Abstract
Sarcopenia is the age-related involuntary loss of skeletal muscle mass and functionality that can lead to the development of disability, frailty and increased health care costs. The development of interventions aimed at preventing and/or treating sarcopenia is complex, requiring the adoption of assumptions and standards that are not well established scientifically or clinically. A number of investigators and clinicians (both from academia and industry) met in Rome (Italy) in 2009 to develop a consensus definition of sarcopenia. Subsequently, in Albuquerque (New Mexico, USA) in 2010, the same group met again to consider the complex issues necessary for designing Phase II clinical trials for sarcopenia. Current clinical trial data indicate that fat-free mass (FFM) parameters are responsive to physical activity/nutritional treatment modalities over short time periods, but pharmacological trials of sarcopenia have yet to show significant efficacy. In order to conduct a clinical trial within a reasonable time frame, groups that model or display accelerated aging and loss of FFM are necessary. Few studies have used acceptable designs for testing treatment effects, sample sizes or primary outcomes that could provide interpretable findings or effects across studies. Dual energy x ray absorptiometry (DXA) is the measure of choice for assessing FFM, but sufficient time is needed for changes to be detected accurately and reliably. A tool set that would allow clinical, basic and epidemiological research on sarcopenia to advance rapidly toward diagnosis and treatment phases should be those reflecting function and strength.
Keywords: Sarcopenia, phase IIB clinical trials, fat-free mass, strength, frailty
Introduction
This was the second meeting of this international group of scientist and geriatricians, many of whom were at a first meeting in Rome in 2009 (1). In Rome, this group proposed a consensus definition of sarcopenia (1): “Sarcopenia is the age-associated loss of skeletal muscle mass and function. Sarcopenia is a complex syndrome that is associated with muscle mass loss alone or in conjunction with increased fat mass. The causes of sarcopenia are multi-factorial and can include disuse, changing endocrine function, chronic diseases, inflammation, insulin resistance and nutritional deficiencies. While cachexia may be a component of sarcopenia, the two conditions are not the same”. There was also consensus that sarcopenia could be effectively targeted by assessing physical functioning in at risk patients.
The purpose of this second meeting by the group in Albuquerque, New Mexico was to consider current scientific and clinical evidence for measured variables and outcomes necessary in the design and development of phase IIB trials based on their consensus definition for sarcopenia. Phase II trials assess treatment effect as case series or randomized controlled trials, and phase IIB trials are designed specifically to study the efficacy of a pharmacological agent or treatment regimen at a prescribed dosage within an economically reasonable time frame. At the meeting in Albuquerque, the topics discussed by the group regarding the design of phase IIB trials included a literature review, target populations, study design (sample size, methodology, etc.), primary outcomes and current experience. The present paper provides a summary of these discussions of the most relevant evidence in support of phase IIB trials for sarcopenia.
A Brief Overview of Sarcopenia
There are numerous reviews of sarcopenia in the literature (2–7). Briefly, adult fat-free mass (FFM) declines with age, and this decline corresponds to concurrent decreases in strength, metabolic rate, aerobic and functional capacity resulting in significant functional, metabolic and health consequences in older adults (7, 8). These declines begin with the atrophy and loss of type II muscle fibers in young adults (9–13) and progresses through adulthood into old age (14). This age-related decline in FFM can lead to sarcopenia due to a complex interaction and degeneration of multifactorial age-related environmental and genetic factors (5, 15) including genetic heritability (16–18), nutritional status (19–24), physical activity (25–28), hormonal changes (29, 30), insulin resistance (31–33), atherosclerosis (34–36) and changes in circulating pro-inflammatory cytokines (37).
To date, there is not a unique, well-accepted, validated, and/or standardized way to identify the presence of sarcopenia in an older person. This deficiency significantly affects each operative definition and leads to different results. Sarcopenia can be quantified by indexing FFM or appendicular FFM both from dual energy x-ray absorptiometry by height squared, fat mass or total mass. Thus, the application of these indices among different age cohorts results in widely varying estimates of the prevalence of sarcopenia of between 30% to 50% in persons over 80 years of age (38) to values of about 10–15% for persons 60 to 70 years of age (38, 39). Reports of a sex-difference in the prevalence of sarcopenia also vary due to differences amongst the indices (40–44).
Sarcopenia is clearly an important geriatric condition (45), and its presence is a key precursor to the development of frailty among geriatric patients, a powerful predictor of late-life disability and a preventable and treatable condition (6, 7, 15, 46). Sarcopenia should also be considered in patients who are bedridden, non-ambulatory, or who cannot rise from a chair unassisted. At the meeting in Rome, there was unanimous agreement that sarcopenia should be evaluated in older adults with clinically observed declines in physical functioning, activities of daily living, strength, health status, those experiencing recurrent falls, recent weight loss, hospitalization, or chronic conditions associated with muscle loss (1). The clinical recognition of sarcopenia would lead to treatment, the safety and efficacy, all of which must first be determined in clinical trials (47). This second meeting in Albuquerque NM addressed these several points.
Designing Phase IIB Trials
Literature Review
A review of the extensive literature on sarcopenia and clinical trials provides limited information as very few clinical trials are currently available on the topic (4, 48). The majority of sarcopenia clinical trials have been intervention trials involving physical activity programs (49–53) and/or dietary supplementation (54–58) as treatments applied to healthy older adults or to those with compromised nutritional and body composition status. A representative list of these is presented in Table 1. Only a few studies included large samples or random or blinded study designs, and the majority were of less than a year's duration (Table 1). However, some positive effects on FFM are reported for the use of resistance training combined with increases in dietary protein and fatty acids (54, 59). Current scientific and clinical trial data indicate that activities that include resistance exercise and intakes of high quality protein above the recommended dietary allowance can help preserve and increase FFM among older adults (58, 60).
Table 1.
Recent Clinical Trials with Exercise or Diet as the Treatment
| N | Random | Intervention | Outcome | Duration | Body Part | |
|---|---|---|---|---|---|---|
| Exercise | ||||||
| Reid et al 2008 (53) | 57 | yes | resistance training | strength DXA | 3 months | legs |
| Hanson et al 2009 (50) | 50 | no | resistance training | function | 5.2 months | legs |
| Kemmler et al 2010 (51) | 246 | yes | resistance exercise | strength DXA treadmill | 18 months | whole body |
| Park et al 2010 (49) | 175 | no | walking | DXA | 12 months | whole body |
| Phillips et | 213 | yes | exercise | function | 12 months | whole body |
| Diet | ||||||
| Riechman et al 2007 (55) | 49 | no | protein exercise | unknown | 17 weeks | whole body |
| Solerte et al 2008 (56) | 41 | yes | amino acids/2day | DXA | 18 months | whole body |
| Symons et al 2009 (57) | 34 | no | protein | protein synthesis | 1 day | whole body |
| Cornish & Chilibeck, 2009 (54) | 51 | yes | ALA, exercise | DXA | 12 weeks | whole body |
| Zak et al 2009 (58) | 91 | yes | protein resistance exercise | strength | 7 weeks | legs |
During the past decade, clinical trials have tested pharmacological interventions toward the treatment of some measurable aspect of sarcopenia such as muscle mass or strength or objectively measured variables related to sarcopenia (61–67). As can be seen in Table 2, these pharmacological trials included small samples, were of short duration and included older adults who were already health compromised or have some existing hormonal or body mass deficiency. Most of these used hormonal treatments and measured FFM or strength as outcomes. Evidence on this topic is strongly limited by the relatively short length of follow-up. In some 6-month studies, hormonal treatments prevented declines in FFM over the course of the study (61, 68), but preventing a decline in FFM is not a complete positive response within so short a time frame considering the level of error in repeated DXA measurements (7). Only two trials were over 12-months duration which is sufficient time to detect real changes in FFM by DXA (62, 63) but none were found.
Table 2.
Recent Pharmacological Clinical Trials
| N | Random | Intervention | Outcome | Duration | Body Part | |
|---|---|---|---|---|---|---|
| Blackman et al 2002 (64) | 132 | yes | GH, sex hormones | DXA strength treadmill | 26 weeks | whole body |
| Kenny et al 2005 (62) | 167 | yes | estradiol | DXA | 3 years | whole body |
| Giannoulis et al 2006 (65) | 80 | yes | GH, testosterone | DXA strength | 6 months | whole body |
| Giannoulis et al 2008 (67) | 21 | yes | GH, testosterone | protein synthesis | 6 months | whole body |
| Nass et al 2008 (63) | 65 | yes | ghrelin | DXA | 24 Months | whole body |
| Atkinson et al 2010 (61) | 30 | yes | testosterone | ultrasound | 6 months | lower leg |
| Jacobsen et al 2010 (66) | 198 | yes | raloxifene | strength BIA | 12 months | whole body |
An outcome assessment of these trials indicates that FFM parameters are responsive to physical activity/nutritional treatment modalities over short time periods (48). The pharmacological trials of sarcopenia have not yet shown any significant efficacy in the treatment of sarcopenia, but some have been successful in addressing changes in FFM by providing stability (4). However, a design issue for all these trials is selecting an outcome measure that is an accepted identifier and measure of sarcopenia. As can be seen in Tables 1 and 2, both muscle mass and strength are tested either for the whole body or for the legs. As a result, no study to date has been able to clearly identify a sample of older adults who can be defined clinically as having “sarcopenia” which is due in part to that lack of consensus on its definition (47). The consensus definition by this group is a step toward the application of a clinical diagnosis for such studies in the future (1).
Target Populations
When selecting the target population for a clinical trial on sarcopenia, several points should be considered. Sarcopenia is a severe manifestation of the age-related loss of FFM over the later decades of life. Thus, in order to conduct a clinical trial within a reasonably short time frame, population groups that can model or display accelerated aging and loss of FFM artificially enhancing the effects of the applied interventions are potentially candidates (69). The following groups were proposed and critiqued with their individual weakness and strengths: healthy sedentary volunteers, older sedendary persons with initial muscle impairment, Progeroid syndromes patients, groups with limited physical activity due to acute illness, and groups with chronic conditions.
A group of healthy adults is not suitable for phase II trials because it lacks the essential characteristics that fit within the constraints of a clinical trial. It would be difficult to recruit healthy individuals except possibly as a control because they tend to have few clinical and biological confounders. Those that are active and with diets of sufficient protein will not potentially respond to treatment (60). A potential subset of healthy volunteers would be those older persons who have initial musculoskeletal impairments, but among this group, the onset of the disabling process limits their use for preventive studies and include numerous age-related comorbidities. However, the selection of these individuals for clinical trials needs to follow rigorous guidelines because the stage of the disabling process with the concomitant clinical and biological abnormalities may significantly modify the study findings. There are potential ethical concerns involved with the inclusion of healthy adults especially regarding the unknown deliterious effects of long-term followup.
Other potential candidates for clinical trials on sarcopenia might be represented by older patients with restricted physical function because of concurrent acute or chronic conditions. By targeting this type of potential participants, investigators should be aware of the interferences that the specific condition, comorbidities, and/or related treatments may exert on the physiological mechanisms of the skeletal muscle, possibly modifying the trajectories of its age-related decline. In this context, it is important to consider that a subject affected by an acute condition may have a specific enhancement of a biological mechanisms (e.g., inflammation) which can bias the evaluation of body composition, driving the model towards a peculiar condition of the skeletal muscle (e.g., predominantly affected by the inflammatory status). Subjects with Progeroid syndromes have also been indicated as potential participants of clinical trials on sarcopenia, but ethical considerations, the relative rarity of these conditions, and the pathological feature of these diseases mimicking the physiological aging process make such possibility difficult to follow.
Study Design
Phase IIB trials for sarcopenia must be designed and conducted with rigorous accepted protocols and recognized measurable and interpretable outcomes. As mentioned above, available literature of clinical trials on sarcopenia presents several major methodological limitations, largely due to the lack of a well-established operative definition. Thus, few studies to date have used acceptable designs for testing treatment effects and/or a sample size that would provide interpretable findings of treatment effects across studies. There is also the problem of the lack of identifiable accepted outcomes for sarcopenia. Without consensus or recommendations on these important design aspects, it is difficult to determine the clinical relevance, inference, efficacy and safety in a phase IIB trial.
For example, the definition of sarcopenia according to criteria that more rapidly change over time may reduce the follow-up duration, but, at the same time, capture aspects of the skeletal muscle loss which are less pertinent to the long-lasting phenomenon of sarcopenia. However, since phase II clinical trials should just demonstrate the efficacy of the proposed intervention on a specific condition, the study design might be more flexible since it is based on surrogates of the condition of interest.
The design of a study on sarcopenia (as a case series, a randomized controlled trial…) will be characterized by the same methodological strength and weaknesses of any other trial on different topics. However, the subtle nature of the sarcopenia phenomenon can present difficulties. For example, the inclusion of a control group is always amenable, but the complexity of skeletal muscle loss can significantly impair identification of the correct reference sample. In fact, such selection can imply the exclusion of the pathophysiological effects of the aging process which is obviously not possible. Consequently, planning a statistical accounting of potential confounders in the subsequent data analyses phase of the trial, and/or the recruitment of young-adult controls can be important to consider.
Pre- versus post-intervention and randomized control trial (RCT) designs have major limitations in regards to phase IIB studies of sarcopenia. For the pre-/post-interventions, design problems include the onset of chronic health problems within samples, regression to the mean and secular changes with age, all of which influence findings. A RCT design would have to address numerous possible confounders both endogenous such as age, gender, race, body composition and exogenous ones such as physical activity, nutritional status, smoking, medications and social support. Clearly a RCT would be the best design for a phase IIB study leading to Phase III studies, but numerous methodological issues remain unresolved (47).
Primary Outcomes
For a phase IIB trial to determine efficacy at a selected dose or treatment effect, it is important that a primary outcome is identified. To be clinically relevant, a certain condition needs to be clearly defined through the validation of feasible and reliable thresholds distinguishing normality versus abnormality. This represents a major limiting issue for the study of sarcopenia. In fact, although several authors have proposed critical cut-points, the heterogeneity of the studied populations, the multitude of assessing instruments of imaging, and (more generally) the lack of consensus currently make sarcopenia difficult to be operatively defined.
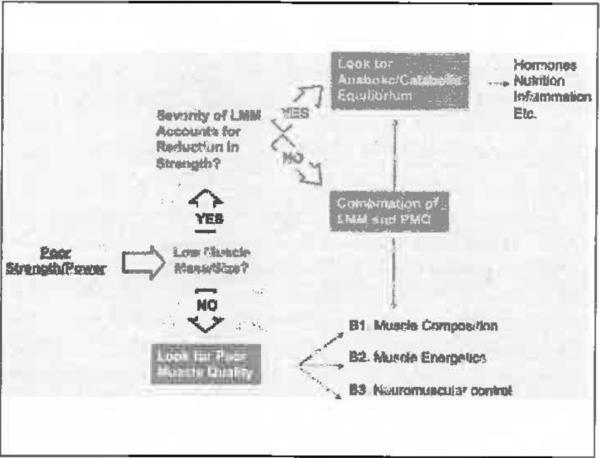
Some recommendations propose cut-points for measures or indices of FFM, for the short physical performance battery or cut-points for walking or gait speed tests (45, 47). These cut-points should be well-established, but others are multisystemic or have exogenous factors that are not specific for skeletal muscle. As an example of the problem, a measure of strength could be considered a primary outcome, but strength is a function of muscle mass and quality (Figure 1). Muscle mass can only partially explain a reduction in strength, and if it is low that leads to additional questions and measures as to the reason for a low measure. If mass is normal, then muscle quality is of concern, and this also leads to several different interrelated factors underlying muscle quality. Employing this type of design in a large sample clearly demonstrates the multi-factorial nature of the cornucopia of measures for sarcopenia, so that the best option might be a syndrome approach to determining muscle impairment.
Figure 1.
An example of strength as a primary outcome
If these measures are adopted, investigators should be aware that they are not directly measuring the skeletal muscle, but rather the effects of its action (Figure 1) with the possible interactions of a multitude of additional systems and apparati involved in functioning. At the same time, a quantitative assessment of skeletal muscle (i.e., measurement of muscle mass) will only partially describe the sarcopenia phenomenon, because mass does not necessarily mirror function (or quality). To date, also considering the preliminary evaluations conducted by Phase II trials, the methodological approach to the sarcopenia definition should be as comprehensive and multidimensional as possible, possibly evaluating the same aspect of skeletal muscle with different tools/instrument (thus to obtain confirmatory evaluations to findings).
Whats is missing in the clinical assessment of sarcopenia is the identification of a clinical measure. Unlike osteoporosis, there is currently the inability to detect or measure clearly a consensus treament effect among individuals or groups with sarcopenia. However, the measurement of bone mineral density of the hip and spine by DXA was realized only after decades of research and clincal trials. Dual energy x-ray absorptiometry is the measure of choice for assessing changes in FFM, and if it is to be used in clinical trials, this will require sufficient time for real changes to be detected accurately and reliably. This is because problems remain for DXA machines in quantifying FFM serially and for the effects of machine and manufacturer differences on findings (70–72). In addition, a decision as to what part of the DXA scan will be used other than whole body as the segmental software of DXA allows regional determinations, but here again the precision and reliability are important design issues (71).
From a scientific and clinically relevant standpoint, the identification, measurement and treatment monitoring of sarcopenia using a single easily administered cost effective test or measure is not currently possible (80). There is no adequate screening tool for sarcopenia and potentially promising tools such as walking speed or ability to rise from a chair have not been analyzed in regards to their sensitivity and specificity in identifying sarcopenia or to measure and monitor any possible response to treatment. If sarcopenia is a decline in muscle mass and function (1), then it would be best to include a test of both as the best assessment of sarcopenia in clinical trials at present.
Consensus
The consensus outcome of the meeting by this group in Albuquerque NM was that phase IIB trials for sarcopenia are possible and should proceed, but there are significant limitations. These include the identification of appropriate and optimal target populations, the management from a design and analysis stand point of the numerous covariates and confounders associated with chronic disease and the normal and pathological aspects of the aging process on metabolism, physiology and composition in test and control groups, and the lack of a set of primary outcomes and a consensus definition (1, 47). If these components were to be prioritized, the lack of a consensus easy, cost-effective clinical measure(s) to identify and quantify changes in sarcopenia is of the highest priority in conjunction with a universally accepted definition. Otherwise, we will continue to apply the many different sets of measures for muscle mass, quality and function. In the interim, a tool or set of tools that would allow clinical, basic and epidemiological research on sarcopenia to advance rapidly toward diagnosis and treatment phases could be those reflecting function and strength such as chair raises and timed walking distances (49, 81, 82). Consequently, clinical trials aimed at preventing or treating sarcopenia in older persons should be as much comprehensive (in terms of adopted approach), cautious, and rigorous as possible in their designs, conduction, and final dissemination of results.
Conclusions
This working group critically considered the scope of sarcopenia as a treatable geriatric condition, proposed a theoretical definition and an assessment of the utility of phase IIB clinical trials for sarcopenia, leading to the following conclusions:
Sarcopenia represents an important prevalent geriatric condition that should receive increased attention from researchers, clinicians, and public health administrators.
A minimal set of screening and assessment tools for sarcopenia needs consensus. In the interim, the documentation and standardization of a basic set of measures or tests within a study could provide that perspective.
Clinical trials for the treatment and possible prevention of sarcopenia are needed and considering the heterogeneity and complexity of the aging process should include controls if at all possible.
The precision and reliability of methodology and outcomes are important to document change as are their sensitivity and specificity in identifying sarcopenia.
Acknowledgements
This material is based upon work supported by the USDA, under agreement No. 58-1950-7-707 Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture. Also supported by the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679).
References
- 1.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definintion: Prevalence, Etiology, and Consequences International Working Group on Sarcopenia. J Am Med Direct Assn. 2011 doi: 10.1016/j.jamda.2011.01.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans WJ. What is Sarcopenia? J Gerontol. 1995;50A(special issue):5–8. [Google Scholar]
- 3.Lang T, Streeper T, Cawthon P, et al. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543–59. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas DR. Sarcopenia. Clin Geriatr Med. 2010;26:331–46. doi: 10.1016/j.cger.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Jones TE, Stephenson KW, King JG, et al. Sarcopenia--mechanisms and treatments. J Geriatr Phys Ther. 2009;32:83–9. [PubMed] [Google Scholar]
- 6.Morley JE. Anorexia, sarcopenia, and aging. Nutrition. 2001;17:660–3. doi: 10.1016/s0899-9007(01)00574-3. [DOI] [PubMed] [Google Scholar]
- 7.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–50. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frontera WR, Hughes VA, Fielding RA, et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 9.Larsson L. Morphological and functional characteristics of the aging skeletal muscle in man. Acta Physiol Scand Suppl. 1978;457(Suppl.):1–36. [PubMed] [Google Scholar]
- 10.Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acat Physiol Scand. 1983;117:469–471. doi: 10.1111/j.1748-1716.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsson L. Morphological and functional characteristics of the ageing skeletal muscle in man. A cross-sectional study. Acta Physiol Scand Suppl. 1978;457:1–36. [PubMed] [Google Scholar]
- 12.Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand. 1983;117:469–71. doi: 10.1111/j.1748-1716.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 13.Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle and Nerve. 1983;6:588–95. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 14.Verdijk LB, Snijders T, Beelen M, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc. 2010;58:2069–75. doi: 10.1111/j.1532-5415.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 15.Weber J, Gillain S, Petermans J. [Sarcopenia: a physical marker of frailty] Rev Med Liege. 2010;65:514–20. [PubMed] [Google Scholar]
- 16.Welle S, Brooks AI, Delchanty JM, et al. Gene expression profile of aging in human muscle. Physiol Genomics. 2003;14:149–59. doi: 10.1152/physiolgenomics.00049.2003. [DOI] [PubMed] [Google Scholar]
- 17.Carey KA, Farnfield MM, Tarquinio SD, Cameron-Smith D. Impaired expression of Notch signaling genes in aged human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2007;62:9–17. doi: 10.1093/gerona/62.1.9. [DOI] [PubMed] [Google Scholar]
- 18.Schrager MA, Roth SM, Ferrell RE, et al. Insulin-like growth factor-2 genotype, fat-free mass, and muscle performance across the adult life span. J Appl Physiol. 2004;97:2176–83. doi: 10.1152/japplphysiol.00985.2003. [DOI] [PubMed] [Google Scholar]
- 19.Chapman IM, Macintosh CG, Morley JE, Horowitz M. The anorexia of ageing. Biogerontology. 2002;3:67–71. doi: 10.1023/a:1015211530695. [DOI] [PubMed] [Google Scholar]
- 20.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 22.Campbell WW, Crim MC, Dallal GE, et al. Increased protein requirements in elderly people: new data and retrospective reassessments. Am J Clin Nutr. 1994;60:501–9. doi: 10.1093/ajcn/60.4.501. [DOI] [PubMed] [Google Scholar]
- 23.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–80. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 25.Kuh D, Bassey EJ, Butterworth S, et al. Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2005;60:224–31. doi: 10.1093/gerona/60.2.224. [DOI] [PubMed] [Google Scholar]
- 26.Hughes VA, Roubenoff R, Wood M, et al. Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr. 2004;80:475–82. doi: 10.1093/ajcn/80.2.475. [DOI] [PubMed] [Google Scholar]
- 27.Hughes VA, Frontera WR, Roubenoff R, et al. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002;76:473–81. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 28.Kortebein P, Ferrando A, Lombeida J, et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. Jama. 2007;297:1772–4. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. 1999;107:123–36. doi: 10.1016/s0047-6374(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 30.Morley JE. Hormones and the aging process. J Am Geriatr Soc. 2003;51:S333–7. doi: 10.1046/j.1365-2389.2003.51344.x. [DOI] [PubMed] [Google Scholar]
- 31.Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mess loss? Diabetes Metab 31 Spec No 2. 2005:5S20–5S26. doi: 10.1016/s1262-3636(05)73648-x. [DOI] [PubMed] [Google Scholar]
- 32.Park SW, Goodpaster BH, Lee JS, et al. Excessive Loss of Skeletal Muscle Mass in Older Adults with Type 2 Diabetes. Diabetes Care. 2009 doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55:1813–8. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 34.McDermctt MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 35.McDermott MM, Guralnik JM, Albay M, et al. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc. 2004;52:405–10. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 36.McDermott MM, Guralnik JM, Ferrucci L, et al. Physical activity, walking exercise, and calf skeletal muscle characteristics in patients with peripheral arterial disease. J Vase Surg. 2007;46:87–93. doi: 10.1016/j.jvs.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 38.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 39.Morley JE, Baumgartner RN, Roubenoff R, et al. Sarcopenia. J Lab Clin Med. 2001;137:231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 40.Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, et al. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25:226–31. doi: 10.1016/s0749-3797(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 41.Gillette-Guyonnet S, Nourhashemi F, Andrieu S, et al. Body composition in French women 75+ years of age: the EPIDOS study. Mech Ageing Dev. 2003;124:311–6. doi: 10.1016/s0047-6374(02)00198-7. [DOI] [PubMed] [Google Scholar]
- 42.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–7. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 43.Tanko LB, Movsesyan L, Mouritzen U, et al. Appendicular lean tissue mass and the prevalence of sarcopenia among healthy women. Metabolism. 2002;51:69–74. doi: 10.1053/meta.2002.28960. [DOI] [PubMed] [Google Scholar]
- 44.Melton LJ, 3rd, Khosla S, Crowson CS, et al. Epidemiology of sarcopenia. Journal of the American Geriatrics Society. 2000;48:625–30. [PubMed] [Google Scholar]
- 45.Rolland Y, Vellas B. [Sarcopenia] Rev Med Interne. 2009;30:150–60. doi: 10.1016/j.revmed.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Morley JE, Kim MJ, Haren MT, et al. Frailty and the aging male. Aging Male. 2005;8:135–40. doi: 10.1080/13685530500277232. [DOI] [PubMed] [Google Scholar]
- 47.Abellan Van Kan G, Chumlea WC, Gillette-Guyonnet S, et al. Clinical trials on sarcopenia - Methodological issues regarding Phase III trials. Clin Trials Geriatr Med. 2011 doi: 10.1016/j.cger.2011.03.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakuma K, Yamaguchi A. Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr Aging Sci. 2010;3:90–101. doi: 10.2174/1874609811003020090. [DOI] [PubMed] [Google Scholar]
- 49.Park H, Park S, Shephard RJ, Aoyagi Y. Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. European Journal of Applied Physiology. 2010;109:953–61. doi: 10.1007/s00421-010-1424-8. [DOI] [PubMed] [Google Scholar]
- 50.Hanson ED, Srivatsan SR, Agrawal S, et al. Effects of strength training on physical function: influence of power, strength, and body composition. J Strength Cond Res. 2009;23:2627–37. doi: 10.1519/JSC.0b013e3181b2297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kemmler W, von Stengel S, Engelke K, et al. Exercise, body composition, and functional ability: a randomized controlled trial. Am J Prev Med. 2010;38:279–87. doi: 10.1016/j.amepre.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 52.Phillips EM, Katula J, Miller ME, et al. Interruption of physical activity because of illness in the Lifestyle Interventions and Independence for Elders Pilot trial. J Aging Phys Act. 2010;18:61–74. doi: 10.1123/japa.18.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reid KF, Callahan DM, Carabello RJ, et al. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res. 2008;20:337–43. doi: 10.1007/bf03324865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cornish SM, Chilibeck PD. Alpha-linolenic acid supplementation and resistance training in older adults. Appl Physiol Nutr Metab. 2009;34:49–59. doi: 10.1139/H08-136. [DOI] [PubMed] [Google Scholar]
- 55.Riechman SE, Andrews RD, Maclean DA, Sheather S. Statins and dietary and serum cholesterol are associated with increased lean mass following resistance training. J Gerontol A Biol Sci Med Sci. 2007;62:1164–71. doi: 10.1093/gerona/62.10.1164. [DOI] [PubMed] [Google Scholar]
- 56.Solerte SB, Gazzaruso C, Boriacasa R, et al. Nutritional supplements with oral amino acid mixtures increases whole-body lean mass and insulin sensitivity in elderly subjects with sarcopenia. Am J Cardiol. 2008;101:69E–77E. doi: 10.1016/j.amjcard.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Dietet Assn. 2009;109:1582–6. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zak M, Swine C, Grodzicki T. Combined effects of functionally-oriented exercise regimens and nutritional supplementation on both the institutionalised and free-living frail elderly (double-blind, randomised clinical trial) BMC Public Health. 2009;9:39. doi: 10.1186/1471-2458-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith GI, Atherton P, Reeds DN, et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–12. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr. 2007;26:696S–703S. doi: 10.1080/07315724.2007.10719650. [DOI] [PubMed] [Google Scholar]
- 61.Atkinson RA, Srinivas-Shankar U, Roberts SA, et al. Effects of testosterone on skeletal muscle architecture in intermediate-frail and frail elderly men. J Gerontol A Biol Sci Med Sci. 2010;65:1215–9. doi: 10.1093/gerona/glq118. [DOI] [PubMed] [Google Scholar]
- 62.Kenny AM, Kleppinger A, Wang Y, Prestwood KM. Effects of ultra-low-dose estrogen therapy on muscle and physical function in older women. J Am Geriatr Soc. 2005;53:1973–7. doi: 10.1111/j.1532-5415.2005.53567.x. [DOI] [PubMed] [Google Scholar]
- 63.Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149:601–11. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blackman MR, Sorkin JD, Munzer T, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. J Am Med Assn. 2002;288:2282–92. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 65.Giannoulis MG, Sonksen PH, Umpleby M, et al. The effects of growth hormone and/or testosterone in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:477–84. doi: 10.1210/jc.2005-0957. [DOI] [PubMed] [Google Scholar]
- 66.Jacobsen DE, Samson MM, Emmelot-Vonk MH, Verhaar HJ. Raloxifene and body composition and muscle strength in postmenopausal women: a randomized, double-blind, placebo-controlled trial. Eur J Endocrinol. 2010;162:371–6. doi: 10.1530/EJE-09-0619. [DOI] [PubMed] [Google Scholar]
- 67.Giannoulis MG, Jackson N, Shojaee-Moradie F, et al. The effects of growth hormone and/or testosterone on whole body protein kinetics and skeletal muscle gene expression in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2008;93:3066–74. doi: 10.1210/jc.2007-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–50. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- 69.Cesari M, Pahor M. Target population for clinical trials on sarcopenia. J Nutr Health Aging. 2008;12:470–8. doi: 10.1007/BF02982708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Litaker MS, Barbeau P, Humphries MC, Gutin B. Comparison of Hologic QDR-1000W and 4500W DXA Scanners in 13- to 18-Year Olds. Obes Res. 2003;11:1545–52. doi: 10.1038/oby.2003.206. [DOI] [PubMed] [Google Scholar]
- 71.Lohman M, Tallroth K, Kettunen JA, Marttinen MT. Reproducibility of dual-energy x-ray absorptiometry total and regional body composition measurements using different scanning positions and definitions of regions. Metabolism. 2009;58:1663–8. doi: 10.1016/j.metabol.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 72.Schoeller D, Tylavski F, Baer DJ, et al. QDR 4500A dual X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;8:1018–1025. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 73.Critchley M. The neurology of old age. Lancet. 1931:1221–30. [Google Scholar]
- 74.Forbes GB. The Adult Decline in Lean Body Mass. Hum Biol. 1976;48:161–171. [PubMed] [Google Scholar]
- 75.Lesser GT, Kumar I, Steele JM. Changes in Body Composition with Age. Ann N Y Acad Sci. 1963;110:578–88. doi: 10.1111/j.1749-6632.1963.tb15781.x. [DOI] [PubMed] [Google Scholar]
- 76.Moore FD, Boyden CM. Body Cell Mass and Limits of Hydration of the Fat-Free Body: Their Relation to Estimated Skeletal Weight. Ann N Y Acad Sci. 1963;110:62–71. doi: 10.1111/j.1749-6632.1963.tb17072.x. [DOI] [PubMed] [Google Scholar]
- 77.Norris AH, Lundy T, Shock NW. Trends in Selected Indices of Body Composition in Men between the Ages 30 and 80 Years. Ann N Y Acad Sci. 1963;110:623–39. doi: 10.1111/j.1749-6632.1963.tb15784.x. [DOI] [PubMed] [Google Scholar]
- 78.Steen B, Birgitta K, Isaksson L, Isaksson B. Body Composition at Age 70, 75, 79 and 81 Years: A Longitudinal Population Study. In: Chandra RK, editor. Nutrition, Immunity and Illness in the Elderly. Pergamon Press; New York, NY: 1985. pp. 49–52. [Google Scholar]
- 79.Wessel JA, Ufer A, Vanhuss WD, Cederquist D. Age Trends of Various Components of Body Composition and Functional Characteristics in Women Aged 20–69 Years. Ann N Y Acad Sci. 1963;110:608–22. doi: 10.1111/j.1749-6632.1963.tb15783.x. [DOI] [PubMed] [Google Scholar]
- 80.Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging. 2010;5:259–70. doi: 10.2147/cia.s6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chale-Rush A, Guralnik JM, Walkup MP, et al. Relationship between physical functioning and physical activity in the lifestyle interventions and independence for elders pilot. J Am Geriatr Soc. 2010;58:1918–24. doi: 10.1111/j.1532-5415.2010.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226–37. doi: 10.1016/j.arr.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]