Abstract
Objective
Over the past 20 years, the incidence of endometrial cancer has increased remarkably in Japan. The number of elderly females has also increased within the population of Japan. We examined the impact of advanced age on the demographic and clinicopathological characteristics in Japanese patients with endometrial cancer.
Methods
Data were collected from 319 surgically treated Japanese females with endometrial cancer from the files of the University Hospital of Occupational and Environmental Health, Yahatanishi-ku, Kitakyushu, Japan, between 1990 and 2010. χ2 tests were performed to evaluate the trends in the variables between two decades (A: 116 cases from 1990–2000) and (B: 203 cases in 2001–2010). The histological subtypes were also evaluated based on the immunohistochemical expressions of p53, estrogen receptor, and Ki-67.
Results
The mean ages ± standard deviation in the decade A group and the decade B group were 57.5 years ± 9.7 years and 61.0 years ± 11.3 years, respectively (P < 0.02). There was an increase in the proportion of patients aged 70 years or older and of high-risk histological tumors including serous carcinoma, clear cell carcinoma, and carcinosarcoma (decade A group and decade B group: 9.5% vs 27.6%, P < 0.001, 10.4% vs 21.6%, P = 0.01, respectively), while the advanced surgical stage (III and IV), obesity (≥25 of body mass index), and nulliparity of the decade A group and decade B group were 23.3% vs 29.1%, P = 0.30, 28.4% vs 33.0%, P = 0.40, and 19.0% vs 21.2%, P = 0.66, respectively. The cancer-specific survival rates in the decade A group and the decade B group were 78.6% and 77.6%, respectively (P = 0.93).
Conclusion
The increase in number of elderly females in the Japanese population is related to the increase in that of high-risk endometrial cancers. A study is needed to investigate prevention strategies and to improve the treatment of elderly patients with high-risk endometrial cancer.
Keywords: endometrial cancer, advanced age, nonendometrioid carcinoma
Introduction
Endometrial cancer is now the most common gynecologic malignancy in the US, with an estimated 43,470 new cases in 2010 and 7950 deaths.1 The number of deaths per year has been increasing despite a relatively stable number of new cases.2 According to the Gynecologic Tumor Committee of the Japan Society of Gynecologic Oncology (JSOG), the total number of endometrial cancer cases from registered institutions was 976 cases in 1983, which increased to 2115 cases in 1994 and reached 6113 cases in 2009.3 The age-adjusted incidence rate of endometrial cancer in Japan was 3.9 in 1990, 5.1 in 2000, and 7.6 in 2006.4 The incidence of endometrial cancer has therefore increased remarkably in Japan.
Advanced age is well recognized as an adverse prognostic factor in patients with endometrial cancer,5 and was reported to be associated with aggressive histological subtypes of tumors, increased tumor grades, and advanced-stage disease.6 In addition, elderly females with endometrial cancer are suggested to receive less aggressive treatment than their younger counterparts.7
In the present study, we determined the histological subtypes of endometrial cancer based on the immunohistochemical findings and tried to show the impact of the increase in the rate of elderly females within the Japanese population on the demographic and clinicopathological characteristics of Japanese patients with endometrial cancer.
Materials and methods
Case selection
The study included 319 Japanese patients with endometrial cancer who had undergone surgery at the University Hospital of Occupational and Environmental Health, Yahatanishi-ku, Kitakyushu, Japan, between 1990 and 2010. These included 263 cases of endometrioid adenocarcinoma and 56 cases of nonendometrioid carcinoma (30 cases of serous carcinoma, eight cases of clear cell carcinoma, and 18 cases of carcinosarcoma). The demographic, clinicopathological, treatment, and survival information of Japanese patients with endometrial cancer was compared for two decades (A: 1990–2000, 116 cases, and B: 2001–2010, 203 cases). All of the studied endometrial cancer specimens were from hysterectomy specimens. A simple total hysterectomy in the decade A group and the decade B group was performed in 81 and 142 patients, an extended hysterectomy (class II) in 33 and 58 patients, and a radical hysterectomy (class III) in two and three patients, respectively (decade A group vs decade B group, P = 0.98).8 When surgeons found a tumor with deep myometrial invasion during the operation, an extended hysterectomy was performed. Radical hysterectomy was performed if the tumor showed a gross cervical involvement. A pelvic lymphadenectomy and a para-aortic lymph node sampling in the decade A group and the decade B group were performed in 99 and 170 patients (P = 0.24), and in 39 and 103 patients (P < 0.01), respectively. The adjuvant chemotherapy was performed when patients exhibited tumor stage IB or above, grade 3 endometrioid tumor, nonendometrioid tumor, or positive lymph-vascular space invasion. The adjuvant radiotherapy was additionally performed for the metastatic lymph nodes. During the period of this study, 15 patients with endometrial carcinoma were treated with primary radiotherapy due to severe complications. These 15 patients were not included in the present study because of little pathologic information of endometrial cancer. In four patients of the decade A group, the mean age ± standard deviation (SD) was 81.5 years ± 5.8 years. Clinical stages I and IV tumors were seen in three patients and one patient, respectively. In eleven patients in the decade B group, the mean age ± SD was 69.1years ± 12.2 years. Clinical stages I, II, and IV tumors were seen in six, two, and three patients, respectively. Tamoxifen-related endometrial cancer was found in three females with breast cancer (two endometrioid adenocarcinomas and a serous carcinoma).
Immunohistochemistry
For the immunohistochemical analysis, 4 μm sections were cut from formalin-fixed paraffin-embedded tissue blocks, deparaffinized in xylene, and rehydrated through sequential changes of alcohol and distilled water. Ki-67 and p53 were detected using the ready-to-use monoclonal antibodies against Ki-67 and p53 (clone MIB-1 and DO-7; Dako Co, Ltd, Kyoto, Japan), respectively. The estrogen receptor α (ERα) was detected using monoclonal antibodies against the ER (clone 6F11, diluted 1:50, Novocastra, Fukuoka, Japan). The slides were heated in an autoclave at 120°C for 5 minutes in 0.01 M citrate buffer (pH = 6.0) before the immunostaining. The slides were then incubated with indicated antibodies for 2 hours at room temperature. Antibody binding was visualized using the EnVision + Dual Link system, and diaminobenzidine was used as a chromogen (Dako Cytomation, Kyoto, Japan). The slides were counterstained with methyl green and mounted.
Interpretation of immunohistochemical preparations
The immunostained slides were analyzed independently by two authors. Slight differences were resolved by simultaneous viewing. The pathologic review was performed in a blinded fashion on all patients. The labeling indices (LIs) of KI-67, p53, and ER were defined as the percentage of the tumor cells with strong nuclear immunoreactivity out of the total number of the tumor cells, respectively.
Statistical analysis
The statistical analyses were carried out using the SPSS software program for Windows, version 18.0.0 (SPSS, Chicago, IL). The outcome data collected included the time from first surgery to the last follow-up or death. Kaplan–Meier curves were generated to examine the overall and cancer-specific survival rates. The log-rank test was used to test differences in survival within variables. The Cox proportional hazards model was used to identify and simultaneously evaluate any independent prognostic factors associated with relative survival. The frequency distributions between categorical variables were compared using the χ2 test. The mean ages of the patients were added to assessment by Student’s t-test. Statistical significance was considered to exist at a value of P < 0.05.
Results
Clinicopathologic features
In the overall study group, the mean ages ± SD of the patients with endometrioid adenocarcinoma, serous carcinoma, clear cell carcinoma, and carcinosarcoma were 57.8 years ± 10.3 years, 69.3 years ± 7.7 years, 69.6 years ± 9.3 years, and 68.3 years ± 9.9 years, respectively.
We divided the study group into two time periods, 1990– 2000 (decade A group) and 2001–2010 (decade B group), to identify the trends in demographic and clinical characteristics (Table 1). The mean ages ± SD in the decade A group and the decade B group were 57.5 years ± 9.7 years and 61.0 years ± 11.3 years, respectively (P < 0.02). For patients with high-risk histologic tumor, the mean ages ± SD in the decade A group and the decade B group were 67.3 years ± 8.4 years and 69.6 years ± 8.5 years, respectively (P = 0.37). The cutoff point of 70 years of age showed significant difference between age distributions of the decade A group and the decade B group (P < 0.001), but those aged 50 years and 60 years showed no significant difference (P = 0.08 and P = 0.34, respectively). The numbers of patients aged 70 years or older with high-risk histologic tumor in the decade A group and the decade B group were three and 24, respectively. Between the two periods, the proportion of patients aged 70 years or older increased from 9.5% to 27.6% (P < 0.001), while the rates of patients with high-risk histologic tumor in the decade A group and the decade B group were 27.3% and 42.9% for patients aged 70 years or older, respectively (P = 0.93). For patients aged less than 70 years, the rates of patients with high-risk histologic tumor in the decade A group and the decade B group were 8.6% and 13.6%, respectively (P = 0.22). There was a significant decrease in the proportion of endometrioid adenocarcinomas with a corresponding increase in the nonendometrioid adenocarcinomas including serous carcinoma, clear cell carcinoma, and carcinosarcoma (P = 0.01). Compared with grade 1 and 2 tumors, the proportion of grade 3 tumors did not change significantly (P = 0.09). Nulliparity, obesity, surgical stage, and myometrial invasion did not change significantly between the decade A group and the decade B group (P = 0.66, 0.40, 0.30, and 0.59, respectively). The rates of para-aortic lymph node sampling and adjuvant chemotherapy increased significantly from 33.6% to 50.7% (P < 0.01) and from 33.6% to 43.8% (P = 0.04), respectively, from the decade A group to the decade B group. The presence of lymph node metastasis was found in 15 cases (12.9%) in the decade A group and 27 cases (13.3%) in the decade B group (P = 0.88).
Table 1.
Demographic, clinicopathologic, and treatment trends
| Characteristics | Decade A group (n = 116) (%)a | Decade B group (n = 203) (%)a | P-value |
|---|---|---|---|
| Age (year) | <0.001 | ||
| <70 | 105 (90.5) | 147 (72.4) | |
| ≥70 | 11 (9.5) | 56 (27.6) | |
| Parity | 0.66 | ||
| 0 | 22 (19.0) | 43 (21.2) | |
| 1–2 | 62 (53.4) | 113 (55.7) | |
| ≥3 | 32 (27.6) | 47 (23.1) | |
| Body mass index | 0.40 | ||
| <25 | 83 (71.6) | 136 (67.0) | |
| ≥25 | 33 (28.4) | 67 (33.0) | |
| Pelvic lymphadenectomy | 99 (85.3) | 170 (83.7) | 0.24 |
| Para-aortic lymph node sampling | 39 (33.6) | 103 (50.7) | <0.01 |
| Median number of nodes (range) | |||
| Pelvic | 14 (1–40) | 13 (1–47) | |
| Para-aortic | 1 (1–7) | 1 (1–14) | |
| Positive lymph nodeb | 0.88 | ||
| Pelvic | 12 (10.3) | 12 (5.9) | |
| Para-aortic | 2 (1.7) | 4 (2.0) | |
| Pelvic and para-aortic | 1 (0.9) | 11 (5.4) | |
| Surgical stagec | 0.30 | ||
| IA | 71 (61.2) | 109 (53.7) | |
| IB | 14 (12.1) | 24 (11.8) | |
| II | 4 (3.4) | 11 (5.4) | |
| IIIA | 7 (6.0) | 19 (9.4) | |
| IIIB | 1 (0.9) | 2 (1.0) | |
| IIIC1 | 9 (7.8) | 12 (5.9) | |
| IIIC2 | 4 (3.4) | 11 (5.4) | |
| IV | 6 (5.2) | 15 (7.4) | |
| Myometrial invasion | 0.59 | ||
| < 1/2 | 80 (69.0) | 134 (66.0) | |
| ≥ 1/2 | 36 (31.0) | 69 (34.0) | |
| Histologyd | 0.01 | ||
| Endometrioid | |||
| Grade 1 | 59 (50.8) | 88 (43.4) | |
| Grade 2 | 32 (27.6) | 45 (22.2) | |
| Grade 3 | 13 (11.2) | 26 (12.8) | |
| Serous | 6 (5.2) | 24 (11.8) | |
| Clear cell | 1 (0.9) | 7 (3.4) | |
| Carcinosarcoma | 5 (4.3) | 13 (6.4) | |
| Adjuvant chemotherapy | 39 (33.6) | 89 (43.8) | 0.04 |
| Adjuvant radiotherapy | 5 (4.3) | 7 (3.4) | 0.70 |
Notes:
Percent denotes proportion of patients during decade;
cases with lymph node metastasis versus cases without lymph node metastasis;
stages I and II versus stages III and IV;
endometrioid versus serous carcinoma, clear cell carcinoma, and carcinosarcoma.
Immunohistochemical findings
The histological subtypes of endometrial carcinoma were diagnosed by hematoxylin and eosin-stained sections. Although we evaluated the histological subtype of endometrial carcinoma using p53, Ki-67,and ER immunohistochemical expressions after the review of hematoxylin and eosin-stained slides, the histological subtypes of endometrial carcinoma were not changed by the immunophenotypes of the tumors except for one tumor (which changed in classification from serous carcinoma to endometrioid adenocarcinoma due to the absence of p53 and a high level of expression of the ER).
In the overall study group, the cases showing more than 50% of LIs for p53 was 41 of 263 endometrioid adenocarcinomas (11.6% of grade 1 tumors, 18.2% of grade 2 tumors, and 28.2% of grade 3 tumors), 29 of 30 serous carcinomas, eight of eight clear cell adenocarcinomas, and 13 of 18 carcinosarcomas. The samples showing more than 10% of LIs for ER were 77 of 263 endometrioid adenocarcinomas (81.0% of grade 1 tumors, 73.7% of grade 2 tumors, and 32.2% of grade 3 tumors), eight of 30 serous carcinomas, none of eight clear cell adenocarcinomas, and three of 18 carcinosarcomas. The samples showing more than 50% of LIs for Ki-67 were 166 of 263 endometrioid adenocarcinomas (54.4% of grade 1 tumors, 72.7% of grade 2 tumors, and 76.9% of grade 3 tumors), 28 of 30 serous carcinomas, seven of eight clear cell adenocarcinomas, and 17 of 18 carcinosarcomas.
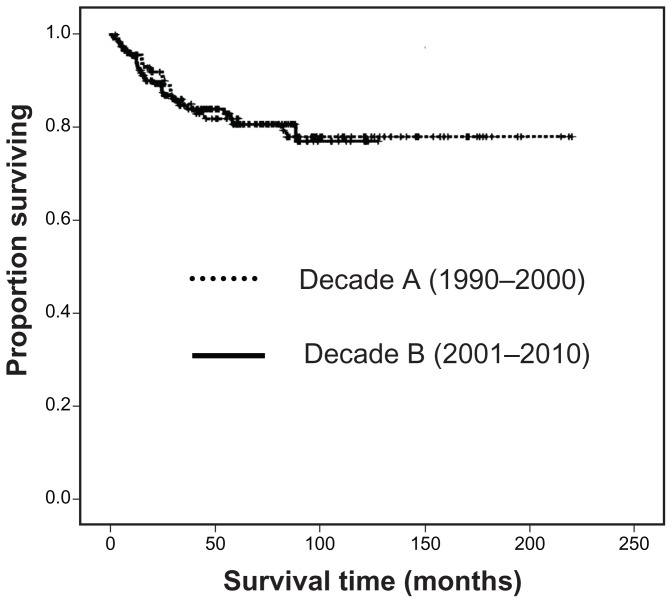
Survival rates
Figure 1 shows that the decade A group and the decade B group had follow-up periods ranging from 3 months to 221 months (median 82 months) with a cancer-specific survival rate of 78.6% and follow-up periods ranging from 2 months to 128 months (median 45 months) with a cancer-specific survival rate of 77.6%, respectively (P = 0.93). The cancer-specific survival rates of patients with endometrioid adenocarcinoma (grades 1, 2, and 3), serous carcinoma, clear cell carcinoma, and carcinosarcoma were 96.8%, 75.3%, 65.6%, 46.2%, 45.2%, and 11.8%, respectively (P < 0.0001). The overall survival rates of patients with endometrioid adenocarcinoma (grades 1, 2, and 3), serous carcinoma, clear cell carcinoma, and carcinosarcoma were 94.6%, 68.8%, 64.5%, 44.1%, 36.6%, and 9.7%, respectively (P < 0.0001). The cancer-specific survival rates of patients with endometrioid tumor and nonendometrioid tumor were 86.2% and 37.2%, respectively (P < 0.001).
Figure 1.
Kaplan–Meier analysis of cancer-specific survival stratified by decade.
On multivariate analyses including variables of age at diagnosis (aged <70 years vs aged 70 years or older), surgical stage (I and II vs III and IV), histology (endometrioid tumors vs nonendometrioid tumors), and p53 expression (<50% of LI vs 50% or more of LIs), surgical stage (hazard ratio [HR] = 2.939, 95% confidence interval [CI] = 5.268–67.738, P-value < 0.001) was a significant independent prognostic factor in the decade A group. Age of diagnosis (HR = 0.948, 95% CI = 1.050–6.349, P-value = 0.039), surgical stage (HR = 1.734, 95% CI = 2.149–14.937, P-value < 0.001), and histology (HR = 2.506, 95% CI = 3.307–45.455, P-value < 0.001) were significant independent prognostic factors in the decade B group.
Discussion
In the present study, the incidence of high-risk histological tumors including serous carcinoma, clear cell carcinoma, and carcinosarcoma was significantly increased, and these cancers were seen most in elderly patients. The incidences of obese or nulliparous patients were not significantly different between the decade A group and the decade B group.
The Surveillance, Epidemiology and End Results (SEER) database of the National Cancer Institute between 1988 and 2001 showed that the incidence of endometrial cancer remained relatively stable in the US, while the number of deaths per year was estimated at an approximate 3000 in the 1980s, 5000 in the 1990s, and over 7000 in 2006. This increased number of deaths was suggested to be partially related to an increased rate of detection of advanced-stage (III and IV) cancer (14.2% of 13,591 patients in 1988– 1992 vs 18.0% of 15,979 patients in 1998–2001, P < 0.001) and high-risk histologies, including serous carcinoma, clear cell carcinoma, and sarcoma (14.7% of 13,591 patients in 1988–1992 vs 17.3% of 15,979 patients in 1998–2001, P < 0.001).2
On the contrary, the analyses of endometrial cancer histological subtypes, based on the database of the National Program of Cancer Registries (NPCR) and SEER programs between 1999 and 2006, showed that the incidence of type I endometrial cancers (endometrioid adenocarcinoma) increased from 12,536 to 22,888, whereas that of type II endometrial cancers (serous carcinoma and clear cell carcinoma) was relatively stable, from 1751 to 2169.9 The authors suggested that the trends in hormone replacement therapy use and the rise of obesity in the US have contributed to the incidence of endometrial cancer, especially among the specific histological subtypes. From a UK population-based registry from 1994 to 2006, the analyses of endometrial cancer types also showed an increase in endometrial cancer incidence that was confined to type I cancers (age-standardized incidence rate from 12.0 per 100,000 in 1994 to 16.3 per 10,000 in 2006, P < 0.001), seen most frequently in the 60–79 year age groups, while the incidence of type II cancer remained static (age-standardized incidence rate from 2.5 per 100,000 in 1994 to 2.2 per 10,000 in 2006, not significant).10 The 5-year relative survival rate in patients with type I cancer improved (not significant, P = 0.95), but that of patients with type II cancer decreased significantly (P = 0.001). The authors compared the increased incidence of type I cancer to an increase in obesity in the UK population and found the relationship between endometrial cancer incidence and obesity to be complex.
Ueda et al2 showed that the increase in mortality in patients with endometrial cancer was related to an increased rate of the detection of advanced-stage cancers. However, the distributions of the surgical stage were not significantly different between the decade A group and the decade B group in the present study. The cancer-specific survival rates of the decade A group and the decade B group also showed no significant differences. Although the patients in the decade B group were more often treated with para-aortic lymph node sampling than those in the decade A group, there were no significant differences in the incidence of the retroperitoneal lymph node metastasis between the decade A group and the decade B group. The increased rate of para-aortic lymph node sampling influenced the increased number of patients with both pelvic and para-aortic lymph node metastases but did not influence the rate of adjuvant chemotherapy. Several studies have shown the benefits of adjuvant chemotherapy for the treatment of early-stage type II endometrial cancer.11,12 However, the median of survival in the decade B group was too short to evaluate the impact of adjuvant chemotherapy on the survival of patients with endometrial cancer.
Only one tumor was diagnosed as an endometrioid adenocarcinoma by the immunohistochemical findings in the present study. The immnohistochemical analysis therefore assured the accuracy of the histological typing of endometrial cancer in the present study.
Conclusion
There has been an increase in the incidence of serous carcinoma, clear cell carcinoma, and carcinosarcoma in Japanese patients with endometrial cancer. The increase in the proportion of elderly females in the total population is probably related to the increase in the diagnosis of high-risk endometrial cancers in Japan. The present study is limited by a small sample-sized institution-based study. A large Japanese population-based study is required.
Footnotes
Disclosure
No author has any conflict of interest to disclose.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics 2010. CA Cancer J Clin. 2011;61(2):133–134. [Google Scholar]
- 2.Ueda SM, Kapp DS, Cheung MK, Shin JY, Osann K, Husain A, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obtet Gynecol. 2008;198(2):218. e1–e6. doi: 10.1016/j.ajog.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 3.Ushijima K. Current status of gynecologic cancer in Japan. J Gynecol Oncol. 2009;20(2):67–71. doi: 10.3802/jgo.2009.20.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Center, Japan. Center for Cancer Control and Information Services. 2011. [Accessed April 16, 2012]. Available from: http://ganjoho.ncc.go.jp/professional/statistics/statistics.html.
- 5.Lachance JA, Everett EN, Greer B, et al. The effect of age on clinical/ pathologic features, surgical morbidity, and outcome in patients with endometrial cancer. Gynecol Oncol. 2006;101(3):470–475. doi: 10.1016/j.ygyno.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Ellenson LH, Ronnett BM, Soslow RA, Zaino RJ, Kurman RJ. Endometrial carcinoma. In: Kruman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s pathology of the female genital tract. New York, NY: Dodrecht Heidelberg; 2011. pp. 417–440. [Google Scholar]
- 7.Ahmed A, Zamba G, DeGeest K, Lynch CF. The impact of surgery on survival of elderly women with endometrial cancer in the SEER program from 1992–2002. Gynecol Oncol. 2008;111(1):35–40. doi: 10.1016/j.ygyno.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 8.Monk BJ, Tewari KS. In: Invasive cervical cancer Clinical gynecologic oncology. DiSaia PJ, Creasman WT, editors. Philadelphia, PA: Mosby; 2007. pp. 78–81. [Google Scholar]
- 9.Duong LM, Wilson JR, Ajani UA, Singh SD, Eheman CR. Trends in endometrial cancer incidence rates in the United States, 1999–2006. J Women’s Health. 2011;20(8):1157–1163. doi: 10.1089/jwh.2010.2529. [DOI] [PubMed] [Google Scholar]
- 10.Evans T, Sany O, Pearmain P, Ganesan R, Blann A, Sundar S. Differential trends in the rising incidence of endometrial cancer by type: data from a UK population-based registry from 1994 to 2006. Br J Cancer. 2011;104(9):1505–1510. doi: 10.1038/bjc.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly M, O’Malley D, Hui P, McAlpine J, Yu H, Rutherford TJ, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98(3):353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Vandenput I, Trovik J, Vergote I, Moerman P, Leunen K, Berteloot P, et al. The role of adjuvant chemotherapy in surgical stages I–II serous and clear cell carcinomas and carcinosarcoma of the endometrium. Int J Gynecol Cancer. 2011;21(2):332–336. doi: 10.1097/IGC.0b013e3182094ded. [DOI] [PubMed] [Google Scholar]