Abstract
Congenital human cytomegalovirus (HCMV) infection can result in lifelong neurological deficits. Seronegative pregnant woman often acquire primary HCMV from clinically asymptomatic, but HCMV-shedding children. Potential age-related differences in viral and immune parameters of primary RhCMV infection were examined in an oral rhesus CMV infection model in specific pathogen free macaques.
Note/Short Paper
Worldwide, human cytomegalovirus (HCMV) infection is the most common congenital infection, affecting ≈ 0.7% of all fetuses. Congenitally infected infants can suffer lifelong neurological sequelae (7, 9, 18), and clinically healthy babies at birth can develop neurological complications in the first years of life. In the US, 0.5–2% of all infants acquire HCMV in utero. Seronegative young infants can acquire HCMV through breast-milk (16) or in day care settings, e.g. through HCMV-contaminated saliva on toys (11, 13). Similar to HCMV-infected adults, infants generally do not develop clinical symptoms upon HCMV acquisition, but, in contrast to adults, shed virus for prolonged periods of time (3). As their infection goes largely unnoticed, HCMV-infected kids can transmit the virus to seronegative pregnant women who either have or do not have preconceptional immunity to HCMV. It is now well-established that HCMV can re-infect HCMV-immune women. In the absence of an HCMV vaccine, interventions aimed at stopping or reducing HCMV shedding in infants could provide an effective means of preventing congenital HCMV infection.
The host factors responsible for prolonged viral shedding in infants are only poorly understood. To overcome sample limitations from young children, we sought to develop an infant rhesus CMV infection model because RhCMV infection in adult macaques is highly similar to human HCMV infection (5, 10). Previously, a direct comparison of RhCMV-specific immune responses between infant and adult macaques in correlation to virological outcome was not possible, because RhCMV is ubiquitous in macaque colonies and >90% of animals have seroconverted by 6 months of age. The generation of Specific Pathogen Free (SPF) macaque colonies in which animals are bred to be free of multiple viruses, including RhCMV (4), now enables controlled RhCMV pathogenesis studies in different age groups.
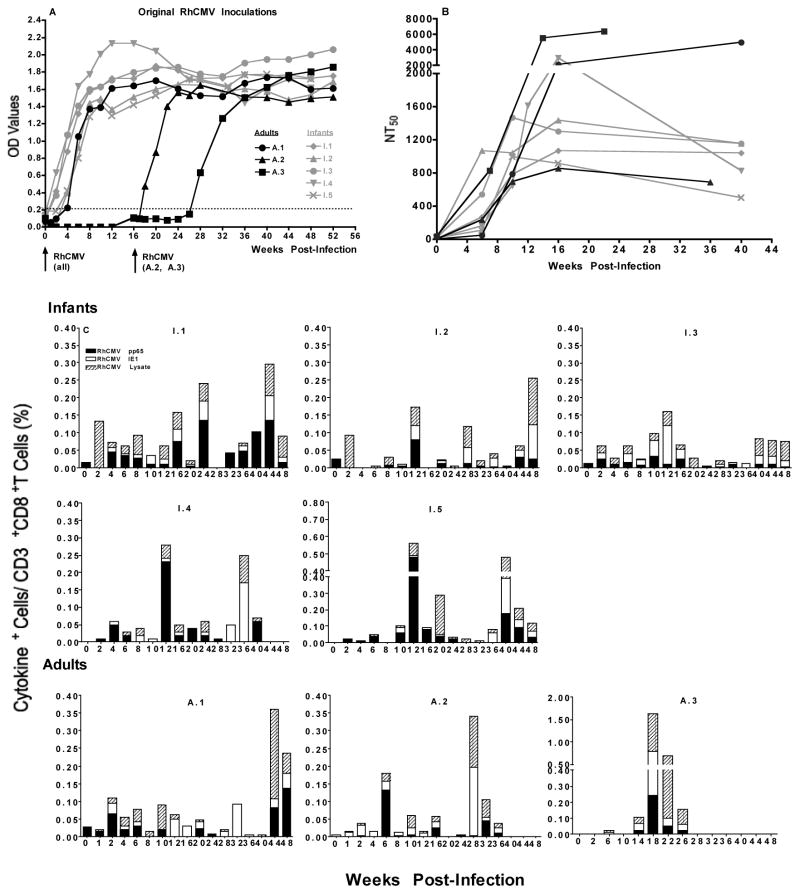
To mimic oral HCMV infection in children, 4-week old infant (n=5) and 5-year old young adult SPF macaques were orally (via the buccal pouch) infected with a natural RhCMV isolate (1x 10^6 PFU/ml) using a blunted syringe, and followed for 1 year. All infants, but only 1 of 3 adult animals, seroconverted within 2–4 weeks (10, 19, 20) (Figure 1A). The 2 seronegative adults received a second RhCMV dose at week 16. One animal seroconverted within 4 weeks, the other animal remained seronegative for 8 weeks, but had RhCMV antibodies by week 12 after the 2nd infection (Figure 1A). The data suggest that infants compared to adults have enhanced susceptibility to oral RhCMV infection. The MHC status of the infants at study entry was unknown, and therefore we cannot make any conclusion about possible genetic factors influencing susceptibility to RhCMV infection or pathogenesis outcome. In addition to binding antibodies, all animals developed RhCMV-specific neutralizing antibodies (1, 2) (Figure 1B). Although two of the adults showed the highest 50% neutralization titers (NT50), no statistically significant differences in NT50 values were detected between infant and adult animals. As all infant and 2 adult animals seroconverted within 4 weeks of RhCMV infection, animal #A3 likely became naturally infected, because the adult animals were co-housed. Animals #A1 and #A2 had qPCR-detectable RhCMV DNA in saliva at a single time point (8, 15) (Table 1), but it could not be conclusively determined whether these animals were actively shedding and transmitted RhCMV to #A3, because the RhCMV copy number was below the cut-off value (100 copies/ml). Consistent with observations in human infant HCMV infection, infant macaques showed pronounced high titer RhCMV shedding in saliva and urine (3, 14, 17, 21) (Table 1).
Figure 1. Immune responses to oral RhCMV infection.
Panel A: RhCMV-specific antibody development. OD values for RhCMV-specific binding antibodies in longitudinally collected plasma samples (1:100 dilution) were determined by ELISA using RhCMV lysate as antigen. Infant animals are depicted in grey symbols and lines and adult animals in black symbols and lines. Arrows below the x-axis indicate the times of oral RhCMV exposure. The weeks post infection for all animals are based on the first oral RhCMV exposure at week 0. The dashed line shows the threshold for a sample to be considered RhCMV antibody positive based on the analysis of a pool of plasma collected from RhCMV-negative animals.
Panel B: Neutralizing antibody titers. Neutralizing antibodies for RhCMV were determined at weeks 0, 6, 10, 16 and 40 post RhCMV infection. For animal A.3 only samples from weeks 0, 7, 14 and 22 were available, assuming that infection occurred between weeks 24–26 or weeks 10–12 after the first or second RhCMV inoculation, respectively. Note that the time points for the adult animals in Panel B were adjusted to reflect the actual time of RhCMV infection (e.g. for animal A.3 week 7 post infection in Panel B corresponds to week 33 in Panel A). Shown are the NT50 titers for individual infant and adult macaques.
Panel C: RhCMV-specific T cell responses in peripheral blood. Although dual cytokine-positive cells were detected, the graph here only shows the sum of single cytokine positive cells (TNF-α+IL-2+IFN-γ) specific for RhCMV pp65 (black bars), RhCMV IE1 (white bars) and/or RhCMV lysate (striped bars) at individual time points post RhCMV infection. The total frequencies of single cytokine positive cells determined by Boolean gating analysis within the CD8+T cell populations in individual animals. Animal numbers are indicated on top of each graph.
Table 1.
RhCMV Detection
| Weeks Post Infection |
0 | 2 | 4 | 6 | 7 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | Compartment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Infants I.1 |
- | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Plasma | |||||
| NS | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | +++ | Saliva | ||||||
| NS | NS | NS | NS | NS | NS | NS | NS | ++ | +++ | NS | + | + | NS | NS | NS | NS | Urine | ||||||
| I.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Plasma | |||||
| NS | - | - | +/− | - | - | - | - | - | - | +/− | - | +/− | +/− | - | +/− | - | Saliva | ||||||
| NS | NS | NS | NS | NS | NS | NS | NS | - | - | NS | +++ | NS | NS | NS | NS | NS | Urine | ||||||
| I.3 | - | - | +/− | +/− | +/− | - | - | - | - | - | - | - | - | - | - | - | Plasma | ||||||
| - | - | - | - | - | - | - | +++ | +/− | ++ | +++ | ++ | ++ | +/− | ++ | + | +/− | Saliva | ||||||
| NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | - | +++ | NS | NS | NS | NS | Urine | ||||||
| I.4 | - | - | - | - | - | - | - | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | Plasma | |||||
| - | - | - | - | - | - | +/− | - | + | NS | ++ | + | +/− | + | + | +/− | NS | Saliva | ||||||
| NS | NS | NS | NS | +/− | NS | + | + | ++ | ++ | + | +++ | NS | + | NS | +++ | NS | Urine | ||||||
| I.5 | - | - | - | - | - | - | +/− | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | Plasma | |||||
| - | + | +/− | ++ | + | ++ | +/− | +/− | +/− | + | - | +/− | +/− | - | +/− | NS | NS | Saliva | ||||||
| NS | NS | NS | NS | NS | NS | +/− | - | NS | + | + | NS | NS | NS | NS | - | NS | Urine | ||||||
|
Adults A.1 |
- | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Plasma | |||||
| - | - | - | +/− | - | - | - | - | - | - | - | - | - | - | - | - | - | Saliva | ||||||
| - | NS | NS | NS | NS | NS | NS | NS | - | - | NS | NS | NS | - | - | NS | NS | Urine | ||||||
| A.2 | - | - | - | - | - | - | - | - | - | - | - | - | - | Plasma | |||||||||
| - | - | - | - | - | - | - | +/− | - | - | - | - | - | Saliva | ||||||||||
| NS | - | - | - | - | NS | - | NS | - | - | NS | NS | NS | Urine | ||||||||||
| A.3 | - | - | - | NT | - | - | - | - | - | Plasma | |||||||||||||
| - | - | - | NT | - | - | - | - | - | Saliva | ||||||||||||||
| NS | - | - | NT | NS | NS | NS | NS | NS | Urine |
NS = no sample available
NT = not tested
less<10 copies (LOD)
 >10, but less <10^4 copies
>10, but less <10^4 copies
 >10^4, but less <10^5 copies
>10^4, but less <10^5 copies
 >10^5 copies
>10^5 copies
Better control of virus shedding in adults could not be explained by antibody responses because binding and neutralizing titers did not differ between infant and adult animals and persisted in both groups throughout the study period. Around the time of seroconversion, all animals showed an increase in Ki67 positive cells within the total CD4 and CD8 T cell populations, with the frequencies of Ki67 positive CD8 T cells being significantly higher in adults than in infants (data not shown). Analysis of antigen-specific T cells in longitudinally collected blood samples by intracellular cytokine staining (IL-2, IFN-γ, TNF-α and CD107) showed that all animals developed CD4 and CD8 T cell responses to whole RhCMV lysate, RhCMV pp65 and RhCMV IE1 (Figure 1C) (1, 2, 12). There were no differences, however, in the magnitude, persistence, or quality of infant and adult T cell responses. Generally, RhCMV-specific T cells produced only a single cytokine, IFN-γ or TNF-α. Dual-positive cytokine responses were detected in 4 of 5 infant and 2 of 3 adult animals within the CD4, and in 4 of 5 infants and 1 of 3 adult animals within the CD8 T cell population (data not shown). These results are in contrast to the age-dependent increase in HCMV-specific T cells (6, 17), and to a similar age-dependency of RhCMV-specific T cell responses in non-SPF macaques (own unpublished data). As 5 year-old macaques are comparable to 15 to 19 year old human teenagers, the age difference between the two animal groups in the current study might have been too small. Alternatively, the SPF status could have affected immune responsiveness.
In this proof-of-concept study, we established an oral RhCMV infection model in infant SPF rhesus macaques. The findings can be summarized as follows: (i) The susceptibility to oral RhCMV infection appears to decline with age, as 2 of 3 older animals required multiple oral RhCMV exposures to become infected, although this route proved to be 100% effective in infants. Future studies should determine host factors enhancing susceptibility to RhCMV infection within the oral microenvironment. (ii) Similar to HCMV infection in humans, infant macaques shed RhCMV more persistently and at higher titers compared to adult macaques. Viral shedding in saliva and urine might represent the most reliable marker to assess control of CMV infection, and efficacy of drug treatments and vaccines. (iii) Larger animal studies are needed to define immune parameters associated with better control of RhCMV in adult compared to young animals. In the limited study here, differences in the magnitude or quality of RhCMV-specific T and B cell responses could not be detected. However, this was the first time SPF animals were available, and as the SPF colony ages, more detailed virological and immunological studies, including tissue analysis, could be performed with larger animals groups.
Acknowledgments
This work was supported by NIH/NIAID grant 1R21DH056051 to K. Abel, the Virology Training Grant T32 AI07419 "Molecular Biology of Viral Diseases" (NIAID, DHHS) to M. dela Pena, and an NIH grant P51RR000169 to the California National Primate Research Center. We are thankful to Joyce Lee, Kim Schmidt, and Koen Van Rompay for technical assistance.
Footnotes
The authors have no conflicting financial interests.
Contributor Information
Myra Grace dela Pena, Email: myra_delapena@med.unc.edu, University of North Carolina, Dept of Microbiology and Immunology, Chapel Hill, North Carolina, USA.
Lisa Strelow, Email: lstrelow@ucdavis.edu, UC Davis, Center for Comparative Medicine, Davis, California, USA.
Peter A. Barry, Email: pabarry@ucdavic.edu, UC Davis, Centre for Comparative Medicine, Davis, California, USA
References
- 1.Abel K, Martinez J, Yue Y, Lacey SF, Wang Z, Strelow L, Dasgupta A, Li Z, Schmidt KA, Oxford KL, Assaf B, Longmate JA, Diamond DJ, Barry PA. Vaccine-induced control of viral shedding following rhesus cytomegalovirus challenge in rhesus macaques. J Virol. 2011;85:2878–2890. doi: 10.1128/JVI.00883-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abel K, Strelow L, Yue Y, Eberhardt MK, Schmidt KA, Barry PA. A heterologous DNA prime/protein boost immunization strategy for rhesus cytomegalovirus. Vaccine. 2008;26:6013–6025. doi: 10.1016/j.vaccine.2008.07.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler SP. Molecular epidemiology of cytomegalovirus: a study of factors affecting transmission among children at three day-care centers. Pediatr Infect Dis J. 1991;10:584–590. [PubMed] [Google Scholar]
- 4.Barry PA, Strelow L. Development of breeding populations of rhesus macaques (Macaca mulatta) that are specific pathogen-free for rhesus cytomegalovirus. Comp Med. 2008;58:43–46. [PMC free article] [PubMed] [Google Scholar]
- 5.Barry PA, William Chang W. Primate betaherpesviruses. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: 2007. [PubMed] [Google Scholar]
- 6.Chen SF, Tu WW, Sharp MA, Tongson EC, He XS, Greenberg HB, Holmes TH, Wang Z, Kemble G, Manganello AM, Adler SP, Dekker CL, Lewis DB, Arvin AM. Antiviral CD8 T cells in the control of primary human cytomegalovirus infection in early childhood. J Infect Dis. 2004;189:1619–1627. doi: 10.1086/383249. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4:725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 8.Huff JL, Eberle R, Capitanio J, Zhou SS, Barry PA. Differential detection of B virus and rhesus cytomegalovirus in rhesus macaques. J Gen Virol. 2003;84:83–92. doi: 10.1099/vir.0.18808-0. [DOI] [PubMed] [Google Scholar]
- 9.Kano Y, Shiohara T. Current understanding of cytomegalovirus infection in immunocompetent individuals. J Dermatol Sci. 2000;22:196–204. doi: 10.1016/s0923-1811(99)00085-7. [DOI] [PubMed] [Google Scholar]
- 10.Lockridge KM, Sequar G, Zhou SS, Yue Y, Mandell CP, Barry PA. Pathogenesis of experimental rhesus cytomegalovirus infection. J Virol. 1999;73:9576–9583. doi: 10.1128/jvi.73.11.9576-9583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall EE, Geballe AP. Multifaceted evasion of the interferon response by cytomegalovirus. J Interferon Cytokine Res. 2009;29:609–619. doi: 10.1089/jir.2009.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marthas ML, Van Rompay KK, Abbott Z, Earl P, Buonocore-Buzzelli L, Moss B, Rose NF, Rose JK, Kozlowski PA, Abel K. Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques. Vaccine. 2011;29:3124–3137. doi: 10.1016/j.vaccine.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noyola DE, Valdez-Lopez BH, Hernandez-Salinas AE, Santos-Diaz MA, Noyola-Frias MA, Reyes-Macias JF, Martinez-Martinez LG. Cytomegalovirus excretion in children attending day-care centers. Arch Med Res. 2005;36:590–593. doi: 10.1016/j.arcmed.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Pass RF, Hutto C. Group day care and cytomegaloviral infections of mothers and children. Rev Infect Dis. 1986;8:599–605. doi: 10.1093/clinids/8.4.599. [DOI] [PubMed] [Google Scholar]
- 15.Sequar G, Britt WJ, Lakeman FD, Lockridge KM, Tarara RP, Canfield DR, Zhou SS, Gardner MB, Barry PA. Experimental coinfection of rhesus macaques with rhesus cytomegalovirus and simian immunodeficiency virus: pathogenesis. J Virol. 2002;76:7661–7671. doi: 10.1128/JVI.76.15.7661-7671.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stagno S, Reynolds DW, Pass RF, Alford CA. Breast milk and the risk of cytomegalovirus infection. N Engl J Med. 1980;302:1073–1076. doi: 10.1056/NEJM198005083021908. [DOI] [PubMed] [Google Scholar]
- 17.Tu W, Chen S, Sharp M, Dekker C, Manganello AM, Tongson EC, Maecker HT, Holmes TH, Wang Z, Kemble G, Adler S, Arvin A, Lewis DB. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 18.Vancikova Z, Dvorak P. Cytomegalovirus infection in immunocompetent and immunocompromised individuals--a review. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:179–187. [PubMed] [Google Scholar]
- 19.Yue Y, Kaur A, Eberhardt MK, Kassis N, Zhou SS, Tarantal AF, Barry PA. Immunogenicity and protective efficacy of DNA vaccines expressing rhesus cytomegalovirus glycoprotein B, phosphoprotein 65-2, and viral interleukin-10 in rhesus macaques. J Virol. 2007;81:1095–1109. doi: 10.1128/JVI.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue Y, Zhou SS, Barry PA. Antibody responses to rhesus cytomegalovirus glycoprotein B in naturally infected rhesus macaques. J Gen Virol. 2003;84:3371–3379. doi: 10.1099/vir.0.19508-0. [DOI] [PubMed] [Google Scholar]
- 21.Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J Infect Dis. 1999;180:702–707. doi: 10.1086/314939. [DOI] [PubMed] [Google Scholar]