INTRODUCTION
Endotoxins or lipopolysaccharides (LPS) are part of the outer membrane of Gram negative bacteria and are shed into the environment after bacteria die.1 Endotoxins are widely distributed in our environment and are potent stimulators of the immune system. Early exposure to endotoxin may result in a decreased TH2 profile2, 3 and may be protective against the development of atopic conditions.2, 4–6 In contrast, possibly as an irritant or a modifier of pulmonary responsiveness to allergens, increased endotoxin exposure has been associated with both the development of early childhood wheezing and increased current asthma symptoms in subjects already diagnosed with asthma.4, 7–15
High levels of endotoxins exist in work environments such as agricultural areas, animal production facilities, waste processing, and textile production.16 In homes, higher levels of endotoxin have been associated with indoor pets, carpeting, air conditioning, and farming exposures.17–19 There are some studies that have demonstrated the presence of endotoxins in daycare centers and schools,20–24 but these studies do not have home levels for comparison of the relative school endotoxin exposure. Our study is designed to evaluate both the school and home environments in a cohort of inner-city children with asthma. Given the important association of endotoxin and asthma, our goal was to evaluate the school endotoxin exposure relative to the home endotoxin exposure in children with asthma. Additionally, we aimed to determine if animal allergen concentrations were associated with endotoxin concentrations.
METHODS
This evaluation of endotoxin exposure in homes and schools is a sub-study of the School Inner-City Asthma Study (SICAS). SICAS is an observational, prospective study evaluating specific factors in both the home and school environments related to asthma morbidity. In this study, 500 elementary school aged children with asthma are being recruited from 35–40 metropolitan schools over 5 years. Students are recruited annually and prospectively followed during the subsequent academic year to monitor asthma morbidity. During the academic year, enrolled students’ school and home environments are sampled for allergen exposure in order to determine school and classroom-specific environmental risk factors, relative to home exposures, associated with asthma morbidity. The primary hypothesis of SICAS is that exposure to indoor allergens in the classroom will increase the risk of asthma morbidity, even after controlling for home allergen exposures.
The focus of the study in this paper is a sub-study made possible through supplemental funding (ACAAI Young Faculty Support Award) obtained to analyze a subset of environmental dust samples for endotoxin. The endotoxin sub-study measured endotoxin concentrations in schools and homes of children with asthma enrolled in SICAS during the study’s first two years. All of the students during this time period were recruited from 12 inner-city elementary schools. Written informed consent was obtained and this study was approved by the Children’s Hospital Boston institutional review board.
School Environment Assessment
In each school, settled dust samples were vacuumed from individual classrooms, gymnasiums, and cafeterias as a measure of school-wide exposure. School settled dust samples were collected twice during the academic year, once in the fall and once in the spring, and were linked to the enrolled student. All students also provided a home bedroom settled dust sample as a measure of home exposure. All school settled dust samples were collected in the same manner. Briefly, an Oreck XL (model BB870-AD) vacuum was used with a dust collector filter (DACI labs, Johns Hopkins, Baltimore, MD) fitted on the inlet hose.25, 26 Vacuuming of the settled dust sample was performed for a total of 6 minutes per sample, 3 minutes on the floor and 3 minutes on other surfaces, such as desks and chairs, as previously described.27 Collected dust was sifted through a 40-mesh metal sieve (>425 micron particle exclusion), aliquoted, and stored at −20°C until extraction.
Home Environmental Assessment
In the home environment, dust samples were collected from the students’ bedrooms in a similar manner as described in previous studies.28 In the students’ bedrooms, the area around the bed was sampled. To do this, dust was vacuumed from both the child’s mattress and from the bedroom floor. Settled dust samples were collected from the subjects’ bedrooms once during the year of observation.
Measurement of Endotoxin and Allergens in Dust Samples
Collected dust was extracted in pyrogene-free water/0.05% Tween 20 and serially diluted for inclusion in the chromogenic Limulus Amoebocyte Lysate (LAL) assay (Lonza, Walkersville, MD) for measurement of endotoxin. The concentration of endotoxin was measured using the units of EU/mg. The lower limits of detection (LLOD) were 0.5 EU/mg for endotoxin. The LLOD for measured allergens was 0.004 μg/g for Fel d 1, 0.012 μg/g for Can f 1, and 0.002 μg/g for Mus m 1.
Seasonal Analysis
For purposes of analysis, the year was divided into “Fall” and “Spring” seasons. The “Fall” season was defined as any time from September to March with the vast majority of samples being collected in September, October, and November. The “Spring” season was defined as any time between April to August with the vast majority of the samples collected in April, May, and June. School samples were collected twice during the year, once in each of the seasons with approximately 6 months between the collections of each sample. Home samples were collected once during the year. These school and home samples were linked to individual children with asthma. Home samples were matched to the school sample that was performed within the same season and the time interval between sampling the home and school was typically within two months.
Statistical Analysis
Medians were calculated for endotoxin levels in each setting (schools and homes) and for each season (fall and spring) in the school samples. Values below the LLOD were assigned the limit of detection for all statistical analyses. Each enrolled subject had personal links that identified their bedroom, their school, and their exact classroom. Statistical analysis was performed on settled dust samples of matched students’ classrooms and bedrooms collected in the same season. Additionally, analyses were conducted on matched fall and spring school samples. Endotoxin concentrations were log transformed so the distributions were approximately normal for parametric statistical tests and correlations. Paired t-tests on log transformed variables were used where p-values are reported. Pearson correlations were computed for log transfored variables to determine levels of associations. Finally, allergen concentrations for cat, dog, and mouse were evaluated as possible predictors of elevated log transformed endotoxin concentrations using generalized linear models to account for the clustering of students within schools (SAS 9.12, Cary, NC).
RESULTS
A total of 347 settled vacuumed dust samples were collected over the two year period. In the subjects’ schools, a total of 229 settled dust samples were collected from multiple rooms in 12 different schools (5 schools in year 1 and 7 schools in year 2). School samples were collected twice during the academic year with 117 settled dust samples collected in the fall and 112 settled dust samples collected in the spring. Also, 118 bedroom settled dust samples were collected.
In the school dust samples, 100% (229 of 229) had detectable levels of endotoxin with a median concentration of 13.4 EU/mg. In the home dust samples, 96.6% (114 of 118) had detectable endotoxin levels with a median concentration of 7.0 EU/mg. Additional information on the overall home and school endotoxin concentrations including levels for different schoolrooms, such as classrooms, cafeterias, and gymnasiums, are included in Table 1.
TABLE 1.
Endotoxin Levels in Schools and Homes of Children with Asthma
| Number of Samples Collected | Samples with Detectable Endotoxin | Median Endotoxin Level | Endotoxin Range | Endotoxin Interquartile Range | |
|---|---|---|---|---|---|
| HOMES (All Bedrooms) | 118 | 96.6% (114) | 7.0 EU/mg | <0.5 – 843.0 EU/mg | 4.6 – 22.5 EU/mg |
| SCHOOLS (All Rooms) | 229 | 100% (229) | 13.4 EU/mg | 0.7 – 360.7 EU/mg | 7.0 – 23.0 EU/mg |
| Different Schoolroom Types: | |||||
| Classrooms | 195 | 100% (195) | 13.8 EU/mg | 0.7 – 360.7 EU/mg | 7.2 – 23.5 EU/mg |
| Cafeterias | 22 | 100% (22) | 16.0 EU/mg | 0.9 – 126.8 EU/mg | 9.0 – 27.8 EU/mg |
| Gymnasiums | 12 | 100% (12) | 5.6 EU/mg | 2.3 – 21.3 EU/mg | 3.2 – 8.3 EU/mg |
A total of 104 enrolled subjects with asthma had matched samples collected from their bedroom and their classroom in the same season. In this subset of matched samples, the classroom endotoxin concentration was significantly higher than the bedroom endotoxin concentration (mean log value = 1.13 vs. 0.99, mean difference = 0.144, 95% CI of difference = 0.009–0.278, p = 0.04, paired t-test). The classroom median endotoxin concentration of 12.5 EU/mg (range = 0.7 – 360.7 EU/mg) was higher than the median bedroom endotoxin concentration of 7.0 EU/mg (range = <0.5 – 843.0 EU/mg). On an individual-student basis, 59% of these subjects (61 of 104) had higher concentrations of endotoxin in their classroom than in their bedroom.
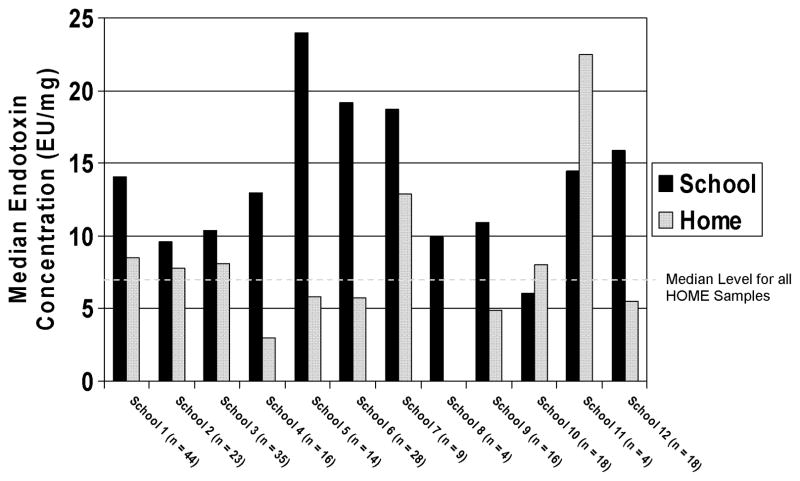
There was wide variability between endotoxin concentrations in dust samples across the 12 schools as seen in Figure 1. The median dust endotoxin concentration at each of the individual schools ranged from 6.6 EU/mg (School 10) to 24.0 EU/mg (School 5). For comparison, Figure 1 also includes the median endotoxin concentration of the homes of students attending that particular school. Additionally, a line on Figure 1 has been drawn to demonstrate the median concentration for dust endotoxin in home bedroom samples (7.0 EU/mg). As depicted in Figure 1, all but one of the schools had a median dust endotoxin concentration that rose above the median dust endotoxin level for home bedroom samples.
FIGURE 1. Median Endotoxin Concentration in Each Inner-City School as Compared to Homes of Students Attending that Particular School.
Black Bars Represent the Median School Endotoxin Concentration
Grey bars represent the Median Home Endotoxin Concentration
Dashed Line Shows Median Home Concentration for All Samples
When comparing school settled dust endotoxin concentrations in the fall to those in the spring, no difference was detected (mean log value = 1.14 vs. 1.09, mean difference = 0.048, 95% CI of difference = −0.054–0.150, p = 0.35, paired samples t-test). For this comparison, 111 rooms within the schools had matching fall and spring samples collected. The median settled dust endotoxin concentrations in fall samples was 14.4 EU/mg (range 0.7 – 360.7 EU/mg) as compared to spring samples in the same rooms with a median settled dust endotoxin concentration of 12.9 EU/mg (range 0.8 – 206.5 EU/mg). Although no difference was detected, there was a weak correlation detected between the fall and spring schoolroom samples (r=0.23).
Finally, dust samples were evaluated to see if higher allergen concentrations were associated with higher endotoxin concentrations. As seen in Table 2, higher concentrations of dog and cat allergens in school dust samples were associated with higher concentrations of endotoxin when adjusting variances for within school variability and repeated observations. There were significant differences in endotoxin concentrations between the schoolrooms in the higher tertiles of cat and dog allergens as compared to the lowest tertile. In contrast, there were no differences in settled dust endotoxin concentration between the schoolrooms when grouped by tertile level of settled dust mouse allergen (data not shown).
TABLE 2.
School Dust Endotoxin Level by Tertile of Allergen Level
| Number | Median Endotoxin Level | Mean Log Endotoxin Level (SD) | |||
|---|---|---|---|---|---|
| CAT ALLERGEN (Fel d 1) | |||||
| Low | ≤ 0.04 μg/g | 76 | 9.6 EU/mg | 0.98 (0.51) | Reference |
| Middle | > 0.04 – < 0.43 μg/g | 76 | 10.7 EU/mg | 1.12 (0.41) | p = 0.019 |
| High | ≥ 0.43 μg/g | 77 | 16.7 EU/mg | 1.23 (0.32) | p < 0.001 |
| DOG ALLERGEN (Can f 1) | |||||
| Low | ≤ 0.012 μg/g | 77 | 9.0 EU/mg | 0.95 (0.51) | Reference |
| Middle | > 0.012 – < 0.15 μg/g | 76 | 13.2 EU/mg | 1.13 (0.42) | p = 0.003 |
| High | ≥ 0.15 μg/g | 76 | 17.0 EU/mg | 1.24 (0.32) | p < 0.001 |
DISCUSSION
In our study, inner-city children with asthma were exposed to higher concentrations of endotoxin in their school classrooms as compared to their home bedrooms. To our knowledge, this is the first study to directly compare school endotoxin exposure to home endotoxin exposure in children with asthma. This result demonstrates that in an urban environment, schools, and not homes, may represent the most significant location for endotoxin exposure. Given that children spend a large proportion of their childhood in school, this endotoxin exposure may substantially contribute to asthma morbidity. Further studies are needed to evaluate the school environment as a cause of asthma morbidity.
Previous studies have found detectable endotoxin in schools, but did not evaluate students’ homes. As expected, Andersson et al discovered that endotoxin levels in daycares and schools were lower than endotoxin levels found in animal sheds;20 however, the home environment provides a better comparison for childhood exposure. Most previous studies have evaluated places of education for the youngest children, such as daycare centers and pre-elementary schools.21, 23, 24 Interestingly, Rullo et al demonstrated that these facilities for younger children had endotoxin levels that were three times higher than endotoxin levels in elementary schools for older children. Our study evaluated elementary schools and found the endotoxin concentrations to be higher than homes. It is possible that if we evaluated daycares or preschool settings, the differential over home endotoxin concentrations may have been even greater.
Another factor important to our study was the inner-city environment. Our comparison showed higher concentrations in urban schools as compared to urban homes, but it is difficult to translate these findings to a rural environment. Morcos et al found that rural schools have higher levels of endotoxin as compared to urban schools.22 Likewise, higher levels of endotoxin have been found in rural homes, especially those homes in close proximity to farm animals.19 Based on these studies, it would be expected that both rural schools and rural homes would have higher exposure of endotoxin, but the comparison between schools and homes in these communities is unknown.
Previous studies have demonstrated that predictors of increased endotoxin levels include pets, carpeting, moisture sources, increased total dust, and increased inhabitants in a living area.17, 18 For our study, the number of residents in the home was not correlated with the bedroom endotoxin concentration (r = 0.02). In the schools, the age of the school was not correlated with the concentration of endotoxin (r = −0.08). There was no correlation between the matched school and home samples when evaluating endotoxin concentrations (r = 0.16). None of our schools had pets, and the majority of our schoolrooms did not have carpeting. It is possible that the increased number of children in each classroom, as compared to a bedroom, could have contributed to our findings. This is supported by our finding that levels of dog and cat allergens were associated with higher concentrations of endotoxin in the schools. Given that there were no pets in the schools, this association may indicate that children are tracking endotoxin into the schools along with pet allergens.
We acknowledge that we have measured endotoxin concentration in the collected dust samples and not total endotoxin load. Previous studies have demonstrated that endotoxin concentration (EU/mg) and endotoxin load (EU/m2) are highly correlated.14, 15 Additionally, endotoxin concentration has been used in primary analyses in a number of previous studies. 9, 11, 14 We present medians rather than means because our dataset has a wide range of values and we do not want a couple of extreme observations to overly influence results that use means. Irrespective of this, p-values were calculated on log transformed data that was normally distributed.
We recognize that our median endotoxin concentrations are lower than those found in previous studies in rural communities19, metropolitan areas3, and inner-city studies.14 Although our median levels are lower than previous studies, our ranges of concentration were wide with some samples having very high concentrations of endotoxin similar to previous reports. Our lower median concentration may be related to a variety of factors including climate, neighborhoods, and relatively few pets in our cohort. Another factor is the complete lack of detectable cockroach allergen in our settled dust samples. We did not have any home or school samples with detectable cockroach allergen in our cohort. Previously, Thorne et al demonstrated that cockroach allergen has been associated with elevated endotoxin levels.29 However, our lower concentrations may also be related to inter-laboratory variations. Due to large inter-laboratory variations between the protocols used for analyses of endotoxins, it is generally recommended that only intra-laboratory results are directly compared.16 As such, our discovered concentrations should not be compared with other studies, but should be compared within our own study. Given this, our lower concentrations do not detract from our primary conclusion that, in our study, there was a higher concentration of endotoxin in the school classrooms as compared to the home bedrooms. We can not, at this time, make a statement regarding the possible implications of these discovered concentrations on asthma morbidity, but we recognize that schools should be considered important when evaluating exposures that may be related to asthma morbidity.
One of our limitations is that our home sample was from the bedroom and not from other rooms in the home. It is possible that other rooms in the home may have higher concentrations of endotoxin due to a number of factors that may be present in non-bedroom areas, such as pets, moisture sources, more foot traffic, and closer proximity to home entrances. We chose the child’s bedroom as this was the site of sampling for previous asthma studies, such as the National Cooperative Inner-City Asthma Study which demonstrated bedroom allergen exposure to be associated with asthma morbidity.30 Additionally, interventional studies have shown that reduction of allergen levels in the bedroom resulted in improved asthma control.31 Focusing on the bedroom makes sense as this is the place were the child spends at least 7–8 hours each night. Within a 24 hour period, this is likely the one room in a home where children spend the most time. This bedroom exposure is comparable to their classroom exposure assuming that they spend approximately 7 hours per day in school. Thus, we designed our study for the main comparison between the classroom and the bedroom.
Another limitation of our study is the potential bias that may arise from selecting a population with asthma. We do not expect their school environments to differ from children without asthma, but it is possible that their home environments may differ. For example, children with asthma and animal allergies may be less likely to have a pet in their home or allow a pet in their bedroom which would possibly decrease the amount of endotoxin in their bedroom. In our study, 24.1% of the enrolled subjects reported having cats or dogs in their home. Additionally, parents of children with asthma may be more likely to keep their child’s bedroom cleaner which may result in less endotoxin compared to non-asthmatic children. Regardless of the possible bias, the importance of the comparison in children with asthma is that it may affect the child’s asthma morbidity. If families of children with asthma are undertaking practices that decrease home endotoxin exposure, then the school endotoxin exposure becomes even more important as parents have little, if any, control over this environment.
Future studies should evaluate if school endotoxin exposure is associated with asthma morbidity, controlling for home exposure. If school-based exposures are found to be important in asthma morbidity, then interventional strategies should be aimed at the schools. For years, physicians have been suggesting tactics to help improve the home environments for children with asthma. Targeting school-based environmental exposures may prove to be a cost-effective way to improve asthma health of many children.
Acknowledgments
Financial Support: Foundation of ACAAI, Young Faculty Support Award (Dr. Phipatanakul)
NIH Grants: R01 AI 073964 and R01 AI 073964-02S1.
Dr. Sheehan received partial support from T32-AI-007512
This work was conducted with support from Harvard Catalyst Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health
Footnotes
Authorship credit: (1) conception and design of the study; (2) data generation (when applicable); (3) analysis and interpretation of the data; and (4) preparation or critical revision of the manuscript.
1, 2, 3, 4: Sheehan, Hoffman, Bailey, Gaffin, Gold, Phipatanakul
2, 3, 4: Fu, Baxi, King, Chapman, Permaul
1, 2, 4: Lane
None of the authors have any conflicts of interest related to this research project.
References
- 1.Zhu Z, Oh SY, Zheng T, Kim YK. Immunomodulating effects of endotoxin in mouse models of allergic asthma. Clin Exp Allergy. Apr;40(4):536–546. doi: 10.1111/j.1365-2222.2010.03477.x. [DOI] [PubMed] [Google Scholar]
- 2.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002 Sep 19;347(12):869–877. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 3.Abraham JH, Finn PW, Milton DK, Ryan LM, Perkins DL, Gold DR. Infant home endotoxin is associated with reduced allergen-stimulated lymphocyte proliferation and IL-13 production in childhood. J Allergy Clin Immunol. 2005 Aug;116(2):431–437. doi: 10.1016/j.jaci.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Celedon JC, Milton DK, Ramsey CD, et al. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007 Jul;120(1):144–149. doi: 10.1016/j.jaci.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karadag B, Ege MJ, Scheynius A, et al. Environmental determinants of atopic eczema phenotypes in relation to asthma and atopic sensitization. Allergy. 2007 Dec;62(12):1387–1393. doi: 10.1111/j.1398-9995.2007.01505.x. [DOI] [PubMed] [Google Scholar]
- 6.Sordillo JE, Hoffman EB, Celedon JC, Litonjua AA, Milton DK, Gold DR. Multiple microbial exposures in the home may protect against asthma or allergy in childhood. Clin Exp Allergy. Jun;40(6):902–910. doi: 10.1111/j.1365-2222.2010.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horick N, Weller E, Milton DK, Gold DR, Li R, Spiegelman D. Home endotoxin exposure and wheeze in infants: correction for bias due to exposure measurement error. Environ Health Perspect. 2006 Jan;114(1):135–140. doi: 10.1289/ehp.7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung TF, Wong YS, Chan IH, et al. Indoor determinants of endotoxin and dust mite exposures in Hong Kong homes with asthmatic children. Int Arch Allergy Immunol. 152(3):279–287. doi: 10.1159/000283039. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001 Feb;163(2):322–328. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- 10.Rennie DC, Lawson JA, Kirychuk SP, et al. Assessment of endotoxin levels in the home and current asthma and wheeze in school-age children. Indoor Air. 2008 Dec;18(6):447–453. doi: 10.1111/j.1600-0668.2008.00543.x. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo MC, Naspitz CK, Fernandez-Caldas E, Lockey RF, Mimica I, Sole D. Endotoxin exposure and symptoms in asthmatic children. Pediatr Allergy Immunol. 1997 Aug;8(3):121–126. doi: 10.1111/j.1399-3038.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z, Sebastian A, Larsson L, Wang Z, Zhang Z, Norback D. Asthmatic symptoms among pupils in relation to microbial dust exposure in schools in Taiyuan, China. Pediatr Allergy Immunol. 2008 Aug;19(5):455–465. doi: 10.1111/j.1399-3038.2007.00664.x. [DOI] [PubMed] [Google Scholar]
- 13.Michel O, Kips J, Duchateau J, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med. 1996 Dec;154(6 Pt 1):1641–1646. doi: 10.1164/ajrccm.154.6.8970348. [DOI] [PubMed] [Google Scholar]
- 14.Perzanowski MS, Miller RL, Thorne PS, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006 May;117(5):1082–1089. doi: 10.1016/j.jaci.2005.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorne PS, Kulhankova K, Yin M, Cohn R, Arbes SJ, Jr, Zeldin DC. Endotoxin exposure is a risk factor for asthma: the national survey of endotoxin in United States housing. Am J Respir Crit Care Med. 2005 Dec 1;172(11):1371–1377. doi: 10.1164/rccm.200505-758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radon K. The two sides of the “endotoxin coin”. Occup Environ Med. 2006 Jan;63(1):73–78. 10. doi: 10.1136/oem.2004.017616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giovannangelo M, Gehring U, Nordling E, et al. Determinants of house dust endotoxin in three European countries - the AIRALLERG study. Indoor Air. 2007 Feb;17(1):70–79. doi: 10.1111/j.1600-0668.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Spiegelman DL, Gold DR, Burge HA, Milton DK. Predictors of airborne endotoxin in the home. Environ Health Perspect. 2001 Aug;109(8):859–864. doi: 10.1289/ehp.01109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waser M, Schierl R, von Mutius E, et al. Determinants of endotoxin levels in living environments of farmers’ children and their peers from rural areas. Clin Exp Allergy. 2004 Mar;34(3):389–397. doi: 10.1111/j.1365-2222.2004.01873.x. [DOI] [PubMed] [Google Scholar]
- 20.Andersson AM, Weiss N, Rainey F, Salkinoja-Salonen MS. Dust-borne bacteria in animal sheds, schools and children’s day care centres. J Appl Microbiol. 1999 Apr;86(4):622–634. doi: 10.1046/j.1365-2672.1999.00706.x. [DOI] [PubMed] [Google Scholar]
- 21.Instanes C, Hetland G, Berntsen S, Lovik M, Nafstad P. Allergens and endotoxin in settled dust from day-care centers and schools in Oslo, Norway. Indoor Air. 2005 Oct;15(5):356–362. doi: 10.1111/j.1600-0668.2005.00381.x. [DOI] [PubMed] [Google Scholar]
- 22.Morcos MM, Morcos WM, Ibrahim MA, Shaheen MA. Environmental exposure to endotoxin in rural and urban Egyptian school children and its relation to asthma and atopy. Minerva Pediatr. Feb;63(1):19–26. [PubMed] [Google Scholar]
- 23.Oldfield K, Siebers R, Crane J. Endotoxin and indoor allergen levels in kindergartens and daycare centres in Wellington, New Zealand. N Z Med J. 2007;120(1248):U2400. [PubMed] [Google Scholar]
- 24.Rullo VE, Rizzo MC, Arruda LK, Sole D, Naspitz CK. Daycare centers and schools as sources of exposure to mites, cockroach, and endotoxin in the city of Sao Paulo, Brazil. J Allergy Clin Immunol. 2002 Oct;110(4):582–588. doi: 10.1067/mai.2002.127511. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell H, Senturia Y, Gergen P, et al. Design and methods of the National Cooperative Inner-City Asthma Study. Pediatr Pulmonol. 1997 Oct;24(4):237–252. doi: 10.1002/(sici)1099-0496(199710)24:4<237::aid-ppul3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 26.Phipatanakul W, Cronin B, Wood RA, et al. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. 2004 Apr;92(4):420–425. doi: 10.1016/S1081-1206(10)61777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smedje G, Norback D, Edling C. Asthma among secondary schoolchildren in relation to the school environment. Clin Exp Allergy. 1997 Nov;27(11):1270–1278. [PubMed] [Google Scholar]
- 28.Arbes SJ, Jr, Sever M, Vaughn B, et al. Feasibility of using subject-collected dust samples in epidemiologic and clinical studies of indoor allergens. Environ Health Perspect. 2005 Jun;113(6):665–669. doi: 10.1289/ehp.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorne PS, Cohn RD, Mav D, Arbes SJ, Zeldin DC. Predictors of endotoxin levels in U.S. housing. Environ Health Perspect. 2009 May;117(5):763–771. doi: 10.1289/ehp.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997 May 8;336(19):1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 31.Kattan M, Stearns SC, Crain EF, et al. Cost-effectiveness of a home-based environmental intervention for inner-city children with asthma. J Allergy Clin Immunol. 2005 Nov;116(5):1058–1063. doi: 10.1016/j.jaci.2005.07.032. [DOI] [PubMed] [Google Scholar]