Abstract
Studies of hematologic abnormalities in HIV infected patients are confounded by a multitude of factors. A retrospective data analysis of SIV infected Rhesus macaques (RM) of Indian origin was performed to determine the prevalence of hematologic abnormalities free of these confounds. Hematologic data from rhesus macaques inoculated with SIV and without antiviral therapy were examined pre-inoculation, and throughout infection and the development of AIDS. Anemia, thrombocytopenia, lymphopenia, eosinophilia, and neutropenia all increased in prevalence with SIV infection. Significant increases in prevalence for both neutropenia and neutrophilia were also detected in SIV-infected macaques. SIV-infected macaques also had lower lymphocyte counts and increased prevalence of lymphopenia compared to non-infected subjects. The prevalence of eosinophilia was significantly increased during SIV infection. Concordance of hematologic abnormalities during SIV infection of macaques with similar changes in HIV infection of humans suggest that, like in HIV infection, hematologic abnormalities are major complications of SIV infection.
INTRODUCTION
Hematologic abnormalities including anemia, lymphopenia, thrombocytopenia, neutropenia, and leukopenia have been reported in HIV infection and AIDS (1-4). However, there is considerable debate whether these abnormalities are caused by HIV infection alone, concurrent opportunistic infections, anti-retroviral therapy (ART), intravenous (IV) drug usage, or other lifestyle factors.
Early studies usually involved patients with advanced HIV disease, often using opportunistic infections as diagnostic criteria, so hematologic changes were often attributed to opportunistic infections such as Pneumocystis carinii pneumonia. Further, early studies were often confounded by the use of anti-microbials and later studies by ART. For example azidothymidine (AZT) trials started in 1986 in the USA, and this and other drugs were eventually shown to have significant side effects including anemia and other hematologic abnormalities (5). An increased risk of anemia in HIV patients naïve for antiviral therapy was observed in African Americans (6). However, the presence of sickle cell or other potential race-linked confounding conditions were not included in the analyses. Also, older patients, African Americans, and males are more likely to become thrombocytopenic during HIV disease (7). HIV patients infected by heterosexual relations were reported to be at higher risk to develop thrombocytopenia compared to homosexuals, bisexual, IV drug users, transfusion recipients, or hemophiliacs (7). Finally, neutropenia is often observed in HIV patients on drug therapy or in late stages of AIDS (8, 9). In summmary, studies of hematologic abnormalities in HIV infected patients are difficult to interpret and it is not known whether pathogenesis involves direct viral destruction of hematopoietic cells and/or precursors or secondary effects from immune dysfunction (i.e. autoimmune hemolytic anemia) caused by the virus, drugs, or concurrent infections (10-14).
The objective of this study was to determine the frequency of hematologic abnormalities in SIV infected macaques during various periods of infection under well-controlled conditions for comparison with HIV infected patients. We hypothesized hematologic changes during progressive non-interventional SIV infection of Rhesus macaques in well-controlled studies without impact of the above confounding factors would be better for evaluating the effects of lentiviral infection in primates, more similar to those of untreated HIV infected patients, and more representive of changes due to viral infection alone. Close approximations in prevalence of hematologic abnormalities between SIV and HIV would further validate the SIV model for hematopoietic studies in HIV infection and AIDS. Currently, SIV infected RM are the premier animal model for studying HIV infection in humans, as SIV-induced disease recapitulates essentially all features of HIV infection and AIDS in the macaque model.
METHODS
Animals and Database Selection
A retrospective longitudinal evaluation of complete blood count (CBC) data collected from RM of Indian origin, inoculated with SIV between January 1986 and December 2006 was conducted at TNPRC. Criteria for inclusion included the following: >1.75 years old at time of inoculation; a single inoculation with pathogenic SIVB670, SIVmac239, or SIVmac251; normal progression to AIDS (≥260 days post-inoculation or DPI); and serial CBC data collected from 0 DPI until humane sacrifice. Further, all animals were devoid of SIV-related experimental therapy or vaccination throughout the study. AIDS criteria were defined based on the 1993 Centers for Disease Control (CDC) guidelines and limited to opportunistic infections (OI), encephalitis, and/or multi-organ lymphoma confirmed by histopathologic examination at necropsy.
Retrospective analysis of the entire TNPRC experimental animal database revealed 66 Indian RM inoculated with a single dose of SIVmac239, 129 Indian RM inoculated with a single dose of SIVmac251, and 427 Indian RM inoculated with a single dose of SIVB670 during the twenty year time period. However, only a subset of animals met the stringent inclusion criteria that excluded animals younger than 1.75 years at inoculation or those that had been; vaccinated/immunized/ and/or challenged with other viruses, treated for SIV or other diseases, treated with ART or other anti-microbials, or were rapid progressors, and finally had serial CBC data collected at all defined stages of infection. Thus only data from ten SIVmac239 infected, six SIVmac251 infected, and ten SIVB670 infected RM were included in this study (Table 1). The 26 infected subjects ranged in age from 1.77-12.2 years of age at inoculation (mean 4.16 years). Four male and six female RM were examined in the SIVmac239 group with an average of 520 DPI (range 307-1113 DPI), six males were examined in the SIVmac251 group with an average of 814 DPI (range 536-1071 DPI), and five male and five females were examined in the SIVB670 group with an average of 454 DPI (range 278-776 DPI). Criteria for diagnosis of AIDS remained fairly consistent between groups except for animal BE53, inoculated with SIVmac251, diagnosed with multi-organ lymphoma. Each infected RM had at least one CBC drawn during each successive stage of infection. For additional normal naïve controls, a single CBC was obtained from thirty-one Indian RM who had never been on a research protocol but awaiting assignment. Twenty-two male and nine female RM were represented in this control group whose ages ranged from 2.24 to 9.72 years (mean of 5.10 years).
Table 1.
SIV Inoculated Rhesus Macaques examined in this study.
| Animal ID | Sex | Age at Infection (years) | SIV Inoculum | Days Post-Inoculation at Sacrifice | Major AIDS defining criteria at necropsy |
|---|---|---|---|---|---|
| BR20 | M | 6.35 | mac239 | 389 | Pneumocystis pneumonia |
| BV01 | F | 4.55 | mac239 | 309 | Pneumocystis pneumonia |
| CE48 | F | 4.29 | mac239 | 307 | Meningoencephalitis |
| CI65 | F | 3.29 | mac239 | 378 | Intestinal mycobacteriosis |
| CK76 | M | 4.78 | mac239 | 364 | Meningoencephalitis |
| DD88 | F | 2.26 | mac239 | 385 | Intestinal mycobacteriosis |
| EE54 | F | 2.75 | mac239 | 364 | Pneumocystis pneumonia |
| I553 | F | 12.2 | mac239 | 646 | Pneumocystis pneumonia |
| P045 | M | 8.46 | mac239 | 1113 | Pneumocystis pneumonia |
| T798 | M | 6.22 | mac239 | 949 | Intestinal mycobacteriosis |
| AE55 | M | 5.1 | mac251 | 658 | Pneumocystis pneumonia |
| BE53 | M | 3.33 | mac251 | 536 | Lymphoma, multi-organ |
| BE65 | M | 3.33 | mac251 | 1071 | Intestinal mycobacteriosis |
| BI33 | M | 5.17 | mac251 | 899 | Pneumocystis pneumonia |
| BT51 | M | 4.03 | mac251 | 990 | Intestinal mycobacteriosis |
| P503 | M | 10.31 | mac251 | 730 | Pneumocystis pneumonia |
| G010 | F | 1.95 | B670 | 390 | Meningoencephalitis |
| G055 | F | 2.31 | B670 | 343 | Meningoencephalitis |
| G143 | M | 1.93 | B670 | 445 | Cryptospordiosis |
| G164 | M | 1.87 | B670 | 776 | Meningoencephalitis |
| J714 | M | 2.02 | B670 | 317 | Pneumocystis pneumonia |
| N073 | M | 3.88 | B670 | 510 | Intestinal mycobacteriosis |
| N217 | F | 9.91 | B670 | 546 | Intestinal mycobacteriosis |
| P337 | M | 2.04 | B670 | 517 | Intestinal mycobacteriosis |
| R544 | F | 7.87 | B670 | 414 | Meningoencephalitis |
| T270 | F | 1.77 | B670 | 278 | Cryptospordiosis |
Hematologic Data and Definitions
Samples were analyzed using an ADVIA 120 hematology analyzer (Bayer; Tarrytown, NY). Abnormalities were defined based upon CBC reference intervals for the Rhesus macaque. Cytopenia or decreased values were defined as the following: leukopenia (total white blood cell (WBC) count <6,600 cells/μL); lymphopenia (absolute lymphocyte count <2,600 cells/μL); monocytopenia (absolute monocyte count <100 cells/μL); neutropenia (absolute neutrophil count <2,200 cells/μL); and thrombocytopenia (TCP) without gross or microscopic evidence of platelet clumping (platelet count < 193,000 cells/μL). Cytosis or cytophilia were defined as the following: leukocytosis (total WBC count >15,500 cells/μL); basophilia (absolute basophil count >100 cells/μL); eosinophilia (absolute eosinophil count >400cells/μL); lymphocytosis (absolute lymphocyte count >8,600 cells/μL); monocytosis (absolute monocyte count >600 cells/μL); and neutrophilia (absolute neutrophil count >5,600 cells/μL).
Anemia was defined as a low hematocrit value (HCT) (hematocrit <34.8%). Anemia was further characterized by mean cell volume (MCV) and mean cell hemoglobin concentration (MCHC) values. Microcytosis was defined as a decreased MCV value (<63.7 fL), normocytosis was defined as a MCV value within the reference interval (63.7-86.9 fL) and macrocytosis was defined as an increased MCV value (>86.9 fL). Anemia was also classified as hypochromic or normochromic by the MCHC value. Hypochromia was defined as a decreased MCHC value (<28.9 g/dL) and normochromia was defined as a MCHC value within the reference interval (28.9-35.4 g/dL).
Statistical Analysis
Hematologic data from pre-inoculation to humane sacrifice for SIV inoculated RM was organized for evaluation and statistical analyses according to viral inoculation or period of infection. Viral inoculation group(s) was identified by individual inoculum with one of three pathogenic strains: SIVB670, SIVmac239, or SIVmac251. Stage of infection was assigned by days post inoculation (DPI) as uninfected or pre-inoculation controls (CTRL), acute stage (1-42 DPI - ACUTE), chronic asymptomatic (43-120 DPI - CHRONIC), and those that progressed to AIDS (>120 DPI). Differences in hematologic values between control macaques and infected RM were compared using the Mann Whitney non-parametric unpaired t test in GraphPad Prism 5 (GraphPad Software; San Diego, CA). Correlations of CBC data were compared using non-parametric Spearman correlation coefficients in GraphPad Prism 5 (GraphPad Software).
Prevalence rate was defined as the percentage of total macaques with a hematologic abnormality. Incidence rate was defined as the percentage of total macaques observed with the first event of a hematologic abnormality within a defined period. Statistical differences in prevalence between the control and infected subjects arranged by inoculum groups or periods were compared using the Tukey Kramer one way analysis of variance (ANOVA) test in GraphInstat 3 (GraphPad Software). Statistical differences in prevalence and incidence between infected subjects arranged by inoculum groups or periods were compared using the Tukey ANOVA test in GraphInStat 3 (GraphPad Software).
Graphs represent the means, and error bars represent the standard error of the mean (SEM). Statistical analyses were considered significant at p value (p) ≤0.05.
RESULTS
Hematologic Differences Between Non-Infected and SIV Infected Rhesus macaques
SIV-infected macaques had significant increases in the prevalence of anemia accompanied by decreased hemoglobin compared to naïve macaques (Fig. 1, Table 2). Platelet counts were decreased in SIV-infected macaques compared to controls as shown by increased incidence and prevalence of thrombocytopenia in infected cohorts (Fig. 3). Significant increases in prevalence for both neutropenia (Fig. 4) and neutrophilia were also detected in SIV-infected macaques. SIV-infected macaques also had significantly low lymphocyte counts and an increased prevalence of lymphopenia compared to non-infected subjects. Increased prevalence of monocytosis and monocytopenia was detected in SIV-infected macaques, although a difference in monocyte counts was not detected compared to naïve macaques. Eosinophilia prevalence was significantly increased during SIV infection with a trend for increased eosinophil counts compared to non-infected RM. Basophilia was noted with significantly increased prevalence in SIV-infected subjects.
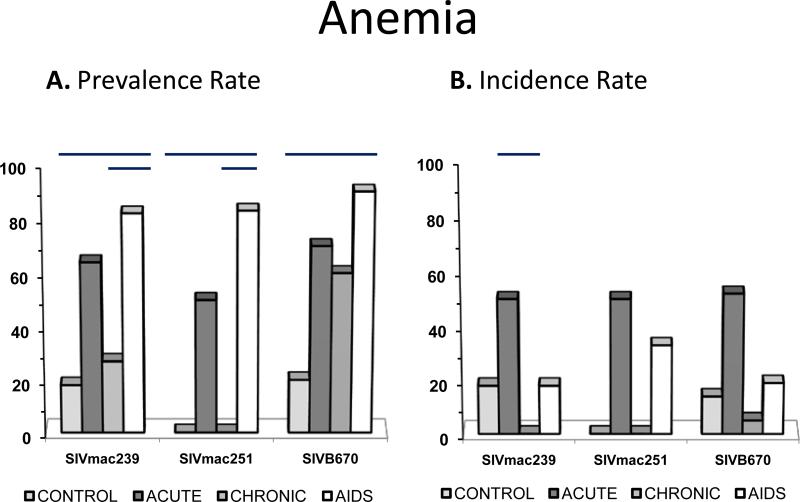
Figure 1. Prevalence and incidence of anemia during SIV infection.
Prevalence rates (A.) and incidence rates (B.) for anemia by period for each viral inoculum. Significance between periods within groups are represented by horizontal lines by ANOVA (p≤0.05).
Table 2.
Prevalence Rate of Hematologic Abnormalities in Naïve and SIV Infected Rhesus macaques
| Hematologic Abnormality | Naïve Subjects (%) | SIV Infected Subjects (%) |
|---|---|---|
| Lymphopenia | 23 | 100* |
| Eosinophilia | 13 | 94* |
| Neutropenia | 0 | 91* |
| Anemia | 3 | 88* |
| Neutrophilia | 10 | 70* |
| Thrombocytopenia | 3 | 66* |
| Monocytosis | 3 | 50* |
| Monocytopenia | 3 | 48* |
| Basophilia | 0 | 22* |
| Microcytosis | 6 | 9 |
| Lymphocytosis | 0 | 3 |
| Hypochromia | 0 | 3 |
| Macrocytosis | 0 | 0 |
p≤0.05
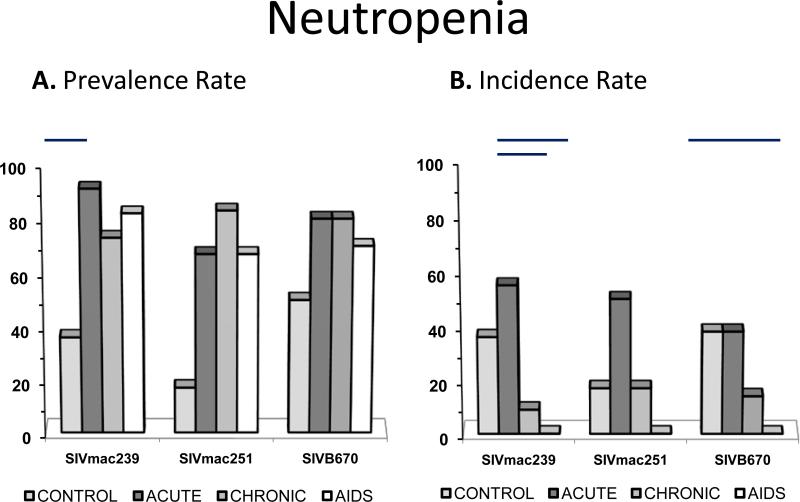
Figure 3. Prevalence and incidence of neutropenia during SIV infection.
Prevalence rates (A.) and incidence rates (B.) for neutropenia by period for each viral inoculum. Significance between periods within groups represented by horizontal lines by ANOVA (p≤0.05).
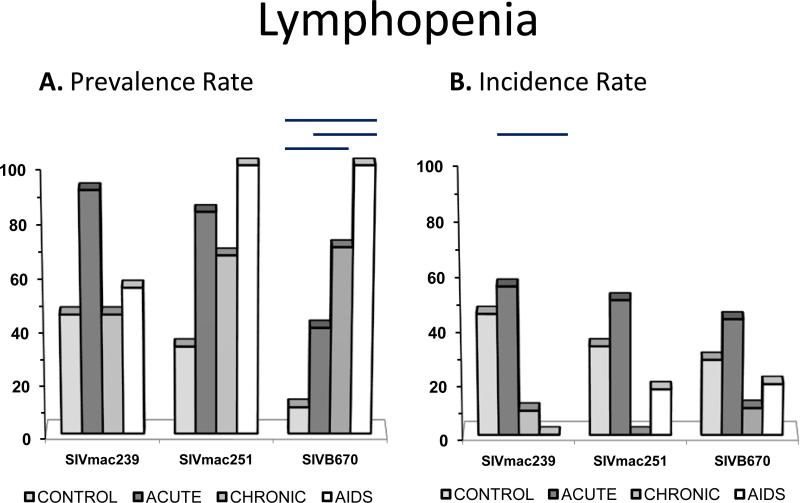
Figure 4. Prevalence and incidence of lymphopenia during SIV infection.
Prevalence rates (A.) and incidence rates (B.) for lymphopenia by period for each viral inoculum. Significance between periods within groups represented by horizontal lines by ANOVA (p≤0.05).
Classification of SIV-infected subjects by inoculum revealed differences when compared by pre-inoculation to post-inoculation CBC data for prevalence of hematologic abnormalities during the course of SIV infection (Table 3). A significant difference was observed between the control group and each SIV inoculum group for prevalence of anemia, TCP, neutropenia, lymphopenia, and eosinophilia. SIVB670 and SIVmac251 infected macaques displayed significant neutrophilia compared to non-infected macaques. Monocytopenia and monocytosis both occurred with increased prevalence in SIVmac239 and SIVB670 infected macaques compared to naïve macaques. Basophilia was only statistically different for SIVB670 macaques.
Table 3.
Significance by p Value Comparing Prevalence Rates of Hematologic Abnormalities for Control and SIV Infected Rhesus macaques sorted by Inoculum
| Hematologic Abnormality | SIVmac239 | SIVmac251 | SIVB670 |
|---|---|---|---|
| Anemia | <0.001 | <0.001 | <0.001 |
| Thrombocytopenia | <0.05 | <0.001 | <0.001 |
| Neutropenia | <0.001 | <0.001 | <0.001 |
| Neutrophilia | NS | <0.01 | <0.001 |
| Lymphopenia | <0.001 | <0.001 | <0.001 |
| Lymphocytosis | NS | NS | NS |
| Monocytopenia | <0.001 | NS | <0.001 |
| Monocytosis | <0.001 | <0.01 | NS |
| Eosinophilia | <0.001 | <0.001 | <0.001 |
| Basophilia | NS | NS | <0.01 |
NS = not significant
Classification of SIV infected subjects by time period revealed differences when compared to control data for prevalence of hematologic abnormalities (Table 4). All time periods showed significant differences compared to naïve macaques for prevalence of neutropenia. Post-inoculation periods significantly differed compared to naive macaques for eosinophilia prevalence. Early infection and AIDS periods showed significant prevalence differences compared to non-infected macaques for anemia and lymphopenia. Only the AIDS period showed a significantly increased prevalence of TCP, monocytopenia, and monocytosis when compared to naïve macaques.
Table 4.
Significance by p Value Comparing Prevalence Rates of Hematologic Abnormalities for Control and SIV Infected Rhesus macaques by stage of infection
| Hematologic Abnormality | Control | Acute | Chronic | AIDS |
|---|---|---|---|---|
| Anemia | NS | <0.001 | NS | <0.001 |
| Thrombocytopenia | NS | NS | NS | <0.001 |
| Neutropenia | <0.05 | <0.001 | <0.001 | <0.001 |
| Neutrophilia | NS | NS | NS | <0.001 |
| Lymphopenia | NS | <0.05 | NS | <0.001 |
| Lymphocytosis | NS | NS | NS | NS |
| Monocytopenia | NS | NS | NS | <0.01 |
| Monocytosis | NS | NS | NS | <0.001 |
| Eosinophilia | NS | <0.001 | <0.001 | <0.001 |
| Basophilia | NS | NS | NS | NS |
NS = not significant
Prevalence and Incidence of Hematologic Values in SIV Infected Rhesus macaques
Incidence rates for hematology changes in SIV infected macaques were calculated to detect the first time a specific hematologic abnormality occurred within a time period by viral inoculum (Table 5). Evaluation of incidence rates aided in the determination of recurrence for a hematologic event. Several changes in circulating cells were noted during SIV, although incidence rates were detected most commonly for anemia, TCP, neutropenia, neutrophilia, lymphopenia, and eosinophilia.
Table 5.
Incidence Rate of Hematologic Abnormalities for SIV infected rhesus macaques
| SIVmac239 (%) | SIVmac251 (%) | SIVB670 (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematologic Abnormality | Total | Ctrl (nml)a | Acute 1-42 dpi | Chronic 43-120 dpi | AIDS >120 dpi | Total | Ctrl (nml) | Acute 1-42 dpi | Chronic 43-120 dpi | AIDS >120 dpi | Total | Ctrl (nml) | Acute 1-42 dpi | Chronic 43-120 dpi | AIDS >120 dpi |
| Anemia | 91 | 18 | 50 | 0 | 18 | 83 | 0 | 50 | 0 | 33 | 90 | 14 | 52 | 5 | 19 |
| TCPb | 45 | 0 | 9 | 9 | 27 | 84 | 0 | 17 | 0 | 67 | 67 | 0 | 10 | 14 | 43 |
| Neutropenia | 100 | 36 | 55 | 9 | 0 | 84 | 17 | 50 | 17 | 0 | 90 | 38 | 38 | 14 | 0 |
| Neutrophilia | 45 | 0 | 9 | 9 | 36 | 83 | 33 | 0 | 0 | 50 | 77 | 10 | 5 | 5 | 57 |
| Lymphopenia | 100 | 45 | 55 | 9 | 0 | 100 | 33 | 50 | 0 | 17 | 100 | 28 | 43 | 10 | 19 |
| Lymphocytosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 5 |
| Monocytopenia | 55 | 9 | 0 | 0 | 45 | 0 | 0 | 0 | 0 | 0 | 62 | 38 | 5 | 0 | 19 |
| Monocytosis | 55 | 0 | 9 | 9 | 36 | 66 | 0 | 0 | 33 | 33 | 47 | 0 | 0 | 14 | 33 |
| Eosinophilia | 91 | 18 | 55 | 9 | 9 | 100 | 0 | 100 | 0 | 0 | 91 | 10 | 57 | 10 | 14 |
| Basophilia | 9 | 0 | 0 | 0 | 9 | 17 | 0 | 0 | 0 | 17 | 40 | 0 | 10 | 5 | 10 |
Ctrl (nml) = Control (normal)
TCP = thrombocytopenia
Prevalence rates for hematologic abnormalities during SIV infection were determined by period for each viral strain (Table 6). All hematologic changes were present in SIVB670 macaques. Prevalence rates for neutropenia and lymphopenia were noted during all periods for all viral strains. Eosinophilia and anemia prevalence rates were the next most common followed by prevalence rates for TCP and neutrophilia.
Table 6.
Prevalence Rate of Hematologic Abnormalities for SIV infected rhesus macaques
| SIVmac239 (%) | SIVmac251 (%) | SIVB670 (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematologic Abnormality | Total | Ctrl (nml)a | Acute 1-42 dpi | Chronic 43-120 dpi | AIDS >120 dpi | Total | Ctrl (nml) | Acute 1-42 dpi | Chronic 43-120 dpi | AIDS >120 dpi | Total | Ctrl (nml) | Acute 1-42 dpi | Chronic 43-120 dpi | AIDS >120 dpi |
| Anemia | 91 | 18 | 64 | 27 | 82 | 83 | 0 | 50 | 0 | 83 | 90 | 20 | 70 | 60 | 90 |
| TCPb | 45 | 0 | 9 | 9 | 27 | 83 | 0 | 17 | 0 | 67 | 70 | 0 | 10 | 40 | 60 |
| Neutropenia | 100 | 36 | 91 | 73 | 82 | 83 | 17 | 67 | 83 | 67 | 90 | 50 | 80 | 80 | 70 |
| Neutrophilia | 45 | 0 | 9 | 9 | 36 | 83 | 33 | 0 | 0 | 83 | 80 | 0 | 10 | 10 | 80 |
| Lymphopenia | 100 | 45 | 91 | 45 | 55 | 100 | 33 | 83 | 67 | 100 | 100 | 10 | 40 | 70 | 100 |
| Lymphocytosis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 10 |
| Monocytopenia | 55 | 9 | 0 | 0 | 45 | 0 | 0 | 0 | 0 | 0 | 90 | 70 | 80 | 80 | 90 |
| Monocytosis | 55 | 0 | 9 | 9 | 55 | 67 | 0 | 0 | 33 | 50 | 30 | 0 | 0 | 0 | 30 |
| Eosinophilia | 91 | 18 | 73 | 73 | 82 | 100 | 0 | 100 | 67 | 83 | 90 | 20 | 40 | 60 | 90 |
| Basophilia | 9 | 0 | 0 | 0 | 9 | 17 | 0 | 0 | 0 | 17 | 40 | 0 | 20 | 10 | 20 |
Ctrl (nml) = Control (normal)
TCP = thrombocytopenia
Hematologic Abnormalities During Progressive SIV Infection
Anemia
Anemia was frequently observed in acute and AIDS periods in SIV infected RM for all inocula (Figure 2). Overall, the anemia was mild, normocytic and normochromic, yet highly prevalent in infected animals. Reticulocyte counts were not available to ascertain regenerative capacity of the anemia, but several causes for anemia were ruled out including hemolysis, renal disease, endocrine disease, bone marrow infiltrative disease, and myelodysplasia. The occurrence of a mild normocytic and normochromic anemia was suggestive of a non-regenerative process such as anemia of chronic inflammatory disease or early iron-deficiency anemia (15, 16).
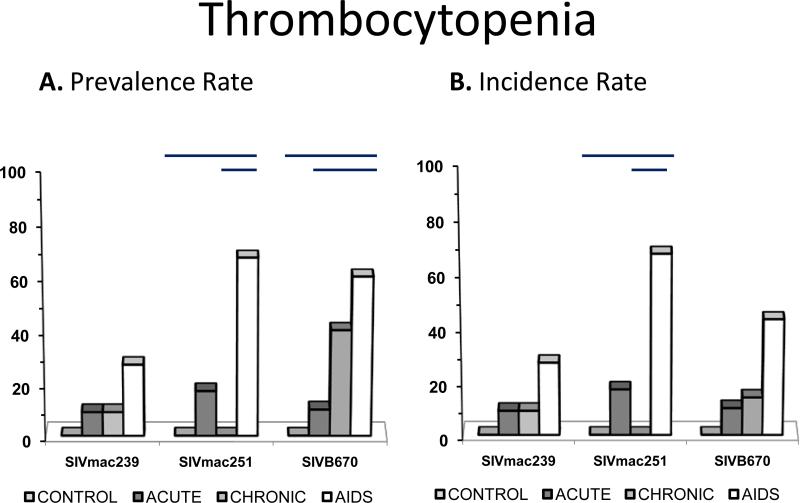
Figure 2. Prevalence and incidence of thrombocytopenia during SIV.
Prevalence rates (A.) and incidence rates (B.) for thrombocytopenia by period for each viral inoculum. Significance between periods within groups represented by horizontal lines by ANOVA (p≤0.05).
Anemia was often recurrent but was rarely persistent in SIV infected RM. The acute period had the highest rate of incidence for anemia (at least 50%) compared to later time periods. Only one subject inoculated with B670 was persistently anemic after inoculation. A mild anemia was detected in animals chronically infected with SIVmac251 and SIVmac239 but varied from mild to severe in SIVB670 subjects as infection progressed. Severe yet transient and recurrent anemia was detected in two subjects chronically infected with SIVB670.
Anemia was not apparent in all periods of infection, but when observed was mostly in AIDS, with lower prevalence in the early period and much less in the chronic non-AIDS period. All except one subject in SIVmac251 and SIVB670 and two subjects in SIVmac239 inoculation groups displayed anemia in the AIDS stage. Interestingly, subjects inoculated with SIVmac251 did not manifest anemia in the chronic period, while a 60% prevalence of anemia was present in the chronic period of SIVB670 inoculated RM.
Thrombocytopenia
Incidence of thrombocytopenia increased with progression to AIDS but the degree of thrombocytopenia did not parallel this finding (Figure 3). Thrombocytopenia in SIV infected RM was moderately prevalent, while rarely recurrent, as it was observed in only three SIVB670 inoculated subjects, including one subject displaying persistent and variably mild to severe thrombocytopenia in chronic and AIDS periods. In the acute period of infection, one subject in each inoculum group was observed with mild thrombocytopenia. Macaques infected with SIVB670 were more likely to demonstrate thrombocytopenia as SIV infection progressed compared to macaques infected with SIVmac239 and SIVmac251.
Thrombocytopenia during the chronic stage was detected in group SIVB670 at 40% prevalence and lower in group SIVmac239 while absent in the SIVmac251 group, similar to prevalence for anemia. During the AIDS stage, all three inoculum groups had thrombocytopenic subjects with prevalence of 27% of SIVmac239 inoculated subjects, 67% of SIVmac251 inoculated subjects, and 60% of SIVB670 inoculated subjects.
Neutropenia
Neutropenia was another frequent finding in SIV-infected macaques, as 90% of infected animals were found to have neutropenia at some period of infection (Figure 4). Incidence of neutropenia was greater than 50% for all time periods post-inoculation and fairly consistent between time periods and inoculum groups but tended to decline with progression of disease. Again, however, although highly prevalent, neutropenia was usually transient, yet often recurrent, although mostly mild to occasionally moderate. All subjects in the SIVmac239 group displayed neutropenia at some stage of infection, and all but one each in both SIVmac251 and SIVB670 groups displayed neutropenia. Neutropenia was always mild in SIVmac251-infected subjects, but often moderate in SIVmac239 and SIVB670 infected subjects.
Lymphopenia
Total prevalence of lymphopenia was highest of all hematologic variances over time by inoculum, but incidence was variable (Figure 5). Essentially every animal experienced lymphopenia at least during some stage of SIV infection. Lymphopenia was present in all inoculum groups at all time periods with at least 40% prevalence ending with 100% prevalence in the progressive (AIDS) stage. Inoculated RM had lymphopenia that was often mild, occasionally moderate, rarely severe, and also rarely persistent in only two subjects infected with SIVmac239 during progressive infection. Subjects inoclated with SIVmac239 had experienced lymphopenia prior to AIDS.
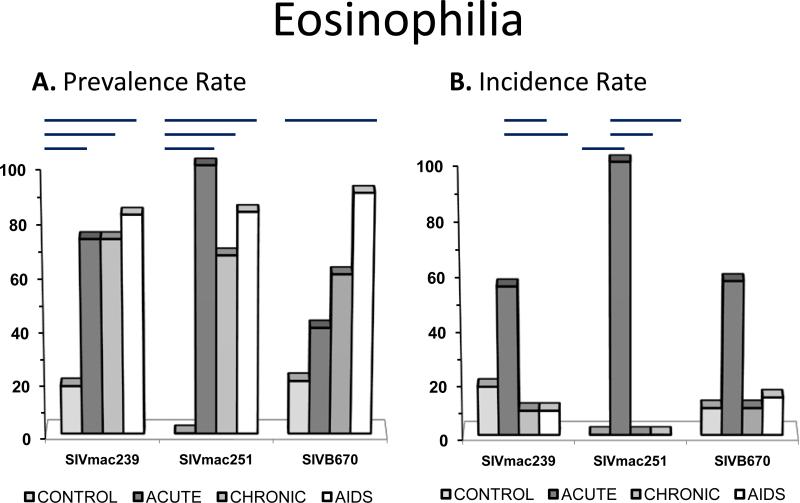
Figure 5. Prevalence and incidence of eosinophilia during SIV infection.
Prevalence rates (A.) and incidence rates (B.) for eosinophilia by period for each viral inoculum. Significance between periods within groups represented by horizontal lines by ANOVA (p≤0.05).
Eosinophilia
Prevalence of eosinophilia was high in SIV infected macaques (Figure 6). Nevertheless, eosinophilia was mild and often recurrent in SIV infected RM but never persistent. At least two subjects from all infected time periods for all inoculums groups displayed eosinophilia. Subjects inoculated with SIVB670 were more likely to be eosinophilic with progression of SIV infected while SIVmac inoculated subjects were consistently eosinophilic.
DISCUSSION
Although several hematologic anomalies were occasionally observed in individual controls, virtually all SIV-infected animals displayed anemia, thrombocytopenia, neutropenia, neutrophilia, lymphopenia, or eosinophilia at some stage of SIV infection. CBC data, prevalence rates, and incidence rates were evaluated between SIV infected and uninfected macaques to identify differences and ascertain relevance of hematologic abnormalities during non-treated SIV infection. Hematologic abnormalities were compared by period and viral group during SIV by prevalence and incidence to identify trends.
Comparison of HCT, hemoglobin, and MCHC as hematologic parameters were significantly lower in SIV-infected macaques than non-infected macaques. Anemia prevalence and incidence were highest in AIDS. Of note, anemia was not present in SIVmac251 infected RM in the chronic stage. The SIV RM model simplifies the ability to rule out many causes for anemia such as drug, diet, and acute or chronic blood loss. However, reticulocyte counts were not available to ascertain regeneration during anemic episodes, so MCHC and MCV were evaluated to classify regenerative anemias.
Initially, MCHC was significantly decreased in SIV infected macaques compared to naïve macaques, but prevalence for hypochromia was low, and a lack of significant differences in MCV between infected and uninfected RM suggested this parameter was not a major feature of SIV infection. In fact, macrocytosis was essentially absent in both naive and SIV infected macaques, with a low prevalence rate for microcytosis.
Platelet count and TCP significantly differed between naïve macaques and SIV infected macaques indicating thrombocytopenia was a relevant hematologic abnormality during SIV infection. TCP prevalence was low compared to other hematologic abnormalities, and early time periods were less likely to manifest this anomaly, with the highest incidence and prevalence of TCP occurring in AIDS stages for all three viruses. Similar to anemia, TCP was not observed in SIVmac251 infected RM during the chronic stage.
A significantly low neutrophil count in SIV infected macaques compared to non-infected macaques was observed, even though the prevalence of both neutropenia and neutrophilia were significantly higher in SIV infected macaques comparatively. Multiple causes could not be discounted for neutrophilia such as epinephrine-mediated physiologic neutrophilia, corticosteroid-induced stress neutrophilia, and inflammation (17). Therefore the significance of this neutrophilia was regarded as equivocal in this study of SIV disease. Neutropenia, however, may be observed during periods of increased tissue demand/ destruction or due to reduced bone marrow production or acute inflammation (17). Absence of neutropenia in naïve macaques and a high occurrence post- SIV inoculation in all viral groups supported neutropenia as being a common hematologic event during SIV. In fact, most infected RM had detectable neutropenia at some point prior to AIDS.
Lymphocyte counts during SIV were significantly lower compared to non-infected macaques, and were in fact observed as the highest prevalence for a hematologic abnormality during SIV infection. Lymphopenia was a hallmark finding in all stages of infection, and for all viral inoculums, yet one SIVB670-infected macaque displayed lymphocytosis in the AIDS stage. Since only this one sample (from 557 CBCs) had lymphocytosis, this was considered rare and possibly attributed to other factors (opportunistic infection, etc.).
The likelihood of circulating monocytes to be elevated or decreased during SIV infection was similar by prevalence rates (50%) resulting in minimal differences in absolute counts compared to naïve macaques. We thus interpreted monocyte count shifts to be non-conclusive during SIV infection, even though significant differences in prevalence were noted for both monocytopenia and monocytosis by group and stage of infection.
Although an increased trend for eosinophilia in SIV infected macaques was noted, a significantly higher prevalence of eosinophilia in infected macaques compared to non-infected macaques was observed, suggesting this was a relevant change associated with SIV infection. Parasite data was evaluated to determine if parasitism alone could account for the eosinophilia. Parasite fecal direct smear and flotation results were available during progressive SIV infection, that included all AIDS diagnoses, with at least one RM evaluated per period. However, only two controls had fecal evaluations on the day of CBC collection. Intestinal parasites (roundworms and protozoa) were detected on all fecal analyses for two SIVmac239 infected RM, two SIVmac251 infected RM, and seven SIVB670 infected RM, in addition to two controls. However, one SIVmac239 infected RM had 3 of 4 fecal results positive for parasites, and 1 fecal examination was negative at 362 DPI. Parasitism could not account for the eosinophilia alone, so this hematologic abnormality was considered pertinent/associated with SIV.
Basophil granulocytes represented <1% of total circulating cells, indicating shifts would minimally impact total WBC, and may be difficult to discern as a specific change in SIV disease. Basophil count and basophilia prevalence in SIV infected macaques was significantly lower than in naïve macaques. Consequently, basophilia was considered an unremarkable event during SIV infection.
Overall, prevalences of hematologic abnormalities in SIV infected RM mirrored what has been described in HIV patients that are not receiving AIDS related therapies (7, 18-28). HIV patients naïve to ART, chemotherapy, radiation therapy, or anti-microbials by self-declaration and/or preceding enrollment in clinical trials were found with the following frequencies of hematologic abnormalities: anemia 10-95%; TCP 10-83%; lymphopenia 64%; neutropenia 17-40%; and eosinophilia 3% (7, 18-28). AIDS patients naïve to antiretroviral therapy also displayed frequencies of 30-88% anemia, TCP 16-66%, lymphopenia 31-83%, neutropenia 8-29%, and eosinophilia 23% (1, 11, 29, 30) which was similarly represented in our prevalence of SIV infected RM with AIDS. Lower values were noted for earlier stages of HIV disease as 6-40% anemia, TCP 0-20%, lymphopenia 15-30%, neutropenia 0-30%, eosinophilia 16-18% (11, 29, 30). Similarly, RM in early (non-AIDS) stages in this study revealed frequencies of anemia, TCP, lymphopenia, neutropenia, and eosinophilia lower than in macaques with AIDS, with the exception of neutropenia, which revealed minimal differences.
Significant differences in incidence and prevalence of hematologic abnormalities were observed among macaques infected with the three different strains of SIV. SIVmac239 infected RM had the highest incidence and prevalence for lymphopenia and neutropenia. SIVB670 inoculated RM had the highest incidence and prevalence for anemia and TCP. SIVmac251 infected RM had the highest incidence and prevalence rates for eosinophilia. The choice to include all three SIV inoculum groups in this retrospective study was that all of these are pathogenic strains of SIVmac used in research. Natural infections of Sooty mangabey monkeys with SIVsm housed at primate centers in the United States were the origin for SIVmac and SIVB670 viruses (reviewed in (31)). SIVmac251 is the prototypic uncloned viral swarm derived from passaging SIVsm through rhesus macaques. SIVmac239 is a highly pathogenic molecular clone derived from SIVmac251, and SIVB670 is an independent pathogenic isolate of SIV that differs from SIVmac251. All three viruses result in CD4 depletion and AIDS and in our study of all SIV infected RM here, normal progressors devoid of therapeutic intervention remarkably recapitulated the incidence and prevalence of several hematologic parameters observed during untreated HIV infection.
This retrospective investigation of natural SIV disease progression in Rhesus macaques revealed prevalence of hematologic abnormalities during progressive SIV infection in Macaca mulatta that mirrored progressive HIV infection in Homo sapiens. These results suggest that, like in HIV infection, lymphopenia, anemia, thrombocytopenia, and neutropenia are major complications of progressive SIV infection. Advantages of the Rhesus macaque model for studies of HIV pathogenesis allow for the control of numerous confounding variables. Further, these results also suggest the viral inoculum and stage of infection are also significant determinants for several hematologic abnormalities and may be relevant in HIV infection.
Supplementary Material
Acknowledgements
We would like to acknowledge Ms. Jean Geddis (TNPRC) for her assistance with mining the database in the retrospective study. This work was supported by NIH grants R01 AI084793,, R01 AI049080, P51 RR000164, and T32 RR021309.
REFERENCES
- 1.Spivak JL, Bender BS, Quinn TC. Hematologic abnormalities in the acquired immune deficiency syndrome. Am J Med. 1984 Aug;77(2):224–8. doi: 10.1016/0002-9343(84)90695-8. [DOI] [PubMed] [Google Scholar]
- 2.Morris L, Distenfeld A, Amorosi E, Karpatkin S. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):714–7. doi: 10.7326/0003-4819-96-6-714. [DOI] [PubMed] [Google Scholar]
- 3.Moyle G. Anaemia in persons with HIV infection: prognostic marker and contributor to morbidity. AIDS reviews. 2002 Jan-Mar;4(1):13–20. [PubMed] [Google Scholar]
- 4.Castella A, Croxson TS, Mildvan D, Witt DH, Zalusky R. The bone marrow in AIDS. A histologic, hematologic, and microbiologic study. Am J Clin Pathol. 1985 Oct;84(4):425–32. doi: 10.1093/ajcp/84.4.425. [DOI] [PubMed] [Google Scholar]
- 5.Fischl MA, Richman DD, Grieco MH, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–91. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998 Jan 1;91(1):301–8. [PubMed] [Google Scholar]
- 7.Sloand EM, Klein HG, Banks SM, Vareldzis B, Merritt S, Pierce P. Epidemiology of thrombocytopenia in HIV infection. European journal of haematology. 1992 Mar;48(3):168–72. doi: 10.1111/j.1600-0609.1992.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 8.Street AM, Gibson J. Managing HIV. Part 5: Treating secondary outcomes. 5.12 HIV and haematological disease. Med J Aust. 1996 Apr 15;164(8):487–8. [PubMed] [Google Scholar]
- 9.Coyle TE. Hematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Med Clin North Am. 1997 Mar;81(2):449–70. doi: 10.1016/s0025-7125(05)70526-5. [DOI] [PubMed] [Google Scholar]
- 10.Treacy M, Lai L, Costello C, Clark A. Peripheral blood and bone marrow abnormalities in patients with HIV related disease. British journal of haematology. 1987 Mar;65(3):289–94. doi: 10.1111/j.1365-2141.1987.tb06855.x. [DOI] [PubMed] [Google Scholar]
- 11.Zon LI, Arkin C, Groopman JE. Haematologic manifestations of the human immune deficiency virus (HIV). British journal of haematology. 1987 Jun;66(2):251–6. doi: 10.1111/j.1365-2141.1987.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 12.Coyle TE. Hematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Med Clin North Am. 1997 Mar;81(2):449–70. doi: 10.1016/s0025-7125(05)70526-5. [DOI] [PubMed] [Google Scholar]
- 13.Olayemi E, Awodu OA, Bazuaye GN. Autoimmune hemolytic anemia in HIV-infected patients: a hospital based study. Ann Afr Med. 2008 Jun;7(2):72–6. doi: 10.4103/1596-3519.55677. [DOI] [PubMed] [Google Scholar]
- 14.Curkendall SM, Richardson JT, Emons MF, Fisher AE, Everhard F. Incidence of anaemia among HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2007 Nov;8(8):483–90. doi: 10.1111/j.1468-1293.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 15.Means RT., Jr. Anemias Secondary to Chronic Disease and Systemic Disorders. In: Greer J, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B, editors. Wintrobe's Clinical Hematology. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 1445–65. [Google Scholar]
- 16.Glader B. Anemia: General Considerations. In: Greer J, Foerster J, Lukens JN, Rodgers GM, Paraskevas F, Glader B, editors. Wintrobe's Clinical Hematology. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 947–78. [Google Scholar]
- 17.Smith GS. Neutrophils. In: Feldman BV, Zinkl JG, Jain NC, editors. Schalm's Veterinary Hematology. 5th ed. Ippincott Williams & Wilkins; Baltimore, Maryland: 2000. pp. 281–307. [Google Scholar]
- 18.Walsh C, Krigel R, Lennette E, Karpatkin S. Thrombocytopenia in homosexual patients. Prognosis, response to therapy, and prevalence of antibody to the retrovirus associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Oct;103(4):542–5. doi: 10.7326/0003-4819-103-4-542. [DOI] [PubMed] [Google Scholar]
- 19.Peltier JY, Lambin P, Doinel C, Courouce AM, Rouger P, Lefrere JJ. Frequency and prognostic importance of thrombocytopenia in symptom-free HIV-infected individuals: a 5-year prospective study. AIDS (London, England) 1991 Apr;5(4):381–4. doi: 10.1097/00002030-199104000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Marroni M, Gresele P, Vezza R, et al. Thrombocytopenia in HIV infected patients. Prevalence and clinical spectrum. Recenti Prog Med. 1995 Mar;86(3):103–6. [PubMed] [Google Scholar]
- 21.Malyangu E, Abayomi EA, Adewuyi J, Coutts AM. Aids is now the commonest clinical condition associated with multilineage blood cytopenia in a central referral hospital in Zimbabwe. Cent Afr J Med. 2000 Mar;46(3):59–61. doi: 10.4314/cajm.v46i3.8525. [DOI] [PubMed] [Google Scholar]
- 22.Erhabor O, Ejele OA, Nwauche CA, Buseri FI. Some haematological parameters in human immunodeficiency virus (HIV) infected Africans: the Nigerian perspective. Niger J Med. 2005 Jan-Mar;14(1):33–8. doi: 10.4314/njm.v14i1.37132. [DOI] [PubMed] [Google Scholar]
- 23.Adediran IA, Durosinmi MA. Peripheral blood and bone marrow changes in patients with acquired immunodeficiency syndrome. Afr J Med Med Sci. 2006 Dec;35(Suppl):85–91. [PubMed] [Google Scholar]
- 24.Alaei K, Alaei A, Mansoori D. Thrombocytopenia in HIV-infected patients, Islamic Republic of Iran. East Mediterr Health J. 2002 Nov;8(6):758–64. [PubMed] [Google Scholar]
- 25.Babadoko AA, Aminu SM, Suleiman AN. Neutropenia and human immunodeficiency virus-1 infection: analysis of 43 cases. Niger J Med. 2008 Jan-Mar;17(1):57–60. doi: 10.4314/njm.v17i1.37357. [DOI] [PubMed] [Google Scholar]
- 26.Mugisha JO, Shafer LA, Van der Paal L, et al. Anaemia in a rural Ugandan HIV cohort: prevalence at enrolment, incidence, diagnosis and associated factors. Trop Med Int Health. 2008 Jun;13(6):788–94. doi: 10.1111/j.1365-3156.2008.02069.x. [DOI] [PubMed] [Google Scholar]
- 27.Sa MS, Sampaio J, Haguihara T, Ventin FO, Brites C. Clinical and laboratory profile of HIV-positive patients at the moment of diagnosis in Bahia, Brazil. Braz J Infect Dis. 2007 Aug;11(4):395–8. doi: 10.1590/s1413-86702007000400004. [DOI] [PubMed] [Google Scholar]
- 28.Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Sep 1;19(1):29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Adewuyi JO, Coutts AM, Latif AS, Smith H, Abayomi AE, Moyo AA. Haematologic features of the human immunodeficiency virus (HIV) infection in adult Zimbabweans. Cent Afr J Med. 1999 Feb;45(2):26–30. doi: 10.4314/cajm.v45i2.8447. [DOI] [PubMed] [Google Scholar]
- 30.Murphy MF, Metcalfe P, Waters AH, et al. Incidence and mechanism of neutropenia and thrombocytopenia in patients with human immunodeficiency virus infection. British journal of haematology. 1987 Jul;66(3):337–40. doi: 10.1111/j.1365-2141.1987.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 31.Apetrei C, Kaur A, Lerche NW, et al. Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. Journal of virology. 2005 Jul;79(14):8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.