Abstract
We evaluated the epidemiology of relapsing fever from 1971 to 2003 in Israel. In civilians, incidence declined from 0.35 to 0.11 cases per 100,000 persons annually; in military personnel it averaged 6.4 cases per 100,000 persons annually. These data imply that the pathogen and vector continue to exist in Israel.
Keywords: relapsing fever, tickborne infections, caves, Israel, Ornithodoros, epidemiology, dispatch
Relapsing fever is caused by infection with Borrelia species that can genetically vary their surface antigens, which causes repeated stimulation of the immune system and recurring episodes of fever. Two distinct types of relapsing fever exist, louseborne relapsing fever and tickborne relapsing fever (TBRF) (1,2). The TBRF Borrelia sp. is transmitted to humans by an infected Ornithodoros tick bite (2).
Human TBRF is generally contracted only in the geographic range of the tick vectors. Ornithodoros species of ticks belong to the Argasidae (soft tick) family that feeds nocturnally. The primary reservoirs of Borrelia spp. are rodents and lagomorphs (2). Many Ornithodoros species have been reported worldwide, each with its characteristic Borrelia species (2–5).
After an incubation period of ≈7 days, TBRF typically begins abruptly with fever, chills, headache, myalgia, arthralgia, and abdominal pain. Death is rare. Diagnosis is usually made by observing spirochetes on peripheral blood smears. Antimicrobial drug treatment is occasionally associated with the Jarisch-Herxheimer reaction (6).
Since 1946, all cases of relapsing fever described in Israel have been tickborne. B. persica, transmitted by Ornithodoros tholozani is thought to be the cause of TBRF in Israel. The O. tholozani tick was first identified in 1937 and is prevalent in Middle Eastern and central Asian countries. It is primarily found in dark, moist areas such as caves and abandoned buildings (7). In Israel, TBRF has traditionally been called cave fever because of the known correlation to cave exposure (8). The only survey conducted in Israel described the incidence of TBRF from 1954 to 1967 and concluded that the incidence of TBRF was declining (9).
The Study
In Israel, all cases of TBRF in civilians are reported to the Ministry of Health and investigated by an epidemiologist nurse. Cases in soldiers are reported to the military health branch of the Surgeon General Headquarters of the Israel Defense Force. An epidemiologic investigation of cases in soldiers is conducted by the reporting physician. We evaluated the demographic, clinical, and geographic data from reports of civilians from 1971 to 2003 and of soldiers from 1975 to 2003.
We included confirmed cases, in which a person had both febrile illness and a positive peripheral blood smear, and associated cases, in which a person had a relapsing febrile illness and was associated with a confirmed case. Tests for trends were conducted with PEPI software, version 4.0 (Sagebrush Press, Salt Lake City, UT, USA). A total of 606 cases were reported during the study years, 283 in civilians, and 323 in soldiers.
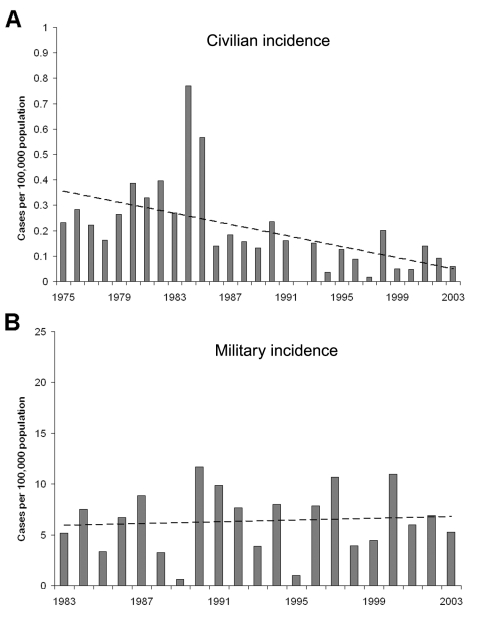
The yearly civilian incidence is shown in Figure 1A. The yearly incidence average in civilians declined from 0.35 cases per 100,000 population from 1975 to 1985 to 0.11 cases per 100,000 population from 1986 to 2003 (p<0.001). The peak in civilian cases in 1984 and 1985 was unexplained; 2 cases occurred in immigrants from Ethiopia 3 months after they arrived in Israel. The incidence of TBRF in the military from 1983 to 2003 has been relatively constant, with an average of 6.4 cases per 100,000 population annually (Figure 1B).
Figure 1.
Incidence of tickborne relapsing fever in Israel. A) among civilians, 1975–2003; B) among soldiers, 1983–2002. Dotted line indicates prevalence.
The patients included 529 men (87%) and 77 women (13%). Of 359 patients for whom this information was available, 304 (85%) were of Jewish origin and 55 (15%) were of non-Jewish origin, similar to the proportions in the Israeli population. Information on exposure circumstances was available for 256 cases. The most commonly reported exposures were visiting caves (64%), visiting abandoned buildings (5%), and engaging in other outdoor activity (19%). In 12% of the cases, exposure to caves was excluded, but the exposure circumstances were not identified. In soldiers, 43% of cases were associated with outdoor exposure, usually related to prolonged periods of lying on the ground. In civilians, 49% were students and 16% were outdoor workers. One hundred cases occurred in clusters with an average of 3.3 (range 2–10) people in each cluster.
Conclusions
Soldiers comprised more than half of the total cases in this study, with an average incidence 36 times greater than in civilians. The higher incidence in soldiers may be due to increased risk of exposure because of their specific activities or better case reporting in the Israel Defense Force.
During the last century in industrialized countries, a significant decline in many infectious diseases has been observed (10). This decline can be attributed to elimination or significant reduction of pathogens or decreased exposure to the pathogens.
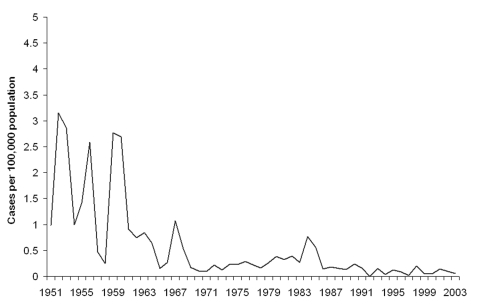
This study has shown a decrease in the incidence of TBRF in civilians, with no similar decrease in soldiers. This decrease becomes even more evident when compared to the incidence in Israel during the 1950s and 1960s (Figure 2). That the incidence has remained similar over time among military personnel indicates that the pathogen and the vector continue to exist in Israel and that soldiers are at a high risk of contracting it because of their activities. Infection has occurred although Israel Defense Force commanders are aware of TBRF and all soldiers and commanders receive annual guidelines on preventing TBRF. Furthermore, entering caves is prohibited, and most TBRF cases in the military (67%) were not due to cave exposure. The declining incidence among civilians is likely due to a decrease in the rate of exposure with increased urbanization. A civilian subpopulation that continues to be at risk is schoolchildren; most cases occurred after field trips.
Figure 2.
Civilian yearly incidence of tickborne relapsing fever, Israel, 1951–2003.
Although TBRF was reported throughout the year, most cases occurred during summer and fall. Possible explanations include higher Ornithodoros tick activity, higher human outdoor activity, and lighter clothes worn during these seasons. TBRF can be contracted throughout Israel, despite the varying climatic conditions (Table). In soldiers, 63% of cases were contracted in the hot, arid, southern regions of Israel. The high number of cases in the southern regions is probably because most military training activity is conducted there. In civilians, more cases were contracted in the northern and central regions of Israel where more persons travel. That 64% of the cases are associated with caves might be explained by the relatively stable conditions in caves that are favorable to ticks.
Table. Geographic distribution (%) of tickborne relapsing fever cases.
| Geographic region | Civilians | Military | Total |
|---|---|---|---|
| Israel | |||
| North | 36 | 18 | 29 |
| Center | 50 | 12 | 35 |
| South | 10 | 63 | 32 |
| Other | |||
| Africa | 4 | 0 | 2 |
| Lebanon | 0 | 7 | 3 |
| Total | 100 | ||
Most cases that occurred in the military were in men. Although women also served in the Israeli military, only a fraction of them served in positions that required outdoor exposure. The illness was also more preponderant in civilian men. This tendency has been observed in previous reports, and likely results from men’s high-risk behavior.
In conclusion, TBRF continues to be endemic in Israel and still poses a considerable hazard for soldiers and civilians. Although TBRF has been substantially reduced in civilians, the incidence in soldiers has not declined. Sufficient information on the habits of the O. tholozani tick and its reservoirs is not available. To reduce the incidence of TBRF, further studies are warranted to accurately map Ornithodoros tick infestations. Further studies are also needed to identify Borrelia reservoirs in Israel and to study the habits of its vector.
Biography
Dr Sidi is a graduate of Sackler School of Medicine, Tel Aviv University, Israel. He served in the medical branch of the Surgeon General Headquarters of the Israel Defense Force. He currently is a medical resident at Jacobi Medical Center, Bronx, New York.
Footnotes
Suggested citation for this article: Sidi G, Davidovitch N, Balicer RD, Anis E, Grotto I, Schwartz E. Tickborne relapsing fever in Israel. Emerg Infect Dis [serial on the Internet]. 2005 Nov [date cited]. http://dx.doi.org/10.3201/eid1111.050521
References
- 1.Goubau PF. Relapsing fevers: a review. Ann Soc Belg Med Trop. 1984;64:335–64. [PubMed] [Google Scholar]
- 2.Dennis DT, Campbell GI. Relapsing fever. In: Braunwald E, Hauser SL, Fauci AS, Longo DL, Kasper DI, Jameson JL, editors. Harrison’s principles of internal medicine. New York: McGraw-Hill; 2001. p. 1058–61. [Google Scholar]
- 3.Dworkin MS, Shoemaker PC, Fritz CL, Doewell ME, Anderson DE. The epidemiology of tick-borne relapsing fever in the United States. Am J Trop Med Hyg. 2002;66:753–8. [DOI] [PubMed] [Google Scholar]
- 4.Rawlings JA. An overview of tick-borne relapsing fever with emphasis on outbreaks in Texas. Tex Med. 1995;91:56–9. [PubMed] [Google Scholar]
- 5.Thompson RS, Burgdorfer W, Russel R, Francis BJ. Outbreak of tick-borne relapsing fever in Spokane County, Washington. JAMA. 1969;210:1045–50. 10.1001/jama.1969.03160320027005 [DOI] [PubMed] [Google Scholar]
- 6.Spach DH, Liles WC, Campbell GL, Quick RE, Anderson DE Jr, Fritsche TR. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936–47. 10.1056/NEJM199309233291308 [DOI] [PubMed] [Google Scholar]
- 7.Megido D. A case survey of acute relapsing fever in Israel [article in Hebrew]. Harefuah. 1984;107:142–5. [PubMed] [Google Scholar]
- 8.Adler S, Theodor O, Schieber H. The transmission of relapsing fever by ticks in Palestine [article in Hebrew]. Harefuah. 1936;10:105–7. [Google Scholar]
- 9.Schwartz T. Master's thesis in public health. Hebrew University, Jerusalem, Israel; 1970. [Google Scholar]
- 10.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–6. 10.1001/jama.281.1.61 [DOI] [PubMed] [Google Scholar]