Abstract
Background
Traumatic spinal cord injury leads to direct myelin and axonal damage and leads to the recruitment of inflammatory cells to site of injury. Although rodent models have provided the greatest insight into the genesis of traumatic spinal cord injury (TSCI); recent studies have attempted to develop an appropriate nonhuman primate model.
Methods
We explored TSCI in a cynomolgus macaque model using a balloon catheter to mimic external trauma to further evaluate the underlying mechanisms of acute TSCI.
Results
Following 1 hour of spinal cord trauma there were focal areas of hemorrhage and necrosis at the site of trauma. Additionally there was a marked increased expression of MRP-8, MMP9, IBA-1, and iNOS in macrophages and microglia at the site of injury.
Conclusions
This data indicates that acute TSCI in the cynomolgus macaque is an appropriate model and that the earliest immunohistochemical changes noted are within macrophage and microglia populations.
Keywords: Immunohistochemistry, inducible nitric oxide synthase, MRP-8, trauma
Introduction
The pathogenesis of acute traumatic spinal cord injury (TSCI) is poorly understood thereby limiting the development of effective treatment options. Current animal models of TSCI include the dog, cat, and various rodent models; however, while these models have led to some understanding of pathophysiologic mechanisms underlying TSCI[2, 14], the need for an animal model to more accurately detail these mechanisms as well as develop and test therapeutics is paramount. In addition, there are important neurophysiologic and anatomical differences between these species and man and, as a result, there has been an attempt to develop a relevant primate model for TSCI. [5, 10, 15]
We have previously described a model of acute spinal cord injury in the cynomolgus macaque (Macaca fascicularis) in which cord lesions are induced by inflating a balloon catheter within the epidural space while the animal is under anesthesia. This procedure produces an acute lesion with detectable hemorrhage and inflammatory infiltrates.[10] In the current study, we continued our evaluation of balloon-catheter induced acute TSCI in the cynomolgus macaque to examine the impact of acute injury on glial and endothelial activation.
Because early treatment of spinal cord injuries is advantageous to optimizing return of neurological function, the time frame and acute inflammatory cascade has been intensively studied in an effort to better understand the possible secondary damage that can occur as a result of the inflammatory cascade. In rodent models of TSCI, neutrophils and macrophages predominate from four to twenty-four hours post-trauma.[11] Although macrophages tend to infiltrate later, following the neutrophils, some research indicates that macrophages arrive at the damaged site immediately after injury.[11] There has been some temporal association with macrophage/microglial number and location with earlier time points characterized by macrophages that predominate in the gray matter followed by the majority of macrophages being in the white matter after several weeks following the original injury.[11] Based on these findings, we hypothesized that there would be rapid microglial/macrophage activation and associated endothelial activation at the site of acute TSCI and that this rapid activation would initiate increased acute phase inflammatory mediators. In this current report, we build upon our previously published model of traumatic spinal cord injury in the cynomolgus macaque and elucidate the earliest alterations in glial, endothelial, and inflammatory cell activation.
Methods
The adult male cynomolgus macaque was housed in a biosafety level 2 facility at the New England Primate Research Center in accordance with The Guide for the Care and Use of Animals and the standards of the Harvard Medical School Standing Committee on Animals and The Association for the Assessment and Accreditation of Laboratory Animal Care. The experimental procedure has been reported previously and is briefly recapitulated here.[10] The animal was placed in deep anesthesia and a dorsal laminectomy was performed. A small catheter was placed in the lumbosacral epidural space and a small balloon was rapidly inflated to mimic a unilateral spinal cord traumatic incident. The animal was anesthetized for one hour during the experimental procedure and immediately following termination of the experiment, the animal was humanely euthanized with pentobarbitol. The spinal cord was immediately collected, fixed in 10% neutral buffered formalin (NBF), embedded in paraffin, sectioned at 5μm, and stained using hematoxylin and eosin (HE). Archived spinal cord sections taken from a normal animal were used as controls.
Following routine HE staining, additional sections from each level of spinal cord (cervical, thoracic, lumbar, and sacral) were prepared for immunohistochemistry. To characterize the spinal cord lesions using immunohistochemical methods we used standard immunoperoxidase staining for macrophage-related protein 8 (MRP-8), ionized calcium binding adaptor molecule 1 (IBA-1), CD16, CD163, monocyte chemoattractant protein 1 (MCP-1), matrix metalloproteinase 9 (MMP-9), tissue transglutaminase (TTG), glucose transporter 1 (GLUT-1), inducible nitric oxide synthase (i-NOS), cyclooxygenase 2 (COX-2), glial fibrillary acidic protein (GFAP), and myelin basic protein (MBP). All immunohistochemical stains were visually evaluated and multiple 20 and 40x fields were counted to determine incidence of immunopositive cells. Formalin fixed, paraffin embedded sections were deparaffinized, rehydrated and subsequently blocked with hydrogen peroxide. Pre-treatment for MRP-8, IBA-1, CD16, CD163, MCP-1, TTG, COX-2, and MBP antibodies involved microwaving for 20 minutes in 0.01M citrate buffer followed by 20 minutes of cooling. Pretreatment for MMP9 and iNOS, involved 5 minutes of proteinase K treatment and antibodies for GLUT-1 and GFAP did not require any pretreatment steps. All steps were followed by a tris-buffered saline (TBS) wash. Prior to application of primary antibodies, all slides were treated with Dako protein block for 10 minutes. Sections were incubated with anti-mouse MRP-8 (Accurate Chemical & Scientific Corporation [Westbury, NY, USA], monoclonal, 1:3200, overnight in refrigerator), anti-human IBA1 (Dako [Carpinteria, CA, USA], polyclonal, 1:1000, 30′ at room temperature), anti-human CD16 (Vector Laboratories [Burlingame, CA, USA], monoclonal, 1:160, overnight in refrigerator), anti-human CD163 (Labvision [Fremont, CA, USA], monoclonal, 1:2000, 60′ at room temperature), anti-human MCP-1 (R&D Systems [Minneapolis, MN, USA], polyclonal, 1:50, 60′ at room temperature), anti-mouse MMP-9 (Abcam [Cambridge, MA, USA], polyclonal, 1:250, 60′ at room temperature), anti-rabbit TTG (Novus Biologicals [Littleton, CO, USA], polyclonal, 1:500, 60′ at room temperature), anti-rabbit GLUT-1 (Millipore [Billerica, MA, USA], polyclonal, 1:5000, 30′ at room temperature), anti-mouse iNOS (Millipore, monoclonal, 1:400, 60′ at room temperature), anti-mouse COX-2 (Dako, monoclonal, 1:50, overnight in refrigerator), anti-mouse GFAP (Thermo Scientific [Fremont, CA, USA], monoclonal, 1:1600, overnight in refrigerator), and anti-human MBP (Dako, polyclonal, 1:1047, 30′ at room temperature). Slides were then incubated with secondary antibody biotinylated goat anti-rabbit (Vector laboratories, [Burlingame, CA, USA], 1:200, 30′ at room temperature) for IBA1, MMP9, TTG, GLUT-1, iNOS, and MBP; biotinylated horse anti-mouse (Vector laboratories, 1:200, 30′ at room temperature) for MRP-8, CD16, CD163, and GFAP; biotinylated rabbit anti-goat for MCP1; and Envision labeled polymer (Dako) for COX-2,. This was followed by 30 minute incubation at room temperature with either Vectastain ABC Elite [Vector laboratories] (MRP-8, IBA1, CD16, CD163, MCP1, MMP9, TTG, iNOS, MBP) or Vectastain ABC standard [Vector laboratories] (GLUT-1, GFAP). All slides were developed with DAB chromagen (Dako) and counterstained with Mayer’s hematoxylin. In all cases, step sections were incubated with isotype-specific irrelevant antibodies for negative controls. Each immunohistochemical stain was performed in a batch to prevent potentially staining irregularities encountered with separate immunohistochemistry runs. Positive controls consisted of sections of encephalitic CNS from an age matched cynomolgus macaque and normal spinal cord from a cynomolgus macaque.
Results
Histology
As described previously, the histologic appearance was typified by hemorrhage, edema, and necrosis at the point of injury and in adjacent spinal cord sections.[10] Rare infiltrates of neutrophils were noted at the site of injury. [10]
Immunohistochemistry
Macrophage and Microglial Markers
IBA-1
IBA-1 is a marker of all subsets of macrophages and microglia. Within the spinal cord of the control animal, IBA-1 positive cells were ubiquitously present throughout the white and grey matter as single, arborizing cells with a prototypical ramified appearance. Analysis of the affected spinal cord revealed increased immunoreactivity in addition to small aggregates of 3–5 positive cells within the white matter. These aggregates lacked the typical ramified appearance, but rather had large, globoid cytoplasmic immunoreactivity. Immunoreactivity was increased throughout the spinal cord; however the focal aggregates predominated within the cervical and cranial thoracic sections (Fig. 1).
Fig. 1.
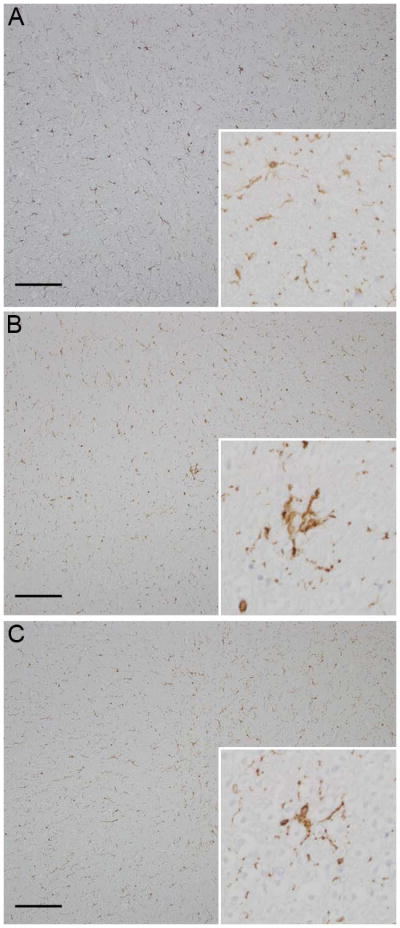
Photomicrograph. (a) Control animal; diffuse Iba-1 immunoreactivity is present (inset, x400); (b) Experimental animal; diffuse Iba-1 immunoreactivity with multifocal foci of increased microglial activation (inset) is present at the site of the traumatic lesion; (c) Experimental animal; diffuse, mild increased immunoreactivity for Iba-1 (inset) in the lumbar spinal cord distal to the traumatic lesion with focus of increased immunoreactivity. Hematoxylin and eosin, bar = 1mm.
MRP-8
Myeloid-related protein-8 (MRP-8) identifies a subset of activated myeloid cells known for their role as activators of Toll-like receptor 4 (TLR4), interleukine-1 receptor- associated kinase-1 (IRAK-1), NFκB, and subsequently TNF-α.[13] Within the spinal cord of the control animal, MRP-8 positive cells were present solely within small parenchymal capillaries (circulating monocytes). In the experimental animal, extravascular aggregates of strongly positive MRP-8 positive cells were present at the site of the spinal cord trauma. Additionally, increased numbers of solitary, extravascular MRP-8 positive cells were present in the sections immediately cranial and caudal to the lesion. Sections of mid- to distal thoracic, lumbar, and sacral spinal cord had only scattered intravascular MRP-8 positive cells (Fig. 2).
Fig. 2.
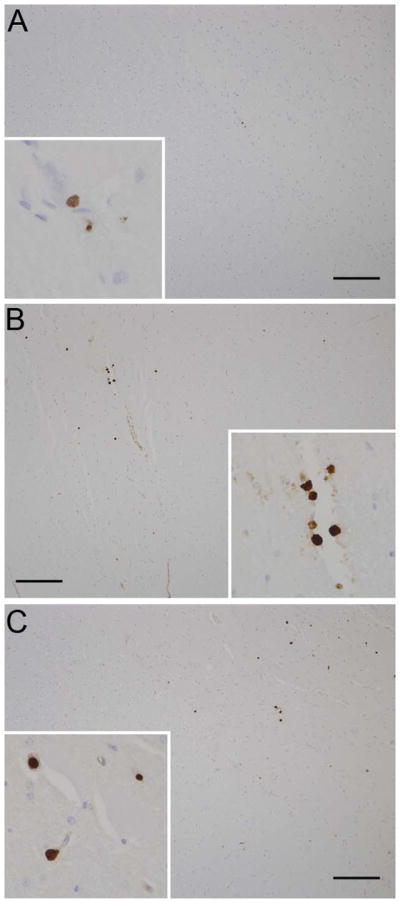
Photomicrograph. (a) Control animal; rare MRP-8 immunoreactive cells are present intravascularly (inset); (b) Experimental animal; increased numbers of MRP-8 immunoreactive cells are present in the cervical spinal cord both in the parenchyma (inset) and around blood vessels; (c) Experimental animal; increased numbers of MRP-8 immunoreactive cells are present in the lumbar spinal cord both in the parenchyma and around blood vessels (inset). Hematoxylin and eosin, bar = 1mm.
CD16
CD16 is a cell surface marker for natural killer cells, neutrophils, and macrophages. Scattered immunoreactive CD16+ cells were limited to the meninges, with no immunopositive cells in the parenchyma in the control as well as the experimental tissues.
CD163
CD163 is a cell surface marker for perivascular macrophages and can be upregulated in chronic cases of encephalitis. Scattered immunoreactive macrophages were present in the meninges with a similar proportion noted in control and experimental tissues.
Recruitment factors
MCP-1
MCP-1 is a myeloid chemotactic protein that aids in the tissue recruitment of macrophages. It was expressed on a small number of cells in the meninges with no difference in the experimental compared to control spinal cord tissues.
MMP-9
Matrix metalloprotease-9 (MMP-9) is a gelatinase which is expressed by subsets of circulating leukocytes, including monocytes, to aid in trafficking across the basement membrane of blood vessels into tissues. In the spinal cord from the control animal, MMP-9 positivity was restricted to the cytoplasm of perivascular macrophages and scattered glial cells. In the experimental spinal cord, there was strong, robust MMP-9 immunoreactivity at the level of injury, particularly in cells morphologically consistent with microglia and macrophages. Increased MMP-9 expression was also present in the spinal cord sections immediately cranial and caudal to the traumatized spinal cord section, but was similar to control levels in more distal segments.
Inducible Nitric Oxide Synthase (iNOS)
In the control animal, minimal to mild immunoreactivity was detected in the microglia, especially in the zone of the glia limitans, and in the endothelial cells of the meningeal blood vessels. In the experimental animal, there was intense immunoreactivity in the microglia population in the grey and white matter and there were solitary, intensely positive cells scattered throughout the white matter (Fig. 3). Double label immunohistochemistry confirmed the dual expression of iNOS and IBA-1 in affected cells confirming their origin as microglia (Fig. 4).
Fig 3.
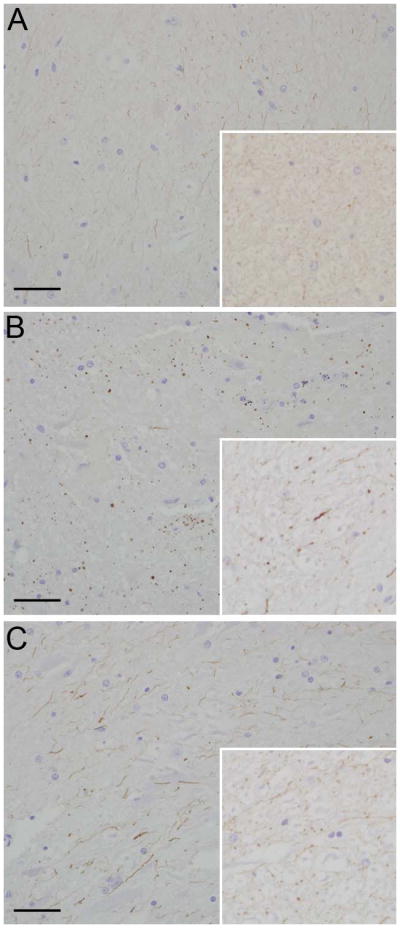
Photomicrograph. (a) Control animal; iNOS immunoreactivity is faint and restricted to microglial processes (inset); (b) Experimental animal; iNOS immunoreactivity is increased and punctate with large globular regions of immunoreactivity in microglial processes at the site of the cervical trauma (inset); (c) Experimental animal; iNOS immunoreactivity is increased in microglial processes in the lumbar spinal cord section (inset). Hematoxylin and eosin, bar = 0.5mm.
Fig 4.
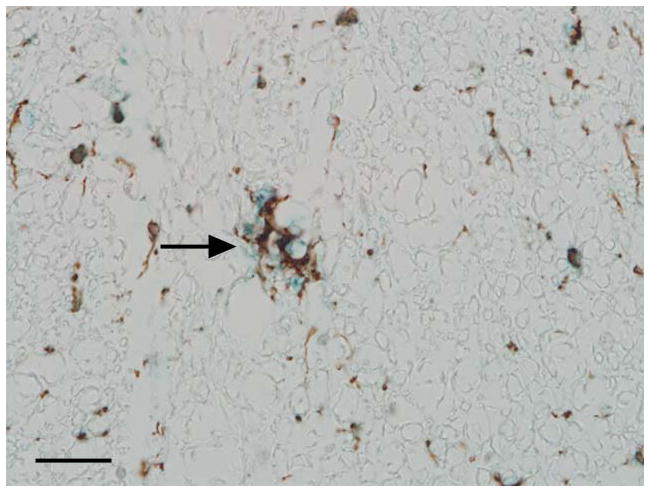
Photomicrograph. Representative image of the affected spinal cord illustrating that microglia are stained with IBA-1 (brown) and iNOS (blue) indicating dual expression of these markers. Immunoperoxidase staining, DAB chromagen (IBA-1) and Vector blue (iNOS), Mayer’s hematoxylin counterstain, bar = 100μm.
Vascular markers
Glucose Transporter-1 and Tissue transglutaminase
There was no change in expression of glucose transporter-1 (glut-1), a brain and spinal cord endothelial specific marker, in the experimental spinal cord sections compared to controls. Likewise, the expression of tissue transglutaminase (tTG), which is upregulated in endothelial cells by thrombin, was unchanged over controls.[1] Both markers identified identical patterns with strong immunoreactivity of endothelial cells in blood vessels of all sizes in the neuroparenchyma and meninges in the control and experimental tissue.
Other Immunohistochemical Markers
Glial fibrillary acidic protein
To determine alterations in the activation state of astrocytes within the experimental spinal cord, sections were analyzed for expression of astrocyte-specific glial fibrillary acidic protein (GFAP). There was strong, robust immunoreactivity in the white and grey matter throughout all sections of the control spinal cord. Identical staining intensity and distribution were noted in the experimental animal.
Myelin Basic Protein
The density of myelin basic protein (MBP) as a marker of axonal and myelin integrity was not different in the spinal cord segments of the experimental animal compared to those of the control.
Discussion
In general, tissue injury results in rapid vascular activation and following activation leukocytes migrate from the vasculature into the tissue following a chemotactic gradient. We examined markers of macrophage subsets using CD16, CD163, and MRP-8 and IBA-1. The animal in the current report had increased numbers of macrophage related protein (MRP-8) positive cells and increased numbers of IBA-1 positive cells not only at the site of damage, but also in spinal cord sites distant from the site of trauma. MRP-8 is a member of the S100 family of proteins and is primarily restricted to cells of the monocyte/macrophage and neutrophil lineage.[7] This marker is expressed both in circulation and in tissue and is restricted to the cytoplasm where it likely plays a role in modifying intracellular calcium levels.[7] In our current study, the aggregates of MRP-8 positive cells were focused at the site of trauma and often were around the areas of hemorrhage suggesting that these MRP-8 positive cells arrived due to vascular damage and associated hemorrhage. There were other areas in which the MRP-8 positive cells were not associated with the hemorrhage and likely recently egressed vasculature in response to the trauma as opposed to leaking out of damaged vessels in conjunction with hemorrhage. There was no histomorphologic evidence to indicate that the MRP-8 positive cells were neutrophils and experimental evidence indicates that the maximal neutrophil influx occurs at 4 hours post injury. [3]
Microglia and macrophages produce reactive oxygen species, which can further damage tissue and worsen pre-existing damage. MMP-9, which is a product of macrophages and microglia, was also upregulated at and around the site of injury in the experimental animal. MMP-9 is a proteolytic enzyme that has a diverse functions involved in tissue remodeling and reorganization. The upregulation at the site of injury illustrates the macrophage/microglial activation. MMP-9 can have many deleterious effects including unwanted tissue remodeling and may further damage tissue. The marked upregulation occurring merely 1 hour post injury certainly suggests an important role for this enzyme and also illustrates the potential utility of finding therapeutic interventions aimed at decreasing the activation of macrophages and microglia during times of spinal cord trauma. IBA-1 is a cell surface marker that is involved in reorganization of the actin cytoskeleton, a process that is directly linked to the activation, migration, and phagocytosis of microglia.[8] Activation of microglia is a common event in spinal cord trauma and is purported to lead to increased tissue damage through the elaboration of proinflammatory substances. Marked microglia activation was noted in the animal described herein and was found to be co-expressed with iNOS indicating expression in microglial cells.
Unfortunately, the presence of activated microglia and macrophages may also cause a negative effect. While the microglia and macrophages are efficient at scavenging debris from ruptured myelin sheaths, disrupted axons, and degenerate neuroglia, they also continue to perpetuate spinal cord damage due to the elaboration of pro-inflammatory molecules including reactive oxygen species (ROS), nitric oxide, and prostanoids.[2] All of these compounds can cause further damage to the spinal cord tissue through a variety of membrane peroxidation, vasodilation, and elaboration of toxic byproducts. The cause of the global microglial activation is not evident by our current research; however it is logical to assume that the spinal cord trauma and resultant vascular disruption would lead to higher levels of inflammatory cytokines being produced, which could lead to global microglial activation.
The acute inflammatory response at the site of traumatic spinal cord injury is of utmost importance in determining the best route of treatment. In rodent models there is a marked influx of polymorphonuclear cells (predominately neutrophils) and macrophages [11]. An inflammatory cascade is initiated almost immediately after the initial injury as the combination of activated microglia and infiltrating neutrophils contributes to the elaboration of pro-inflammatory compounds with dynamic effects on vasodilation and expression of vascular adhesion molecules. 3[3, 6, 10, 11] The upregulation of various selectins, including ICAM-1 and VCAM, allows for the rapid influx of additional blood monocytes which further the inflammatory cascade.[11, 12] Although macrophages can continue to perpetuate the inflammatory cascade, recent data suggest that macrophages also have an important role in normal healing and function of neurons. Macrophage subpopulations appear to have a distinct role in supporting neuronal sprouting at sites of injury.[14] There is also marked macrophage heterogeneity within the brain and spinal cord and it is likely that different subpopulations of macrophages play different roles supporting normal neuroglia function.[9, 14] There are many spinal cord contusion models that have been developed that attempt to bridge the gap in knowledge between the early and late phases of spinal cord injury. Unfortunately most of these animal models, especially the rodent models, don’t necessarily reflect the physiology and pathology that occurs in humans afflicted with the same condition (Table 1).[15]
Table 1.
Comparison of acute traumatic spinal cord injury
| Species | Dura Comprised | Histologic Findings | Immunohistochemistry Findings |
|---|---|---|---|
| Rat | Yes | Focal hemorrhage and malacia | Upregulation of microglia within 12 hours |
| Cynomolgus macaque | No | Areas of hemorrhage and malacia; mild influx of neutrophils | Immediate upregulation of macrophage related proteins (MRP-8, MMP- 9, and iNOS) |
| Human | No | Focal hemorrhage and malacia | Increased influx of neutrophils and microglia activation within first 24–48 hours |
Interestingly in the current study, we did not appreciate increased immunoreactivity of CNS endothelial activation markers, most notably tissue transglutaminase and glucose transporter-1; however, there was strong, robust immunoreactivity for inducible nitric oxide synthase (iNOS) at the site of injury and in the spinal cord segments immediately adjacent to the site of trauma. iNOS is typically either not expressed or expressed at very low levels in the brain; however the production of cytokines can lead to its expression in microglia and astrocytes.[4] Once iNOS is expressed, continuously high levels of nitric oxide are produced which can lead to further tissue damage through toxic byproducts, specifically peroxynitrite.[4] Additionally, nitric oxide is potentially toxic to neurons and has been implicated in neuronal death.
Lastly, the markers of GFAP, myelin basic protein, and neurofilament were unchanged between the control animal and the experimental animal. This is not entirely surprising as astrocytosis is generally thought of as a chronic process that does not initiate until several days following injury. Similarly, myelin and axonal damage, although occurring immediately at the time of injury does not begin to manifest morphologically until several days following the traumatic event. This is due to continuous elaboration of toxic metabolites that perpetuate axonal and myelin damage that was initiated by the trauma.
In conclusion, the cynomolgus macaque model of traumatic spinal cord injury shows great promise in elucidating the mechanisms of the immediate post-injury pathogenesis. Early markers of neuroinflammation in the current study included MRP-8, MMP-9, iNOS, and IBA-1 indicating the importance of both tissue and infiltrating macrophages in causing and potentially perpetuating spinal cord damage. Elucidating the mechanisms of early spinal cord injury has major implications on the potential treatments that are used to ameliorate the condition.
Acknowledgments
This research was supported, in part, by RR00168.
Footnotes
Disclosure:
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specific in this paper.
References
- 1.Auld GC, Ritchie H, Robbie LA, Booth NA. Thrombin upregulates tissue transglutaminase in endothelial cells: a potential role for tissue transglutaminase in stability of atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2001;21:1689–1694. doi: 10.1161/hq1001.097063. [DOI] [PubMed] [Google Scholar]
- 2.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 3.Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- 4.Conti A, Miscusi M, Cardali S, Germano A, Suzuki H, Cuzzocrea S, Tomasello F. Nitric oxide in the injured spinal cord: synthases cross-talk, oxidative stress and inflammation. Brain Res Rev. 2007;54:205–218. doi: 10.1016/j.brainresrev.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 6.Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 7.Hessian PA, Edgeworth J, Hogg N. MRP-8 and MRP-14, two abundant Ca(2+)-binding proteins of neutrophils and monocytes. J Leukoc Biol. 1993;53:197–204. [PubMed] [Google Scholar]
- 8.Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- 9.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nesathurai S, Graham WA, Mansfield K, Magill D, Sehgal P, Westmoreland SV, Prusty S, Rosene DL, Sledge JB. Model of traumatic spinal cord injury in Macaca fascicularis: similarity of experimental lesions created by epidural catheter to human spinal cord injury. J Med Primatol. 2006;35:401–404. doi: 10.1111/j.1600-0684.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 11.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Schwab JM, Brechtel K, Nguyen TD, Schluesener HJ. Persistent accumulation of cyclooxygenase-1 (COX-1) expressing microglia/macrophages and upregulation by endothelium following spinal cord injury. J Neuroimmunol. 2000;111:122–130. doi: 10.1016/s0165-5728(00)00372-6. [DOI] [PubMed] [Google Scholar]
- 13.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 14.Wu D, Miyamoto O, Shibuya S, Okada M, Igawa H, Janjua NA, Norimatsu H, Itano T. Different expression of macrophages and microglia in rat spinal cord contusion injury model at morphological and regional levels. Acta Med Okayama. 2005;59:121–127. doi: 10.18926/AMO/31950. [DOI] [PubMed] [Google Scholar]
- 15.Young W. Spinal cord contusion models. Prog Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. [DOI] [PubMed] [Google Scholar]


