscular overgrowth of an entire limb with diffuse pink macular cutaneous staining. The imaging and histopathology are that of a diffuse arteriovenous malformation.(39) In venous malformation, the most common intramuscular vascular anomaly, abnormal thin- and thick-walled veins, sometimes with a nodular distribution, are present; however, abnormal arteries are absent.(23) Some venous malformations, so-called ossified intramuscular hemangioma, have abundant bone formation, but they lack other characteristics of PHOST.(11) Adipocytic and fibrous components in arteriovenous and venous malformations are seen, but usually, in contrast to PHOST, they are minor. The 3 types of intramuscular “hemangioma” described by Allen and Enzinger may be considered in the differential diagnosis of PHOST. The “small-vessel” type has characteristic lobules of small vessels with capillary morphology that do not occur in PHOST. We are of the opinion that the “large-vessel” and “mixed” types are venous malformations, although some might be PHOST (vide infra).(1) Lymphatic malformations are rarely intramuscular, and usually have thinner walls, contain proteinaceous material, lymphocytes, a few macrophages and generally no blood. An increase in fibroadipose tissue and lymphoid aggregates or follicles is frequently seen, but abnormal arterial or venous components are absent. The clinical and imaging picture is distinct in Klippel-Trenaunay syndrome, the combined capillary-lymphatic-venous malformation.(39) The capillary component typically lies along the lateral aspect of the leg in the distribution of the anomalous vein of Servelle. Histopathologically, the large veins with irregular smooth muscle hyperplasia may mimic the venous component of PHOST; however, the cutaneous and subcutaneous lymphatic component is dominant in Klippel-Trenaunay syndrome, the subcutaneous fibroadipose tissue overgrowth is diffuse and usually uniform, the skeletal muscle is almost never involved, and there is no abnormal arterial component. Enlarged feeding arteries and draining veins of congenital or infantile hemangioma may bear a superficial resemblance to PHOST, but they present at birth or early infancy, respectively, have characteristic capillary lobules and are virtually never intramuscular.(5, 12)
We have encountered case reports, as well as cases in large series of intramuscular or soft tissue vascular lesions that are reminiscent of PHOST. Some lesions reported as “infiltrating angiolipoma” of muscle and soft tissue, intramuscular hemangioma, complex vascular malformation and angiomatosis could in fact be PHOST.(1, 3, 16, 28, 48, 54)
The adipocytic lesions which commonly occur in PTHS deserve comment.(2, 21, 26) They can be single or multiple, encapsulated or infiltrative, some reaching extraordinary prominence. While some are composed exclusively of adipocytes and are called lipoma, others contain a minor vascular component, described as angiomatous, lymphangiomatous and lymphangiomyomatous.(2, 31) The illustrations of the angiomatous component in these reports are similar to the venous component of PHOST. One patient in our study also had a “lipoma” with abnormal venous clusters similar to those in PHOST. This combination of components is another manifestation of the connective tissue overgrowths in PTHS and probably contributes to the diverse terminology applied to these lesions. In contrast to ordinary lipomas which are immunopositive for HMGA2, the resected lipomatous lesions depicted in Figure 10, were negative indicating a different pathogenesis.(9) Thus, PHOST is only one of several vascular anomalies and connective tissue overgrowths that occur in PTHS, some of which have more than one tissue type. PHOST, however, is the most complex and its diverse constitutive elements allows its recognition. The possibility exists that purely or almost exclusively adipocytic lesions in PTHS are at one end of the spectrum of PHOST. Additional vascular lesions encountered by us in patients with PTHS were arteriovenous fistula and lymphatic malformation. Perhaps the arteriovenous fistula is also a restricted expression of PHOST.
The role of PTEN mutations in the development of PHOST is not fully understood. PTEN encodes a dual specificity phosphatase that antagonizes the action of phosphatidylinositol 3-kinase (PI3K), thereby providing the central negative regulation of the PI3K/AKT signaling pathway. This ubiquitous pathway influences basic cellular functions including cell cycle progression, cell growth, survival, and migration.(45, 50) Thus, PTEN functions as a classical tumor suppressor gene with somatic loss or inactivation in many sporadic malignancies. Patients with germline PTEN mutations are predisposed to malignancies, predominantly epithelial, but also mesenchymal and hematopoietic.(7)
PTEN affects cell growth and/or migration of the various components of PHOST.(15, 20, 24, 29, 38, 41, 47, 57, 58, 61) A loss of PTEN-mediated regulation provides a plausible mechanism for the formation of PHOST. It is unclear if hemizygosity is sufficient or if a “second-hit” is required for development of PHOST. As in normal tissue controls, we found that all PHOST expressed PTEN by immunohistochemistry, suggesting hemizygosity. However, since many patients have mutations that express an abnormal PTEN (45), immunohistochemistry lacks sensitivity to detect biallelic inactivation. PTEN loss from biallelic mosaicism has been documented in the soft tissue overgrowth lesions in a subset of PHTS.(6, 52, 60) This is analogous to the biallelic loss of TSC in tuberous sclerosis, which also results in upregulation of the AKT/mTOR pathway and appears necessary for hamartoma formation in this syndrome.(33, 43)
It is important to recognize PHOST because of its association with PTHS. The clinical manifestations of PTHS may not be readily apparent and PHOST may be the initial presentation. Histopathologic recognition of PHOST should prompt a detailed clinical evaluation, including family history, as well as possible genetic testing for a PTEN mutation.
Acknowledgments
We are grateful to Dr. George Murphy (Brigham and Women’s Hospital) for performing PTEN immunochemistry.
Footnotes
Disclosures: None of the authors have conflicts of interest or funding to disclose.
References
- 1.Allen PW, Enzinger FM. Hemangioma of skeletal muscle. An analysis of 89 cases. Cancer. 1972;29:8–22. doi: 10.1002/1097-0142(197201)29:1<8::aid-cncr2820290103>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Bannayan GA. Lipomatosis, angiomatosis, and macrencephalia. A previously undescribed congenital syndrome. Arch Pathol. 1971;92:1–5. [PubMed] [Google Scholar]
- 3.Beham A, Fletcher CD. Intramuscular angioma: a clinicopathological analysis of 74 cases. Histopathology. 1991;18:53–59. doi: 10.1111/j.1365-2559.1991.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 4.Bennett KL, Mester J, Eng C. Germline epigenetic regulation of KILLIN in Cowden and Cowden-like syndrome. JAMA. 2010;304:2724–2731. doi: 10.1001/jama.2010.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495–510. doi: 10.1007/s10024-003-2134-6. [DOI] [PubMed] [Google Scholar]
- 6.Caux F, Plauchu H, Chibon F, et al. Segmental overgrowth, lipomatosis, arteriovenous malformation and epidermal nevus (SOLAMEN) syndrome is related to mosaic PTEN nullizygosity. Eur J Hum Genet. 2007;15:767–773. doi: 10.1038/sj.ejhg.5201823. [DOI] [PubMed] [Google Scholar]
- 7.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241:184–196. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MM, Jr, Turner JT, Biesecker LG. Proteus syndrome: misdiagnosis with PTEN mutations. Am J Med Genet A. 2003;122A:323–324. doi: 10.1002/ajmg.a.20474. [DOI] [PubMed] [Google Scholar]
- 9.Cviko A, Klingensmith W, Calicchio M, et al. HMGA2 immunoreactivity in pediatric lipomatous tumors (Abstract) Mod Pathol. 2009;22:322. [Google Scholar]
- 10.Ekinci S, Karnak I, Balci S, et al. Bannayan-riley-ruvalcaba syndrome from the point of view of the pediatric surgeon. Eur J Pediatr Surg. 2006;16:209–213. doi: 10.1055/s-2006-924203. [DOI] [PubMed] [Google Scholar]
- 11.Engelstad BL, Gilula LA, Kyriakos M. Ossified skeletal muscle hemangioma: radiologic and pathologic features. Skeletal Radiol. 1980;5:35–40. doi: 10.1007/BF00347096. [DOI] [PubMed] [Google Scholar]
- 12.Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647–1654. doi: 10.1097/00006534-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Erkek E, Hizel S, Sanly C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639–643. doi: 10.1016/j.jaad.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Fargnoli MC, Orlow SJ, Semel-Concepcion J, et al. Clinicopathologic findings in the Bannayan-Riley-Ruvalcaba syndrome. Arch Dermatol. 1996;132:1214–1218. [PubMed] [Google Scholar]
- 15.Furgeson SB, Simpson PA, Park I, et al. Inactivation of the tumour suppressor, PTEN, in smooth muscle promotes a pro-inflammatory phenotype and enhances neointima formation. Cardiovasc Res. 86:274–282. doi: 10.1093/cvr/cvp425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Crussi F, Enneking WF, Arean VM. Infiltrating angiolipoma. J Bone Joint Surg Am. 1966;48:1111–1124. [PubMed] [Google Scholar]
- 17.Gorlin RJ, Cohen MM, Jr, Condon LM, et al. Bannayan-Riley-Ruvalcaba syndrome. Am J Med Genet. 1992;44:307–314. doi: 10.1002/ajmg.1320440309. [DOI] [PubMed] [Google Scholar]
- 18.Greene AK, Perlyn CA, editors. Clinics in Plastic Surgery. Philadelphia: W.B. Saunders Co; 2011. [Google Scholar]
- 19.Gujrati M, Thomas C, Zelby A, et al. Bannayan-Zonana syndrome: a rare autosomal dominant syndrome with multiple lipomas and hemangiomas: a case report and review of literature. Surg Neurol. 1998;50:164–168. doi: 10.1016/s0090-3019(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 20.Hamada K, Sasaki T, Koni PA, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanssen AM, Fryns JP. Cowden syndrome. J Med Genet. 1995;32:117–119. doi: 10.1136/jmg.32.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi Y, Ohi R, Tomita Y, et al. Bannayan-Zonana syndrome associated with lipomas, hemangiomas, and lymphangiomas. J Pediatr Surg. 1992;27:722–723. doi: 10.1016/s0022-3468(05)80100-9. [DOI] [PubMed] [Google Scholar]
- 23.Hein KD, Mulliken JB, Kozakewich HP, et al. Venous malformations of skeletal muscle. Plast Reconstr Surg. 2002;110:1625–1635. doi: 10.1097/01.PRS.0000033021.60657.74. [DOI] [PubMed] [Google Scholar]
- 24.Hernando E, Charytonowicz E, Dudas ME, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 25.Higginbottom MC, Schultz P. The Bannayan syndrome: an autosomal dominant disorder consisting of macrocephaly, lipomas, hemangiomas, and risk for intracranial tumors. Pediatrics. 1982;69:632–634. [PubMed] [Google Scholar]
- 26.Hobert JA, Eng C. PTEN hamartoma tumor syndrome: an overview. Genet Med. 2009;11:687–694. doi: 10.1097/GIM.0b013e3181ac9aea. [DOI] [PubMed] [Google Scholar]
- 27.Howard EL, Tenant LB, Upton J, et al. PTEN-Associated Vascular Anomaly (abstract) Mod Pathol. 2005;18:301. [Google Scholar]
- 28.Howat AJ, Campbell PE. Angiomatosis: a vascular malformation of infancy and childhood. Report of 17 cases. Pathology. 1987;19:377–382. doi: 10.3109/00313028709103887. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh SC, Chen NT, Lo SH. Conditional loss of PTEN leads to skeletal abnormalities and lipoma formation. Mol Carcinog. 2009;48:545–552. doi: 10.1002/mc.20491. [DOI] [PubMed] [Google Scholar]
- 30.Jennings WC, Say B. Steroid treatment of soft-tissue tumors in Bannayan syndrome. Plast Reconstr Surg. 1988;82:362–363. doi: 10.1097/00006534-198808000-00039. [DOI] [PubMed] [Google Scholar]
- 31.Klein JA, Barr RJ. Bannayan-Zonana syndrome associated with lymphangiomyomatous lesions. Pediatr Dermatol. 1990;7:48–53. doi: 10.1111/j.1525-1470.1990.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 32.Lacey JV, Jr, Mutter GL, Ronnett BM, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Res. 2008;68:6014–6020. doi: 10.1158/0008-5472.CAN-08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesma E, Sirchia SM, Ancona S, et al. The methylation of the TSC2 promoter underlies the abnormal growth of TSC2 angiomyolipoma-derived smooth muscle cells. Am J Pathol. 2009;174:2150–2159. doi: 10.2353/ajpath.2009.080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 35.Marsh DJ, Coulon V, Lunetta KL, et al. Mutation spectrum and genotype-phenotype analyses in Cowden disease and Bannayan-Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet. 1998;7:507–515. doi: 10.1093/hmg/7.3.507. [DOI] [PubMed] [Google Scholar]
- 36.Marsh DJ, Kum JB, Lunetta KL, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461–1472. doi: 10.1093/hmg/8.8.1461. [DOI] [PubMed] [Google Scholar]
- 37.Miles JH, Zonana J, McFarlane J, et al. Macrocephaly with hamartomas: Bannayan-Zonana syndrome. Am J Med Genet. 1984;19:225–234. doi: 10.1002/ajmg.1320190204. [DOI] [PubMed] [Google Scholar]
- 38.Moon SK, Kim HM, Kim CH. PTEN induces G1 cell cycle arrest and inhibits MMP-9 expression via the regulation of NF-kappaB and AP-1 in vascular smooth muscle cells. Arch Biochem Biophys. 2004;421:267–276. doi: 10.1016/j.abb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. 2000;37:517–584. doi: 10.1016/s0011-3840(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 40.Naidich JJ, Rofsky NM, Rosen R, et al. Arteriovenous malformation in a patient with Bannayan--Zonana syndrome. Clin Imaging. 2001;25:130–132. doi: 10.1016/s0899-7071(01)00241-8. [DOI] [PubMed] [Google Scholar]
- 41.Nho RS, Xia H, Diebold D, et al. PTEN regulates fibroblast elimination during collagen matrix contraction. J Biol Chem. 2006;281:33291–33301. doi: 10.1074/jbc.M606450200. [DOI] [PubMed] [Google Scholar]
- 42.Ni Y, Zbuk KM, Sadler T, et al. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83:261–268. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niida Y, Stemmer-Rachamimov AO, Logrip M, et al. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am J Hum Genet. 2001;69:493–503. doi: 10.1086/321972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuss DD, Aeling JL, Clemons DE, et al. Multiple hamartoma syndrome (Cowden’s disease) Arch Dermatol. 1978;114:743–746. [PubMed] [Google Scholar]
- 45.Orloff MS, Eng C. Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene. 2008;27:5387–5397. doi: 10.1038/onc.2008.237. [DOI] [PubMed] [Google Scholar]
- 46.Palencia R, Ardura J. [Bannayan syndrome with intracranial arteriovenous malformations] An Esp Pediatr. 1986;25:462–466. [PubMed] [Google Scholar]
- 47.Pore N, Liu S, Haas-Kogan DA, et al. PTEN mutation and epidermal growth factor receptor activation regulate vascular endothelial growth factor (VEGF) mRNA expression in human glioblastoma cells by transactivating the proximal VEGF promoter. Cancer Res. 2003;63:236–241. [PubMed] [Google Scholar]
- 48.Rao VK, Weiss SW. Angiomatosis of soft tissue. An analysis of the histologic features and clinical outcome in 51 cases. Am J Surg Pathol. 1992;16:764–771. doi: 10.1097/00000478-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Riley HD, Smith WR. Macrocephaly, pseudopapilledema, and multiple hemangiomata: A previously undescribed heredofamilial syndrome. Pediatrics. 1960;26:293–300. [Google Scholar]
- 50.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 51.Sharabi SE, Koshy JC, Hollier LH., Jr Multiple, Recurrent, Refractory Vascular Malformations as the Primary Presenting Feature of a PTEN Mutation. Pediatr Dermatol. 28:466–467. doi: 10.1111/j.1525-1470.2010.01298.x. [DOI] [PubMed] [Google Scholar]
- 52.Tan MH, Mester J, Peterson C, et al. A Clinical Scoring System for Selection of Patients for PTEN Mutation Testing Is Proposed on the Basis of a Prospective Study of 3042 Probands. Am J Hum Genet. 2011;88:42–56. doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tan WH, Baris HN, Burrows PE, et al. The spectrum of vascular anomalies in patients with PTEN mutations: implications for diagnosis and management. J Med Genet. 2007;44:594–602. doi: 10.1136/jmg.2007.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tews DS, Goebel HH, Heffner RR, et al. Hamartoma of the triceps surae muscle. Acta Neuropathol. 1997;94:91–94. doi: 10.1007/s004010050677. [DOI] [PubMed] [Google Scholar]
- 55.Turnbull MM, Humeniuk V, Stein B, et al. Arteriovenous malformations in Cowden syndrome. J Med Genet. 2005;42:e50. doi: 10.1136/jmg.2004.030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weary PE, Gorlin RJ, Gentry WC, Jr, et al. Multiple hamartoma syndrome (Cowden’s disease) Arch Dermatol. 1972;106:682–690. [PubMed] [Google Scholar]
- 57.White ES, Atrasz RG, Hu B, et al. Negative regulation of myofibroblast differentiation by PTEN (Phosphatase and Tensin Homolog Deleted on chromosome 10) Am J Respir Crit Care Med. 2006;173:112–121. doi: 10.1164/rccm.200507-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang G, Sun Q, Teng Y, et al. PTEN deficiency causes dyschondroplasia in mice by enhanced hypoxia-inducible factor 1alpha signaling and endoplasmic reticulum stress. Development. 2008;135:3587–3597. doi: 10.1242/dev.028118. [DOI] [PubMed] [Google Scholar]
- 59.Zhou XP, Marsh DJ, Hampel H, et al. Germline and germline mosaic PTEN mutations associated with a Proteus-like syndrome of hemihypertrophy, lower limb asymmetry, arteriovenous malformations and lipomatosis. Hum Mol Genet. 2000;9:765–768. doi: 10.1093/hmg/9.5.765. [DOI] [PubMed] [Google Scholar]
- 60.Zhou XP, Waite KA, Pilarski R, et al. Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway. Am J Hum Genet. 2003;73:404–411. doi: 10.1086/377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zundel W, Schindler C, Haas-Kogan D, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]