SUMMARY
Non-apoptotic forms of cell death may facilitate the selective elimination of some tumor cells or be activated in specific pathological states. The oncogenic RAS-selective lethal small molecule erastin triggers a unique iron-dependent form of non-apoptotic cell death that we term ferroptosis. Ferroptosis is dependent upon intracellular iron, but not other metals, and is morphologically, biochemically and genetically distinct from apoptosis, necrosis and autophagy. We identify the small molecule ferrostatin-1 as a potent inhibitor of ferroptosis in cancer cells and glutamate-induced cell death in organotypic rat brain slices, suggesting similarities between these two processes. Indeed, erastin, like glutamate, inhibits cystine uptake by the cystine/glutamate antiporter (system xc−), creating a void in the antioxidant defenses of the cell, ultimately leading to iron-dependent, oxidative death. Thus, activation of ferroptosis results in the non-apoptotic destruction of certain cancer cells, while inhibition of this process may protect organisms from neurodegeneration.
Keywords: erastin, iron, reactive oxygen species, cell death, necrosis, RNAi, excitotoxic, glutamate, cystine, SLC7A11
INTRODUCTION
Cell death is crucial for normal development, homeostasis and the prevention of hyper-proliferative diseases such as cancer (Fuchs and Steller, 2011; Thompson, 1995). It was once thought that almost all regulated cell death in mammalian cells resulted from the activation of caspase-dependent apoptosis (Fuchs and Steller, 2011; Thompson, 1995). More recently this view has been challenged by the discovery of several regulated non-apoptotic cell death pathways activated in specific disease states, including poly(ADP-ribose) polymerase-1 (PARP-1) and apoptosis inducing factor 1 (AIF1)-dependent parthanatos, caspase-1-dependent pyroptosis and receptor interacting protein kinase 1 (RIPK1)-dependent necroptosis (Bergsbaken et al., 2009; Christofferson and Yuan, 2010; Wang et al., 2009). We hypothesized that additional regulated forms of non-apoptotic cell death likely remain to be discovered that mediate cell death in other developmental or pathological circumstances.
The RAS family small GTPases (HRAS, NRAS and KRAS) are mutated in ~30% of all cancers (Vigil et al., 2010). Finding compounds that are selectively lethal to RAS-mutant tumor cells is therefore a high priority. We previously identified two structurally unrelated small molecules, named erastin and RSL3, that were selectively lethal to oncogenic RAS-mutant cell lines, and which we refer to together as RAS-selective lethal (RSL) compounds (Dolma et al., 2003; Yang and Stockwell, 2008). Using affinity purification, we identified voltage dependent anion channels 2 and 3 (VDAC2/3) as direct targets of erastin (Yagoda et al., 2007), but not RSL3. ShRNA and cDNA overexpression studies demonstrated that VDAC2 and VDAC3 are necessary, but not sufficient, for erastin-induced death (Yagoda et al., 2007), indicating that additional unknown targets are required for this process.
The type of cell death activated by the RSLs has been enigmatic. Classic features of apoptosis, such as mitochondrial cytochrome c release, caspase activation and chromatin fragmentation, are not observed in RSL-treated cells (Dolma et al., 2003; Yagoda et al., 2007; Yang and Stockwell, 2008). RSL-induced death is, however, associated with increased levels of intracellular reactive oxygen species (ROS) and is prevented by iron chelation or genetic inhibition of cellular iron uptake (Yagoda et al., 2007; Yang and Stockwell, 2008). In a recent systematic study of various mechanistically unique lethal compounds, the prevention of cell death by iron chelation was a rare phenomenon (Wolpaw et al., 2011), suggesting that few triggers can access iron-dependent lethal mechanisms.
We have explored the hypothesis that RSLs, such as erastin, activate a lethal pathway that is different from apoptosis, necrosis and other well-characterized types of regulated cell death. We find that erastin-induced death involves a unique constellation of morphological, biochemical and genetic features, which led us to propose the name ferroptosis as a description for this phenotype. We identified a specific small molecule inhibitor of ferroptosis (ferrostatin-1) that prevents ferroptosis in cancer cells, as well as glutamate-induced cell death in postnatal rat brain slices. Our results suggest an underlying similarity between diverse forms of iron-dependent, non-apoptotic death and that the manipulation of ferroptosis may be exploited to selectively destroy RAS-mutant tumor cells or to preserve neuronal cells exposed to specific oxidative conditions.
RESULTS
Erastin triggers oxidative, iron-dependent cell death
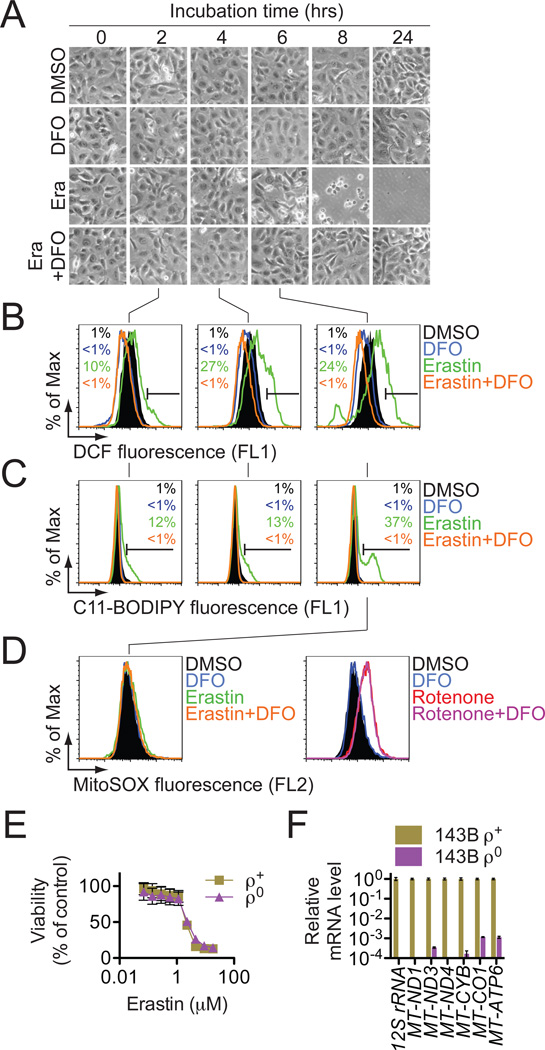
RSL-induced cell death is a poorly characterized process involving the accumulation of ROS derived from an unknown source and the inhibition of cell death by iron chelation (Yagoda et al., 2007; Yang and Stockwell, 2008). We observed that these two processes were linked. Treatment of NRAS-mutant HT-1080 fibrosarcoma cells with the RSL molecule erastin (10 µM) resulted in a time-dependent increase in cytosolic and lipid ROS beginning at 2 hours, as assayed by flow cytometry using the fluorescent probes H2DCFDA and C11-BODIPY, respectively (Figure 1B,C). This increase in ROS preceded cell detachment and overt death, which began at 6 hours (Figure 1A). ROS accumulation and cell death were suppressed by co-treatment with the iron chelator deferoxamine (DFO, 100 µM) (Figure 1A–C), while incubation with three different exogenous sources of iron, but not by other divalent transition metal ions (Cu2+, Mn2+, Ni2+, Co2+), potentiated erastin-induced death (Figure S1A,B). As cell death occurred in erastin-treated cells following a prolonged period of ROS accumulation and was suppressed by antioxidants (see below), our data suggest that the overwhelming, iron-dependent accumulation of ROS is what kills these cells.
Figure 1. Erastin-induced death triggers the accumulation of cytosolic ROS whose production can be inhibited by DFO.
(A) Visualization of HT-1080 cell viability over time +/− erastin (Era, 10 µM) and deferoxamine (DFO, 100 µM). (B,C) Cytosolic and lipid ROS production assessed over time (2,4 and 6 hrs) by flow cytometry using H2DCFDA and C11-BODIPY. (D) Mitochondrial ROS assessed in HT-1080 cells treated for 6 hrs with erastin+/−DFO, as above, or with rotenone (250 nM)+/−DFO. In (A–D) representative data from one of four experiments is shown. (E) Erastin-induced death in 143B ρ0 and ρ+ cells. (F) mtDNA-encoded transcript levels in ρ0 and ρ+ cells. Results in (E) and (F) are mean+/−SD from one of three representative experiments.
Since two erastin targets, VDAC2 and VDAC3, reside in the mitochondria, we hypothesized that erastin-induced death involved aberrant ROS production by the mitochondrial electron transport chain (ETC). However, in erastin-treated (10 µM, 6 hrs) HT-1080 cells, we observed no increase in MitoSOX-sensitive mitochondrial ROS production (Figure 1D, left). The ETC complex I inhibitor rotenone (250 nM, 6 hrs) enhanced MitoSOX-sensitive ROS production, but in a manner that was insensitive to DFO (Figure 1D, right). Furthermore, KRAS-mutant 143B osteosarcoma cells incapable of ETC-dependent ROS formation, due to the depletion of mitochondrial DNA (mtDNA)-encoded transcripts (ρ0 cells), were as sensitive to erastin and RSL3 as matched mtDNA-wild-type (ρ+) cells (Figures 1E,F, S1C–E). Thus, erastin-induced cell death in human cancer cells involves DFO-sensitive ROS accumulation and can occur in cells lacking a functional ETC. We named this iron-dependent death phenotype ferroptosis.
Ferroptosis is distinct from known forms of cell death
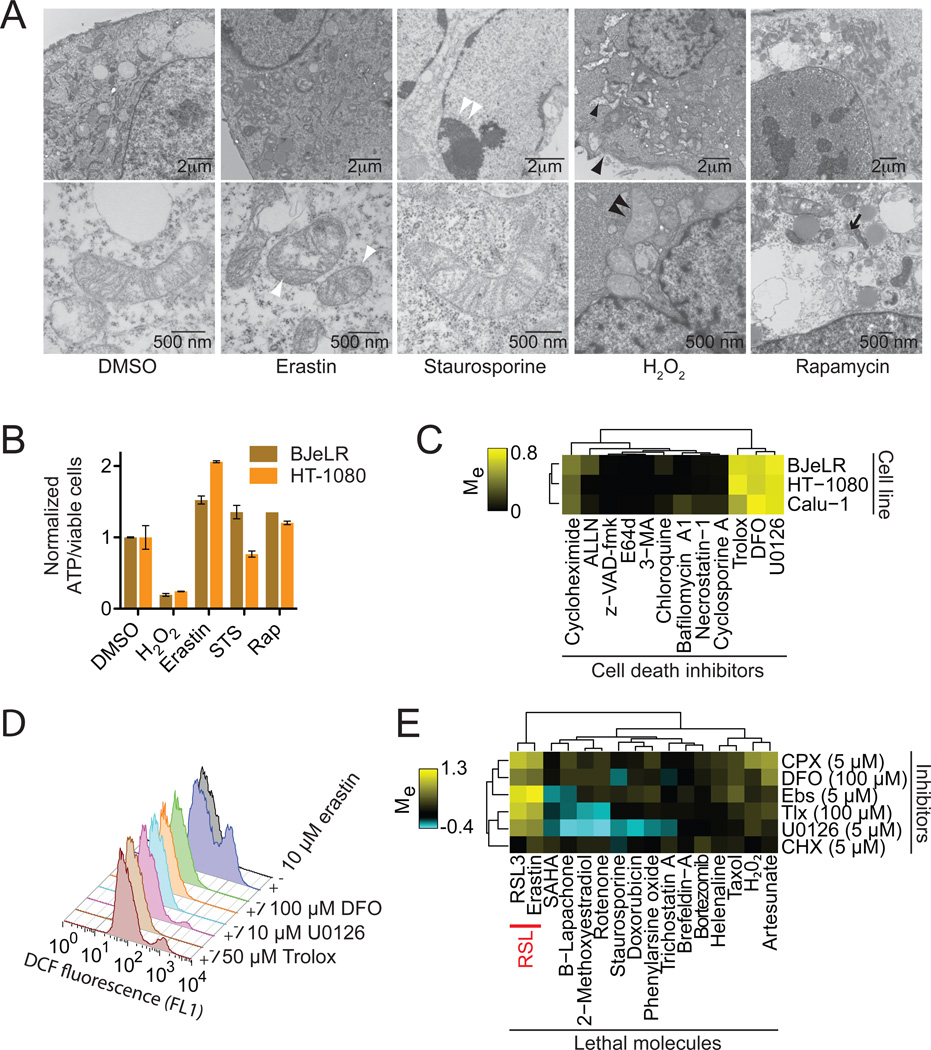
We examined whether ferroptosis shared morphological, bioenergetic or other similarities with apoptotic or necrotic death, or with autophagy. Using transmission electron microscopy, we observed that HRAS-mutant BJeLR engineered tumor cells treated with erastin exhibited none of the characteristic morphologic features associated with staurosporine (STS)-induced apoptosis (e.g. chromatin condensation and margination), hydrogen peroxide (H2O2)-induced necrosis (e.g. cytoplasmic and organelle swelling, plasma membrane rupture) or rapamycin-induced autophagy (e.g. formation of double-membrane enclosed vesicles) (Figure 2A). The lone distinctive morphological feature of erastin-treated cells involved mitochondria that appeared smaller than normal, with increased membrane density, consistent with our previous report (Yagoda et al., 2007) (Figure 2A). With respect to bioenergetics, we observed substantial depletion of intracellular ATP in BJeLR and HT-1080 cells treated with H2O2, but not erastin, STS or rapamycin (Figure 2B), distinguishing ferroptosis from various forms of necrosis that involve bioenergetic failure.
Figure 2. Erastin-induced oxidative death is iron-dependent.
(A) Transmission electron microscopy of BJeLR cells treated with DMSO (10 hrs), erastin (37 µM, 10 hrs), staurosporine (STS, 0.75 µM, 8 hrs), H2O2 (16 mM, 1 hr) and rapamycin (Rap, 100 nM, 24 hr). Single white arrowheads: shrunken mitochondria; paired white arrowheads: chromatin condensation; black arrowheads: cytoplasmic and organelle swelling, plasma membrane rupture; black arrow: formation of double-membrane vesicles. A minimum of 10 cells per treatment condition were examined. (B) Normalized ATP levels in HT-1080 and BJeLR cells treated as in (A) with the indicated compounds. Representative data (mean+/−SD) from one of three independent experiments is shown. (C) Modulatory profiling of known small molecule cell death inhibitors in HT-1080, BJ-eLR and Calu-1 cells treated with erastin (10 µM, 24 hrs). (D) Effect of inhibitors on H2DCFDA-sensitive ROS production in HT-1080 cells treated for 4 hours. (E) Modulatory profiling of ciclopirox olamine (CPX), DFO, ebselen (Ebs), trolox (Tlx), U0126 and CHX on oxidative and non-oxidative lethal agents.
Using a variation of our recently reported modulatory profiling strategy (Wolpaw et al., 2011), we tested the ability of twelve established small molecule cell death inhibitors to prevent ferroptosis in HT-1080, BJeLR and KRAS-mutant Calu-1 non-small cell lung cancer cells. We computed the modulatory effect (Me) for each inhibitor (tested in a 10-point, 4-fold dilution series) on the normalized viability of cells treated with a lethal dose of erastin (Me<0: death sensitization; Me = 0: no effect; Me>0: death rescue). The resulting values were clustered hierarchically in an unsupervised fashion and displayed as a heatmap. Using this approach, we observed that erastin-induced death was not consistently modulated by inhibitors of caspase, cathepsin or calpain proteases (z-VAD-fmk, E64d or ALLN), RIPK1 (necrostatin-1), cyclophilin D (cyclosporin A) or lysosomal function/autophagy (bafilomycin A1, 3-methyladenine, chloroquine), compounds known to inhibit forms of apoptosis, necrosis and autophagic cell death (Figure 2C).
DFO, the anti-oxidant trolox, the MEK inhibitor U0126 and, to a weaker extent, the protein synthesis inhibitor cycloheximide (CHX), all rescued from erastin-induced death in HT-1080, BJeLR and Calu-1 cells (Figure 2C) (Yagoda et al., 2007). These inhibitors were also effective at preventing erastin-induced ferroptosis in both wild-type and apoptosis-deficient Bax/Bak double knockout (DKO) mouse embryonic fibroblasts (Figures S2A,B), indicating that ferroptosis can be activated in human-derived and mouse-derived cells and is independent of the core apoptotic machinery regulated by Bax and Bak. DFO, trolox and U0126 all prevented the accumulation of H2DCFDA-sensitive ROS in erastin-treated HT-1080 cells (Figure 2D), demonstrating that these inhibitors act to prevent death upstream or at the level of ROS production. Since trolox, U0126 and the membrane permeable iron chelator 2,2-bipyridyl could be added to HT-1080 cells up to 6 hours after erastin and still confer substantial protection from death (Figure S2C), ferroptosis likely requires continuous iron-dependent ROS formation over an extended period of time to trigger death.
Finally, in a modulatory profiling experiment that tested the ability of DFO, trolox, U0126, CHX, the membrane permeable iron chelator ciclopirox olamine (CPX) and the glutathione peroxidase mimetic ebselen (Ebs) to modulate the lethality of erastin, RSL3 or sixteen other mechanistically distinct lethal compounds thought to kill cells through various ROS-dependent and ROSindependent mechanisms, we observed that erastin and RSL3 formed a distinct cluster, separate from all other inducers of cell death (Figure 2E). Together, these data support the hypothesis that RSL-induced ferroptosis is a novel death phenotype distinct from apoptosis, various forms of necrosis and autophagy.
Ferroptosis is regulated by a distinct set of genes
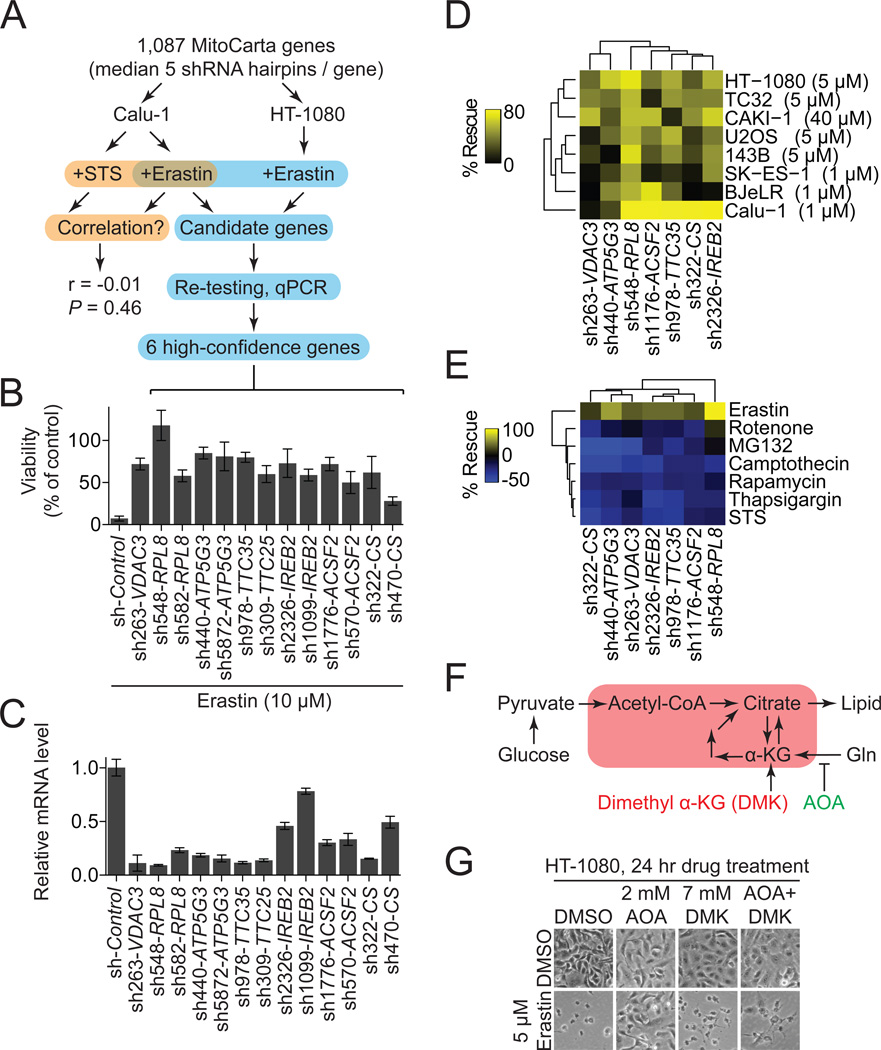
To explore the genetic basis of ferroptosis, we sought to identify genes uniquely required for this process. We focused on the potential role of the mitochondria, as this organelle displayed an aberrant morphology in erastin-treated cells (Figure 2A). Mitochondrial gene function was perturbed using a custom arrayed shRNA library targeting 1,087 genes (median 5 hairpins/gene), most of which (901, 88%) encode predicted mitochondrial proteins (Pagliarini et al., 2008) (Figure 3A). Using this library, we first compared the genetic suppressibility of erastin (7.3 µM)-induced ferroptosis and STS (1 µM)-induced apoptosis in Calu-1 cells (Figure 3A). Across all 5,817 informative hairpins, we observed no significant correlation between those shRNAs that rescued from erastin-induced ferroptosis and from STS-induced apoptosis (Spearman rank sum test, r = −0.01, P = 0.46), confirming that distinct genetic networks govern erastin-induced ferroptosis and STS-induced apoptosis.
Figure 3. Erastin-induced ferroptosis exhibits a unique genetic profile.
(A) Outline of the MitoCarta shRNA screen and confirmation pipeline. (B,C) Six high confidence genes required for erastin-induced ferroptosis. (B) Viability of HT-1080 cells infected with shRNAs for 72 hours and treated with erastin (10 µM, 24 hrs). (C) mRNA levels for hairpins shown in (B) determined using RT-qPCR. Data in (B) and (C) are mean+/−SD from one of three experiments. (D,E) Effect of shRNA-mediated silencing of high-confidence genes using the best hairpin identified by mRNA silencing efficiency in (C) on cell viability. (E) Viability of various cell lines treated with a lethal dose of erastin (indicated in brackets) for 24 hours. (E) Viability of HT-1080 cells treated with various death-inducing or cytostatic compounds. For (D) and (E) % rescue was computed relative to each shRNA alone+DMSO. (F) Cartoon outline of glutamine (Gln) metabolism. Red box indicates mitochondria. (G) Images of HT-1080 cells treated with aminooxyacetic acid (AOA) +/− dimethyl alphaketoglutarate (DMK) +/− erastin.
Next, we performed a second erastin resistance screen in HT-1080 cells and, using a rigorous confirmation pipeline, identified six high-confidence genes supported by at least two independent shRNAs per gene that are required for erastin-induced ferroptosis in both HT-1080 and Calu-1 cells—RPL8 (ribosomal protein L8), IREB2 (iron response element binding protein 2), ATP5G3 (ATP synthase F0 complex subunit C3), CS (citrate synthase), TTC35 (tetratricopeptide repeat domain 35) and ACSF2 (acyl-CoA synthetase family member 2) (Figure 3B,C). Consistent with the established CHX- and DFO-sensitive nature of erastin-induced ferroptosis, RPL8 encodes a component of the 60S ribosomal subunit presumably required for translation, and IREB2 encodes a master regulator of iron metabolism. We further validated these results, showing that shRNA-mediated silencing of IREB2 and the IREB2 negative regulator FBXL5 (Salahudeen et al., 2009; Vashisht et al., 2009) resulted in reciprocal changes in the expression of the known iron uptake, metabolism and storage genes TFRC, ISCU, FTH1, FTL and in erastin sensitivity (Figure S3A–C). These results provide confidence in the quality of the screening and confirmation procedures.
To establish the generalizability of the results obtained in HT-1080 and Calu-1 cells, we tested the effects of silencing these genes in HT-1080, Calu-1 and six additional cell lines treated with erastin. Silencing of these six high confidence genes using the most effective hairpin for each gene, defined by mRNA silencing levels in HT-1080 cells (Figure 3C), conferred ≥20% rescue in 79% (38/48) of shRNA-cell line combinations (Figure 3D). Thus, these genes appear to be broadly required for erastin-induced ferroptosis. We next tested whether silencing of these genes specifically attenuated erastin-induced ferroptosis, or more broadly modulated a variety of lethal effects. Silencing of these six genes conferred protection against erastin-induced ferroptosis (≥40% rescue for 6/6 hairpins), but not cell death/cytostasis induced by STS, rotenone, rapamycin, the proteasome inhibitor MG132, the DNA-damaging agent camptothecin or the Ca2+-dependent ATPase inhibitor thapsigargin (≥40% rescue for 0/6 hairpins) (Figure 3E). Together, these data support the hypothesis that a unique genetic network governs erastin-induced ferroptosis compared to other forms of cell death.
Both ACSF2 and CS are implicated in the regulation of mitochondrial fatty acid metabolism (Mullen et al., 2011; Watkins et al., 2007), and we wondered whether this process could contribute to ferroptosis. In cancer cells, fatty acid synthesis is in part dependent upon the metabolism of glutamine (Gln) to alpha-ketoglutarate, a process that can be inhibited by the small molecule transaminase inhibitor aminooxyacetic acid (AOA) (Wise et al., 2008) (Figure 3F). In cell culture media containing both glucose and Gln, we found that AOA (2 mM) rescued both HT-1080 and BJeLR cells from erastin-induced ferroptosis (Figures 3F, S3D), mimicking the effects of silencing CS and ACSF2. In AOA-treated HT-1080 cells, the lethality of erastin was restored by co-incubation with dimethyl alpha ketoglutarate (DMK), which provides the downstream metabolite whose production from Gln is blocked by AOA (Wise et al., 2008) (Figure 3F,G). An unrelated modulator of mitochondrial function not predicted to directly affect Gln metabolism, dichloroacetic acid (DCA), had no effect on erastin-induced ferroptosis (Figure S3D). These results suggest that a Gln- CS- and ACSF2-dependent lipid synthesis pathway could supply a specific lipid precursor required for ferroptosis.
Identification of ferrostatin-1 as a small molecule inhibitor of ferroptosis
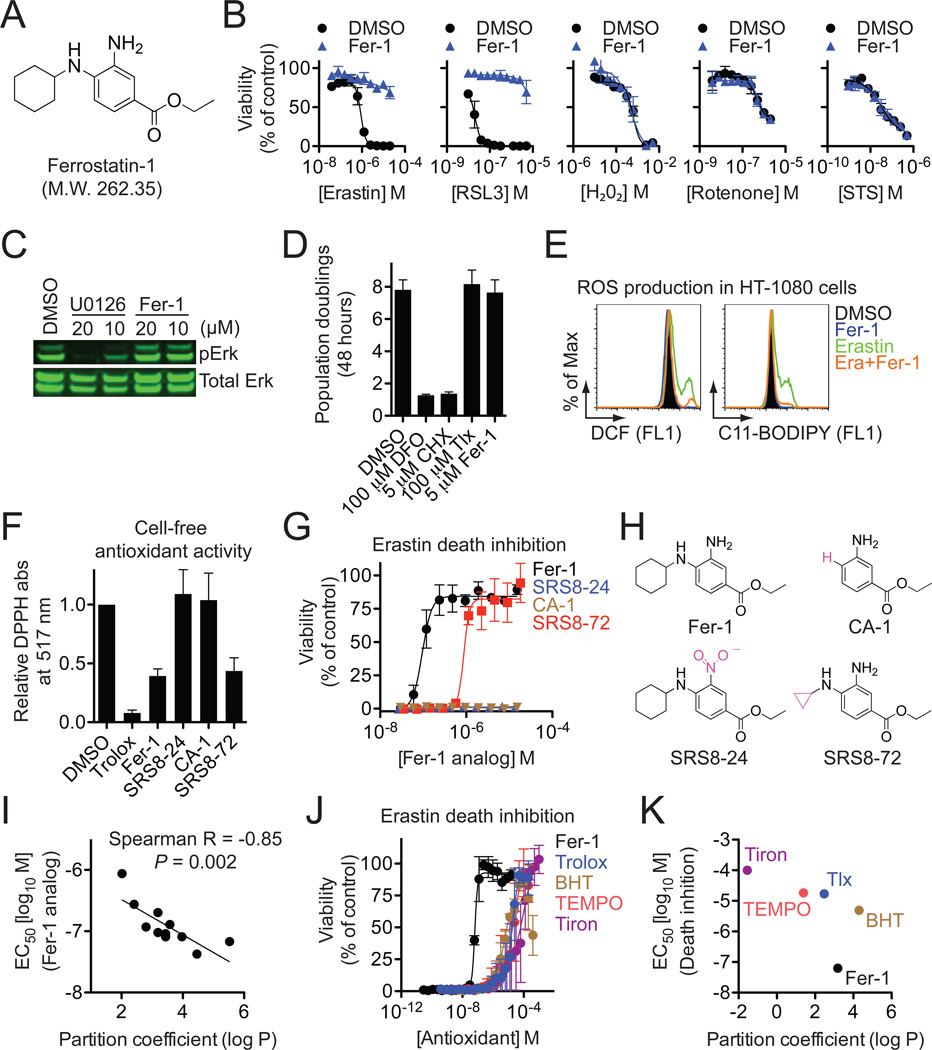
One ultimate aim is to investigate the potential role of ferroptosis in vivo and we therefore sought to identify a potent and specific drug-like small molecule inhibitor of this process. To overcome the inherent limitations of many individual small molecule collections (Macarron et al., 2011), we assembled a custom screening library of 9,517 small molecules derived from a starting pool of 3,372,615 commercially available compounds that were filtered in silico on the basis of drug-likeness, solubility, scaffold diversity and other parameters. Screening of this ‘lead-optimized compound’ (LOC) library and subsequent confirmation studies identified a compound we named ferrostatin-1 (Fer-1) as the most potent inhibitor of erastin-induced ferroptosis in HT-1080 cells (EC50=60 nM) (Figures 4A, S4A,B). To our knowledge, the activity for Fer-1 has not previously been reported in any biological system. We performed a total synthesis of Fer-1 (see Supplemental Experimental Procedures) and used this material to confirm the activity of Fer-1 and demonstrate that it specifically inhibited RSL-induced death, but not cell death induced by other oxidative lethal compounds and apoptosis-inducing agents (Figures 4B, 6A).
Figure 4. Identification and characterization of Ferrostatin-1.
(A) Structure of ferrostatin-1 (Fer-1). (B) Effect of resynthesized Fer-1 (0.5 µM) on the lethality of various compounds in HT-1080 cells. (C) Effect of Fer-1 and U0126 on ERK phosphorylation in HT-1080 cells. (D) Effect of DFO, CHX, trolox (Tlx) and Fer-1 on HT-1080 cell proliferation over 48 hours as assessed by Vi-Cell. (E) Effect of Fer-1 (0.5 µM) on erastin (10 µM)-induced ROS production in HT-1080 cells (4 hr treatment). (F) Cell-free antioxidant potential monitored by changes is the absorbance at 517 nm of the stable radical DPPH. (G) Dose-response relationship for inhibition of erastin (10 µM, 24 hrs)-induced death in HT-1080 cells by Fer-1 and analogs. (H) Correlation between predicted partition coefficient (log P) and the ability of various Fer-1 analogs to prevent erastin-induced death. (I) Dose-response relationship for inhibition of erastin (10 µM, 24 hrs)-induced death by various antioxidants. (J) Plot of predicted partition coefficient (log P) and ability of various antioxidants to prevent erastin-induced death. Data in (B), (D), (F), (G) and (J) represents mean+/−SD from one of three representative experiments.
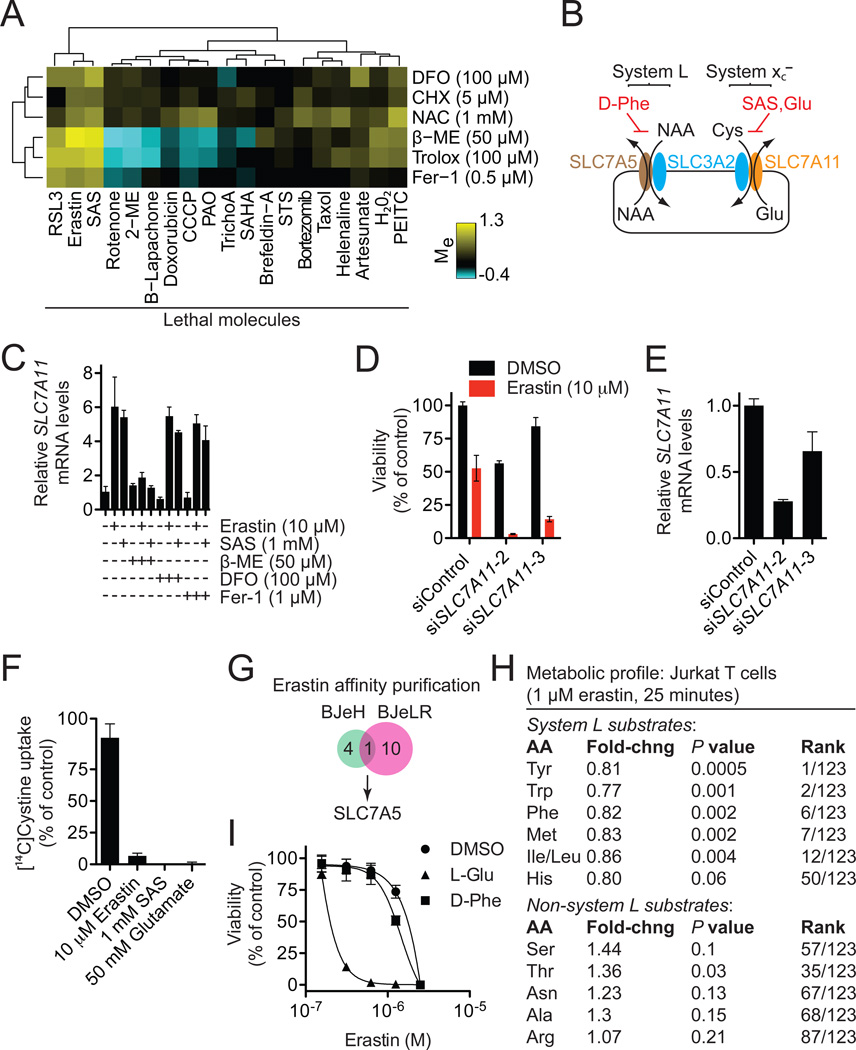
Figure 6. Erastin inhibits the activity of system xc−.
(A) Modulatory profile of HT-1080 cells treated with different lethal compounds and inhibitors. (B) Cartoon depicting the composition and function of system L and system xc−. Cys: cystine, NAA: neutral amino acids. (C) SLC7A11 mRNA levels in compound (6 hrs)-treated HT-1080 cells determined by RT-qPCR. (D,E) Effect of silencing SLC7A11 using siRNA on erastin (10 µM, 8 hrs)-induced death (D) and mRNA levels (E) in HT-1080 cells. (F) Na+-independent [14C]-cystine uptake by HT-1080 cells in response to various drugs. (G) Identification of SLC7A5 as the lone target identified by erastin affinity purification in both BJeH and BJeLR cells. (H) Metabolic profiling of system L and non-system L substrate amino acid levels in erastin-treated Jurkat cells. (I) Effect of L-glutamic acid (L-Glu, 12.5 mM) and Dphenylalanine (D-Phe, 12.5 mM) on erastin-induced death in HT-1080 cells.
We next sought to define the Fer-1 mechanism of action. Fer-1 did not inhibit ERK phosphorylation or arrest the proliferation of HT-1080 cells, suggesting that it does not inhibit the MEK/ERK pathway, chelate iron or inhibit protein synthesis (Figure 4C,D). Fer-1 did, however, prevent erastin-induced accumulation of cytosolic and lipid ROS (Figure 4E). Moreover, similar to the positive control antioxidant trolox, Fer-1 readily oxidized the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) under cell free conditions, a test of intrinsic antioxidant potential (Figure 4F). Substitution of the primary aromatic amine for a nitro group (SRS8–24), or elimination of the N-cyclohexyl moiety (CA-1), destroyed the antioxidant capability of Fer-1, as well as its ability to prevent erastin (10 µM)-induced death in HT-1080 cells (Figure 4F–H). Thus, both aromatic amines are required for Fer-1 to prevent RSL-induced death, a function plausibly linked to its ability to scavenge free radicals.
Our results suggested that lipid ROS were crucial for erastin-induced death. We therefore hypothesized that Fer-1 was a lipid ROS scavenger, with the N-cyclohexyl moiety serving as a lipophilic anchor within biological membranes. Consistent with this hypothesis, in a series of ten Fer-1 analogs, where the number of carbons in the N-substituted cyclic moiety was systematically varied, we observed a significant correlation between the predicted lipophilicity (octanol-water partition coefficient, log P) and the erastin-death-suppressing ability of each molecule (Spearman R = −0.85, P = 0.002) (Figures 4I, S4C). Of note, SRS8–72, a Fer-1 analog with N-cyclopropyl in place of N-cyclohexyl, which was an order of magnitude less potent than Fer-1 at preventing death, nonetheless retained equivalent intrinsic antioxidant capability in the cell-free DPPH assay (Figures 4F–H, S4C). Thus, the N-cyclohexyl moiety likely enables Fer-1 to prevent ferroptosis by promoting the tethering of Fer-1 within lipid membranes, as opposed to influencing the intrinsic antioxidant potential of this molecule.
Intriguingly, lipid partitioning alone does not appear to be sufficient to account for the potency of Fer-1. Fer-1 has similar predicted lipophilicity, but much greater erastin-suppressing potency than two canonical lipophilic antioxidants (trolox and butylated hydroxyltoluene [BHT]), while being both considerably more lipophilic and more potent than two representative soluble antioxidants (Tiron, TEMPO) (Figure 4J,K). Both trolox and BHT are phenolic antioxidants, while Fer-1 contains an aromatic amine. We hypothesize that this difference may confer a unique profile of radical reactivity upon Fer-1 that is better tuned to the RSL mechanism.
Fer-1 prevents glutamate-induced neurotoxicity
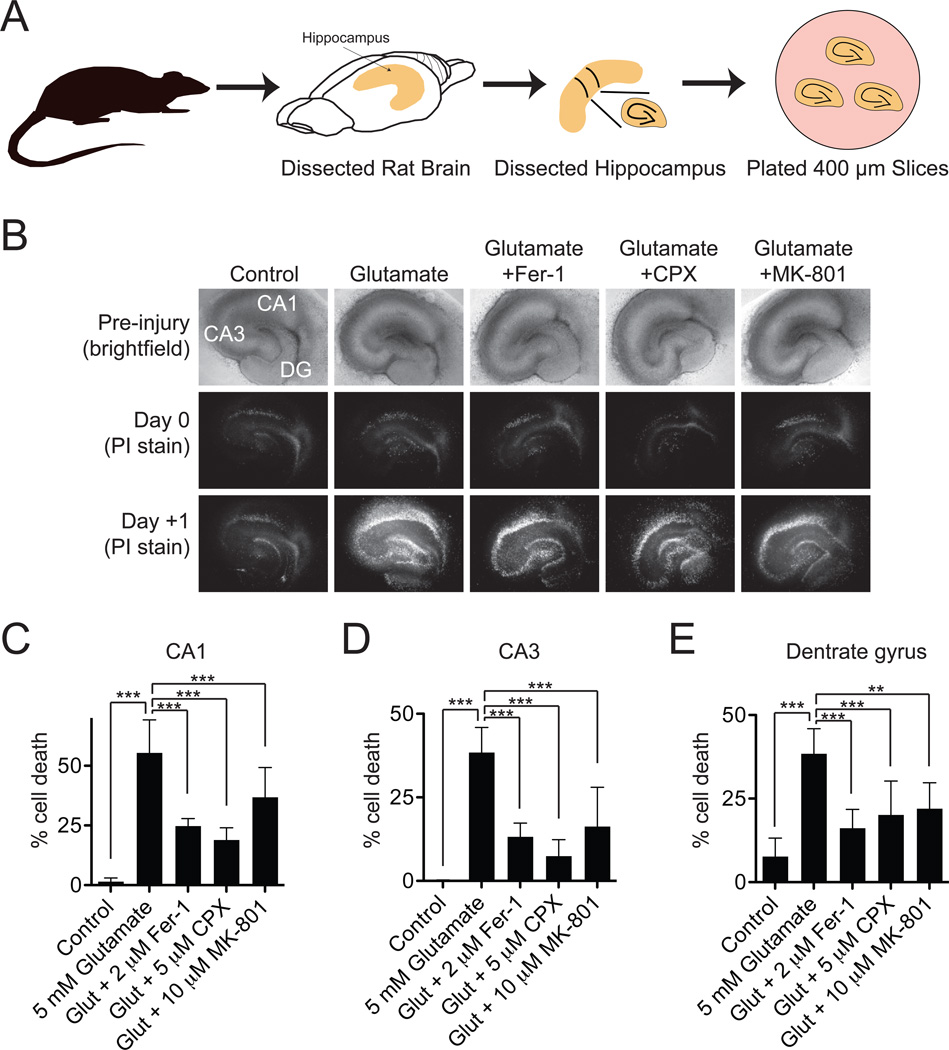
Excitotoxic cell death that occurs in the nervous system in epilepsy, stroke and other trauma situations has also been described as an oxidative, iron-dependent process (Cheah et al., 2006; Choi, 1988; Murphy et al., 1989). We hypothesized that excitotoxic death could be related to erastin-induced ferroptosis. We tested this hypothesis using a rat organotypic hippocampal slice culture (OHSC) model that closely resembles the hippocampus in vivo by preserving the integrity of neuronal connections, both inhibitory and excitatory, and their supporting cells, including astrocytes and microglia (Lossi et al., 2009). OHSCs have proven to be ideal complex preparations for lead-compound identification and validation (Noraberg et al., 2005; Sundstrom et al., 2005), capable of predicting in vivo efficacy (Cater et al., 2007; Morrison et al., 2002).
OHSCs were treated with a lethal excitotoxic stimulus (5 mM L-glutamate, 3 hrs) that mimics the consequences of stroke and neurodegenerative disease (Morrison et al., 2002; Sundstrom et al., 2005) (Figure 5A). These slices were co-incubated with glutamate and vehicle alone or with glutamate plus Fer-1 (2 µM), the iron chelator CPX (5 µM) or, as a positive control, the NMDA receptor antagonist MK-801 (10 µM). We analyzed the effects of these compound treatments on propidium iodide (PI) uptake, as an indicator of cell death, 24 hours following the end of glutamate treatment, in 3 defined regions of the OHSCs: the dentate gyrus (DG), the CA1 and the CA3 fields of the hippocampus. A two-way analysis of variance (ANOVA) suggested significant differences for both brain region (F2,75 = 19.23, P < .0001) and compound treatment (F4,75 = 67.8, P < .0001) factors. Focusing on the compound treatment effect, Bonferroni post-tests indicated that glutamate induced significant cell death in all three regions of the brain, and that this death was attenuated significantly and to an almost identical extent by co-treatment with Fer-1, CPX or MK-801 (P < .001 for all interactions except glutamate+MK-801 within the DG, P < .01) (Figure 5B–E). These results suggest that glutamate-induced death in OHSCs and erastin-induced death in cancer cells share in common a core lethal mechanism that can be inhibited by iron chelation or Fer-1.
Figure 5. Effects of Fer-1 on excitotoxic cell death in organotypic hippocampal slice cultures.
(A) Cartoon outline of hippocampal slice procedure. (B) Bright-field and fluorescent images of PI staining of treated hippocampal slices. Slices were treated with glutamate (5 mM, 3 hrs) +/− Fer-1 (2 µM), CPX (5 µM) or MK-801 (10 µM). Representative images from 1 one 6 slices per condition are shown. (C–E) Quantification of the effects depicted in (B). Data were analyzed using a two-way ANOVA (brain region × drug treatment) followed by Bonferroni post-tests. *: P < .05, **: P < .01, ***: P < .001.
Erastin inhibits system xc−
CPX and Fer-1 suppressed erastin-induced death in cancer cells and glutamate-induced toxicity in OHSCs, consistent with a common iron- and ROS-dependent death execution mechanism. We wondered whether any death-initiating mechanisms could also be shared between these two processes.
Glutamate-induced death in brain cells can be initiated by calcium influx through ionotropic glutamate receptors and through competitive inhibition of cystine uptake by the Na+-independent cystine/glutamate antiporter, system xc− (Choi, 1988; Murphy et al., 1989). The calcium chelators BAPTA-AM, Fura-2 and EGTA had no effect on erastin-induced death (Figure S5A) (Wolpaw et al., 2011), arguing against a role for Ca2+ influx in this process. However, we observed striking clustering of erastin and sulfasalazine (SAS), a specific inhibitor of system xc− (Gout et al., 2001), in a modulatory profile of 19 oxidative and non-oxidative lethal molecules generated in HT-1080 cells (Figure 6A). If blockade of system xc−-mediated cystine import can trigger ferroptosis, then providing this metabolite to cells through an alternative means should rescue from death. Indeed, β-mercaptoethanol (β-ME), which can circumvent the inhibition of system xc− by promoting cystine uptake through an alternative pathway (Ishii et al., 1981), strongly inhibited cell death in HT-1080 cells induced by erastin, SAS and glutamate (Figures 6A, S5B). As predicted by these results, SAS, like erastin, behaved as an oncogenic RAS-selective lethal (RSL) compound, albeit with considerably lower potency than erastin (Figure S5C). This is nonetheless noteworthy, as SAS is an FDA-approved drug not previously shown to demonstrate such activity.
System xc− is a disulfide-linked heterodimer composed of SLC7A11 (xCT) and SLC3A2 (4F2hc, CD98hc) (Sato et al., 1999) (Figure 6B). Inhibition of system xc− can lead to a compensatory transcriptional up-regulation of SLC7A11 (Lo et al., 2008). Consistent with this, we observed substantial upregulation of SLC7A11 in HT-1080 cells treated with erastin or SAS, an effect suppressed by β-ME, but not DFO or Fer-1 (Figure 6C). Further confirming the relevance of system xc− to erastin-induced ferroptosis, siRNA-mediated silencing of SLC7A11 with two independent siRNAs sensitized HT-1080 cells to erastin-induced death (Figures 6D,E), while transfection of HT-1080 cells with a plasmid encoding DDK-tagged SLC7A11 conferred protection from erastin- and SAS-induced death (Figure S5D). Given these results, we directly examined the uptake of [14C]-cystine into HT-1080 cells. Erastin (10 µM), glutamate (50 mM) and SAS (1 mM) abolished the Na+-independent uptake of [14C]-cystine, while RSL3 had no effect on this process (Figures 6F, S5E).
How does erastin inhibit system xc−? Analysis of affinity purification data (Yagoda et al., 2007) identified SLC7A5 (LAT1, 4F2lc, CD98lc) as the lone protein bound by an active erastin affinity analog in lysates from both HRAS-wildtype BJeH and HRAS-mutant BJeLR cells (Figure 6G). SLC7A5 (like SLC7A11) is one of six light chains that bind SLC3A2 to form amino acid transporters of differing substrate selectivity. The SLC7A5/SLC3A2 complex (system L) transports large, neutral amino acids (Kanai and Endou, 2003) (Figure 6B). In a profile of 123 metabolites from human Jurkat T lymphocytes treated with erastin (1 µM, 25 min) (Ramanathan and Schreiber, 2009), highly significant decreases were observed in the levels of system L substrates (Kanai and Endou, 2003), while the levels of non-system L substrates were unchanged or increased (Figure 6H). However, unlike inhibition of system xc− using excess glutamate (12.5 mM), inhibition of system L using excess D-phenylalanine (12.5 mM) (Kanai and Endou, 2003) did not strongly sensitize to erastin (Figure 6I). Together, this suggests that erastin inhibits system L-mediated amino acid uptake, but that this does not contribute directly to ferroptosis. Rather, erastin binding to SLC7A5 or the SLC7A5/SLC3A2 complex interferes with cystine uptake by the SLC3A2/SLC7A11 complex in trans.
NAPDH oxidases provide one source of death-inducing ROS in erastin-treated cells
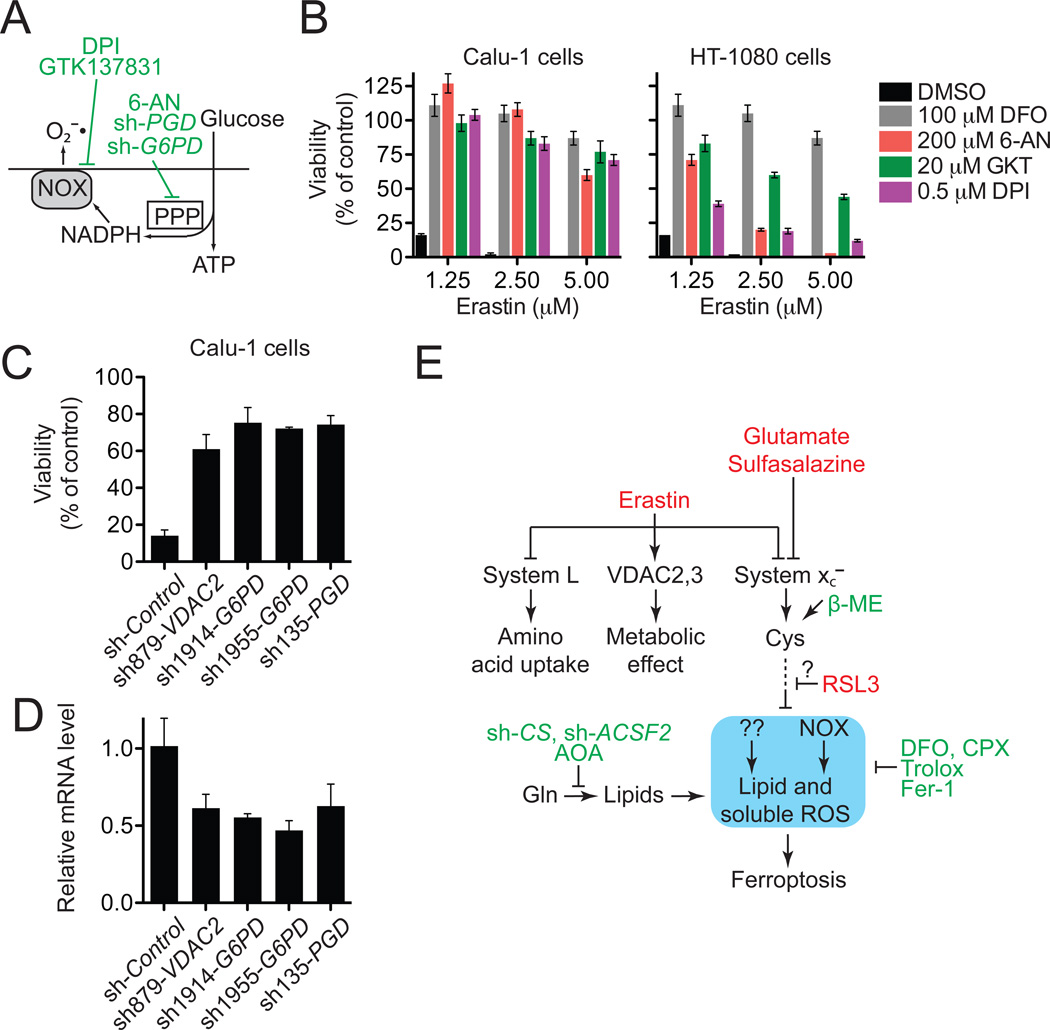
Blocking system xc− inhibits cysteine-dependent glutathione (GSH) synthesis and inhibits the trans-plasma membrane cysteine redox shuttle (Banjac et al., 2008; Ishii et al., 1981). Both effects impair cellular antioxidant defenses, thereby facilitating toxic ROS accumulation. Having ruled out the mitochondrial ETC as a source of death-inducing ROS in erastin-treated cells (Figure 1D–F), we examined the role of the NADPH oxidase (NOX) family of superoxide-producing enzymes (NOX1–5, DUOX1,2), which are up-regulated in several RAS-mutant tumors (Kamata, 2009). Erastin-induced ferroptosis was strongly suppressed in Calu-1 cells by the canonical NOX inhibitor diphenylene iodonium (DPI), the NOX1/4 specific inhibitor GKT137831 (Laleu et al., 2010) and an inhibitor of the NADPH-generating pentose phosphate pathway (PPP), 6-aminonicotinamde (6-AN) (Figure 7A,B). Given that Calu-1 cells express NOX1 at much higher levels than NOX4 (Figure S6A), NOX1 is the most likely candidate to mediate the observed NOX-dependent lethal effects in these cells. Additionally, shRNAmediated silencing of two PPP enzymes, glucose-6-phosphate dehydrogenase (G6PD) and phosphoglycerate dehydrogenase (PGD), also prevented erastin-induced ferroptosis in Calu-1 cells to the same extent as silencing of VDAC2 (Figure 7C,D). 6-AN also prevented cell death as well as ROS production in BJeLR cells (Figure S6B,C), suggesting an important role for this pathway is these cell types. On the other hand, NOX and PPP inhibitors were only partially effective at preventing erastin-induced ferroptosis in HT-1080 cells (Figure 7B), indicating that other pathways, in addition to the PPP/NOX pathway, can contribute to the onset of death in erastin-treated cells, once the appropriate conditions have been set by the inhibition of system xc−.
Figure 7. Role of NOX in erastin-induced death.
(A) Outline of NOX pathway. Inhibitors are shown in green. (B) Effect of NOX pathway inhibitors on erastin-induced death in Calu-1 and HT-1080 cells. GKT: GKT137831. (C,D) Effect of shRNA silencing of the PPP enzymes glucose-6-phosphate dehydrogenase (G6PD) and phosphogluconate dehydrogenase (PGD) on viability of erastin (2.5 µM)-treated Calu-1 cells. Infection with shRNA targeting VDAC2 was used as a positive control. Relative mRNA levels in (D) were assessed by qPCR following shRNA knockdown. Data in (B), (C) and (D) represents mean+/−SD. (E) Model of ferroptosis pathway. The core ferroptotic lethal mechanism is highlighted in blue.
DISCUSSION
Ferroptotic death is morphologically, biochemically and genetically distinct from apoptosis, various forms of necrosis, and autophagy. This process is characterized by the overwhelming, iron-dependent accumulation of lethal lipid ROS (Figure 7E, blue outline). Unlike other forms of apoptotic and non-apoptotic death (Christofferson and Yuan, 2010; Jacobson and Raff, 1995), this requirement for ROS accumulation appears to be universal. In at least some cells, NOX-family enzymes make important contributions to this process. Indeed, although we cannot exclude the possibility of a death-inducing protein or protein complex activated downstream of ROS accumulation, we posit that the executioners of death in cancer cells undergoing ferroptosis are these ROS themselves. An important prediction of this model is that under anoxic conditions ferroptosis will be inactive. However, even here, agents such as erastin that prevent uptake of essential amino acids by system L are likely to be toxic to cells.
Using an shRNA library targeting most known genes encoding mitochondrial proteins (Pagliarini et al., 2008) we identified specific roles for RPL8, IREB2, ATP5G3, TTC35, CS and ACSF2 in erastin-induced ferroptosis. A plausible new hypothesis to emerge from these data is that CS and ACSF2 are required to synthesize a specific lipid precursor necessary for death (Figure 7E). Just as important, the high-resolution of the arrayed approach (1 hairpin/well, minimum 5 hairpins/gene) provides confidence that the various mitochondrial genes not identified in our screen, including many implicated in apoptotic and non-apoptotic death (BID, BAK1, BAX, AIFM1, PPIF, HTRA2, ENDOG, PGAM5), are truly not required for erastin-induced ferroptosis. This screening collection will be a valuable resource for future studies of the role of the mitochondria in cell physiology.
In cancer cells, inhibition of system xc−-mediated cystine uptake by erastin, SAS or glutamate may be sufficient to initiate iron-dependent ferroptosis. Inhibition of system xc− is, however, not necessary: RSL3 does not inhibit cystine uptake and yet triggers an otherwise similar iron and ROS-dependent ferroptototic death program. Thus, RSL3 likely modulates the activity of a target lying downstream of or in parallel to system xc− (Figure 7E). Importantly, this may enable RSL3 to activate ferroptosis in cells or conditions where cystine uptake via system xc− is not limiting for survival. Lanperisone, another recently identified oncogenic RAS-selective lethal small molecule that causes non-apoptotic, iron-dependent death in mouse Kras-mutant tumor cells (Shaw et al., 2011), may also inhibit the function of system xc− or another target in the ferroptotic pathway. Other compounds that behave as RSLs, such as PEITC, oncrasin and piperlongumine (Guo et al., 2008; Raj et al., 2011; Trachootham et al., 2006), trigger mitochondrial cytochrome C release, caspase activation and other features of apoptosis not observed in cancer cells undergoing ferroptosis. Certain tumor cells are highly resistant to apoptosis (Ni Chonghaile et al., 2011). Thus, agents such as erastin, RSL3 and lanperisone that can trigger non-apoptotic death may exhibit a unique spectrum of clinical activity.
In some brain cell populations, inhibition of system xc− by glutamate triggers oxidative cell death dependent on iron and lipid ROS, but also Ca2+ influx, mitochondrial damage, mitochondrial ROS production and chromatin fragmentation (Li et al., 1997; Murphy et al., 1989; Ratan et al., 1994; Tan et al., 1998; Yonezawa et al., 1996). These latter events are not required for RSL-induced ferroptosis in cancer cells, perhaps because heightened activity of NOX or other pro-oxidant enzymes, or basally altered membrane lipid composition, is sufficient to promote death in the absence of these additional features. Regardless, the oxidative death pathways triggered in cancer cells and brain cells by blockade of cystine uptake both appear to access a core iron- and ROS-dependent ferroptotic mechanism, accounting for the ability of Fer-1 and CPX to attenuate death in both cases (Figure 7E).
The specific role of iron in ferroptosis remains unclear. Ferroptosis cannot be explained by a simple increase in H202-dependent, iron-catalyzed ROS production (i.e. Fenton chemistry), as H202-induced death is distinct from RSL-induced ferroptosis (Figures 1,2). Rather, our results are most consistent with one or more iron-dependent enzymes functioning as part of the core, oxidative lethal mechanism. The void created in the antioxidant defenses of the cell by the inhibition of cystine uptake by erastin may be required to unleash the activity of these enzymes. Thus, for better or worse, the aberrantly elevated levels of iron observed in some cancer cells (Pinnix et al., 2010) and pathological neuronal populations (Duce et al., 2010; Lei et al., 2012) may predispose to ferroptotic death in situations of cystine or cysteine limitation.
Experimental Procedures
Analysis of reactive oxygen species production
The day before the experiment, 200,000 cells/well were seeded in 6-well dishes (Corning). The day of the experiment, cells were treated with test compounds for the indicated times, then harvested by trypsinization, resuspended in 500 µL Hanks Balanced Salt Solution (HBSS, Gibco) containing either H2DCFDA (25 µM), C11-BODIPY(581/591) (2 µM) or MitoSOX (5 µM) (all from Molecular Probes, Invitrogen) and incubated for 10 minutes at 37°C in a tissue culture incubator. Cells were then resuspended in 500 µL of fresh HBSS, strained through a 40 µM cell strainer (BD Falcon) and analyzed using a flow cytometer (FACSCalibur or Accuri C6, BD Biosciences), equipped with 488-nm laser for excitation. Data were collected from the FL1 (H2DCFDA, C11-BODIPY) or FL2 channel (MitoSOX). A minimum of 10,000 cells were analyzed per condition.
Cancer cell viability measurements
Cell viability was typically assessed in 384-well format by Alamar Blue (Invitrogen) fluorescence (ex/em 530/590) measured on a Victor3 platereader (Perkin Elmer). In some experiments, Trypan Blue dye exclusion counting was performed using an automated cell counter (ViCell, Beckman-Coulter). Cell viability under test conditions is reported as a percentage relative to the negative control treatment.
shRNA Screening
An arrayed collection of 6,528 shRNA hairpins derived from The RNAi Consortium (TRC) collection targeting 1,087 genes, kindly provided by Vamsi Mootha and Joshua Baughman (MIT), was screened in 384-well plate format (Corning) using both Calu-1 and HT-1080 cells. ShRNAs targeting GFP and RFP, randomly distributed through each plate, served as negative controls. 400 cells per well were infected in duplicate for 48 hours with 2 µL shRNA-containing viral supernatant, selected for 24 hours in puromycin (1.5 µg/mL), then treated with DMSO, erastin (7.3 µM) or STS (1 µM) for 24 hours. Cell viability was determined using Alamar Blue. For each hairpin within each treatment condition, a cell death rescue score was computed as the ratio of the average viability of the two replicates to the average viability of the within-plate negative controls. These scores were used to compare the effects between compounds. To identify genes required for ferroptosis, individual hairpins were scored as hits if they displayed an average death suppression ≥ 3 median average deviations from the median within-plate or screen-wide negative control values. 51 candidate genes were identified with the same two (or more) unique hairpins per gene called as hits in both the Calu-1 and HT-1080 screens. For each candidate gene, confirmation studies using RT-qPCR analysis of mRNA silencing was performed in HT-1080 cells using freshly prepared virus as described in more detail in the Supplemental Experimental Protocols.
[14C]-cystine uptake assay
200,000 HT-1080 cells/well were seeded overnight in 6-well dishes (Corning). The next day, cells were washed twice in pre-warmed Na+-free uptake buffer (137 mM choline chloride, 3 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM D-glucose, 0.7 mM K2HPO4, 10 mM HEPES, pH 7.4), then incubated for 10 minutes at 37°C in 1 mL of uptake buffer, to deplete cellular amino acids. At this point, in each well the buffer was replaced with 600 µL uptake buffer containing compound and 0.12 µCi (80–110 mCi/mmol) of L-[3,3'-14C]-cystine (Perkin Elmer) and incubated for 3 minutes at 37°C. Cells were then washed three times with ice-cold uptake buffer and lysed in 500 µL 0.1M NaOH. To this lysate was added 15 mL of scintillation fluid and radioactive counts per minute were obtained using a scintillation counter. All measurements were performed in triplicate for each condition.
Statistical Analyses
All statistical analyses were performed using Prism 5.0c (GraphPad Software).
Article Highlights.
Certain lethal compounds trigger iron-dependent cell death, called ferroptosis
Ferroptosis is distinct from apoptosis, necrosis and autophagy
Ferroptosis can be initiated by inhibition of cystine uptake by system xc−
Impairment of cystine uptake leads to the production of lethal lipid ROS
Supplementary Material
Acknowledgements
We thank Vamsi Mootha, Joshua Baughman and David Root for sharing the custom shRNA library, Kristy Brown and Elma Zaganjor for assistance with electron microscopy, Darnelle Delva for help with qPCR, Rohitha SriRamaratnam for help with cell death assays, Eric Schon for providing the 143B cell lines, Craig Thompson for providing Bax−/− Bak−/− MEFs and Patrick Page (GenKyoTex S.A.) for providing GKT137831. We thank David Clarke for comments on the manuscript. Certain shRNA collections used in this work were generated with the assistance of the Scientific Planning and Allocation of Resources Committee (SPARC, to Vamsi Mootha). NSF grant CHE-0840451 supported the purchase and operation of equipment used in the chemical characterization of ferrostatin-1. M.R.L. was supported by a fellowship from the National Science Foundation. K.M.L was supported by the Medical Scientist Training Program (Columbia University). S.J.D. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research. This research was supported by grants from the US National Institutes of Health (5R01CA097061, 5R01GM085081 and R01CA161061), the Arnold and Mabel Beckman Foundation and NYSTAR. B.R.S. is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kolle P, Tschoep K, Issels RD, Daniel PT, et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cater HL, Gitterman D, Davis SM, Benham CD, Morrison B, 3rd, Sundstrom LE. Stretch-induced injury in organotypic hippocampal slice cultures reproduces in vivo post-traumatic neurodegeneration: role of glutamate receptors and voltage-dependent calcium channels. J Neurochem. 2007;101:434–447. doi: 10.1111/j.1471-4159.2006.04379.x. [DOI] [PubMed] [Google Scholar]
- Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, 3rd, Papadopoulos V, Snyder SH. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Current Opinion in Cell Biology. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, et al. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- Guo W, Wu S, Liu J, Fang B. Identification of a small molecule with synthetic lethality for K-ras and protein kinase C iota. Cancer Res. 2008;68:7403–7408. doi: 10.1158/0008-5472.CAN-08-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Bannai S, Sugita Y. Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. The Journal of biological chemistry. 1981;256:12387–12392. [PubMed] [Google Scholar]
- Jacobson MD, Raff MC. Programmed cell death and Bcl-2 protection in very low oxygen. Nature. 1995;374:814–816. doi: 10.1038/374814a0. [DOI] [PubMed] [Google Scholar]
- Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009;100:1382–1388. doi: 10.1111/j.1349-7006.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Endou H. Functional properties of multispecific amino acid transporters and their implications to transporter-mediated toxicity. J Toxicol Sci. 2003;28:1–17. doi: 10.2131/jts.28.1. [DOI] [PubMed] [Google Scholar]
- Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, et al. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. Journal of medicinal chemistry. 2010;53:7715–7730. doi: 10.1021/jm100773e. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nature medicine. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- Lo M, Ling V, Wang YZ, Gout PW. The xc-cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. British journal of cancer. 2008;99:464–472. doi: 10.1038/sj.bjc.6604485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossi L, Alasia S, Salio C, Merighi A. Cell death and proliferation in acute slices and organotypic cultures of mammalian CNS. Prog Neurobiol. 2009;88:221–245. doi: 10.1016/j.pneurobio.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, Green DV, Hertzberg RP, Janzen WP, Paslay JW, et al. Impact of high-throughput screening in biomedical research. Nature reviews Drug discovery. 2011;10:188–195. doi: 10.1038/nrd3368. [DOI] [PubMed] [Google Scholar]
- Morrison B, 3rd, Pringle AK, McManus T, Ellard J, Bradley M, Signorelli F, Iannotti F, Sundstrom LE. L-arginyl-3,4-spermidine is neuroprotective in several in vitro models of neurodegeneration and in vivo ischaemia without suppressing synaptic transmission. Br J Pharmacol. 2002;137:1255–1268. doi: 10.1038/sj.bjp.0704986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, Deberardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011 doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnix ZK, Miller LD, Wang W, D'Agostino R, Jr, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med. 2010;2 doi: 10.1126/scisignal.3001127. 43ra56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ramanathan A, Schreiber SL. Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci U S A. 2009;106:22229–22232. doi: 10.1073/pnas.0912074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan RR, Murphy TH, Baraban JM. Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem. 1994;62:376–379. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- Salahudeen AA, Thompson JW, Ruiz JC, Ma HW, Kinch LN, Li Q, Grishin NV, Bruick RK. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science. 2009;326:722–726. doi: 10.1126/science.1176326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. The Journal of biological chemistry. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Shaw AT, Winslow MM, Magendantz M, Ouyang C, Dowdle J, Subramanian A, Lewis TA, Maglathin RL, Tolliday N, Jacks T. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom L, Morrison B, 3rd, Bradley M, Pringle A. Organotypic cultures as tools for functional screening in the CNS. Drug discovery today. 2005;10:993–1000. doi: 10.1016/S1359-6446(05)03502-6. [DOI] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. The Journal of Cell Biology. 1998;141:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Vashisht AA, Zumbrennen KB, Huang X, Powers DN, Durazo A, Sun D, Bhaskaran N, Persson A, Uhlen M, Sangfelt O, et al. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science. 2009;326:718–721. doi: 10.1126/science.1176333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol. 2009;218:193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acylcoenzyme A synthetase genes in the human genome. J Lipid Res. 2007;48:2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw AJ, Shimada K, Skouta R, Welsch ME, Akavia UD, Pe'er D, Shaik F, Bulinski JC, Stockwell BR. Modulatory profiling identifies mechanisms of small molecule-induced cell death. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1106149108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chemistry & biology. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa M, Back SA, Gan X, Rosenberg PA, Volpe JJ. Cystine deprivation induces oligodendroglial death: rescue by free radical scavengers and by a diffusible glial factor. J Neurochem. 1996;67:566–573. doi: 10.1046/j.1471-4159.1996.67020566.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.