Abstract
Pentraxins are a family of evolutionarily conserved pattern-recognition proteins that are made up of five identical subunits. Based on the primary structure of the subunit, the pentraxins are divided into two groups: short pentraxins and long pentraxins. C-reactive protein (CRP) and serum amyloid P-component (SAP) are the two short pentraxins. The prototype protein of the long pentraxin group is pentraxin 3 (PTX3). CRP and SAP are produced primarily in the liver while PTX3 is produced in a variety of tissues during inflammation. The main functions of short pentraxins are to recognize a variety of pathogenic agents and then to either eliminate them or neutralize their harmful effects by utilizing the complement pathways and macrophages in the host. CRP binds to modified low-density lipoproteins, bacterial polysaccharides, apoptotic cells, and nuclear materials. By virtue of these recognition functions, CRP participates in the resolution of cardiovascular, infectious, and autoimmune diseases. SAP recognizes carbohydrates, nuclear substances, and amyloid fibrils and thus participates in the resolution of infectious diseases, autoimmunity, and amyloidosis. PTX3 interacts with several ligands, including growth factors, extracellular matrix component and selected pathogens, playing a role in complement activation and facilitating pathogen recognition by phagocytes. In addition, data in gene-targeted mice show that PTX3 is essential in female fertility, participating in the assembly of the cumulus oophorus extra-cellular matrix. PTX3 is therefore a nonredundant component of the humoral arm of innate immunity as well as a tuner of inflammation. Thus, in conjunction with the other components of innate immunity, the pentraxins use their pattern-recognition property for the benefit of the host.
Pentraxins
Pentraxins are a family of phylogenetically conserved, pattern-recognition proteins and a host-defense-related component of the innate immune system.1–5 Based on the length of their primary structure, the pentraxins are divided into two groups: short pentraxins and long pentraxins. Short pentraxins include C-reactive protein (CRP) and serum amyloid P component (SAP). The first discovered long pentraxin, known as pentraxin 3 (PTX3), serves as the prototype protein of the long pentraxin group. The term pentraxin was first assigned to CRP for its pentagonal appearance of five subunits in electron micrographs.6 Pentraxins (Table 1) recognize a wide range of altered-self and nonself pathogenic substances and lead to protection of the host.
Table 1.
The pattern-recognition property of pentraxins involved in host-defense
| Pentraxin | Recognition | Pathologic Implication |
|---|---|---|
| CRP | Bacteria | Microbial infection |
| Parasites | Parasitic infection | |
| Apoptotic cells | Autoimmunity | |
| Necrotic cells | Autoimmunity | |
| Damaged cells | Autoimmunity | |
| Nuclear materials | Autoimmunity | |
| Modified LDL | Atherosclerosis | |
| Fibronectin | Cancer | |
| SAP | Bacteria | Microbial infection |
| Apoptotic cells | Autoimmunity | |
| Nuclear materials | Autoimmunity | |
| β-amyloid | Amyloidosis | |
| PTX3 | Bacteria | Microbial infection |
| Apoptotic cells | Autoimmunity | |
| Oophorous matrix | Female fertility |
Short Pentraxins: CRP and SAP
CRP, the most characteristic acute phase protein in humans, was discovered in 1929.7 The plasma concentration of CRP rises in both chronic and acute inflammatory conditions.7,8 SAP was discovered in 1965 as an amyloid protein of plasma (P) origin and is not an acute phase protein in humans.9 SAP is associated with the deposits that characterize amyloid fibrils in systemic amyloidosis, Alzheimer’s disease and the transmissible spongiform encephalitis.10 In mouse, which is a widely used animal to determine the in vivo functions of short pentraxins, SAP is the acute phase protein.11 CRP is only a trace serum protein in mice and not an acute phase protein.
Phylogeny
Both CRP and SAP have been found in all vertebrates where they have been sought.12,13 CRP is also found in the hemolymph of invertebrates such as the arthropod Limulus polyphemus and the mollusc Achatina fulica.14,15 At least one short pentraxin is present in all vertebrates as well as in some invertebrates. Humans have got both CRP and SAP. The pattern-recognition property of the short pentraxins towards a wide range of substances of biological importance has been conserved throughout evolution although their acute phase nature is species-specific. For example, in contrast to humans and mice, in rats CRP is constitutively expressed at relatively high levels and is only a minor acute phase protein.16
Metabolism
The major site of CRP synthesis is liver.17 In vitro, in human hepatoma cells, cytokines IL-6 and IL-1 are the main inducers of CRP expression.18–20 Nitric oxide has been shown to reduce the induction of CRP production by cytokines.21 Expression of CRP mRNA in the tissues other than the liver has also been reported.22,23 In mice transgenic for human CRP, the CRP gene has been shown to be under hormonal control while in hamsters, SAP gene is under hormonal control.24,25 In healthy persons, the median concentration of CRP is 0.8 mg/L but, following an acute-phase stimulus, this may increase to more than 500 mg/L. The half-life of CRP is about 19 h in humans.26 SAP is also produced exclusively by hepatocytes and turnover rapidly with plasma half-life of 24 h.27 SAP and CRP are both resistant to proteolysis. SAP, which has localized to the deposits, persists there for prolonged periods without being catabolized.10
Structure
CRP has five identical, 206 amino acid long subunits of 23 kD each.28,29 A single internal disulfide bond is present in each subunit.29 There is no carbohydrate present in human CRP and there are no potential N-glycosylation sites either. The subunits are held together through noncovalent interactions and are arranged in pentameric symmetry.30 Each subunit is made up of two antiparallel β-sheets and a single short α-helix, and is characterized by the presence of a cleft that extends from about the center of the subunit to its edge at the central pore of the pentamer. The overall dimensions of the CRP pentamer are about 102 Å outside diameter with a central pore diameter of 30 Å and a subunit diameter of 36 Å.30 SAP, on the other hand, is a glycoprotein made up of five noncovalently attached subunits of 23 kd each.31,32 SAP is a single pentamer in serum but under certain conditions, SAP forms decamers by stacking of two pentamers.33 The complete glycoprotein structure is important for the functions of SAP.10
Binding to Calcium and Phosphocholine
CRP is called so because it reacts with C-polysaccharide of the cell wall of Streptococcus pneumoniae.7 The reaction of CRP and C-polysaccharide requires calcium ions.34 CRP binds two Ca2+ through the two overlapping Ca2+-binding sites present on each subunit.30 The two Ca2+ in CRP are coordinated by Asp60, Asn61, and by residues (Glu138, Gln139, Asp140, Glu147 and Gln150) in a loop. This loop, in the absence of bound Ca2+, moves away from the main body of the CRP molecule exposing an otherwise hidden site of proteolysis. Bound Ca2+ are integral structural elements of CRP and protect CRP from proteolytic cleavage.35
Phosphocholine (PCh) is the principal Ca2+-dependent ligand of CRP.36 The PCh-containing substances, to which CRP binds, are present in many prokaryotes and in all eukaryotes and include pneumococcal C-polysaccharide, damaged, necrotic and apoptotic cells, altered lipid bilayers, and pulmonary surfactant lipids.2–4,37 CRP also binds in a Ca2+-dependent manner to phosphoethanolamine and other phosphate monoesters.2–4 A PCh-binding site, located next to the Ca++-binding sites, is present on each CRP subunit. Since the subunits have same orientation in the assembled pentamer, all five PCh-binding sites fall on the same face of the CRP pentamer. The PCh-binding site consists of a critical hydrophobic pocket formed by residues Leu64, Phe66 and Thr76, and two Ca2+ that are bound to CRP.38 The phosphate group of PCh directly coordinates with the two Ca2+. Phe66 and Glu81 in CRP provide contacts to the choline moiety of PCh that lies within the hydrophobic pocket.38–41
Like CRP, SAP also binds two Ca2+ through the two overlapping Ca2+-binding sites present on each subunit.32 Ca2+-bound SAP is also protease-resistant.42 Since SAP binds to phosphoethanolamine, it can bind to necrotic and late apoptotic cells in Ca2+-dependent manner.43 SAP pentamers are also capable of interacting with CRP pentamers to form decamers but only in Ca2+-free conditions, indicating that CRP and SAP do not interact in vivo.44,45
Binding to Bacteria and Protective Role against Microbial Infections
CRP binds a wide variety of bacteria including several serotypes of S. pneumoniae, Haemophilus influenzae, and Neisseriae spp.46–49 In vitro, CRP has been shown to promote phagocytosis of PCh-expressing bacteria and to block the attachment of such bacteria to the receptors for platelet-activating factor (PAF) on the host cells.48,50
Human CRP protects mice against fatal infection with S. pneumoniae.51–53 Employing complement C3 knockout mice and complement depletion using cobra venom factor, it has been shown that a functioning complement system is required for full CRP-mediated protection.51–53 However, the protection is independent of naturally occurring anti-pneumococcal antibody.54 CRP also protects mice from infection with Salmonella typhimurium, a pathogen to which CRP is not known to bind.55
SAP binds to several bacteria via lipopolysachharide (LPS) and prevents LPS-mediated complement activation.56 SAP binds LPS and thereby protects the host from LPS toxicity.57,58 However, for certain organisms to which SAP binds, such as S. pyrogens and rough strains of E. coli, SAP enhances virulence by protecting the bacteria against phagocytosis.59 SAP is not involved in resistance against TNFα-induced lethal hepatitis shock.60 SAP enhances the ability of mouse macrophages to kill Listeria monocytogenes and this activity is not associated with the binding of SAP to the pathogen.61 It has been shown that SAP is protective in infection with organisms to which it does not bind.59
Binding to Lipoproteins: Implications for Atherosclerosis
Another major CRP-ligand of tremendous biological significance is modified low-density lipoprotein (LDL). CRP readily gets complexed in a Ca2+-dependent manner to modified (oxidized and enzymatically-treated) LDL but not to native LDL.62–65 Binding of CRP to LDL is mediated by the PCh-binding site in CRP that interacts with the PCh and cholesterol moieties present on LDL.64,65 Consistent with the interaction between CRP and LDL in vitro, CRP has been found deposited and colocalized with LDL in human atherosclerotic lesions.66,67 CRP is thus capable of covering certain properties of modified LDL such as complement activation. It has been shown that CRP protects the host from complement activation by LDL.68 However, CRP has not been found to be either atheroprotective or proatherogenic in mouse models of atherosclerosis.69–71 Although the denatured CRP has been shown to be atheroprotective in mice, the role of intact CRP in the development of atherosclerosis is not clear.72
The serum level of CRP is raised in atherosclerosis. Measurement of serum CRP is recommended for use as an indicator of arterial inflammation and predictor of future cardiovascular events.73 Statins that lower cholesterol levels have also been shown to lower CRP levels.21 Recent data have indicated that the measurement of serum CRP levels alone, at least in individuals on statin-therapy, is not beneficial.
Binding to Nuclear Constituents: Role in Protection against Autoimmunity
The presence of CRP in the nuclei of synoviocytes and histiocytes in the patients with rheumatoid arthritis led to the finding that CRP binds nuclear materials such as chromatin, histones, small nuclear ribonucleoproteins, nuclear envelope proteins, and nucleosome core particles.74–77 The interaction is Ca2+-dependent and involves the PCh-binding site of CRP.75–77 CRP, however, does not bind chromatin in serum suggesting that this interaction occurs only if CRP or chromatin is deposited at sites of inflammation.78
SAP binds to histones and chromatin, and also to DNA. The binding is Ca2+-dependent and does not occur in serum.78,79 SAP binds chromatin in vivo also; it has been detected with the nuclear deposits in skin biopsies from lupus patients.80 CRP and SAP have a nuclear localization signal and thus their transport into nuclei is possible.81 The primary role of CRP and SAP is believed to be the disposal of nuclear materials released in the extracellular environment by apoptotic and necrotic cells, thereby preventing the hazard of autoimmunity.77–79
In lupus-prone mice, CRP delays the onset of nephritis and increases clearance and prevents accumulation of immune complexes in the renal cortex. It also decreases autoantibody levels that reduces autoimmune manifestations and thus prolongs the survival.82–84 Another important aspect of the role of short pentraxins in autoimmunity is their ability to bind immobilized IgG and immune complexes.85,86 SAP-deficient mice degrade chromatin more rapidly than normal, have enhanced antibody response to exogenous chromatin, and develop anti-chromatin autoimmunity and glomerulonephritis, a phenotype resembling lupus.87,88 Thus SAP, by controlling chromatin degradation, prevents glomerulonephritis although anti-nuclear antibodies are formed.
Binding to Polycations and Extracellular Matrix Proteins
For binding to certain ligands, CRP does not require Ca2+. Such ligands include polycations like protamine, leukocyte cationic proteins, and a variety of arginine-rich and lysine-rich cationic molecules, and extracellular matrix (ECM) proteins like fibronectin (Fn).89–92 Instead, Ca2+ inhibits these interactions at the physiological pH. SAP also binds to Fn.93 The Ca2+-binding site of CRP participates in binding to Fn and polycations.92 On Fn, the C-terminal domain including the cell-binding and heparin-binding regions is involved in binding to CRP.91 The CRP-Fn interaction may explain in part the selective deposition of CRP at sites of tissue injury and may play a role in the formation of ECM needed for tissue repair.
No binding occurs between soluble CRP and Fn. The maximum interaction between CRP and Fn occurs at pH 5.0 and this interaction is not inhibited by Ca2+.90,92 Since CRP circulates in the blood in its Ca2+-bound form, it can interact with Fn exclusively at the ECM of the inflammatory sites including carcinomas where the pH goes down.90,92 Because CRP, Fn and the ECM have been implicated in cancer, it has been proposed that CRP-Fn interactions may change the architecture of ECM to modify the course of the disease progression.92 In mouse models of melanoma, CRP is tumoricidal and inhibits metastases.94
Binding to Carbohydrate Structures on the Parasite Surface
CRP also reacts with bacterial polysaccharides that do not contain PCh, with several galactans through galactose and phosphate residues, and to substances derived from fungi and yeast.85,95–98 CRP also binds, in a Ca2+-dependent manner, to certain parasites such as Plasmodium falciparum, Hymenopepis diminuta, and Leishmania donovani through either PCh or carbohydrates on their surfaces.99–102 Binding of CRP to L. donovani induces their developmental transformation.103 The PCh-binding site of CRP participates in binding of CRP to carbohydrate moieties.41
The carbohydrate-binding property of SAP is shown by the binding of SAP to agarose, microbial polysaccharides, aggregated IgG, Type IV collagen, calumenin, shiga toxin 2, lactate, influenza virus hemagglutinin, heparin, 6-phosphorylated mannose, 3-sulfated saccharides, and glycosaminoglycans.104–108 Most of these interactions require Ca2+. The interaction of CRP and SAP with carbohydrates occur best at mildly acidic pH.85,109
The binding of SAP to glycosaminoglycans neutralizes its anticoagulant effect.110 Heparin, in the presence of SAP, is a better inhibitor of thrombin-catalyzed conversion of fibrinogen to fibrin than heparin alone, and thus SAP also inhibits fibrin polymerization.111 Heparin and lactic acid prevent SAP self-association, however, lactic acid does not dissociate SAP-heparin complex. Finally, SAP has been shown to enhance refolding of denatured lactate dehydrogenase and thus SAP also acts as a chaperone.112
Binding to Platelet-Activating Factor
CRP binds to PAF, -O-alkyl-sn-2-O-acetyl-n-glycero-3-phosphocholine, probably through the PCh group in PAF.113,114 CRP inhibits PAF-induced platelet-aggregation, inhibits binding of PAF to platelets, and prevents capture of neutrophils by platelets.115–118 In vivo, CRP protects mice from lethal challenge with PAF.114,119 The platelets treated with CRP have been shown to kill immature form of Schistosoma mansoni in vitro and confer protection against S. mansoni in rats.120
Binding to Complement C1q and Modulation of the Classical Complement Pathway
Ligand-complexed CRP binds C1q, the first component of the classical pathway of complement, and activates the classical pathway of complement.121,122 CRP-initiated activation of complement leads to the assembly of an effective C3 convertase and generates anaphylotoxins C3a and C4a and the opsonins C4b, C3b, and iC3b. CRP-complexes do not result in the formation of an effective C5-convertase, and therefore prevent generation of pro-inflammatory molecules like C5a and also avoid the assembly of membrane-damaging, terminal membrane attack complex of the complement pathway.123
The C1q-binding site on CRP is located in the cleft regions present one on each subunit and is formed by Tyr175, Asp112, Glu88, His38, and Asn158. Residues Asp112 and Tyr175 appear to provide contacts with C1q.124,125 The CRP-binding site on C1q is located on the globular region of C1q.126–128 SAP, in addition to binding to C1q, also binds to C4b-binding protein.129 Aggregated SAP and ligand-bound SAP activate the classical pathway of complement.130,131 The C1q-binding site on SAP is not known.
Complement activation by CRP-complexes participates in phagocytosis of apoptotic cells.132 CRP attenuates the formation of the membrane attack complex on the surfaces of apoptotic cells, thereby protecting the cells from lysis. This effect is achieved by the recruitment of factor H, a complement regulatory protein that accelerates the decay of the C3 and C5 convertases.132 The factor H-dependent inhibitory effect of CRP on activation of the alternative pathway of complement by S. pneumoniae and artificially sensitized erythrocytes has been observed.133–135 Interestingly, the property of CRP to activate the classical pathway of complement is irrelevant for the protection of mice from pneumococcal infection.136
Binding to the IgG Receptors FcγRI and IIa and Interaction with Phagocytes
CRP interacts with phagocytic cells through the IgG receptors, FcγRI (CD64) and FcγRII (CD32), in heat-inactivated plasma, in cell culture medium, and in buffered solutions.137–143 The exact physiological conditions in which this interaction can occur in vivo are unclear but it is reasonable to believe that either CRP should be ligand-complexed or the phagocytic cells should be immobilized. FcγRIIa, the low affinity IgG receptor providing stimulatory signals in the cells, is the high affinity receptor for CRP.139 In contrast, FcγRI, the high affinity IgG receptor providing inhibitory signals in the cells, is the low affinity receptor for CRP.138 The binding of CRP to FcγR is Ca2+-dependent, specific, saturable, reversible, and rapid with a half-life of 3 min.137–143 PCh does not inhibit CRP-FcR interaction.142 Because IgG inhibits binding of CRP to FcγR, it is likely that the sites on FcγR that bind IgG and CRP are similar.141 A hydrophobic region present in the cleft on the CRP molecule provides the contact amino acids for FcγR. These amino acids are Thr173, Asn186, Lys114, and Leu176.144 Thus the binding sites on CRP for FcγR and for C1q are discrete but overlapping.
CRP affects the functions of the phagocytic cells such as augmentation of FcR-dependent aggregated IgG-mediated respiratory burst activity.145 Upon binding to monocytic cells, CRP is internalized and degraded.146 Although CRP enhances the phagocytosis of a variety of Gram-negative pathogens in vitro, it has been shown that the binding ability of CRP for FcγR is not required for protection of mice from pneumococcal infection.147 However, binding of CRP to FcγR is required for the protection of mice from LPS toxicity.148 CRP induces macrophage tumoricidal activity but it is not known whether CRP-FcγR interactions are involved.149 CRP also binds mouse FcγR.150–152 SAP also binds to human and mouse FcγR.153,154
Binding of SAP to Amyloid Fibrils and Role in Amyloidosis
SAP binds to β-amyloid peptide in a Ca2+-dependent manner and inhibits the formation of amyloid fibrils.155,156 SAP has been shown to induce cell death in primary cultures of rat cerebral cortex suggesting that SAP may play a role in the development of Alzheimer’s disease.157 Binding of SAP to amyloid fibrils prevents proteolysis of the amyloid fibrils in vitro and thus enhances induction of amyloidosis in vivo as shown by delayed and reduced amyloid deposition in SAP-deficient mice.158,159 The interference with binding of SAP to amyloid fibrils in vivo may promote regression of the deposits.160 SAP-deficient mice do not necessarily develop severe autoimmune disease; they have high antinuclear antibodies but no glomerulonephritis.88
Long Pentraxin: PTX3
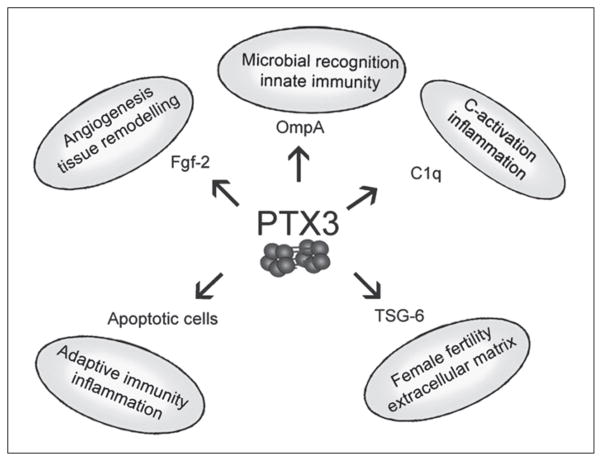
In the 1990s a new member of the pentraxin family was cloned in endothelial cells stimulated with IL-1 or in fibroblasts treated with TNFα.161,162 The prototypic long pentraxin 3 (PTX3) is characterized by a C-terminal domain sharing a high degree of homology with short pentraxins, associated to an unrelated long N-terminal domain. In spite of the sequence homology, PTX3 differs from CRP and SAP in terms of gene organization and chromosomal localization as well as cellular source, inducing-stimuli, and ligand-binding properties.1,163 PTX3 interacts with C1q, the growth factor FGF2, the ECM component TSG-6 and selected pathogens (Fig. 1).164–169 Recent data in gene-targeted mice show that PTX3 has complex nonredundant functions in vivo, ranging from female fertility and innate immune response against diverse microorganisms, to the assembly of ECM.1,167,168,170–173 PTX3 is highly conserved in evolution suggesting that the results obtained in animal models are likely to be informative for the function of PTX3 in humans. In humans, PTX3 plasma levels are very low in normal condition (≤2 ng/ml) but increase rapidly in several pathological conditions raising the possibility that PTX3 may have a diagnostic and prognostic role.
Figure 1.
The long pentraxin PTX3 acts as a soluble multifunctional protein. The multifunctional properties of PTX3 involve interaction with a number of different ligands such as the complement component C1q, the growth factor FGF2, the extracellular matrix component TSG-6, late apoptotic cells and outer membrane proteins of Gram-negative bacteria. Interaction of PTX3 with its ligands is essential for the multifunctional properties exerted by this pentraxin in microbial recognition, discrimination between self and nonself, tissue remodelling, and tuning of the inflammatory response. Binding of PTX3 to plastic-immobilized C1q induces activation of the classical complement pathway and the angiogenic activity of FGF2 in vivo and in vitro is blocked by PTX3. In addition PTX3 is an integral component of the extracellular matrix and plays a crucial role in female fertility.
Gene and Protein Organization
The human ptx3 gene is located on chromosome 3, band q25 and is organized in three exons coding respectively for the leader peptide, the N-terminal domain and the C-terminal pentraxin like-domain of the protein. PTX3 protein is 381 amino acids long, including a signal peptide of 17 amino acids, and it has a predicted molecular weight of 40,165 Da. The C-terminal domain contains the pentraxin signature, two conserved cysteines (Cys210 and Cys271) and a N-linked glycosylation site at Asn220. PTX3 protomers are assembled to form multimers predominantly of 440 kDa apparent molecular mass, corresponding to decamers. In contrast to what was observed for CRP and SAP pentamers, the decameric form of PTX3 is dependent upon interchain disulfide bonds, as demonstrated by nonreducing SDS-PAGE.164
A murine ptx3 has been also described: the murine gene is located on chromosome 3, in a region (q24-28) homologous to human chromosome 3q, and has the same genomic organization in three exons and two introns as the human counterpart.174 Human and murine PTX3 are highly conserved: both proteins are 381 amino acids long and share 82% of identical amino acids and 92% of conserved amino acids.
A model for PTX3 tertiary folding has been proposed based in part on the sequence homology with CRP and SAP. According to this model, PTX3 pentraxin domain well accommodates on the tertiary fold of SAP, with almost all of the β-strands and the α-helical segments conserved.174
PTX3 is produced by a variety of cell types, including mononuclear phagocytes, dendritic cells (DC), fibroblasts, endothelial cells, smooth muscle cells, adipocytes, synovial cells, chondrocytes and cells of epithelial origin, such as renal and alveolar cells.161,162,175–182 Resting cells generally do not release appreciable levels of the protein but they can be triggered to produce PTX3 by primary inflammatory signals, such as TNFα and IL-1β, toll-like receptor (TLR) ligands and microbial moieties, such as LPS, lipoarabinomannans and outer membrane proteins.1,169
Myelomonocytic DCs are major producer of PTX3 in vitro in response to TLR engagement while plasmacytoid DCs do not produce PTX3.183 On the other side, PTX3 can regulate the maturation program of DCs as well as the secretion of soluble factors such as IL-10 and TNF-α, behaving as a flexible regulator of the function of this cell population.184 IFNγ and IL-10 play divergent effects on regulation of PTX3 production by DCs: IFN-γ, which has generally a synergistic effect with LPS, inhibits LPS induction of PTX3 while IL-10 amplifies the response to LPS, TLR ligands and IL-1β.185,186 Production of PTX3 by IL-10-treated DCs is likely to be associated with matrix deposition, considering the role of IL-10 in the induction of a genetic program related to tissue remodeling.
Role in Female Fertility
Ptx3-deficiency in mice is associated with a severe defect in female fertility.168,173 Infertility is due to fertilization failure in vivo and is associated with an abnormal cumulus oophorus expansion. The oocyte develops normally in the absence of PTX3 and can be fertilized in vitro, but the unstable cumulus ECM, in which cumulus cells are uniformly dispersed instead of radiating out from a central oocyte, accounts for fertilization defect in vivo. Cumulus granulosa cells express PTX3 mRNA during the preovulatory period, upon the induction by hormonal ovulatory stimuli as follicle stimulating hormone or human chorionic gonadotropin, and by oocyte derived soluble factors, in particular a member of the TGFβ family, growth differentiation factor-9.167,173 The protein localizes in the ECM, where it plays a crucial role in the assembly of the hyaluronic acid (HA)-rich matrix of the cumulus oophorus.167,187 PTX3 does not interact directly with HA, but it binds TSG-6, which participates in the assembly of the HA-rich matrix. PTX3/TSG-6 complexes might thus serve as an anchoring site for multiple HA molecules, thereby substantially forming a multi-molecular complex that acts as a ‘node’ for cross-linking HA chains. Therefore, PTX3 plays a nonredundant role as structural constituent of the cumulus oophorus ECM essential for in vivo fertilization.
Human cumulus cells express PTX3 as well, and PTX3 protein is present in human cumulus matrix, suggesting that this molecule might have the same role in human female fertility.167,187 Studies have been conducted to assess the potential role of PTX3 as diagnostic marker for oocyte quality: real-time PCR data showed a relatively higher abundance of PTX3 mRNA in cumulus cells from fertilized oocytes compared with cumulus cells from unfertilized oocytes;187 by contrast, PTX3 levels in follicular fluids did not correlate with oocyte quality.188
Results collected over the years on PTX3 levels in patients with a series of inflammatory and infectious disorders (see below) outlined a role of this protein as marker of pathology and prognostic factor, in particular, for conditions reflecting the involvement of the vascular bed. Endothelial dysfunction is a prominent feature of preeclampsia, an important cause of maternal as well as perinatal morbidity and mortality, which may arise in the third trimester of gestation. The pathogenesis of this disease, still not completely defined, is characterized by an excessive maternal inflammatory response; accordingly, significantly higher levels of PTX3 have been found in preeclampsia compared to normal pregnancies.189
Role in Innate Immunity, Inflammation and Apoptotic Cell Clearence
Pathogen recognition is a common feature among the members of the pentraxin family including PTX3 and efforts have been made in order to identify the molecular moieties recognized on bacterial surface. PTX3 does not bind LPS as well as lipoteichoic acid, N-acetylmuramyl-L-alanyl-D-isoglutamine, exotoxin A and enterotoxins A and B. However, it binds with high affinity to recombinant outer membrane protein A from Klebsiella pneumoniae (KpOmpA). KpOmpA binds and activates macrophages and DCs in a TLR2-dependent way, activating a genetic program that includes induction of PTX3. PTX3 in turn binds KpOmpA, and plays a crucial role in the amplification of the inflammatory response to this microbial protein, as demonstrated by the impairment of the inflammatory response induced by KpOmpA in ptx3-deficient mice.169
PTX3 plays an important role in defence against selected pathogens such as Aspergillus fumigatus. This can be explained, at least in part, by an opsonic effect of PTX3, facilitating ingestion of conidia by macrophages.168 Macrophages from PTX3-overexpressing mice have an improved phagocytic activity towards zymosan and Paracoccidioides brasiliensis.170 Moreover, recombinant PTX3 binds to zymosan and P. brasiliensis, and functions as an opsonin, thereby increasing the phagocytic activity of peritoneal macrophages from wild-type animals. These findings provide evidence for a role of PTX3 as a functional ancestor of antibodies and imply the existence of a receptor for this molecule. Accordingly, a binding site has been observed on murine macrophages as well as human mononuclear phagocytes and DCs (Bottazzi, unpublished observations).
Studies in ptx3-deficient mice suggest that the role played by PTX3 in innate resistance is nonredundant and relevant in selected fungal and bacterial infections (A. fumigatus, P. aeruginosa, S. typhimurium) and irrelevant in others (L. monocytogenes, S. aureus, polymicrobic intra-abdominal sepsis) (Garlanda, unpublished observations).168 In particular, extreme susceptibility was observed to invasive pulmonary aspergillosis which was associated with the lack of development of appropriate and protective Th1 anti-fungal responses and to an unbalanced cytokine profile skewed towards a Th2 response.168 The specificity of the defect and the therapeutic potential of PTX3 could be demonstrated by the complete protective effect following treatment with recombinant PTX3.168,190 Variable susceptibility to different pathogens suggests that PTX3 deficiency does not cause a generalized immunodeficiency, and that PTX3 is involved in recognition and resistance against specific microorganisms.
Ptx3 overexpressing and deficient mice were used to evaluate the role of PTX3 in inflammatory conditions. Ptx3 overexpression increases resistance to LPS toxicity and cecal ligation and puncture,171 but induces an exacerbated inflammatory response and reduces survival rate following intestinal ischemia reperfusion injury.172 In a model of kainate-induced seizures, ptx3-deficient mice had more widespread and severe IL-1-induced neuronal damage. In this model PTX3 confers resistance to neurodegeneration, possibly by binding to dying neurons and rescuing them from otherwise irreversible damage.191
Like other members of the pentraxin family,132,192 PTX3 binds apoptotic cells inhibiting their recognition by DCs.184,193 Binding occurs late in the apoptotic process and modulates cytokine production by DC. In addition, preincubation of apoptotic cells with PTX3 enhances C1q binding and C3 deposition on the cell surface, suggesting a role for PTX3 in the complement-mediated clearance of apoptotic cells.165 Moreover, in the presence of dying cells, PTX3 may contribute to editing recognition of apoptotic self versus infectious non self and restricts the cross presentation of antigens derived from dying cells.184 These results suggest that PTX3 has a dual role in the protection against pathogens and in the control of autoimmunity.
Role in Human Pathology
The results obtained in gene-modified mice together with the similarities to a widely used marker of inflammation such as CRP, have given impetus to efforts aimed to investigate the role of PTX3 in diverse human pathologies. PTX3 is expressed at very high levels in the heart of rodents, after systemic administration of microbial products and inflammatory cytokines or ligation of the left coronary artery to model acute myocardial infarction (AMI) (Latini, unpublished observations).174 PTX3 is present in atherosclerotic lesions and is induced by oxidized LDL in smooth muscle cells, moreover PTX3 increases tissue factor expression by mononuclear cells, potentially playing a role in thrombogenesis and wound healing.178,194–196 In this context a pilot study was conducted in a small group of patients with AMI, showing that PTX3 plasma levels increase rapidly reaching a peak 6–8 hours after the onset of symptoms.196 In the same context, plasma CRP increased, but it peaked much later, between 24 and 48 hours after symptom onset. Because CRP is produced mainly by the liver in response to IL-6 and PTX3 by the heart and vasculature in response to primary inflammatory stimuli, it was hypothesized that PTX3, rather than CRP, could be an acute-phase reactant more closely related to cardiac injuries such as AMI and therefore could be a sensitive and specific prognostic indicator in this context.197 This hypothesis was confirmed in a recent prospective study on a large group of patients with AMI where it has been shown that PTX3 is an earlier and stronger prognostic marker of death compared to other accepted markers of myocardial necrosis such as creatine kinase and troponin T.198
PTX3 blood levels, barely detectable in normal conditions (1–2 ng/ml), increase rapidly and dramatically (200–800 ng/ml) during a range of pathological conditions others than AMI. Plasma levels of PTX3 are increased in diverse infectious disorders, including sepsis, A. fumigatus infections, active pulmonary tuberculosis and dengue virus infection.168,199–201 The higher levels of PTX3 observed in patients with pulmonary tuberculosis or dengue are associated with disease severity and possibly with clinical outcome. Patients with active vasculitis have significantly higher plasma levels of PTX3 than patients with quiescent disease.202 PTX3 inhibition of macrophage phagocytosis of late apoptotic cells could in part explain the phenomenon of leukocytoclasia observed in small vessel vasculitis. PTX3 is also expressed at high levels in patients with systemic juvenile idiopathic arthritis.203
A general feature common to all these pathologies is the rapidity of PTX3 increase compared to CRP: CRP is made in the liver in response primarily to IL-6 while PTX3 is produced locally by a number of different cells in response to proinflammatory signals and thus representing a rapid marker for local activation of inflammation and innate immunity.
Data collected so far in a number of different pathologies indicate a correlation between PTX3 plasma levels and severity of disease, suggesting a possible role of PTX3 as marker of pathology. It remains to be elucidated whether the impressive correlation with outcome and severity actually reflects a role in the pathogenesis of damage, for instance by amplifying the complement and coagulation cascades.
Conclusion
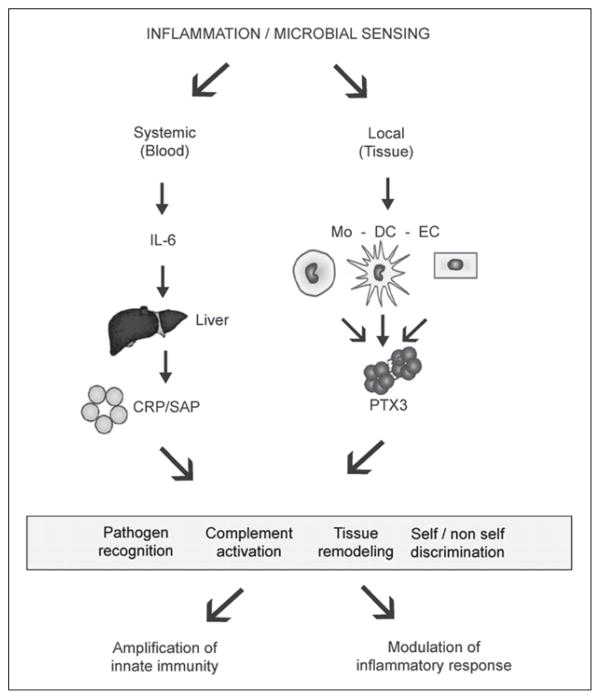
Most suggested functions of short pentraxins CRP and SAP are based on their pattern-recognition characteristics seen in vitro (Table 1). The data on the exact physiological situations in vivo where the short pentraxins would interact with their ligands are beginning to emerge. Likewise, the functional consequences of the recognition properties of CRP and SAP are subjects of current research interests (Fig. 2). Of most importance are the values of CRP in bacterial infections, atherosclerosis and autoimmunity and the value of SAP in amyloidosis. The contribution of the two known effector functions of short pentraxins, that is complement activation and phagocytosis, is under intensive investigation in mouse models of human diseases where these pentraxins have been implicated.
Figure 2.
Pentraxins in innate immunity. Inflammation and microbial sensing induce both local and systemic responses characterized by the production of different members of the pentraxin family. Systemic response involves production by the liver of the prototypic short pentraxin CRP and SAP while local response involves production by macrophages, dendritic cells and endothelial cells of the long pentraxin PTX3. Both short and long pentraxins recognize pathogens, activate the classical complement cascade, participate in tissue remodelling and in self/ non self discrimination, outlining the role of these proteins in the amplification of innate immunity and in the modulation of inflammatory response.
Gene targeting of the prototypic, evolutionarily conserved, long pentraxin PTX3 has unequivocally defined the role of this molecule at the crossroad of innate immunity, inflammation, matrix deposition and female fertility (Fig. 1).1 Moreover, recent progress has further defined the structure, regulation, microbial recognition and in vivo function of PTX3.
PTX3 is a component of the complex and complementary network of cellular and humoral pattern recognition receptors involved in the recognition and response to microbial elements and damaged tissues, in tuning inflammatory reactions, in discriminating between infectious nonself and apoptotic self. Translational efforts suggest that PTX3 may represent a new marker of innate immunity and inflammation, rapidly reflecting tissue and vascular bed involvement.
Acknowledgments
This work was supported by National Institutes of Health, USA, and Associazione Italiana per la Ricerca sul Cancro (AIRC), Ministero Istruzione Università e Ricerca (MIUR), European Commission.
References
- 1.Garlanda C, Bottazzi B, Bastone A, et al. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A. CRP after 2004. Mol Immunol. 2005;42:927–930. doi: 10.1016/j.molimm.2004.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volanakis JE. Human C-reactive protein: Expression, structure, and function. Mol Immunol. 2001;38:189–197. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 4.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 5.Skinner M, Cohen AS. Amyloid P component. Methods Enzymol. 1988;163:523–536. doi: 10.1016/0076-6879(88)63048-5. [DOI] [PubMed] [Google Scholar]
- 6.Osmand AP, Friedenson B, Gewurz H, et al. Characterization of C-reactive protein and the complement subcomponent C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins) Proc Natl Acad Sci USA. 1977;74:739–743. doi: 10.1073/pnas.74.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tillett WS, Francis T., Jr Serological reactions in pneumonia with a nonprotein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–571. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschfield GM, Pepys MB. C-reactive protein and cardiovascular disease: New insights from an old molecule. QJM. 2003;96:793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- 9.Cathcart ES, Comerford FR, Cohen AS. Immunological studies on a protein extracted from human secondary amyloid. New Engl J Med. 1965;273:143–146. doi: 10.1056/NEJM196507152730306. [DOI] [PubMed] [Google Scholar]
- 10.Pepys MB, Rademacher TW, Amatayakul-Chantler S, et al. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Natl Acad Sci USA. 1994;91:5602–5606. doi: 10.1073/pnas.91.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehead AS, Zahedi K, Rits M, et al. Mouse C-reactive protein: Generation of cDNA clones, structural analysis, and induction of mRNA during inflammation. Biochem J. 1990;266:283–290. doi: 10.1042/bj2660283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepys MB, Dash AC, Fletcher TC, et al. Analogues in other mammals and in fish of human plasma proteins, C-reactive protein and amyloid P component. Nature. 1978;273:168–170. doi: 10.1038/273168a0. [DOI] [PubMed] [Google Scholar]
- 13.Ying SC, Marchalonis JJ, Gewurz AT, et al. Reactivity of anti-human C-reactive protein and serum amyloid P component monoclonal antibodies with limulin and pentraxins of other species. Immunology. 1992;76:324–330. [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen NY, Suzuki A, Boykins RA, et al. The amino acid sequence of Limulus C-reactive protein: Evidence of polymorphism. J Biol Chem. 1986;261:10456–10465. [PubMed] [Google Scholar]
- 15.Agrawal A, Mitra S, Ghosh N, et al. C-reactive protein in hemolymph of a mollusc, Achatina fulica Bowdich. Indian J Exp Biol. 1990;28:788–789. [PubMed] [Google Scholar]
- 16.De Beer FC, Baltz ML, Munn EA, et al. Isolation and characterization of C-reactive protein and serum amyloid P component in the rat. Immunology. 1982;45:55–70. [PMC free article] [PubMed] [Google Scholar]
- 17.Hurlimann J, Thorbecke GJ, Hochwald GM. The liver as the site of C-reactive protein formation. J Exp Med. 1966;123:365–378. doi: 10.1084/jem.123.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganapathi MK, Rzewnicki D, Samols D, et al. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep3B cells. J Immunol. 1991;147:1261–1265. [PubMed] [Google Scholar]
- 19.Agrawal A, Samols D, Kushner I. Transcription factor c-Rel enhances C-reactive protein expression by facilitating the binding of C/EBPβ to the promoter. Mol Immunol. 2003;40:373–380. doi: 10.1016/s0161-5890(03)00148-2. [DOI] [PubMed] [Google Scholar]
- 20.Voleti B, Agrawal A. Regulation of basal and induced expression of C-reactive protein through an overlapping element for OCT-1 and NF-κB on the proximal promoter. J Immunol. 2005;175:3386–3390. doi: 10.4049/jimmunol.175.5.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voleti B, Agrawal A. Statins and nitric oxide reduce C-reactive protein production while inflammatory conditions persist. Mol Immunol. 2006;43:891–896. doi: 10.1016/j.molimm.2005.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould JM, Weiser JN. Expression of C-reactive protein in the human respiratory tract. Infect Immun. 2001;69:1747–1754. doi: 10.1128/IAI.69.3.1747-1754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabs WJ, Busse M, Kruger S, et al. Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney Int. 2005;68:2103–2110. doi: 10.1111/j.1523-1755.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 24.Szalai AJ, Van Ginkel FW, Dalrymple SA, et al. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J Immunol. 1998;160:5294–5299. [PubMed] [Google Scholar]
- 25.Coe JE, Ross MJ. Amyloidosis and female protein in the Syrian hamster: Concurrent regulation by sex hormones. J Exp Med. 1990;171:1257–1267. doi: 10.1084/jem.171.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–1357. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins PN, Wootten R, Pepys MB. Metabolic studies of radioiodinated serum amyloid P component in normal subjects and patients with systemic amyloidosis. J Clin Invest. 1990;86:1862–1869. doi: 10.1172/JCI114917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotschlich EC, Edelman GM. C-reactive protein: A molecule composed of subunits. Proc Natl Acad Sci USA. 1965;54:558–566. doi: 10.1073/pnas.54.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveria EB, Gotschlich EC, Liu TY. Primary structure of human C-reactive protein. J Biol Chem. 1979;254:489–502. [PubMed] [Google Scholar]
- 30.Shrive AK, Cheetham GM, Holden D, et al. Three-dimensional structure of human C-reactive protein. Nature Struct Biol. 1996;3:346–354. doi: 10.1038/nsb0496-346. [DOI] [PubMed] [Google Scholar]
- 31.Hamazaki H. Structure and significance of N-linked sugar unit of human serum amyloid P component. Biochim Biophys Acta. 1990;1037:435–438. doi: 10.1016/0167-4838(90)90047-j. [DOI] [PubMed] [Google Scholar]
- 32.Emsley J, White HE, O’Hara BP, et al. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 33.Sørensen IJ, Andersen O, Nielsen EH, et al. Native human serum amyloid P component is a single pentamer. Scand J Immunol. 1995;41:263–267. doi: 10.1111/j.1365-3083.1995.tb03562.x. [DOI] [PubMed] [Google Scholar]
- 34.Abernethy TJ, Avery OT. The occurrence during acute infections of a protein not normally present in the blood. I. Distribution of the reactive protein in patients’ sera and the effect of calcium on the flocculation reaction with C-polysaccharide of pneumococcus. J Exp Med. 1941;73:173–182. doi: 10.1084/jem.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinoshita CM, Ying SC, Hugli TE, et al. Elucidation of a protease-sensitive site involved in the binding of calcium to C-reactive protein. Biochemistry. 1989;28:9840–9848. doi: 10.1021/bi00451a044. [DOI] [PubMed] [Google Scholar]
- 36.Volanakis JE, Kaplan MH. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971;136:612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- 37.Nauta AJ, Daha MR, Van Kooten C, et al. Recognition and clearance of apoptotic cells: A role for complement and pentraxins. Trends Immunol. 2003;24:148–154. doi: 10.1016/s1471-4906(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 38.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure. 1999;7:169–177. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal A, Xu Y, Ansardi D, et al. Probing the phosphocholine-binding site of human C-reactive protein by site-directed mutagenesis. J Biol Chem. 1992;267:25352–25358. [PMC free article] [PubMed] [Google Scholar]
- 40.Agrawal A, Lee S, Carson M, et al. Site-directed mutagenesis of the phosphocholine-binding site of human C-reactive protein: Role of Thr76 and Trp67. J Immunol. 1997;158:345–350. [PubMed] [Google Scholar]
- 41.Agrawal A, Simpson MJ, Black S, et al. A C-reactive protein mutant that does not bind to phosphocholine and pneumococcal C-polysaccharide. J Immunol. 2002;169:3217–3222. doi: 10.4049/jimmunol.169.6.3217. [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita CM, Gewurz AT, Siegel JN, et al. A protease-sensitive site in the proposed Ca2+-binding region of human serum amyloid P component and other pentraxins. Protein Sci. 1992;1:700–709. doi: 10.1002/pro.5560010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bijl M, Horst G, Bijzet J, et al. Serum amyloid P component binds to late apoptotic cells and mediates their uptake by monocyte-derived macrophages. Arthrit Rheumat. 2003;48:248–254. doi: 10.1002/art.10737. [DOI] [PubMed] [Google Scholar]
- 44.Christner RB, Mortensen RF. Specificity of the binding interaction between human serum amyloid P-component and immobilized human C-reactive protein. J Biol Chem. 1994;269:9760–9766. [PubMed] [Google Scholar]
- 45.Aquilina JA, Robinson CV. Investigating interactions of the pentraxins serum amyloid P component and C-reactive protein by mass spectrometry. Biochem J. 2003;375:323–328. doi: 10.1042/BJ20030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mold C, Rodgers CP, Kaplan RL, et al. Binding of human C-reactive protein to bacteria. Infect Immun. 1982;38:392–395. doi: 10.1128/iai.38.1.392-395.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Beaufort AJ, Langermans JAM, Matze-Van Der Lans AM, et al. Difference in binding of killed and live Streptococcus pneumoniae serotypes by C-reactive protein. Scand J Immunol. 1997;46:597–600. doi: 10.1046/j.1365-3083.1997.d01-171.x. [DOI] [PubMed] [Google Scholar]
- 48.Weiser JN, Pan M, McGowan KL, et al. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serino L, Virji M. Genetic and functional analysis of the phosphorylcholine moiety of commensal Neisseria lipopolysaccharide. Mol Microbiol. 2002;43:437–448. doi: 10.1046/j.1365-2958.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 50.Kindmark CO. Stimulating effect of C-reactive protein on phagocytosis of various species of pathogenic bacteria. Clin Exp Immunol. 1971;8:941–948. [PMC free article] [PubMed] [Google Scholar]
- 51.Yother J, Volanakis JE, Briles DE. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in mice. J Immunol. 1982;128:2374–2376. [PubMed] [Google Scholar]
- 52.Mold C, Nakayama S, Holzer TJ, et al. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med. 1981;154:1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szalai AJ, Briles DE, Volanakis JE. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J Immunol. 1995;155:2557–2563. [PubMed] [Google Scholar]
- 54.Szalai AJ. The antimicrobial activity of C-reactive protein. Microbes Infect. 2002;4:201–205. doi: 10.1016/s1286-4579(01)01528-3. [DOI] [PubMed] [Google Scholar]
- 55.Szalai AJ, VanCott JL, McGhee JR, et al. Human C-reactive protein is protective against fatal Salmonella enterica serovar typhimurium infection in transgenic mice. Infect Immun. 2000;68:5652–5656. doi: 10.1128/iai.68.10.5652-5656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Haas CJC, van Leeuwen EMM, Van Bommel T, et al. Serum amyloid P component bound to Gram-negative bacteria prevents lipopolysaccharide-mediated classical pathway complement activation. Infect Immun. 2000;68:1753–1759. doi: 10.1128/iai.68.4.1753-1759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Haas CJC, Van Der Tol ME, Van Kessel KPM, et al. A synthetic lipopolysaccharide-binding peptide based on amino acids 27–39 of serum amyloid P component inhibits lipopolysaccharide-induced responses in human blood. J Immunol. 1998;161:3607–3615. [PubMed] [Google Scholar]
- 58.De Haas CJC, Van Der Zee R, Benaissa-Trouw B, et al. Lipopolysaccharide (LPS)-binding synthetic peptides derived from serum amyloid P component neutralize LPS. Infect Immun. 1999;67:2790–2796. doi: 10.1128/iai.67.6.2790-2796.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noursadeghi M, Bickerstaff MC, Gallimore JR, et al. Role of serum amyloid P component in bacterial infection: Protection of the host or protection of the pathogen. Proc Natl Acad Sci USA. 2000;97:14584–14589. doi: 10.1073/pnas.97.26.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Molle W, Hochepied T, Brouckaert P, et al. The major acute-phase protein, serum amyloid P component, in mice is not involved in endogenous resistance against tumor necrosis factor alpha-induced lethal hepatitis, shock, and skin necrosis. Infect Immun. 2000;68:5026–5029. doi: 10.1128/iai.68.9.5026-5029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh PP, Gervais F, Skamene E, et al. Serum amyloid P-component-induced enhancement of macrophage listericidal activity. Infect Immun. 1986;52:688–694. doi: 10.1128/iai.52.3.688-694.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Beer FC, Soutar AK, Baltz ML, et al. Low density lipoprotein and very low density lipoprotein are selectively bound by aggregated C-reactive protein. J Exp Med. 1982;156:230–242. doi: 10.1084/jem.156.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhakdi S, Torzewski M, Klouche M, et al. Complement and atherogenesis: Binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler Thromb Vasc Biol. 1999;19:2348–2354. doi: 10.1161/01.atv.19.10.2348. [DOI] [PubMed] [Google Scholar]
- 64.Chang MK, Binder CJ, Torzewski M, et al. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci USA. 2002;99:13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taskinen S, Kovanen PT, Jarva H, et al. Binding of C-reactive protein to modified low-density-lipoprotein particles: Identification of cholesterol as a novel ligand for C-reactive protein. Biochem J. 2002;367:403–412. doi: 10.1042/BJ20020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reynolds GD, Vance RP. C-reactive protein immunohistochemical localization in normal and atherosclerotic human aortas. Arch Pathol Lab Med. 1987;111:265–269. [PubMed] [Google Scholar]
- 67.Sun H, Koike T, Ichikawa T, et al. C-reactive protein in atherosclerotic lesions: Its origin and patho-physiological significance. Am J Pathol. 2005;167:1139–1148. doi: 10.1016/S0002-9440(10)61202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhakdi S, Torzewski M, Paprotka K, et al. Possible protective role for C-reactive protein in atherogenesis: Complement activation by modified lipoproteins halts before detrimental terminal sequence. Circulation. 2004;109:1870–1876. doi: 10.1161/01.CIR.0000124228.08972.26. [DOI] [PubMed] [Google Scholar]
- 69.Hirschfield GM, Gallimore JR, Kahan MC, et al. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc Natl Acad Sci USA. 2005;102:8309–8314. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reifenberg K, Lehr HA, Baskal D, et al. Role of C-reactive protein in atherogenesis: Can the apolipoprotein E knockout mouse provide the answer? Arterioscler Thromb Vasc Biol. 2005;25:1641–1646. doi: 10.1161/01.ATV.0000171983.95612.90. [DOI] [PubMed] [Google Scholar]
- 71.Trion A, De Maat MP, Jukema JW, et al. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-leiden/human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:1635–1640. doi: 10.1161/01.ATV.0000171992.36710.1e. [DOI] [PubMed] [Google Scholar]
- 72.Schwedler SB, Amann K, Wernicke K, et al. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2005;112:1016–1023. doi: 10.1161/CIRCULATIONAHA.105.556530. [DOI] [PubMed] [Google Scholar]
- 73.Libby P, Ridker PM. Inflammation and atherosclerosis: Role of C-reactive protein in risk assessment. Am J Med. 2004;116:9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Gitlin JD, Gitlin JI, Gitlin D. Localization of C-reactive protein in synovium of patients with rheumatoid arthritis. Arthrit Rheum. 1977;20:1491–1499. doi: 10.1002/art.1780200808. [DOI] [PubMed] [Google Scholar]
- 75.Robey FA, Jones KD, Tanaka T, et al. Binding of C-reactive protein to chromatin and nucleosome core particles: A possible physiological role of C-reactive protein. J Biol Chem. 1984;259:7311–7316. [PubMed] [Google Scholar]
- 76.Du Clos TW. The interaction of C-reactive protein and serum amyloid P component with nuclear antigens. Mol Biol Rep. 1996;23:253–260. doi: 10.1007/BF00351177. [DOI] [PubMed] [Google Scholar]
- 77.Shephard EG, Smith PJ, Coetzee S, et al. Pentraxin binding to isolated rat liver nuclei. Biochem J. 1991;279:257–262. doi: 10.1042/bj2790257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butler PJG, Tennent GA, Pepys MB. Pentraxin-chromatin interactions: Serum amyloid P component specifically displaces H1-type histones and solubilizes native long chromatin. J Exp Med. 1999;172:13–18. doi: 10.1084/jem.172.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pepys MB, Butler PJG. Serum amyloid P component is the major calcium-dependent specific DNA binding protein of the serum. Biochem Biophys Res Comm. 1987;148:308–313. doi: 10.1016/0006-291x(87)91111-9. [DOI] [PubMed] [Google Scholar]
- 80.Breathnach SM, Kofler H, Sepp N, et al. Serum amyloid P component binds to cell nuclei in vitro and to in vivo deposits of extracellular chromatin in systemic lupus erythematosus. J Exp Med. 1989;170:1433–1438. doi: 10.1084/jem.170.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DuClos TW, Mold C, Stump RF. Identification of a polypeptide sequence that mediates nuclear localization of the acute phase protein C-reactive protein. J Immunol. 1990;145:3869–3875. [PubMed] [Google Scholar]
- 82.DuClos TW, Zlock LT, Hicks PS, et al. Decreased autoantibody levels and enhanced survival of (NZB x NZW) F1 mice treated with C-reactive protein. Clin Immunol Immunopathol. 1994;70:22–27. doi: 10.1006/clin.1994.1005. [DOI] [PubMed] [Google Scholar]
- 83.Rodriguez W, Mold C, Kataranovski M, et al. Reversal of ongoing proteinuria in autoimmune mice by treatment with C-reactive protein. Arthritis Rheum. 2005;52:642–650. doi: 10.1002/art.20846. [DOI] [PubMed] [Google Scholar]
- 84.Szalai AJ, Weaver CT, McCrory MA, et al. Delayed lupus onset in (NZB x NZW) F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum. 2003;48:1602–1611. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- 85.Kottgen E, Hell B, Kage A, et al. Lectin specificity and binding characteristics of human C-reactive protein. J Immunol. 1992;149:445–453. [PubMed] [Google Scholar]
- 86.Brown MR, Anderson BE. Receptor-ligand interactions between serum amyloid P component and model soluble immune complexes. J Immunol. 1993;151:2087–2095. [PubMed] [Google Scholar]
- 87.Bickerstaff MCM, Botto M, Hutchinson WL, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nature Med. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 88.Soma M, Tamaoki T, Kawano H, et al. Mice lacking serum amyloid P component do not necessarily develop severe autoimmune disease. Biochem Biophys Res Comm. 2001;286:200–205. doi: 10.1006/bbrc.2001.5364. [DOI] [PubMed] [Google Scholar]
- 89.Potempa LA, Siegel JN, Gewurz H. Binding reactivity of C-reactive protein for polycations. II. Modulatory effects of calcium and phosphocholine. J Immunol. 1981;127:1509–1514. [PubMed] [Google Scholar]
- 90.Salonen EM, Vartio T, Hedman K, et al. Binding of fibronectin by the acute phase reactant C-reactive protein. J Biol Chem. 1984;259:1496–1501. [PubMed] [Google Scholar]
- 91.Tseng J, Mortensen RF. Binding of human C-reactive protein to plasma fibronectin occurs via the phosphorylcholine-binding site. Mol Immunol. 1988;25:679–686. doi: 10.1016/0161-5890(88)90103-4. [DOI] [PubMed] [Google Scholar]
- 92.Suresh MV, Singh SK, Agrawal A. Interaction of calcium-bound C-reactive protein with fibronectin is controlled by pH: In vivo implications. J Biol Chem. 2004;279:52552–52557. doi: 10.1074/jbc.M409054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tseng J, Mortensen RF. Binding specificity of mouse serum amyloid P-component for fibronectin. Immunol Invest. 1986;15:749–761. doi: 10.3109/08820138609036360. [DOI] [PubMed] [Google Scholar]
- 94.Barna BP, Eppstein DA, Thomassen MJ, et al. Therapeutic effects of a synthetic peptide of C-reactive protein in preclinical tumor models. Cancer Immunol Immunother. 1993;36:171–176. doi: 10.1007/BF01741088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higgenbotham JD, Heidelberger M, Gotschlich EC. Degradation of a pneumococcal type-specific polysaccharide with exposure of group-specificity. Proc Natl Acad Sci USA. 1970;67:138–142. doi: 10.1073/pnas.67.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kempka G, Toos PH, Kolb-Bachofen V. A membrane-associated form of C-reactive protein is the galactose-specific particle receptor on rat liver macrophages. J Immunol. 1990;144:1004–1009. [PubMed] [Google Scholar]
- 97.Baldo BA, Fletcher TC, Pepys J. Isolation of a peptide-polysaccharide from the dermatophyte Epi-dermophyton floccosum and a study of its reaction with human C-reactive protein and mouse anti-phosphorylcholine myeloma serum. Immunology. 1977;32:831–842. [PMC free article] [PubMed] [Google Scholar]
- 98.Jensen TDB, Schønheyder H, Andersen P, et al. Binding of C-reactive protein to Aspergillus fumigatus fractions. J Med Microbiol. 1986;21:173–177. doi: 10.1099/00222615-21-2-173. [DOI] [PubMed] [Google Scholar]
- 99.Pied S, Nussler A, Pontet M, et al. C-reactive protein protects against preerythrocytic stages of malaria. Infect Immun. 1989;57:278–282. doi: 10.1128/iai.57.1.278-282.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taylor K, Hoole D. Interactions between rat C-reactive protein and adult Hymenolepis diminuta. Parasitology. 1997;115:297–302. doi: 10.1017/s0031182097001418. [DOI] [PubMed] [Google Scholar]
- 101.Pritchard DG, Volanakis JE, Slutski GM, et al. C-reactive protein binds Leishmanial excreted factors. Proc Soc Exp Biol Med. 1985;178:500–503. doi: 10.3181/00379727-178-rc4. [DOI] [PubMed] [Google Scholar]
- 102.Culley FJ, Harris RA, Kaye PM, et al. C-reactive protein binds to a novel ligand on Leishmania donovani and increases uptake into human macrophages. J Immunol. 1996;156:4691–4696. [PubMed] [Google Scholar]
- 103.Bee A, Culley FJ, Alkhalife IS, et al. Transformation of Leishmania mexicana metacyclic promastigotes to amastigote-like forms mediated by binding of human C-reactive protein. Parasitology. 2001;122:521–529. doi: 10.1017/s0031182001007612. [DOI] [PubMed] [Google Scholar]
- 104.Hind CRK, Collins PM, Baltz ML, et al. Human serum amyloid P component, a circulating lectin with specificity for the cyclic 4,6-pyruvate acetal of galactose. Biochem J. 1985;225:107–111. doi: 10.1042/bj2250107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loveless RW, Floyd-O’Sullivan G, Raynes JG, et al. Human serum amyloid P is a multispecific adhesive protein whose ligands include 6-phosphorylated mannose and the 3-sulphated saccharides galactose, M-acetylgalactosamine and glucuronic acid. EMBO J. 1992;11:813–819. doi: 10.1002/j.1460-2075.1992.tb05118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown MR, Anderson BE. Receptor-ligand interactions between serum amyloid P component and model soluble immune complexes. J Immunol. 1993;151:2087–2095. [PubMed] [Google Scholar]
- 107.Li XA, Hatanaka K, Guo L, et al. Binding of serum amyloid P component to heparin in human serum. Biochim Biophys Acta. 1994;1201:142–148. doi: 10.1016/0304-4165(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 108.Zahedi K. Characterization of the binding of serum amyloid P to type IV collagen. J Biol Chem. 1996;271:14897–14902. doi: 10.1074/jbc.271.25.14897. [DOI] [PubMed] [Google Scholar]
- 109.Danielsen B, Sørensen IJ, Nybo M, et al. Calcium-dependent and -independent binding of the pentraxin serum amyloid P component to glycosaminoglycans and amyloid proteins: Enhanced binding at slightly acid pH. Biochim Biophys Acta. 1997;1339:73–78. doi: 10.1016/s0167-4838(96)00218-x. [DOI] [PubMed] [Google Scholar]
- 110.Williams EC, Huppert BJ, Asakura S. Neutralization of the anticoagulant effects of glycosaminoglycans by serum amyloid P component: Comparison with other plasma and platelet proteins. J Lab Clin Med. 1992;120:159–167. [PubMed] [Google Scholar]
- 111.Meyers K, Smith R, Williams EC. Inhibition of fibrin polymerization by serum amyloid P component and heparin. Thromb Haemost. 1987;57:345–348. [PubMed] [Google Scholar]
- 112.Coker AR, Purvis A, Baker D, et al. Molecular chaperone properties of serum amyloid P component. FEBS Lett. 2000;473:199–202. doi: 10.1016/s0014-5793(00)01530-1. [DOI] [PubMed] [Google Scholar]
- 113.Filep J, Földes-Filep E. Effects of C-reactive protein on human neutrophil granulocytes challenged with n-formyl-methionyl-leucyl-phenylalanine and platelet-activating factor. Life Sci. 1989;44:517–524. doi: 10.1016/0024-3205(89)90613-9. [DOI] [PubMed] [Google Scholar]
- 114.Xia D, Samols D. Transgenic mice expressing rabbit C-reactive protein are resistant to endotoxemia. Proc Natl Acad Sci USA. 1997;94:2575–2580. doi: 10.1073/pnas.94.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kilpatrick JM, Virella G. Inhibition of platelet-activating factor by rabbit C-reactive protein. Clin Immunol Immunopathol. 1985;37:276–281. doi: 10.1016/0090-1229(85)90159-x. [DOI] [PubMed] [Google Scholar]
- 116.Vigo C. Effect of C-reactive protein on platelet-activating factor-induced platelet aggregation and membrane stabilization. J Biol Chem. 1985;260:3418–3422. [PubMed] [Google Scholar]
- 117.Filep JG, Hermán F, Kelemen E, et al. C-reactive protein inhibits binding of platelet-activating factor to human platelets. Thromb Res. 1991;61:411–421. doi: 10.1016/0049-3848(91)90655-g. [DOI] [PubMed] [Google Scholar]
- 118.Khreiss T, Jozsef L, Potempa LA, et al. Opposing effects of CRP isoforms on shear-induced neutrophil-platelet adhesion and neutrophil aggregation in whole blood. Circulation. 2004;110:2713–2720. doi: 10.1161/01.CIR.0000146846.00816.DD. [DOI] [PubMed] [Google Scholar]
- 119.Black S, Wilson A, Samols D. An intact phosphocholine-binding site is necessary for transgenic rabbit C-reactive protein to protect mice against challenge with platelet-activating factor. J Immunol. 2005;175:1192–1196. doi: 10.4049/jimmunol.175.2.1192. [DOI] [PubMed] [Google Scholar]
- 120.Bout D, Joseph M, Pontet M, et al. Rat resistance to schistosomiasis: Platelet-mediated cytotoxicity induced by C-reactive protein. Science. 1986;231:153–156. doi: 10.1126/science.3079916. [DOI] [PubMed] [Google Scholar]
- 121.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin, and sphingomyelin. J Immunol. 1974;112:2135–2147. [PubMed] [Google Scholar]
- 122.Siegel J, Rent R, Gewurz H. Interactions of C-reactive protein complexes with the complement system. I. Protamine-induced consumption of complement in acute phase sera. J Exp Med. 1974;140:631–647. doi: 10.1084/jem.140.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berman S, Gewurz H, Mold C. Binding of C-reactive protein to nucleated cells leads to complement activation without cytolysis. J Immunol. 1986;136:1354–1359. [PubMed] [Google Scholar]
- 124.Agrawal A, Volanakis JE. Probing the C1q-binding site on human C-reactive protein by site-directed mutagenesis. J Immunol. 1994;152:5404–5410. [PubMed] [Google Scholar]
- 125.Agrawal A, Shrive AK, Greenhough TJ, et al. Topology and structure of the C1q-binding site on C-reactive protein. J Immunol. 2001;166:3998–4004. doi: 10.4049/jimmunol.166.6.3998. [DOI] [PubMed] [Google Scholar]
- 126.Gaboriaud C, Juanhuix J, Gruez A, et al. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. 2003;278:46974–46982. doi: 10.1074/jbc.M307764200. [DOI] [PubMed] [Google Scholar]
- 127.Kishore U, Ghai R, Greenhough TJ, et al. Structural and functional anatomy of the globular domain of complement protein C1q. Immunol Lett. 2004;95:113–128. doi: 10.1016/j.imlet.2004.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roumenina LT, Ruseva MM, Zlatarova A, et al. Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: Mutational studies using recombinant globular head regions of human C1q A, B and C chains. Biochemistry. 2006;45:4093–104. doi: 10.1021/bi052646f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Evans TC, Jr, Nelsestuen GL. Dissociation of serum amyloid P from C4b-binding protein and other sites by lactic acid: Potential role of lactic acid in the regulation of pentraxin function. Biochemistry. 1995;34:10440–10447. doi: 10.1021/bi00033a016. [DOI] [PubMed] [Google Scholar]
- 130.Hicks PS, Saunero-Nava L, Du Clos TW, et al. Serum amyloid P component binds to histones and activates the classical complement pathway. J Immunol. 1992;149:3689–3694. [PubMed] [Google Scholar]
- 131.Ying SC, Gewurz AT, Jiang H, et al. Human serum amyloid P component oligomers bind and activate the classical complement pathway via residues 14–26 and 76–92 of the A chain collagen-like region of C1q. J Immunol. 1993;150:169–176. [PubMed] [Google Scholar]
- 132.Gershov D, Kim S, Brot N, et al. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: Implications for systemic autoimmunity. J Exp Med. 2000;192:1353–1363. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jarva H, Jokiranta TS, Hellwage J, et al. Regulation of complement activation by C-reactive protein: Targeting the complement inhibitory activity of factor H by an interaction with short consensus repeat domains 7 and 8–11. J Immunol. 1999;163:3957–3962. [PubMed] [Google Scholar]
- 134.Mold C, Gewurz H, Du Clos TW. Regulation of complement activation by C-reactive protein. Immunopharmacology. 1999;42:23–30. doi: 10.1016/s0162-3109(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 135.Giannakis E, Male DA, Ormsby RJ, et al. Multiple ligand binding sites on domain seven of human complement factor H. Int Immunopharmacol. 2001;1:433–443. doi: 10.1016/s1567-5769(00)00040-0. [DOI] [PubMed] [Google Scholar]
- 136.Suresh MV, Singh SK, Ferguson DA, Jr, et al. Role of the property of C-reactive protein to activate the classical pathway of complement in protecting mice from Streptococcus pneumoniae infection. J Immunol. 2006;176:4369–74. doi: 10.4049/jimmunol.176.7.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Müller H, Fehr J. Binding of C-reactive protein to human polymorphonuclear leukocytes: Evidence for association of binding sites with Fc receptors. J Immunol. 1986;136:2202–2207. [PubMed] [Google Scholar]
- 138.Marnell LL, Mold C, Volzer MA, et al. C-reactive protein binds to FcγRI in transfected COS cells. J Immunol. 1995;155:2185–2193. [PubMed] [Google Scholar]
- 139.Bharadwaj D, Stein MP, Volzer M, et al. The major receptor for C-reactive protein on leukocytes is Fcγ receptor II. J Exp Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chi M, Tridandapani S, Zhong W, et al. C-reactive protein induces signaling through FcγRIIa on HL-60 granulocytes. J Immunol. 2002;168:1413–1418. doi: 10.4049/jimmunol.168.3.1413. [DOI] [PubMed] [Google Scholar]
- 141.Bodman-Smith KB, Melendex AJ, Campbell I, et al. C-reactive protein-mediated phagocytosis and phospholipase D signalling through the high-affinity receptor for immunoglobulin G (FcγRI) Immunology. 2002;107:252–260. doi: 10.1046/j.1365-2567.2002.01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manolov DE, Rocker C, Hombach V, et al. Ultrasensitive confocal fluorescence microscopy of C-reactive protein interacting with FcγRIIa. Arterioscler Thromb Vasc Biol. 2004;24:2372–2377. doi: 10.1161/01.ATV.0000147407.17137.02. [DOI] [PubMed] [Google Scholar]
- 143.Heuertz RM, Schneider GP, Potempa LA, et al. Native and modified C-reactive protein bind different receptors on human neutrophils. Int J Biochem Cell Biol. 2005;37:320–335. doi: 10.1016/j.biocel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 144.Bang R, Marnell L, Mold C, et al. Analysis of binding sites in human C-reactive protein for FcγRI, FcγRIIa, and C1q by site-directed mutagenesis. J Biol Chem. 2005;280:25095–25102. doi: 10.1074/jbc.M504782200. [DOI] [PubMed] [Google Scholar]
- 145.Zeller JM, Landay AL, Lint TF, et al. Enhancement of human peripheral blood monocyte respiratory burst activity by aggregated C-reactive protein. J Leukoc Biol. 1986;40:769–783. doi: 10.1002/jlb.40.6.769. [DOI] [PubMed] [Google Scholar]
- 146.Tebo JM, Mortensen RF. Internalization and degradation of receptor bound C-reactive protein by U-937 cells: Induction of H2O2 production and tumoricidal activity. Biochim Biophys Acta. 1991;1095:210–216. doi: 10.1016/0167-4889(91)90101-3. [DOI] [PubMed] [Google Scholar]
- 147.Mold C, Rodic-Polic B, DuClos TW. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fcγ receptors. J Immunol. 2002;168:6375–6381. doi: 10.4049/jimmunol.168.12.6375. [DOI] [PubMed] [Google Scholar]
- 148.Mold C, Rodriguez W, Rodic-Polic B, et al. C-reactive protein mediates protection from lipopolysaccharide through interactions with FcγR. J Immunol. 2002;169:7019–7025. doi: 10.4049/jimmunol.169.12.7019. [DOI] [PubMed] [Google Scholar]
- 149.Barna BP, Thomassen MJ, Wiedemann HP, et al. Modulation of human alveolar macrophage tumoricidal activity by C-reactive protein. J Biol Response Mod. 1988;7:483–487. [PubMed] [Google Scholar]
- 150.Mortensen RF, Duszkiewicz JA. Mediation of CRP-dependent phagocytosis through mouse macrophage Fc-receptors. J Immunol. 1977;119:1611–1616. [PubMed] [Google Scholar]
- 151.Zahedi K, Tebo JM, Siripont J, et al. Binding of human C-reactive protein to mouse macrophages is mediated by distinct receptors. J Immunol. 1989;142:2384–2392. [PubMed] [Google Scholar]
- 152.Stein MP, Mold C, DuClos TW. C-reactive protein binding to murine leukocytes requires Fcγreceptors. J Immunol. 2000;164:1514–1520. doi: 10.4049/jimmunol.164.3.1514. [DOI] [PubMed] [Google Scholar]
- 153.Bharadwaj D, Mold C, Markham E, et al. Serum amyloid P component binds to Fc gamma receptors and opsonizes particles for phagocytosis. J Immunol. 2001;166:6735–6741. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- 154.Mold C, Gresham HD, DuClos TW. Serum amyloid P component and C-reactive protein mediate phagocytosis through murine FcγRs. J Immunol. 2001;166:1200–1205. doi: 10.4049/jimmunol.166.2.1200. [DOI] [PubMed] [Google Scholar]
- 155.Hamazaki H. Ca2+-dependent binding of human serum amyloid P component to Alzheimer’s β-amyloid peptide. J Biol Chem. 1995;270:10392–10394. doi: 10.1074/jbc.270.18.10392. [DOI] [PubMed] [Google Scholar]
- 156.Janciauskiene S, García De Frutos P, Carlemalm E, et al. Inhibition of Alzheimer β-peptide fibril formation by serum amyloid P component. J Biol Chem. 1995;270:26041–26044. doi: 10.1074/jbc.270.44.26041. [DOI] [PubMed] [Google Scholar]
- 157.Urbányi Z, Lakics V, Erdö SL. Serum amyloid P component-induced cell death in primary cultures of rat cerebral cortex. Eur J Pharmacol. 1994;270:375–378. doi: 10.1016/0926-6917(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 158.Togashi S, Lim SK, Kawano H, et al. Serum amyloid P component enhances induction of murine amyloidosis. Lab Invest. 1997;77:525–531. [PubMed] [Google Scholar]
- 159.Botto M, Hawkins PM, Bickerstaff MCM, et al. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nature Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 160.Tennent GA, Lovat LB, Pepys MB. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis. Proc Natl Acad Sci USA. 1995;92:4299–4303. doi: 10.1073/pnas.92.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Breviario F, d’Aniello EM, Golay J, et al. Interleukin-1-inducible genes in endothelial cells: Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 162.Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804–1812. [PubMed] [Google Scholar]
- 163.Bottazzi B, Garlanda C, Salvatori G, et al. Pentraxins as a key component of innate immunity. Curr Opin Immunol. 2006;18:10–15. doi: 10.1016/j.coi.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 164.Bottazzi B, Vouret-Craviari V, Bastone A, et al. Multimer formation and ligand recognition by the long pentraxin PTX3: Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817–32823. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 165.Nauta AJ, Bottazzi B, Mantovani A, et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33:465–473. doi: 10.1002/immu.200310022. [DOI] [PubMed] [Google Scholar]
- 166.Rusnati M, Camozzi M, Moroni E, et al. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood. 2004;104:92–99. doi: 10.1182/blood-2003-10-3433. [DOI] [PubMed] [Google Scholar]
- 167.Salustri A, Garlanda C, Hirsch E, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 168.Garlanda C, Hirsch E, Bozza S, et al. Nonredundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 169.Jeannin P, Bottazzi B, Sironi M, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 170.Diniz SN, Nomizo R, Cisalpino PS, et al. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J Leukoc Biol. 2004;75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 171.Dias AA, Goodman AR, Dos Santos JL, et al. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol. 2001;69:928–936. [PubMed] [Google Scholar]
- 172.Souza DG, Soares AC, Pinho V, et al. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. 2002;160:1755–1765. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Varani S, Elvin JA, Yan C, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- 174.Introna M, Alles VV, Castellano M, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–1872. [PubMed] [Google Scholar]
- 175.Vidal Alles V, Bottazzi B, Peri G, et al. Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes. Blood. 1994;84:3483–3493. [PubMed] [Google Scholar]
- 176.Polentarutti N, Bottazzi B, Di Santo E, et al. Inducible expression of the long pentraxin PTX3 in the central nervous system. J Neuroimmunol. 2000;106:87–94. doi: 10.1016/s0165-5728(00)00214-9. [DOI] [PubMed] [Google Scholar]
- 177.Goodman AR, Levy DE, Reis LF, et al. Differential regulation of TSG-14 expression in murine fibroblasts and peritoneal macrophages. J Leukoc Biol. 2000;67:387–395. doi: 10.1002/jlb.67.3.387. [DOI] [PubMed] [Google Scholar]
- 178.Klouche M, Peri G, Knabbe C, et al. Modified atherogenic lipoproteins induce expression of pentraxin-3 by human vascular smooth muscle cells. Atherosclerosis. 2004;175:221–228. doi: 10.1016/j.atherosclerosis.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 179.Abderrahim-Ferkoune A, Bezy O, Chiellini C, et al. Characterization of the long pentraxin PTX3 as a TNFα-induced secreted protein of adipose cells. J Lipid Res. 2003;44:994–1000. doi: 10.1194/jlr.M200382-JLR200. [DOI] [PubMed] [Google Scholar]
- 180.Luchetti MM, Piccinini G, Mantovani A, et al. Expression and production of the long pentraxin PTX3 in rheumatoid arthritis. Clin Exp Immunol. 2000;119:196–202. doi: 10.1046/j.1365-2249.2000.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nauta AJ, de Haij S, Bottazzi B, et al. Human renal epithelial cells produce the long pentraxin PTX3. Kidney Int. 2005;67:543–553. doi: 10.1111/j.1523-1755.2005.67111.x. [DOI] [PubMed] [Google Scholar]
- 182.Dos Santos CC, Han B, Andrade CF, et al. DNA microarray analysis of gene expression in alveolar epithelial cells in response to TNFα, LPS, and cyclic stretch. Physiol Genomics. 2004;19:331–342. doi: 10.1152/physiolgenomics.00153.2004. [DOI] [PubMed] [Google Scholar]
- 183.Doni A, Peri G, Chieppa M, et al. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells. Eur J Immunol. 2003;33:2886–2893. doi: 10.1002/eji.200324390. [DOI] [PubMed] [Google Scholar]
- 184.Baruah P, Propato A, Dumitriu IE, et al. The pattern recognition receptor PTX3 is recruited at the synapse between dying and dendritic cells and edits the cross-presentation of self, viral and tumor antigens. Blood. 2006;107:151–8. doi: 10.1182/blood-2005-03-1112. [DOI] [PubMed] [Google Scholar]
- 185.Polentarutti N, Picardi G, Basile A, et al. Interferon-γ inhibits expression of the long pentraxin PTX3 in human monocytes. Eur J Immunol. 1998;28:496–501. doi: 10.1002/(SICI)1521-4141(199802)28:02<496::AID-IMMU496>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 186.Doni A, Mosca M, Bottazzi B, et al. Regulation of PTX3, a key component of the humoral innate immunity, in human dendritic cells: Stimulation by IL-10 and inhibition by IFNγ. J Leukoc Biol. 2006;79:797–802. doi: 10.1189/jlb.0905493. [DOI] [PubMed] [Google Scholar]
- 187.Zhang X, Jafari N, Barnes RB, et al. Studies of gene expression in human cumulus cells indicate pentraxin 3 as a possible marker for oocyte quality. Fertil Steril. 2005;83:1169–1179. doi: 10.1016/j.fertnstert.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 188.Paffoni A, Ragni G, Doni A, et al. Follicular fluid levels of the long pentraxin PTX3. J Soc Gynecol Invest. 2006;13:226–31. doi: 10.1016/j.jsgi.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 189.Cetini I, Cozzi V, Pasqualini F, et al. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:1347–53. doi: 10.1016/j.ajog.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 190.Gaziano R, Bozza S, Bellocchio S, et al. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother. 2004;48:4414–4421. doi: 10.1128/AAC.48.11.4414-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ravizza T, Moneta D, Bottazzi B, et al. Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: Evidence for a protective role in seizure-induced neurodegeneration. Neuroscience. 2001;105:43–53. doi: 10.1016/s0306-4522(01)00177-4. [DOI] [PubMed] [Google Scholar]
- 192.Familian A, Zwart B, Huisman HG, et al. Chromatin-independent binding of serum amyloid P component to apoptotic cells. J Immunol. 2001;167:647–654. doi: 10.4049/jimmunol.167.2.647. [DOI] [PubMed] [Google Scholar]
- 193.Rovere P, Peri G, Fazzini F, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96:4300–4306. [PubMed] [Google Scholar]
- 194.Rolph MS, Zimmer S, Bottazzi B, et al. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–e14. doi: 10.1161/01.atv.0000015595.95497.2f. [DOI] [PubMed] [Google Scholar]
- 195.Napoleone E, Di Santo A, Bastone A, et al. Long pentraxin PTX3 up-regulates tissue factor expression in human endothelial cells: A novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol. 2002;22:782–787. doi: 10.1161/01.atv.0000012282.39306.64. [DOI] [PubMed] [Google Scholar]
- 196.Napoleone E, Di Santo A, Peri G, et al. The long pentraxin PTX3 up-regulates tissue factor in activated monocytes: Another link between inflammation and clotting activation. J Leukoc Biol. 2004;76:203–209. doi: 10.1189/jlb.1003528. [DOI] [PubMed] [Google Scholar]
- 197.Peri G, Introna M, Corradi D, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 198.Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 199.Muller B, Peri G, Doni A, et al. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–1407. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 200.Mairuhu AT, Peri G, Setiati TE, et al. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol. 2005;76:547–552. doi: 10.1002/jmv.20397. [DOI] [PubMed] [Google Scholar]
- 201.Azzurri A, Sow OY, Amedei A, et al. IFN-γ-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 202.Fazzini F, Peri G, Doni A, et al. PTX3 in small-vessel vasculitides: An independent indicator of disease activity produced at sites of inflammation. Arthritis Rheum. 2001;44:2841–2850. doi: 10.1002/1529-0131(200112)44:12<2841::aid-art472>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 203.Pascual V, Allantaz F, Arce E, et al. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]