Abstract
The underlying molecular mechanisms of drug abuse and addiction behaviors are poorly understood. C. elegans provide a simple, whole animal model with conserved molecular pathways well suited for studying the foundations of complex diseases. Historically, chemotaxis has been a measure used to examine sensory approach and avoidance behavior in worms. Chemotaxis can be modulated by previous experience, and cue-dependent conditioned learning has been demonstrated in C. elegans, but such conditioning with drugs of abuse has not been reported. Here we show that pairing a distinctive salt cue with a drug (cocaine or methamphetamine) results in a concentration-dependent change in preference for the cue that was paired with the drug during conditioning. Further, we demonstrate that pairing of either drug with a distinctive food type can also increase preference for the drug-paired food in the absence of the drug. Dopamine-deficient mutants did not develop drug-paired, cue-conditioned responses. The findings suggest that, like vertebrates, C. elegans display a conditioned preference for environments containing cues previously associated with drugs of abuse, and this response is dependent on dopamine neurotransmission. This model provides a new and powerful method to study the genetic and molecular mechanisms that mediate drug preference.
Keywords: Caenorhabditis elegans, chemosensory cue conditioning, stimulants, addiction, drug reward
Drug addiction is a multi-faceted condition that may interfere with an individual’s ability to function normally within society. On a neurobiological level, it is believed that drugs of abuse can alter synaptic plasticity to produce behaviors geared toward increasing compulsive drug self-administration (Chen et al., 2009; Koob & Volkow, 2010). The biological systems already in place to sustain the organism (e.g., feeding) and survival of the species (e.g., sex), or the systems that are governed by natural rewards, can by “hijacked” by drugs to initiate and perpetuate drug taking behavior. The molecular mechanisms underlying various aspects of drug-induced changes in behavior remain unclear. To dissect these changes, animal models are utilized to characterize particular traits of addiction.
Animal models provide opportunities to ethically and experimentally control studies of complex human diseases such as addiction. The majority of paradigms have used vertebrate models and have been developed to investigate the rewarding or positive reinforcing properties of drugs, including self-administration, brain stimulation reward, drug discrimination and conditioned place preference (CPP) (Shippenberg & Koob, 2002). In classical conditioning, unconditioned stimuli (UCS) are paired with specific environmental stimuli. After repeated pairings of a UCS with a specific stimulus in conditioning sessions, the stimulus itself becomes rewarding, even in the absence of the UCS. A large number of studies have demonstrated acquisition of CPP using many different drugs of abuse, including cocaine, amphetamine, and meth-amphetamine in many vertebrate animals, including humans (For review, see Tzechentke, 2007).
Caenorhabditis elegans (C. elegans) is a simple animal model that can give insight into complex behaviors. C. elegans are able to sense environmental stimuli and employ approach or avoidance behavior to food, odors, and even light (Ward et al., 2008) through different neuronal systems (Bargmann, 2006). C. elegans was also the first multi-cellular organism to have its entire genome sequenced and many conserved gene systems exist between humans and these animals (Bargmann, 1998; Kaletta & Hengartner, 2006). Genes that are implicated in the actions of drugs of abuse are mostly conserved across species (Wolf & Heberlein, 2003). Like the conserved mechanisms of learning and memory seen in C. elegans (for review, see Ardiel & Rankin, 2010), it is possible that some of the mechanisms underlying the rewarding, reinforcing, and addictive properties of drugs of abuse in humans are also present in C. elegans.
As with vertebrates, C. elegans form associations between neutral stimuli and biologically relevant stimuli (or appetitive stimuli) through associative learning. (Law et al., 2004; Wen et al., 1997). C. elegans are able to discriminate between two equally preferred salt ion cues (Na+ and Cl−) and learn to associate the specific cue previously paired with food by preferentially selecting that “salt environment” following conditioning (Wen et al., 1997). This is important because it shows that C. elegans are able to produce behaviors that suggest a learned association between an unconditioned stimulus and a conditioned stimulus. The current study paired psychostimulant drugs and environmental cues with the hypothesis that C. elegans will demonstrate a preference for an environment containing a cue previously associated with a drug. This study suggests that chemosensory cue conditioning using drugs in C. elegans may be utilized to better understand the mechanisms underlying addiction.
Methods
Materials
All reagents and assay materials were purchased from Sigma Aldrich and Fisher Scientific. Methamphetamine (MAP), dopamine (DA), and cocaine hydrochloride salts were purchased from Sigma Aldrich (St. Louis, MO).
Culture and maintenance of strains
The N2 Bristol WT strain was used in all assays. The cat-1(e1111) and cat-2(e1112) mutant strains along with the N2 WT worms were obtained from the laboratory of Richard Nass (Indiana University School of Medicine). All animals were maintained at 20°C and all general culturing techniques are described by Nass and Hamza (2007). Worms were grown with E. coli (strain NA22) as a food source unless otherwise noted. Young adult worms were used for conditioning and testing to control for any effects of different sensitivities and responses to drugs at varying developmental stages. Worm populations were age synchronized as described by Nass and Hamza (2007). A bleach and sodium hydroxide solution was used to lyse gravid adults and release the eggs into solution. The eggs were allowed to hatch overnight in an M9 buffer and plated 18 hours later as L1-stage larvae. Conditioning and testing began approximately 72 hours post-plating the L1 worms.
Apparatus
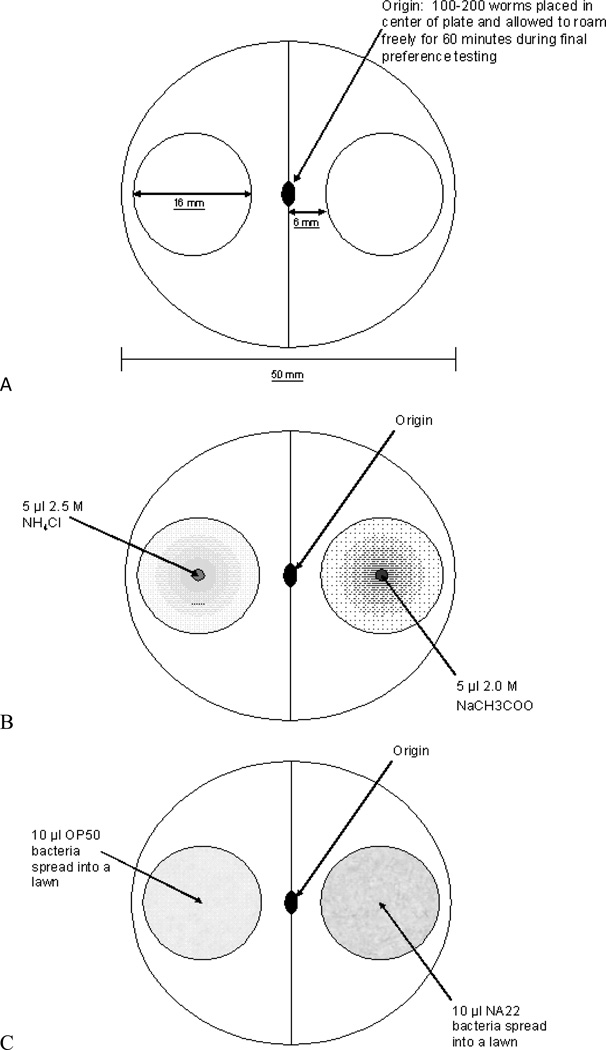
In each of the experiments, the worms were conditioned and tested on 60 mm petri dishes. Worms were viewed and photographed with an Amscope LED stereomicroscope with an 8 MP USB digital camera (Model MD1800, Amscope.com). The worms were counted as they appeared in 16 mm diameter circular sectors of the plate drawn in a pattern on the bottom of the petri dish (See Figure 1).
Figure 1.
A. Conditioned cue assay plate. A grid was drawn on the bottom of all assay plates so that all testing plates were uniform. For each assay, both cue gradients were photographed so that the worms present in the environments could be counted. B. Salt cue conditioning assay plate. A point source of salt ion was placed in the center of each circle on either side of the center line. On one side, a 5 µl drop of 2.0 M NaCH3COO was placed in the center of the circle. On the other side, a 5 µl drop of 2.5 M NH4Cl was placed in the center of the circle. The drops were placed 3 hours prior to testing to allow the solution to diffuse into a gradient. C. Food context conditioning assay plate. 10 µl of OP50 E. coli bacterial broth was placed in the center of one circle and the opposite circle contained 10 µl of NA22 E. coli bacterial broth. The bacterial culture was spread with a sterile glass rod to cover the entire 16 mm diameter circle. The bacterial lawns were allowed to incubate overnight and were used for testing the following day.
Salt ion conditioning and testing plates
Chemosensory conditioning was completed with either a “sodium” or “chloride” cue, similar to previous studies (Rankin, 2000; Wen et al., 1997). Sodium acetate (NaCH3COO) was used to establish the sodium cue and ammonium chloride (NH4Cl) for the chloride cue. These chemosensory cues have been used in previous associative learning protocols as the Na+ and Cl− (Rankin, 2000; Wen et al., 1997). Furthermore, additional studies have shown their counterparts (acetate and ammonium ions respectively) are also attractants in their own right (Frokjaer-Jensen et al., 2008). However, for the purposes of this study, sodium acetate will be referred to as the Na+ cue and ammonium chloride as the Cl− cue. NGM agar minus the NaCl was used as the plain context for salt ion conditioning. When conditioning required an ion environment, 6.15 g/L NaCH3COO or 4.01 g/L NH4Cl was substituted for NaCl to produce 75 mM “Na+” or “Cl−” plates, respectively (Wen et al., 1997). Cocaine or MAP hydrochloride was added to cooled agar immediately before pouring at desired concentrations.
Chemosensory cue assay plates were made by taking salt-free NGM agar plates and preparing salt gradients with one 5 µl drop of each ion-spotting solution (2.0 M NaCH3COO and 2.5 M NH4Cl) at the center of either gridded circle (see Figure 1). The drops were placed 3 hours before testing began to allow for the ion gradient to diffuse. The spotting solutions were frequently changed due to greater variability in results with age (Rankin, 2000; Wen et al., 1997).
When determining whether stimulant chemosensory cue conditioning could be rescued in the cat-1 and cat-2 strains, both conditioning and testing plates were made as normal; however, a dopamine hydrochloride solution was mixed with agar just prior to pouring plates to reach final concentrations in the range of 5–25 mM dopamine. Conditioning and testing with dopamine plates occurred immediately following the cooling of the agar to prevent oxidation. Procedures for dopamine-rescued conditioning are the same as described in the next section.
Salt ion conditioning procedure
Synchronized, young adult worms were washed from their maintenance plates into 15-ml conical tubes and centrifuged for one minute at 2000 rpm. The supernatant was removed, leaving the pelleted worms in the tube and 10 ml DI water was added. The worms were gently mixed, centrifuged, and the supernatant removed. One washing cycle consisted of adding water, mixing, centrifugation, and supernatant removal. Worms began conditioning following three washing cycles, see Figure 2 for a procedure timeline. When assigned to the CS+ condition first, worms were placed on conditioning plates designed to combine a particular concentration of drug with a constant exposure to one salt stimulus. The CS− condition would repeat the procedure used with the CS+ salt using the alternate salt ion (CS−) only in the absence of any drug. After the final wash, worms were transferred to their first conditioning plate in 20 µl aliquots and excess water was absorbed with a Kimwipe to spread the worms over the conditioning plate and to prevent large clumps of animals. Worms remained on their conditioning plates undisturbed for 15 minutes. Worms were then washed from the conditioning plates with 1 ml DI water followed by three washing cycles. After the initial conditioning session (either the CS+ or CS− condition) and washes, worms were transferred to the opposite CS condition for 15 minutes. One conditioning cycle consisted of a CS+ session and a CS− session. Two conditioning cycles were completed for all groups unless otherwise specified. Following conditioning, worms underwent three washing cycles and were then placed onto assay plates by micro-pipetting a 10 µl drop containing approximately 100–200 worms to the center of the assay plate. Excess water was gently wicked away and worms were allowed to roam freely on the assay plates for 60 minutes. After 60 minutes, the assay plates were photographed and the worms counted within the 16 mm diameter circular gradients containing the chemosensory cues. A final preference index (PI) score was calculated to determine the strength of the preference for a particular cue (whether it be the cue that had been previously paired with the drug or the control cue). The PI score was calculated as a percentage of worms in the CS+ gradient from the total number of worms in both the CS+ and CS− gradients. Worms that remained in the center of the plate or outside the gradient circles were not included in the calculation. Data are presented as mean ± SEM. One- or two-way ANOVAs with Tukey’s post-hoc analyses were used to compare groups as necessary.
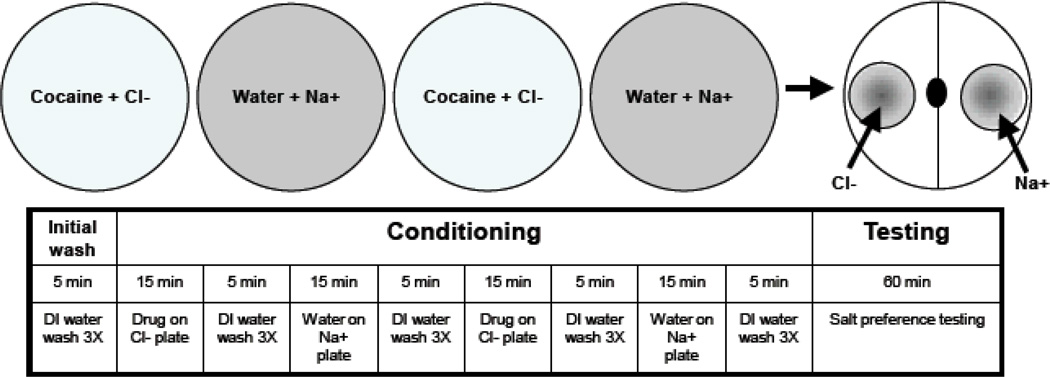
Figure 2.
Salt Cue Conditioning Procedure. This timeline shows the conditioning and testing procedure when pairing the Cl− salt chemosensory cue with cocaine.
Food conditioning and testing plates
NGM agar (as described in Nass & Hamza, 2007) was used for food conditioning. The NGM plates were produced by filling 60 mm petri dishes with 10 ml regular NGM agar (17 g/l bactoagar, 2.5 g/l bactopeptone, 3 g/l NaCl). NA22 and OP50 strains of E. coli were used when the conditioning required exposure to a food source. 20 µl of either NA22 or OP50 bacterial culture (NA22: 2 g tryptone, 1.2 g yeast extract, 0.625 g NaCl, and 125 ml water autoclaved and then inoculated with NA22 and incubated; OP50: 1.25 g tryptone, 0.625 g yeast extract, 0.625 g NaCl, 25 mg streptomycin, and 125 ml water autoclaved and then inoculated with OP50 and incubated) were pipetted onto hardened NGM plates and spread evenly across the plate to form a consistent lawn. The bacterial lawn was allowed to dry and incubated overnight at 37.5°C. Plates were ready for conditioning the following day. Food conditioning followed the same protocol as salt conditioning except just prior to worms being placed on conditioning plates, they were exposed to their designated drug concentration. Drug exposure consisted of 1 ml drug solution being placed into the tube containing the worm pellet left after the initial washing cycles. The worms were gently mixed with the solution and then dropped onto the conditioning plate in 20 µl aliquots. The drug solution was not wicked away and worms were allowed to freely roam in the bacterial lawn for the 15-minute conditioning period. Conditioning and testing proceeded as described above. Food conditioning assay plates were made by spreading 10 µl of bacterial culture (OP50 or NA22) within the 16 mm diameter circles on each side of the NGM assay plates (see Figure 1). The bacterial circles were allowed to incubate overnight before testing.
Control Groups
Naïve controls
Animals were washed from maintenance plates and immediately tested on either salt ion or food-type assay plates without any conditioning trials.
UCS alone
Worms were placed on salt-free NGM plates that had been washed with 50 µM MAP or 50 µM cocaine for 30 minutes. The animals were then tested for salt ion or food cue preference.
CS1− ↔ CS2−. Water controls
Animals went through salt or food conditioning as described with the following exception: no presentation of any unconditioned stimulus (e.g., drug).
UCS → CS1− ↔ CS2−
The unpaired controls were conducted by placing animals in a 50 µM MAP or 50 µM cocaine drug solution for 15 minutes and then went through salt or food conditioning and testing as normal without any drug being presented during conditioning.
E. coli paired with salt ions
To show that chemosensory conditioning could be produced using salt ions as conditioned stimuli, OP50 was used as the unconditioned stimulus. Conditioning proceeded just as described above for salt ion conditioning except that the drug was replaced by a thin layer of OP50 (incubated overnight) spread onto the designated (food assigned to either Na+ or Cl−) salt ion plate.
Results
N2 wild-type worms show salt cue conditioning
The feeding (Avery, 1993; Greer et al., 2008) and chemotaxis (Bargmann & Horvitz, 1991; Bargmann et al., 1993; Mori, 1999; Sengupta, 2007; Ward, 1973) behavior of C. elegans, as well as the circuitry underlying these behaviors (Chatterjee & Sinha, 2008; de Bono & Maricq, 2005; Schafer, 2005) is well-described, making these behaviors well-suited for studying associative conditioning. The first experiment was conducted to determine if C. elegans could form associative connections to the Na+ and Cl− cues by pairing OP50 food with each separately. The salt concentrations selected were previously shown to produce a balanced response resulting in no preference for either ion (Wen et al., 1997), and this was confirmed with naïve N2 worms (t(18) = −.26, p = .79; student’s t-test; Figure 3). This allowed for an “unbiased” apparatus for testing conditioning effects on final preference, because at baseline there is no partial attraction for a particular side.
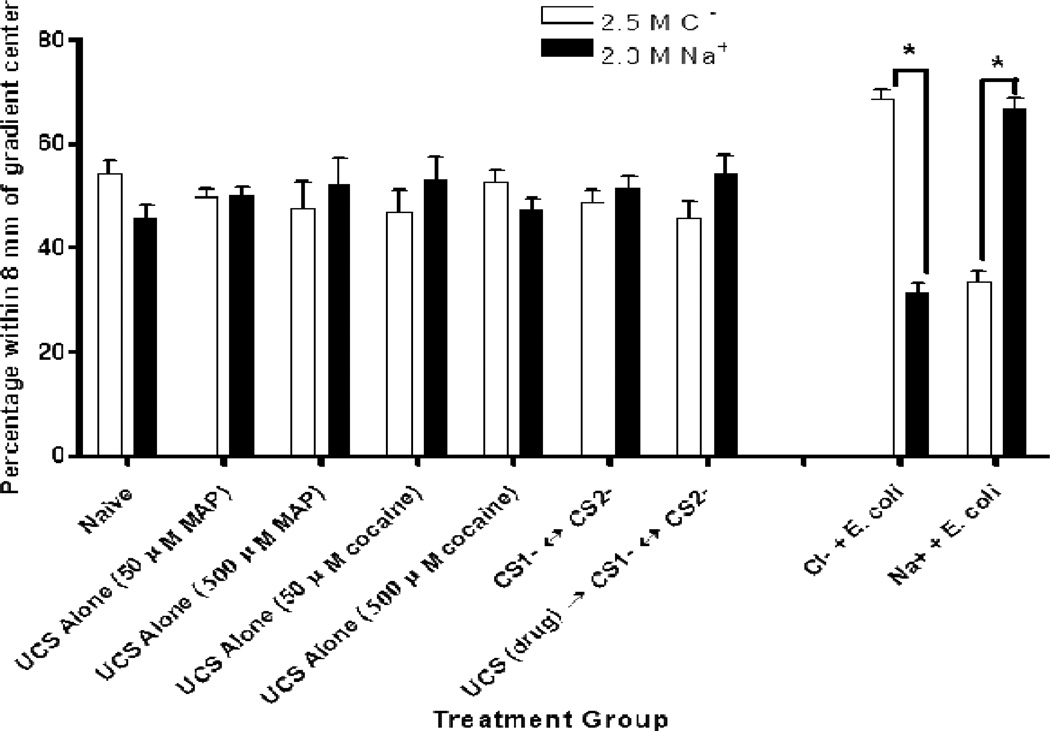
Figure 3.
Salt Cue Conditioning Controls. The data presented on the right show that preference for the ion predicting food was significantly greater than preference for the ion that had not previously been paired with food (Cl− as CS+: t(19) = 10.88, p < .001; Na+ as CS+: t(19) = 7.45, p < .001). Further, a comparison of Na+ preference for naïve controls (far left) and Na+ preference for positive controls (on the right) revealed significant differences (for Na+ as CS−: t(37) = 6.02, p < .001; for Na+ as CS+: t(37) = 4.49, p < .001). All negative controls on the left revealed no significant differences in final salt ion preference: naïve, t(18) = −.27, p = .79; 50 µM MAP alone, t(5) = 0.08, p = .94, 500 µM MAP alone, t(5) = .46, p = .67; 50 µM cocaine alone, t(5) = −.72, p = .51, 500 µM cocaine alone, t(5) = .99, p = .42; CS1− ↔ CS2− or water controls, t(23) = −.28, p = .78; and UCS → CS1− ↔ CS2− or unpaired drug controls, t(10) = −1.26, p = .24. Bars represent means ± SEM (n values from left to right: 19, 6, 6, 6, 6, 24, 11, 20, and 20 plates).
The worms tested in all assays were not food-deprived. It has been found that food deprivation results in a significantly greater final preference for the salt ion that had cued food availability (Wen et al., 1997), however, because there was no food deprivation for drug conditioning, worms were not starved in these positive controls. Following food-paired conditioning, the worms showed a robust preference for the salt ion that had been paired with the food (Figure 3, right). When the OP50 was paired with the chloride ion, there was a significant preference for chloride during testing, t(19) = 10.88, p < .001. However, when the OP50 was paired with the sodium ion, there was a significant preference for sodium during testing, t(19) = −7.45, p < .001. A two-way ANOVA (CS+ order×CS+ ion-type) revealed no effects of CS+ order (whether the food was paired first or second during conditioning), F(1, 20) = .09, p = .77, or any effect of CS+ ion-type (whether the food was paired with sodium or chloride), F(1, 20) = 3.25, p = .09 (data not graphed). Further, the attrition rate once worms were placed onto assay plates averaged 4.58 ± .45%. Most of the worms that were placed on testing plates went to one of the two salt (or food) environments.
C. elegans responds to cocaine and methamphetamine salt cue conditioning in a dose-dependent manner
C. elegans has been found to respond dose-dependently to drugs of abuse, including ethanol (Mitchell et al., 2007; Davis et al., 2008; Mitchell et al., 2010), cocaine (Ward et al., 2008) and amphetamine (Carvelli et al., 2010). Having demonstrated that chemosensation is amenable to use for classical conditioning, we altered the assay to determine whether changing the natural UCS (food) to a drug, known to cause behavioral changes in C. elegans, could also induce a conditioned response. Previously, C. elegans has shown an aversive response to a cue that has been paired with starvation (Saeki et al., 2001); thus, it was important to determine whether a cue that was presented second in order (i.e., increased time from being taken off maintenance plates and feeding) would elicit an aversive response rather than the approach response for the CS+. The presence of an order effect could also suggest an adaptation to the first cue, resulting in less responsiveness to the second cue. Three-way ANOVAs (CS+ order×Drug-paired Salt Ion×Concentration) showed no effect of CS+ order, either for cocaine [F(1, 82) = .27, p = .60] or MAP [F(1, 87) = 3.35, p = .08], thus data were collapsed across CS+ order. Two-way ANOVAs (Drug-Paired Salt Ion×Concentration) showed no differences in which salt ion was paired with drug, whether it was for cocaine [F(1, 82) = 1.21, p = .27] or MAP [F(1, 87) = 3.49, p = .06]. For each drug-type, data were collapsed across CS+-type. Worms conditioned with the stimulant cocaine displayed a concentration-dependent effect on preference for the ion that predicted the occurrence of drug during conditioning, F(6, 82) = 4.62, p < .01 (Figure 4). Tukey’s post hoc analyses showed that the 50 µM cocaine treatment during conditioning resulted in a significantly greater preference for the drug-paired ion compared with naïve controls and vehicle/water controls (both p < .01). No other cocaine concentration levels were found to be significantly different from controls.
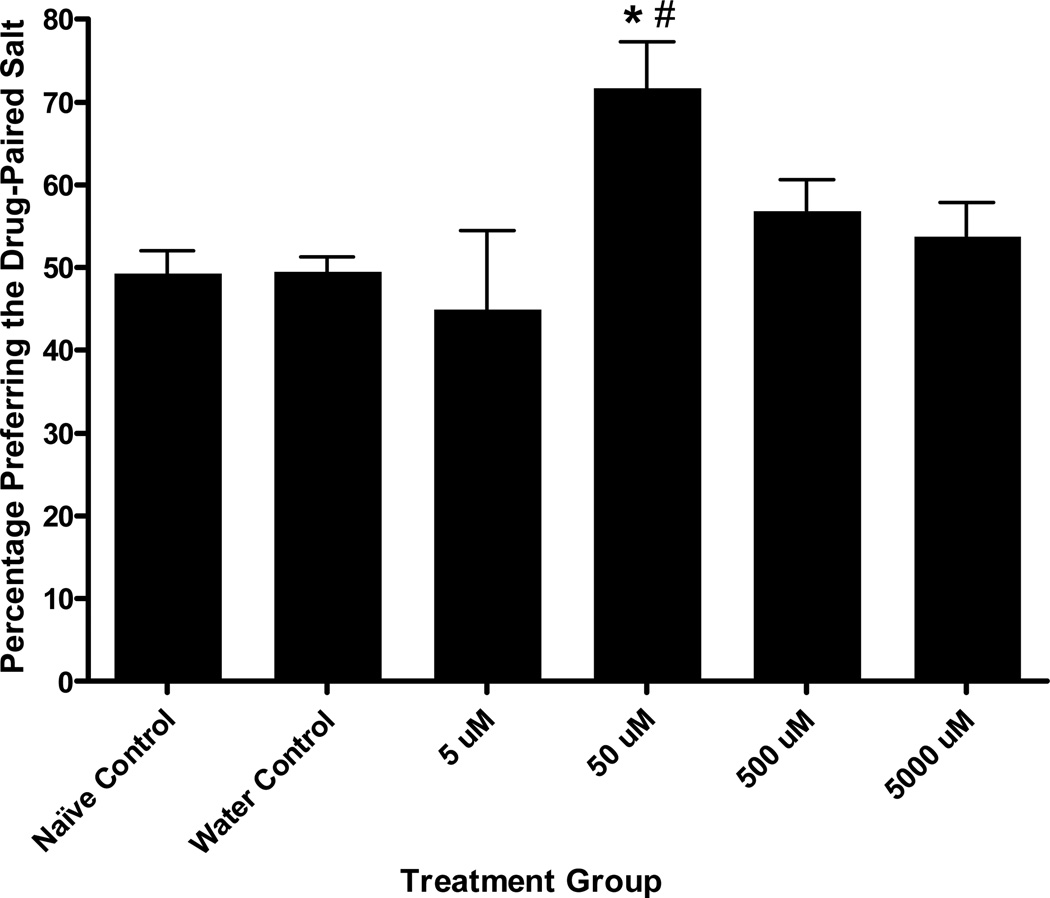
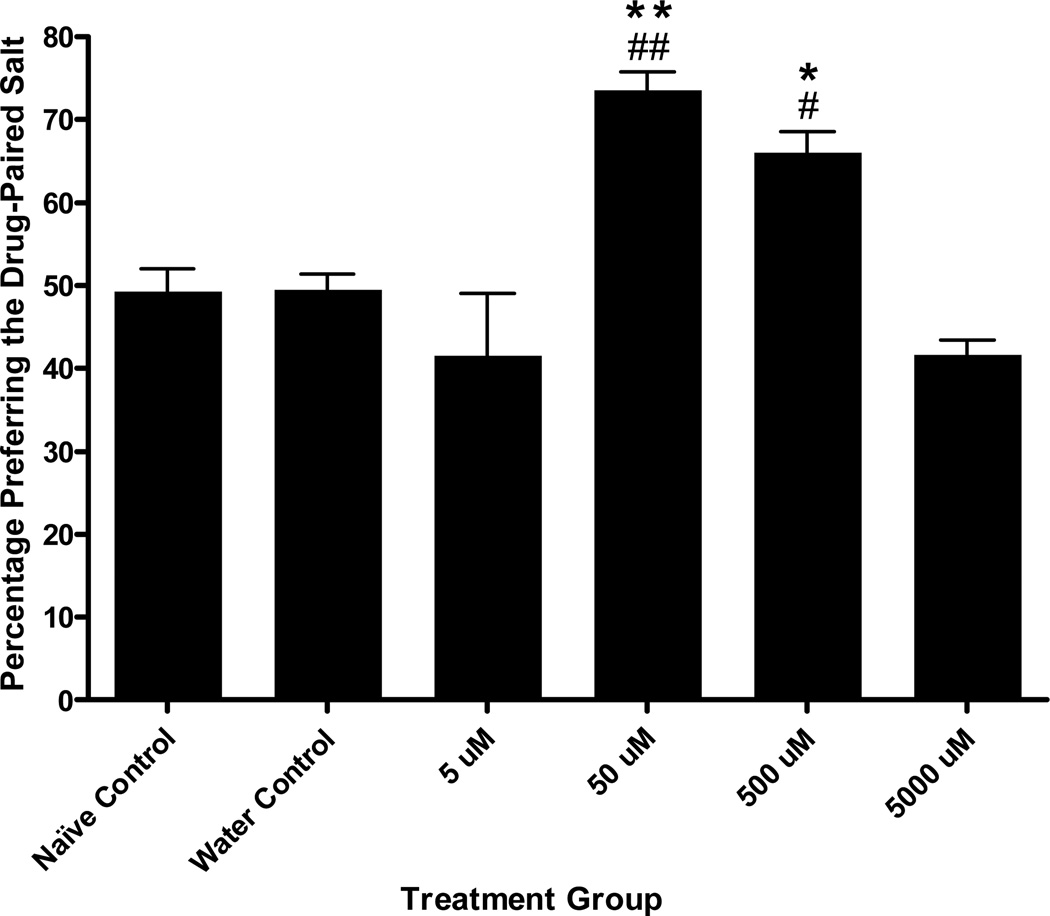
Figure 4.
Cocaine Conditioned Salt Cue Preference. A one-way ANOVA revealed a significant effect of treatment group on final preference for the drug-paired salt cue, F (6, 82) = 4.62, p < .001. Tukey’s post hoc analyses showed that the 50 µM cocaine treatment during conditioning resulted in a significant increase in preference for the drug-paired chemosensory cue compared with naïve controls and water controls. No other concentration group differed significantly in preference from either control group. Bars represent means ± SEM (n values from left to right: 19, 24, 8, 13, 8, and 8 plates). *p < .01, compared to naïve controls; # p < .01, compared to water controls
Similar to cocaine, MAP also induced a conditioned preference. A one-way ANOVA revealed that worms conditioned with MAP showed a concentration-dependent preference for the ion paired with MAP during conditioning, F(6, 87) = 16.20, p < .01 (Figure 5). Tukey’s post hoc analyses revealed that both the 50 µM and 500 µM MAP treatment groups showed significantly higher preference for the drug-paired salt ion cue compared with naïve controls (50 µM: p < .001; 500 µM: p = .01) and vehicle/water controls (50 µM: p < .001; 500 µM: p < .01). No other MAP treatment groups were significantly different from either control. Cocaine and MAP conditioned preference testing occurred concurrently, thus water controls (i.e., drug concentration of 0 µM) were the same for both drugs.
Figure 5.
MAP Conditioned Salt Cue Preference. A one-way ANOVA showed a significant effect of treatment group on final preference for the drug-paired salt context, F (6, 87) = 16.20, p < .01. Tukey’s post hoc analyses showed that both the 50 µM and 500 µM MAP treatment groups resulted in significantly higher preference for the drug-paired chemosensory cue compared with controls while no other concentration groups differed from controls. Bars represent means ± SEM (n values from left to right: 19, 24, 10, 15, 10, and 5 plates). *p < .05, compared to naïve controls; **p < .001, compared to naïve controls; # p < .01, compared to water controls; ## p < .001, compared to water controls
Several negative controls were conducted to rule out alternative explanations for the observed conditioning (Figure 3, left). To determine whether presentation of the drug was changing final preference between sodium and chloride, worms were exposed to MAP or cocaine without any conditioning or pairing with salt ions (UCS alone, Figure 3, left). Worms exposed to a 50 µM MAP UCS alone showed no preference for one ion-type over the other, t(5) = .08, p = .94, nor did worms exposed to the 500 µM MAP UCS, t(5) = .46, p = .67. Likewise, worms exposed to 50 µM cocaine alone did not prefer a particular salt ion, t(5) =.72, p = .51, nor did worms exposed to the 500 µM cocaine UCS, t(5) = .99, p = .42. A second negative control consisted of animals undergoing the entire conditioning process without the presentation of any UCS. Worms exposed to both CS ions sequentially, with no UCS presentation, did not show any final preference for either CS ion during testing, t(23) = −.27, p = .78. The final negative control analysis was determination of backward conditioning effects. These unpaired controls had presentation of drug prior to exposure to both CS ions and showed no preference for either CS ion at testing, t(10) = −1.26, p = .24.
Dopamine-deficient worms do not show salt cue conditioning
Dopamine plays a role in addiction-related behavior (for review, see Koob & Volkow, 2010). In C. elegans, dopamine has been found to be important in several behavioral plasticities including mechanosensory behavior plasticity (Sanyal et al., 2004), gustatory plasticity (Hukema et al., 2008), state-dependent learning (Bettinger & McIntire, 2004), and ethanol-induced plasticity (Lee et al., 2009; Mitchell et al., 2010). The cat-1 mutants are defective in vesicle packaging of dopamine (and other neuromodulators, including serotonin) because the cat-1 gene encodes a vesicular monoamine transporter (Duerr et al., 1999). The cat-2 mutants lack the gene encoding tyrosine hydroxylase which is needed for the synthesis of dopamine (Lints & Emmons, 1999). Both mutants have decreased levels of dopamine compared to N2 worms (Sanyal et al., 2004). Because dopamine is involved in both drug-induced behavior and drug-dependent learning in C. elegans, we determined whether dopamine neurotransmission played a role in drug-induced chemosensory cue conditioning.
At 50 µM concentrations, both MAP and cocaine were shown to induce preference for the salt ion cue that they had been paired with during conditioning for N2 worms (Figure 4 and Figure 5). When cat-1 and cat-2 mutants were conditioned with 50 µM MAP, one-way ANOVAs showed no effects of treatment on preference for the drug-paired ion [Figure 6; cat-1: F(3, 34) = 0.25, p = .86; cat-2: F(3, 40) = 1.76, p =.17]. A two-way ANOVA (Strain×Treatment) revealed a main effect of strain on final preference for the drug-paired salt cue, F(2, 158) = 26.71, p < .001, a main effect of treatment on final preference, F(3, 158) = 5.87, p < .001, and a significant interaction between strain and treatment on final salt preference, F(6, 158) = 3.15, p < .01. Tukey’s post hoc analyses showed no differences between the strains in either of the control treatment groups. However, N2 worms were found to have significantly higher preference for the drug-paired salt cue than either of the dopamine-deficient mutants when exposed to 50 µM cocaine during conditioning (compared to cat-1, p < .001; compared to cat-2, p < .01). Additionally, N2 worms showed greater preference for the drug-paired cue than the mutants when conditioned with 50 µM MAP (compared to cat-1, p < .001; compared to cat-2, p < .01).
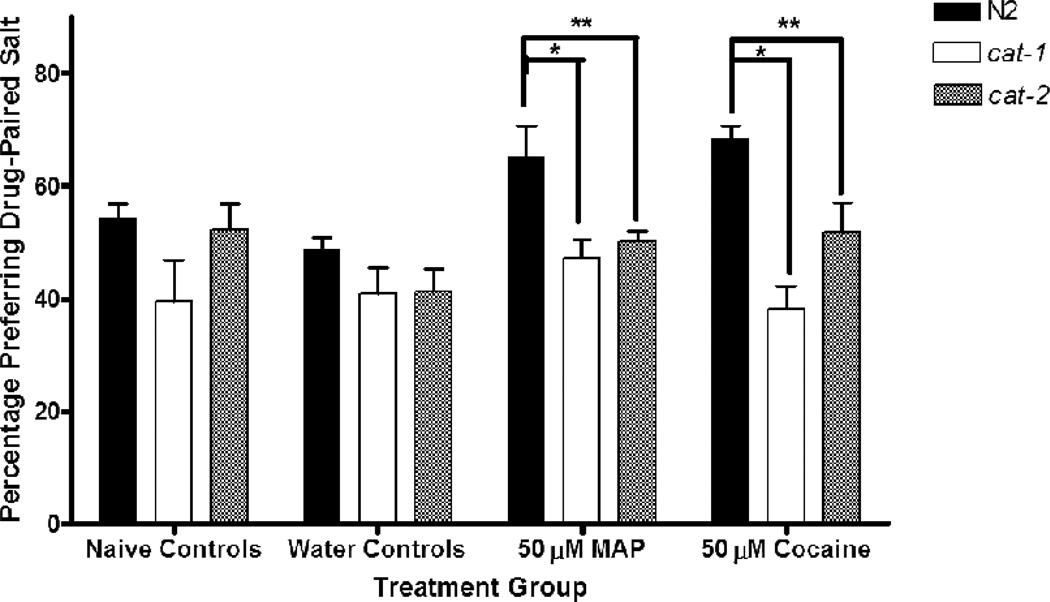
Figure 6.
Comparing N2 Wild-type with Dopamine Deficient Mutants in Stimulant Conditioned Salt Cue Preference. A two-way factorial ANOVA showed a main effect of strain on final preference for the drug-paired salt cue [F (2, 138) = 21.99, p < .001], a main effect of treatment [F (3, 138) = 5.01, p < .01], but no interaction between strain and treatment. Tukey’s post hoc analyses showed no differences between the strains in either of the control treatment groups. However, N2 wild-type worms were found to have significantly higher preference for the drug-paired salt cue than either of the dopamine-deficient mutants when exposed to 50 µM cocaine during conditioning (compared to cat-1, p < .001; compared to cat-2, p = .03). Additionally, N2 worms showed greater preference for the drug-paired cue than the mutants when conditioned with 50 µM MAP (compared to cat-1, p = .001; compared to cat-2, p = .004). Bars represent means ± SEM (n values from left to right: 14, 19, 14, 16, 7, 8, 12, 8, 7, 11, 13, and 10 plates). * p < .05; ** p < .001
Similar to N2 worms, the cat-1 and cat-2 mutants showed no differences in final ion preference during UCS alone and unpaired negative controls (50 µM cocaine UCS alone, t(5) = .83, p = .44; 50 µM MAP UCS alone, t(5) = .73, p = .49; 50 µM cocaine unpaired controls, t(9) = .35, p = .74; 50 µM MAP unpaired controls: t(9) = 1.72, p = .12; data not graphed). However, unlike N2 worms, who showed a robust response for a salt cue that had been paired with food during conditioning (see Figure 3), neither the cat-1 or cat-2 strains showed any learning when using food cues as the UCS (data not graphed).
Additionally, we attempted to rescue the stimulant learning in the cat-1 and cat-2 strains by adding exogenous dopamine to the conditioning plates. N2, cat-1, and cat-2 strains were tested for a learned association between a salt cue and 50 µM cocaine or 50 µM MAP, while both the CS+ and CS− conditioning plates and testing plates contained a range from 5–25 mM dopamine. The addition of dopamine to conditioning and testing plates resulted in the rescue of both cocaine and MAP salt ion conditioning for both the cat-1 and cat-2 mutants when compared to their water controls (see Table 1). These findings differ from the N2 strain, where demonstration of learned association between the MAP cue and the salt ion cue occurs whether additional dopamine is present or not, although exogenous dopamine appeared to block the learned association between cocaine and the ion cues (Table 1). Perhaps this block with cocaine learning occurs because the exogenous dopamine induces overstimulation, thus negating the effectiveness of cocaine to serve as an unconditioned stimulus.
Table 1.
Average percentage ± SEM of worms per plate preferring the drug-paired salt cue.
| Strain | Group | Cocaine | MAP |
|---|---|---|---|
| N2 | Control | 48.70 ± 2.13 | 48.70 ± 2.13 |
| Drug | 69.13 ± 5.32 * | 66.07 ± 2.07 * | |
| Drug + DA | 55.35 ± 2.98 | 60.51 ± 2.34 * | |
| cat-1 | Control | 45.32 ± 3.11 | 45.32 ± 3.11 |
| Drug | 38.15 ± 3.68 | 44.56 ± 3.87 | |
| Drug + DA | 60.32 ± 5.25 * | 57.92 ± 1.51 * | |
| cat-2 | Control | 46.21 ± 3.41 | 46.21 ± 3.41 |
| Drug | 50.72 ± 1.87 | 51.65 ± 5.09 | |
| Drug + DA | 61.00 ± 3.62 * | 55.78 ± 9.31 * |
N = 8+ for each cell. Control = Water Controls; MAP = Methamphetamine; Drug = 50 µM drug paired with salt cue during conditioning; Drug + DA = Dopamine present on both CS+ and CS− plates during conditioning and present on final salt preference assay plates.
Average percentage of worms preferring cue paired with drug during conditioning, p < 0.05 vs. water controls (Tukey’s t-test).
Food cues can also be used as conditioned stimuli
In previous chemosensory cue conditioning studies with C. elegans, salt cues were used as the conditioned stimuli to pair with unconditioned stimuli such as food. However, it has been shown that C. elegans demonstrate choice behavior when presented with multiple different bacterial strain options (Zhang et al., 2005; Shtonda & Avery, 2006). We developed a conditioned food cue assay similar to the conditioned salt cue assay where the “neutral” cues during conditioning and in final preference testing became the E. coli strains OP50 and NA22. However, initial observation using naïve controls, water controls, unpaired controls, and drug alone controls revealed that the worms did demonstrate a baseline preference for the NA22 bacteria over the OP50 (see Figure 7 for negative controls: for naïve controls: preference for OP50 = 38.35 ± 2.61%, preference for NA22 = 61.65 ± 2.61%, t(15) = −4.47, p < .01; for CS1− <--> CS2− (water) controls: preference for OP50 = 43.32 ± 1.68%, preference for NA22 = 56.68 ± 1.68%, t(34) = −3.97, p < .01; for 50 µM cocaine alone controls: preference for OP50 = 38.77 ± 2.56%, preference for NA22 = 61.23 ± 2.56%, t(9) = −3.40, p < .05; for 50 µM MAP drug alone controls: preference for OP50 = 44.11 ± 1.52%, preference for NA22 = 55.89 ± 1.52%, t(9) = 2.99, p < .05; for 50 µM cocaine unpaired controls: preference for OP50 = 41.33 ± 2.04%, preference for NA22 = 58.67 ± 2.04%, t(9) = −4.25, p < .01; for 50 µM MAP unpaired controls: preference for OP50 = 38.77 ± 3.70%, preference for NA22 = 61.23 ± 3.70%, t(9) = −3.04, p < .01). Thus, for the food cue conditioning experiments, the least preferred food type (OP50) was used as the conditioned stimulus to determine if drug conditioning could overcome the baseline preference for NA22 over OP50.
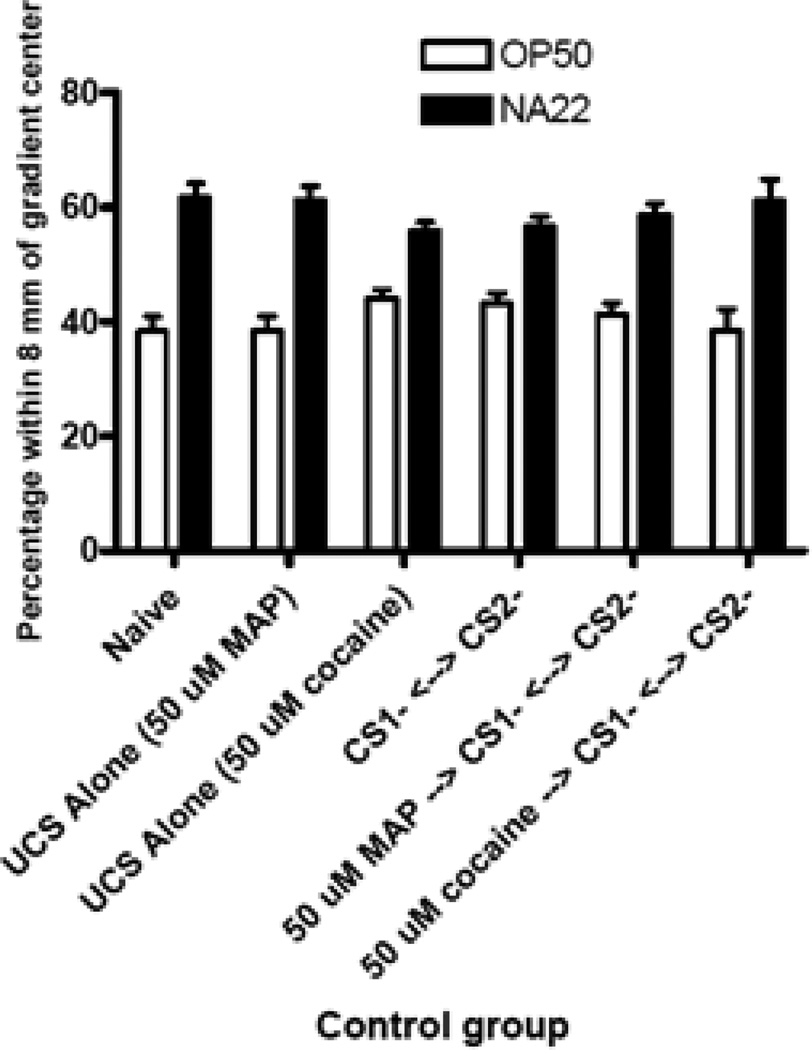
Figure 7.
Food Cue Conditioning Negative Controls. All controls revealed significant differences in bacterial strain preference, each control preferring the NA22 bacteria: naïve, t(15) = −4.47, p < .01; for CS1− <--> CS2− controls, t(34) = −3.97, p < .01; for UCS Alone (50 uM cocaine), t(9) = − 3.40, p < .05; for UCS Alone (50 uM MAP) controls, t(9) = 2.99, p < .05; for 50 µM cocaine unpaired controls, t(9) = −4.25, p < .01; for 50 µM MAP unpaired controls, t(9) = −3.04, p < .05. Bars represent means ± SEM (n values from left to right: 16, 35, 10, 10, 10, and 10 plates).
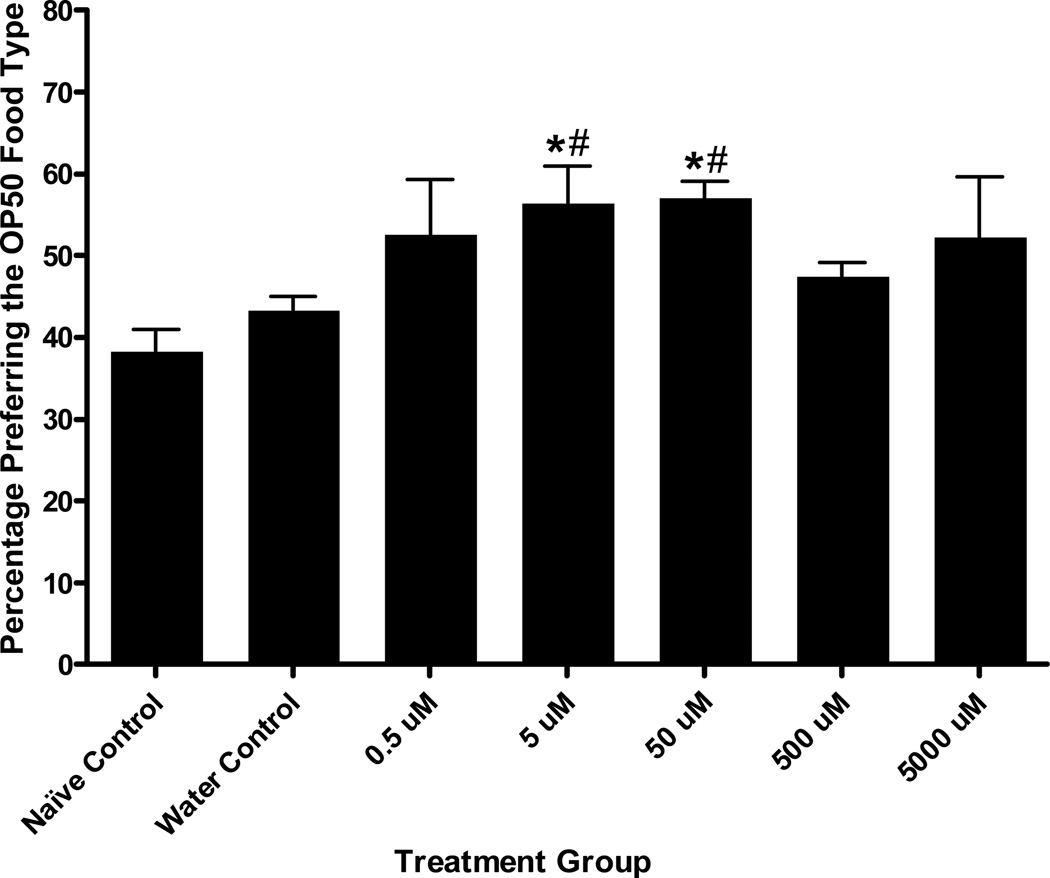
Pairing MAP with OP50 during conditioning produced a significant effect on OP50 preference during testing, F(6, 93) = 2.43, p < .04 (Figure 8). Because no order effects were seen during salt ion conditioning (i.e., preference was not altered due to CS+ being presented first or second), all food conditioning experiments were completed using the CS+ (OP50) as the first cue. Further, more replicates were completed at drug concentrations that elicited effects during salt ion conditioning. Tukey’s post hoc analyses revealed a significant increase in preference for the drug-paired food cue for the 50 µM MAP treatment group over naïve controls (p < .01) and a trend towards increased preference compared to water controls (p = .08). No other treatment groups were found to be significantly different from either control. Similarly, when cocaine was the UCS, a one-way ANOVA showed a significant effect of treatment group on final preference for the drug-paired food cue, F(6, 94) = 7.55, p < .001 (Figure 9). Tukey’s post hoc analyses showed that the 5 µM and 50 µM cocaine groups showed significantly greater preference for the drug-paired food than either of the controls (5 µM: compared to naïve controls, p < .001, compared to water controls p < .01; 50 µM: compared to naïve controls, p < .001, compared to water controls, p < .01). No other treatment groups were different from either control.
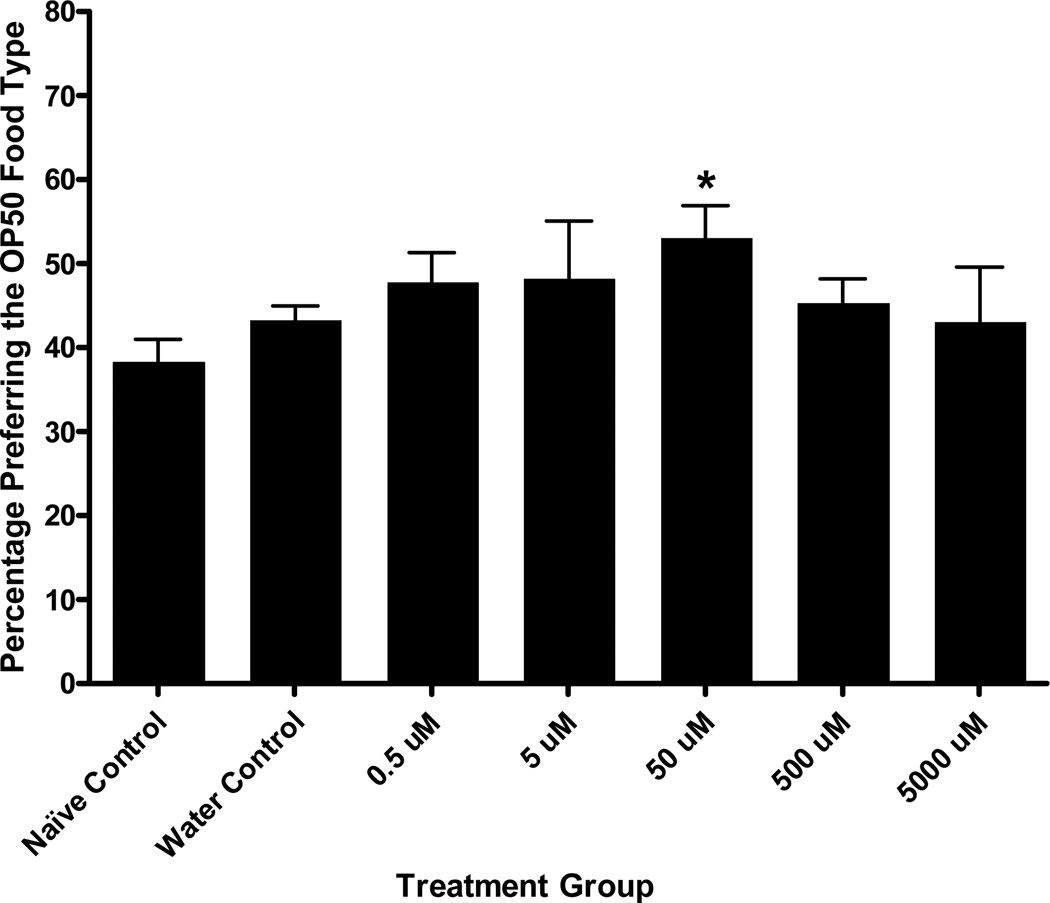
Figure 8.
MAP Conditioned Food Cue Preference. These assays represent a pairing of MAP with the food-type OP50. A one-way ANOVA showed a significant effect of treatment group on final preference for the drug-paired food cue, F (6, 93) = 2.43, p < .04. Tukey’s post hoc analyses showed that the 50 µM MAP treatment during conditioning caused a significant increase in preference for the drug-paired chemosensory cue compared with naïve controls and a trend towards significantly greater preference over water controls (p = .08). The controls were not significantly different from any other treatment groups. Bars represent means ± SEM (n values from left to right: 16, 35, 4, 4, 16, 15, and 4 plates). * p < .01 over naïve controls
Figure 9.
Cocaine Conditioned Food Cue Preference. These assays represent a pairing of the cocaine unconditioned stimulus with the OP50 food-type. A one-way ANOVA showed a significant effect of treatment group on final preference for the drug-paired food cue, F (6, 94) = 7.55, p < .001. Tukey’s post hoc analyses showed that the 5 µM and 50 µM cocaine groups showed significantly higher final preference for the food paired with drug than either of the control groups. No other treatment levels were significantly different from water controls. Bars represent means ± SEM (n values from left to right: 16, 35, 4, 11, 13, 12, and 4 plates). *p < .001 compared to naïve controls; # p < .01 compared to water controls
Discussion
Associative learning in C. elegans has previously been confined to pairing cues with appetitive stimuli (i.e., food; Morrison & van der Kooy, 1997; Wen et al., 1997) or aversive stimuli (e.g., starvation: Nuttley et al., 2002; Saeki et al., 2001). This study expanded the unconditioned stimuli to include stimulant drugs that are known to be robust unconditioned stimuli in vertebrate (for comprehensive reviews, see Cunningham et al., 2006; Tzschentke, 2007) and other invertebrate models (Huber et al., 2011; Kusayama & Watanabe, 2000; for ethanol, see Kaun et al., 2011). A simple choice measurement was used to show drugconditioned learning in C. elegans, employing the same principles used to study drug reward in higher level organisms. Previous studies have shown C. elegans respond behaviorally to cocaine and amphetamine, and these responses are at least partially dependent on serotonergic and dopaminergic neurotransmission (Carvelli et al., 2010; Ward et al., 2009). Using similar concentrations, we have shown that C. elegans can associate these drugs with chemosensory cues and that preference for these cues increases following repeated pairings. Using chemosensory cues, C. elegans showed an increased preference for the salt ion that was paired with cocaine or MAP (see Figures 4, 5). Further, the worms show that different bacterial cultures can be used as chemosensory cues in associative learning. C. elegans showed increased preference for the baseline less-preferred food when that food had been paired with cocaine or MAP during conditioning (see Figures 8, 9). The current findings provide evidence of possible reward mechanisms in C. elegans that are activated by stimulants. The prospect for modeling some complex behavioral states such as “motivation” in C. elegans is debatable (Mitchell et al., 2010). However, the development of preference for a cue that was previously paired with drugs, suggests that some conserved aspects of reward behavior may also be evident in C. elegans (Cunningham et al., 2006; Tzschentke, 2007). Moreover, the current data are also consistent with recent findings in which drosophila melanogaster demonstrate increased preference responses to cues that had been previously paired with ethanol (Kaun et al., 2011). Together, these findings support the hypothesis that some mechanisms mediating the rewarding properties of drugs of abuse may be conserved in invertebrates. Thus, invertebrates may prove to be valuable model organisms in the study the neurobiological basis of addiction.
The observed stimulant-induced associative learning was dose-dependent. This suggests it was not just the presence of drug that altered the preference for one chemosensory cue over the other, but rather the effects on the internal state of the worm which was the UCS. If it was solely the presence of drug during conditioning that changed chemotaxis, then the increased preference would have been observed at all concentrations. Further, previous exposure to stimulants alone, with no pairing of salt ion cues, did not alter future preference for either ion over naïve controls (see Figure 3). These findings suggest that the drugs themselves are not altering preference, but that alterations in the internal states produced by the drugs are being associated with learned cues as evidenced by subsequent behavioral changes observed in the absence of the drug.
Drug-paired classical conditioning can be used as a paradigm for the measurement of the rewarding properties of drugs because when animals seek out environments previously associated with drugs, it is believed to be because the drugs were rewarding. We developed our assay from previous conditioning procedures where food was used as the UCS (Wen et al., 1997). It is possible that drug rewards and food rewards are fundamentally different when used as UCS (Spiteri et al., 2000). It has been argued that the preference observed after food conditioning is a measurement of the consummatory properties of the food, while druginduced CPP is measuring the affective properties from the drug (Spiteri et al., 2000). However, in this study, both the food UCS and drug UCS were given simultaneously with the chemosensory cues, so all final preference choices should be considered a reflection of the association with both the consummatory and affective properties of the UCS’s. Further, unpaired controls showed that no associative learning occurred when the worms were exposed to the UCS at a different timepoint than presentation of the chemosensory cue.
In addition to the development of a C. elegans measurement of drug conditioned preference, we also were able to demonstrate that the drug conditioning occurred with different types of conditioned stimuli. Previous conditioning literature has used salt ions, odorants, and temperature as “neutral” stimuli or cues for associative conditioning (for review, see Ardiel & Rankin, 2010). It has also been shown that worms do exhibit dietary choice behavior (Shtonda & Avery, 2006). The present study used the ability of C. elegans to sense differences in food choices as cues for pairing with cocaine and MAP unconditioned stimuli. It was found that no conditioning was observed when the drugs were paired with the NA22 bacteria. All the N2 young adults tested had been raised on NA22 bacteria and when tested for preference, both acutely and following non-drug paired conditioning controls, showed a higher preference for NA22 than OP50. It is possible that there is just a ceiling effect of preference shown during testing for NA22 and the pairing of any rewarding UCS just could not increase preference. It is also possible that the effects of “dwelling” could be affecting final preference ratios. Worms typically will slow down when entering a bacterial lawn (de Bono & Maricq, 2005), which may limit the ability of the worms to leave that area and go to another, although it has been reported that in a 2-choice assay between two bacterial types, worms quickly went to the chosen food environment and did not leave the lawn (Zhang et al., 2005). However, consistent with the current results, if drugs of abuse in this paradigm are indeed producing these effects by activating reward systems already in place for natural rewards (e.g., food), one might expect worms to show similar responses to the drug-associated cues as they would for food.
The mechanisms underlying addiction are still not fully understood. The dopamine reward pathway is thought to be involved, particularly in the plasticity of behavior going from drug use to drug abuse (Chen et al., 2009; Koob & Volkow, 2010). Previously, associative learning in C. elegans has been found to be modulated by monoaminergic systems (Hukema et al., 2008) and dopamine-deficient mutants were found to be impaired in state-dependent learning (Bettinger & McIntire, 2004). In the current study, two DA-deficient mutants were tested and did not demonstrate cocaine or MAP conditioned salt cue preference at concentrations seen in N2 wild-type worms. These data are consistent with findings in drosophila in which dopamine neurotransmission was required for the development of a conditioned preference response using ethanol as the UCS (Kaun et al., 2011). Further, in the current study, neither the cat-1 or cat-2 mutants showed a conditioned response when using food as the UCS, although dopamine-deficient mutants have been found to have problems sensing food (Duerr et al., 1999; Sawin et al., 2000). Ward et al. (2009) found that the cat-2 mutants did not differ from N2 worms in their locomotor response to cocaine exposure; instead, they found that serotonin modulated cocaine-induced locomotion changes. Further, cat-2 mutants also have been found to be normal in olfactory learning from pre-exposure to pathogenic bacteria [a conditioned taste aversion (CTA)-like paradigm], while the serotonin system largely moderated the aversive learning response (Zhang et al., 2005). However, we found that cat-2 mutants showed no cocaine conditioned preference. This suggests that the acute behavioral effects of cocaine and the conditioned preference plasticity effects could be regulated by different pathways and also that different types of associative learning (conditioned aversion vs. conditioned preference) could be regulated by different systems. Similar to the current findings, monoaminergic-pathway mutants in vertebrates also have shown alterations in stimulant-induced classically conditioned behavior (Budygin et al., 2004; Sora et al., 2001). In different learning paradigms, learning responses have been rescued in these dopamine-deficient strains by exposure to exogenous dopamine (Matsuura et al., 2010). Consistent with these findings, exposure to exogenous dopamine rescues the conditioned preference response in both strains using either cocaine or MAP as the UCS. It is unclear why the increase in the learned response was more robust in the cat-1 strain as compared to the cat-2 strain. These strains have been shown to have different intracellular amounts of dopamine present (Sanyal et al., 2004), possibly resulting in different adaptations in dopamine processing or receptor systems that may impact the effectiveness of rescue via exogenous dopamine exposure. Regardless, these results support the hypothesis that the dopaminergic system is involved in the stimulant associative learning response in C. elegans.
Chemosensory cue conditioning appears to be a useful assay for the analysis of drug-paired associative learning in C. elegans. The strength of the design is testing in the absence of any drug which can confound interpretations in many other addiction-related animal models. We have demonstrated that C. elegans develops cocaine and MAP conditioned cue preference. By studying addiction-related behaviors in a simple nervous system that is amenable to identification of molecules, neurons, and circuits, we may be able to better understand the mechanisms underlying addictions and identify novel regulators of this reward-based plasticity.
Acknowledgments
This research was supported by NIAAA Training Grant AA07462 awarded to Heather N. Musselman.
The authors thank Dr. James M. Murphy for providing helpful comments on the manuscript
References
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133(4):897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardiel EL, Rankin CH. An elegant mind: learning and memory in Caenorhabditis elegans. Learning and Memory. 2010;17:191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Chemosensation in C. elegans. In: WormBook, editor. The C. elegans Research Community, WormBook. 2006. doi/10.1895/wormbook.1.123.1, http://www.wormbook.org. [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- Bettinger JC, McIntire SL. State-dependency in C. elegans. Genes, Brain and Behavior. 2004;3:266–272. doi: 10.1111/j.1601-183X.2004.00080.x. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Brodie MS, Sotnikova TD, Mateo Y, John CE, Cyr M, Gainetdinov RR, Jones SR. Dissociation of rewarding and dopamine transporter-mediated properties of amphetamine. Proc Natl Acad Sci USA. 2004;101(20):7781–7786. doi: 10.1073/pnas.0401418101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Matthies DS, Galli A. Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Molecular Pharmacology. 2010;78:151–156. doi: 10.1124/mol.109.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nature Neuroscience. 2004;10:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Sinha S. Understanding the mind of a worm: hierarchical network structure underlying nervous system function in C. elegans. Progress in Brain Research. 2008;168:145–153. doi: 10.1016/S0079-6123(07)68012-1. [DOI] [PubMed] [Google Scholar]
- Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system. Annals of the New York Academy of Sciences. 2010;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learning & Memory. 1997;4:179–191. doi: 10.1101/lm.4.2.179. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nature Protocols. 2006;1(4):1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- Davis JR, Li Y, Rankin CH. Effects of developmental exposure to ethanol on Caenorhabditis elegans. Alcoholism: Clinical and Experimental Research. 2008;32(5):853–867. doi: 10.1111/j.1530-0277.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. Journal of Neuroscience. 1999;19(1):72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Ailion M, Lockery SR. Ammonium-acetate sensed by gustatory and olfactory neurons in Caenorhabditis elegans. PLoS One. 2008;3(6):e2467. doi: 10.1371/journal.pone.0002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer ER, Perez CL, Van Gilst MR, Lee BH, Ashrafi K. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metabolism. 2008;8(2):118–131. doi: 10.1016/j.cmet.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Panksepp JB, Nathaniel T, Alcaro A, Panksepp J. Drug-sensitive reward in crayfish: an invertebrate model system for the study of SEEKING, reward, addiction, and withdrawal. Neuroscience and Biobehavioral Reviews. 2011 doi: 10.1016/j.neubiorev.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukema RK, Rademakers S, Jansen G. Gustatory plasticity in C. elegans involves integration of negative cues and NaCl taste mediated by serotonin, dopamine, and glutamate. Learning and Memory. 2008;15:829–836. doi: 10.1101/lm.994408. [DOI] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nature Reviews Drug Discovery. 2006;5(5):387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. A Drosophila model for alcohol reward. Nature Neuroscience. 2011;14(5):612–619. doi: 10.1038/nn.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Li TK. What is addiction? Alcohol Research & Health. 2008;31(2):93–95. [PMC free article] [PubMed] [Google Scholar]
- Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000;11(113):2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- Law E, Nuttley WM, van der Kooy D. Contextual taste cues modulate olfactory learning in C. elegans by an occasion-setting mechanism. Curr Biol. 2004;14:1303–1308. doi: 10.1016/j.cub.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Lee J, Jee C, McIntire SL. Ethanol preference in C. elegans. Genes, Brain and Behavior. 2009;8:578–585. doi: 10.1111/j.1601-183X.2009.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints R, Emmons SW. Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development. 1999;126(24):5819–5831. doi: 10.1242/dev.126.24.5819. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Oda T, Hayashi G, Sugisaki D, Ichinose M. Enhancement of chemotactic response to sodium acetate in the nematode Caenorhabditis elegans. Zoological Science. 2010;27(8):629–637. doi: 10.2108/zsj.27.629. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Bull K, Glautier S, Hopper NA, Holden-Dye L, O’Connor V. The concentration-dependent effects of ethanol on Caenorhabditis elegans behaviour. The Pharmacogenomics Journal. 2007;7:411–417. doi: 10.1038/sj.tpj.6500440. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Mould R, Dillon J, Glautier S, Andrianakis I, James C, Pugh A, Holden-Dye L, O’Connor V. A differential role for neuropeptides in acute and chronic adaptive responses to alcohol: behavioural and genetic analysis in Caenorhabditis elegans. PLoS ONE. 2010;5(5):e10422. doi: 10.1371/journal.pone.0010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I. Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annual Review of Genetics. 1999;33:399–422. doi: 10.1146/annurev.genet.33.1.399. [DOI] [PubMed] [Google Scholar]
- Morrison GE, van der Kooy D. Cold shock before associative conditioning blocks memory retrieval, but cold shock after conditioning blocks memory retention in Caenorhabditis elegans. Behavioral Neuroscience. 1997;111(3):564–578. doi: 10.1037//0735-7044.111.3.564. [DOI] [PubMed] [Google Scholar]
- Nass R, Hamza I. The nematode C. elegans as a model to explore toxicology in vivo: solid and axenic growth culture conditions and compound exposure parameters. Curr Protocols Toxicology. 2007:1.9.1–1.9.18. doi: 10.1002/0471140856.tx0109s31. [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Atkinson-Leadbeater KP, van der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99(19):12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH. Context conditioning in habituation in the nematode Caenorhabditis elegans. Behavioral Neuroscience. 2000;114(3):496–505. [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. The Journal of Experimental Biology. 2001;204:1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hebert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HHM. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. The EMBO Journal. 2004;23:473–482. doi: 10.1038/sj.emboj.7600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Schafer WR. Deciphering the neural and molecular mechanisms of C. elegans behavior. Current Biology. 2005;15:R723–R729. doi: 10.1016/j.cub.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Sengupta P. Generation and modulation of chemosensory behaviors in C. elegans. Pflugers Arch. 2007;454:721–734. doi: 10.1007/s00424-006-0196-9. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Koob GF. Recent advances in animal models of drug addiction. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 1381–1397. [Google Scholar]
- Shtonda BB, Avery L. Dietary choice behavior in Caenorhabditis elegans. The Journal of Experimental Biology. 2006;209:89–102. doi: 10.1242/jeb.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li X-F, Wei H-B, Wichems C, Lesch K-P, Murphy DL, Uhl GR. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98(9):5300–5305. doi: 10.1073/pnas.091039298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri T, Le Pape G, Agmo A. What is learned during place preference conditioning? A comparison of food- and morphine-induced reward. Psychobiology. 2000;28:367–382. [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addiction Biology. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Ward A, Walker VJ, Feng Z, Xu XZS. Cocaine modulates locomotion behavior in C. elegans. PLoS ONE. 2009;4(6):e5946. doi: 10.1371/journal.pone.0005946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc. Nat. Acad. Sci. USA. 1973;70(3):817–821. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JYM, Kumar N, Morrison G, Rambaldini G, Runciman S, Rousseau J, van der Kooy D. Mutations that prevent associative learning in C. elegans. Behavioral Neuroscience. 1997;111(2):354–368. doi: 10.1037//0735-7044.111.2.354. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Heberlein U. Invertebrate models of drug abuse. Journal of Neurobiology. 2003;54(1):161–178. doi: 10.1002/neu.10166. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]