Abstract
Development of the metanephric kidney in mammals requires complex reciprocal tissue interactions between the ureteric epithelium and the mesenchyme. It is believed that Gdnf, produced in the metanephric mesenchyme, activates Ret signaling in the Wolffian duct to initiate the formation of the metanephros. However, the molecular mechanism for induction of Gdnf in the metanephric mesenchyme is not completely defined. Previous studies demonstrated that during the early stages of kidney development, loss of Osr1, Eya1, Pax2 or Wt1 gene function in the metanephric mesenchyme compromises the formation of the kidney. Moreover, it has been shown that the Hox11-Eya1-Pax2 complex activates the expression of Six2 and Gdnf in the metanephric mesenchyme to drive nephrogenesis. Here, we demonstrate that the orphan nuclear receptor chicken ovalbumin upstream promoter transcription factor II (COUP-TFII, also known as Nr2f2) is required for the specification of the metanephric mesenchyme. Deletion of COUP-TFII at E7.5 results in improper differentiation of the metanephric mesenchyme and absence of essential developmental regulators, such as Eya1, Six2, Pax2 and Gdnf. Importantly, we show that COUP-TFII directly regulates the expression of both Eya1 and Wt1 in the metanephric mesenchyme. Our findings reveal, for the first time, that COUP-TFII plays a central role in the specification of metanephric fate and in the maintenance of metanephric mesenchyme proliferation and survival by acting as a crucial regulator of Eya1 and Wt1 expression.
Keywords: COUP-TFII, Metanephric mesenchyme, Eya1, Wt1, Mouse
INTRODUCTION
The mammalian kidney originates from the intermediate mesoderm and develops through three distinct stages: pronephros, mesonephros and metanephros. Organogenesis of the permanent kidney occurs through reciprocal interactions between the Wolffian (mesonephric) duct and the metanephric mesenchyme. At embryonic day (E) 10.5 in mouse, the metanephric mesenchyme secretes glial cell line-derived neurotrophic factor (Gdnf), which induces the Wolffian duct to invade the metanephric mesenchyme and induce adjacent mesenchymal cells to condense. These condensed metanephric mesenchyme cells then induce ureteric bud outgrowth and branching, leading to the formation of nephrons (Dressler, 2006; Saxén, 1987). Specifically, Gdnf promotes ureteric bud outgrowth by binding to its receptor tyrosine kinase (Ret) and co-receptor Gdnf family receptor alpha-1 (Gfrα1) on the surface of the ureteric bud (Durbec et al., 1996; Moore et al., 1996; Sainio et al., 1997; Vainio and Lin, 2002).
Although it is well established that the Gdnf-Ret signal transduction pathway initiates metanephric induction, the detailed mechanism of this signal pathway is still not well understood. During the metanephric mesenchyme condensation, many marker genes, including Osr1 (James et al., 2006), Pax2 (Torres et al., 1995), Eya1 (Sajithlal et al., 2005), Wt1 (Kreidberg et al., 1993), Six1 (Xu et al., 2003), Six2 (Self et al., 2006) and the Hox11 paralogous group (Hoxa11, Hoxc11 and Hoxd11) (Wellik et al., 2002), are expressed in the metanephric mesenchyme. Ablation of any of these genes in mice leads to renal agenesis, indicating that these genes are essential for the proper formation of the kidney. Osr1 and Eya1, the two earliest genes expressed in kidney precursor cells, sit atop the signal cascade and activate expression of other marker genes. Osr1 is expressed first. Mice lacking Osr1 do not form metanephric mesenchyme and do not express Eya1, Six2, Pax2, Sall1 and Gdnf. Eya1 is expressed downstream of Osr1 and is required for renal genesis (Xu et al., 1999). Eya proteins have no intrinsic DNA-binding domain but can localize to the nucleus and function as transcriptional co-activators (Ohto et al., 1999). Eya1 not only works with Six1 and Pax2 to regulate Gdnf expression, but also complexes with Hox11 paralogous proteins (including Hoxa11, Hoxc11 and Hoxd11) and Pax2. The Hox11-Eya1-Pax2 complex binds to the Six2 enhancer to induce the expression of Six2, which, in turn, mediates Six2 and Gdnf activation (Gong et al., 2007). Thus, Eya1 acts as a key regulator of Gdnf expression and, hence, determination of metanephric fate within the intermediate mesoderm (Sajithlal et al., 2005).
Chicken ovalbumin upstream promoter-transcription factor I and II (COUP-TFI and COUP-TFII; Nr2f1 and Nr2f2, respectively – Mouse Genome Informatics) are members of the nuclear receptor superfamily (Qiu et al., 1994). Although biochemical studies indicate that these two factors have similar DNA-binding and transcriptional activity in vitro, the patterns of COUP-TFI and COUP-TFII expression are distinct from each other. COUP-TFII is expressed in the mesenchyme of developing organs and is shown to play a key role in their organogenesis, cell fate determination, cell differentiation, angiogenesis and metabolic homeostasis (Kim et al., 2009; Kurihara et al., 2007; Li et al., 2009; Lin et al., 2010; Qin et al., 2010; Qin et al., 2008; Tang et al., 2010; Xie et al., 2011; You et al., 2005a; You et al., 2005b). During kidney development, COUP-TFII expression is detectable in the condensed mesenchyme and derivates, including pretubular aggregates, comma-shaped bodies and S-shaped bodies. Finally, in the adult mouse kidney, COUP-TFII is found in the distal tubules, podocytes and the epithelial cells of the Bowman’s capsule (Suh et al., 2006). These observations imply that COUP-TFII might play an important role in the genesis of the kidney and maintenance of its function. Here, we provide new insights into the role of COUP-TFII in the formation of the metanephric mesenchyme. Not only is COUP-TFII expressed in the undifferentiated kidney precursor cells, it is also indispensable for the establishment and maintenance of the metanephric mesenchyme. It acts as a crucial link in determination of metanephric fate through its recruitment to the promoter of the Eya1 gene and direct regulation of Eya1 transcription. In addition, COUP-TFII directly regulates the transcription of Wt1, an essential factor for maintaining metanephric mesenchyme cell survival (Davies et al., 2004; Kreidberg et al., 1993). Therefore, our studies clearly indicate that COUP-TFII is a key early regulator of the formation of metanephric mesenchyme and the subsequent formation and differentiation of the kidney.
MATERIALS AND METHODS
Animals
Generation of the floxed COUP-TFII mice and COUP-TFII-lacZ knock-in mice was as described previously (Takamoto et al., 2005). The Rosa26-Cre-ERT2/+ knock-in mouse strain was provided by T. Ludwig (Columbia University, New York City, USA) (de Luca et al., 2005). For inducible deletions during embryonic stages, 2 mg tamoxifen (Tam), dissolved in corn oil (Sigma-Aldrich; 10 mg/ml), was injected intraperitoneally into pregnant females at E7.5 or E8.5. Embryos from littermates were collected at indicated stages. All mouse strains were maintained in a mixed genetic background and received standard rodent chow.
X-gal staining
Whole-mount X-gal staining of embryos (up to E10.5) was performed according to published methods (Takamoto et al., 2005).
Histology, immunofluorescence and in situ hybridization
All the histology and immunofluorescence staining of paraffin-embedded slides were performed as described (You et al., 2005a). Antibodies used were: anti-COUP-TFII (R&D, 1:2000), anti-Pax2 (Covance, 1:500), anti-Ki67 (BD Biosciences, 1:400), anti-β-galactosidase (Biogenesis, 1:1500), anti-Wt1 (Santa Cruz, 1:500), anti-Gdnf (Santa Cruz, 1:200) and anti-Six2 (Proteintech group, 1:400). Non-radioactive in situ hybridization was carried out as described previously (Bramblett et al., 2004). The RNA probe for mouse Osr1 was provided by Thomas M. Schultheiss (Harvard University, Boston, USA) and the mouse Eya1 probe was as described (Xu et al., 1999). Cell apoptosis was detected by cleaved Caspase 3 (Cell Signaling, 1:500) staining or by TUNEL assay (Roche, In Situ Cell Death Detection Kit) according to the manufacturer’s instructions.
Kidney organ culture and staining
The metanephric mesenchymes containing the Wolffian duct from 10.5 days post-coitum (dpc) mouse embryos were cultured on transwell filters as described (Shakya et al., 2005). The Wolffian duct and ureteric bud were visualized by staining with anti-E-Cadherin (BD Biosciences, 1:200).
Cell lines and transfection
Human embryonic kidney 293 (HEK 293) cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. The rat inducible metanephric mesenchyme (RIMM-18) cell line was provided by Alan O. Perantoni (National Institutes of Health, NCI, Bethesda, MD, USA) and the culture conditions were as described (Levashova et al., 2003).
RNA interference and quantitative real-time RT-PCR assay
The siRNA oligonucleotides were purchased from Thermo Fisher Scientific or Applied Biosystems/Ambion. The target sequences for rat siRNA are described in supplementary material Table S1. RNA interference experiments were carried out as described (Lin et al., 2010). The primer sequences are listed in supplementary material Table S2. Means for mRNA levels in control and knockdown cells were compared using Student’s t-test.
Chromatin immunoprecipitation (ChIP) and PCR
ChIP assays were carried out with an EZ ChIP Chromatin Immunoprecipitation Kit (Millipore) by following the manufacturer’s protocol. Primers are shown in supplementary material Table S3.
Luciferase assays
The human EYA1 and WT1 promoter regions, which contained the putative Sp1-binding sites, were amplified from human BAC clone RP11-160C13 (Children’s Hospital Oakland Research Institute, CA, USA) and CTD-2083A15 (Invitrogen) separately. The PCR primers for amplifying the promoter region or for generating the Sp1-binding site mutations are as shown in supplementary material Table S4. The amplified promoter fragments were cloned into the pGL2-basic-luc vector (Promega). HEK 293 cells were transfected with pGL2-Eya1-basic-luc or pGL2-Wt1-luc using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Statistics
Statistical analysis was carried out by Student’s t-test. P values of less than 0.05 were considered significant.
RESULTS
COUP-TFII is expressed specifically in kidney precursor cells
We showed previously that COUP-TFII is expressed in the metanephric blastema at E11.5 and later in the developing nephron, nephrogenic cortex and stromal cells. In the adult kidney, high COUP-TFII expression is also detectable in the distal tubules, podocytes and epithelial cells of Bowman’s capsule (Suh et al., 2006). Nonetheless, the expression pattern of COUP-TFII in kidney precursor cells has not been determined. Here, we employed a COUP-TFII-specific antibody to investigate its expression pattern in the nephrogenic mesoderm from E9.5 to E10 (Fig. 1A-C). At E9.5, COUP-TFII staining was observed in the intermediate mesoderm and other mesenchyme cells surrounding the Wolffian duct (Fig. 1A). Using Pax2 to mark the Wolffian duct, we demonstrated that COUP-TFII is only expressed in the intermediate mesoderm and not in the duct (Fig. 1B). Half a day later, at E10, COUP-TFII expression was observed in the nephrogenic mesenchyme region surrounding the Wolffian duct and in the urogenital ridge (Fig. 1C). These results indicate that COUP-TFII is expressed in kidney precursor cells.
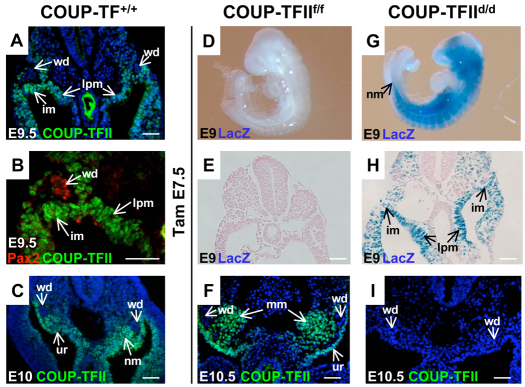
Fig. 1.
Expression pattern and conditional deletion of COUP-TFII in kidney precursor cells. (A) Transverse section of wild-type E9.5 mouse embryo shows that COUP-TFII is expressed in the intermediate mesoderm (im), lateral plate mesoderm (lpm) and mesenchyme cells surrounding the Wolffian duct (wd). (B) High magnification view indicates that COUP-TFII is not expressed in the Wolffian ducts, which are stained positively with Pax2. (C) Transverse section of an E10 embryo shows that COUP-TFII is expressed in the nephrogenic mesenchyme (nm) and urogenital ridge (ur), but not in the Wolffian duct (wd). (D-I) Tamoxifen was administered at E7.5 to induce COUP-TFII deletion and analyzed at E9. The whole-mount X-gal staining indicates that COUP-TFII is deleted throughout the whole mutant embryo, including the nephrogenic mesenchyme (nm) (G). The transverse section also shows deletion of COUP-TFII in the intermediate mesoderm and lateral plate mesoderm of the mutant embryo (E,H). At E10.5, COUP-TFII expression is detected in the urogenital ridge and metanephric mesenchyme (mm) (F). COUP-TFII expression is totally lost in the mutant embryo three days after Tam administration (I). DAPI counterstain for nuclei (blue) was applied for A, C, F and I. Scale bars: 50 μm.
Inducible deletion of COUP-TFII in kidney precursor cells
In order to bypass early embryonic lethality of COUP-TFII homozygous mutant at E10.5, we conditionally inactivated COUP-TFII by crossing the COUP-TFII floxed mouse strain (COUP-TFIIf/f) (Takamoto et al., 2005) with Rosa26-Cre-ERT2/+, a strain that harbors a tamoxifen-inducible Cre recombinase under the control of the ubiquitously active ROSA26 promoter (de Luca et al., 2005). Rosa26-Cre-ERT2/+;COUP-TFIIf/f males (hereafter referred to as COUP-TFIId/d) were intercrossed with COUP-TFIIf/f females, and a single dosage of 2 mg Tam was injected intraperitoneally into pregnant dams to induce COUP-TFII deletion. Specifically, we performed Tam injection at E7.5. In our mouse model, a lacZ gene inserted into the COUP-TFII locus is turned on when the COUP-TFII gene is deleted. One and half a days after Tam treatment, COUP-TFII was deleted throughout the whole body of COUP-TFIId/d mutant embryos, including the nephrogenic mesenchyme (Fig. 1D,G). Further examination of X-gal staining of transverse sections at the nephrogenic mesenchyme indicated that deletion of COUP-TFII occurred in the intermediate mesoderm and lateral plate mesoderm of COUP-TFIId/d mutant embryos (Fig. 1E,H). Using the COUP-TFII-specific antibody, we showed that COUP-TFII is highly expressed in the urogenital ridge and the developing condensed nephrogenic mesenchyme, but not in the Wolffian duct of the COUP-TFIIf/f control at E10.5 (Fig. 1F). In COUP-TFII mutants, expression was undetectable (Fig. 1I). These data indicate the Tam-inducible deletion system effectively deletes COUP-TFII in kidney progenitor cells. Unfortunately, deletion of COUP-TFII at E7.5 led to embryonic lethality at ∼E11, which limits our study to embryos before E11 (data not shown).
COUP-TFII is essential for metanephric mesenchyme formation but not for the formation of mesonephros
During the formation of the metanephros, the metanephric mesenchyme appears morphologically as an aggregate of the nephrogenic mesenchyme cells at the caudal end of the nephrogenic cord at E10.5 (Fig. 2A,C). This structure is largely missing in COUP-TFIId/d mutant embryos (Fig. 2B,D). In addition, serial sections through the entire metanephric mesenchyme region were examined with littermates of the same somite number. The results clearly show that the metanephric mesenchyme is not formed in the COUP-TFIId/d mutant (supplementary material Fig. S1). As the paired box-containing transcription factor Pax2 is a key regulator of kidney development (Torres et al., 1995), we investigated whether the expression of this gene is compromised. In the mouse kidney, the expression of Pax2 is initiated in the intermediate mesoderm and maintained throughout the development of the pronephros and mesonephros (Bouchard et al., 2000). Pax2 is expressed in the induced metanephric mesenchyme and in the Wolffian duct of the metanephros in the controls (Fig. 2E). By contrast, Pax2 expression is totally lost in the nephrogenic mesenchyme area, whereas it is intact in the Wolffian duct of the COUP-TFIId/d mutants (Fig. 2F). lacZ-positive cells, which denote COUP-TFII ‘expressing cells’ in the COUP-TFIId/d mutants, indicated that some ‘COUP-TFII expressing’ metanephric mesenchyme cells surrounding the Wolffian duct are still present (Fig. 2F).
Fig. 2.
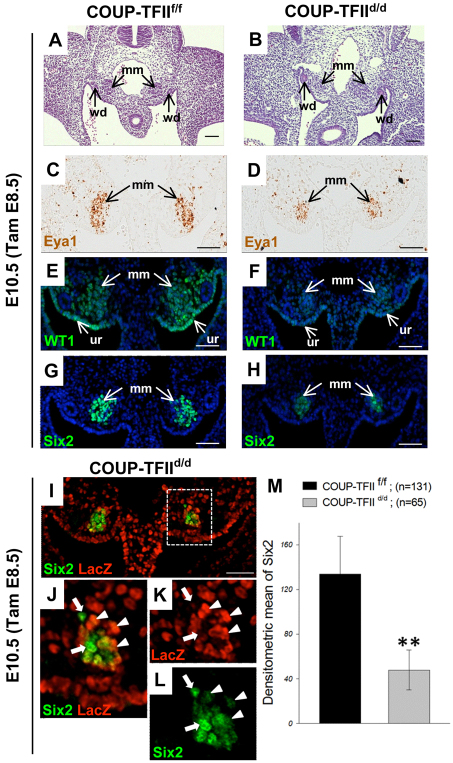
COUP-TFII is essential for the metanephric mesenchyme formation. Tamoxifen was administered at E7.5 to delete COUP-TFII. (A-D) Transverse sections of metanephric mesenchyme (mm) from these embryos were stained with hematoxylin and eosin at E10.5. The Wolffian duct (wd) and condensed metanephric mesenchyme (mm) are seen in the COUP-TFIIf/f control (A,C). The metanephric mesenchyme is completely absent in the COUP-TFIId/d mutant embryo (B,D). (E-J) At E10.5, the metanephric mesenchyme of COUP-TFIIf/f control embryos expresses Pax2, Six2 and Gdnf (E,G,I). By contrast, these three gene products are undetectable in the metanephric mesenchyme region of COUP-TFIId/d null mutants (F,H,J,). However, the Pax2 expression in the Wolffian duct remains (F). lacZ staining indicates that COUP-TFII is deleted in the mesenchyme cells (F). Boxed areas in I and J are shown in insets. (K,L) Kidney organ cultures from the metanephric mesenchyme of COUP-TFIIf/f control (K) and COUP-TFIId/d mutant (L) embryos indicated that the ureteric bud (ub) outgrowth, visualized by E-cadherin staining, was detected from the Wolffian duct of the COUP-TFIIf/f control (K), but not from the COUP-TFIId/d mutant embryo (L). DAPI counterstain for nuclei (blue) was applied for E-J. Scale bars: 50 μm.
In addition to Pax2, the homeobox gene Six2 is also specifically expressed in the metanephric mesenchyme before ureteric bud outgrowth (Xu et al., 1999) and is required to activate Gdnf expression by directly binding to its promoter during kidney development (Brodbeck et al., 2004). Therefore, we examined Six2 expression in COUP-TFII mutants. As indicated in Fig. 2G,H, Six2 expression is not detectable in the mutant metanephric mesenchyme. As both Pax2 and Six2 lie upstream of Gdnf, which is essential for promoting the ureteric bud outgrowth (Costantini and Shakya, 2006), we investigated whether Gdnf expression in the nephrogenic mesenchyme cells is also compromised in the mutants. Gdnf is detectable in the cytoplasm of the metanephric mesenchyme in control animals (Fig. 2I), but is not detected in the COUP-TFIId/d mutants (Fig. 2J). Together, these results strongly implied that COUP-TFII is likely to regulate the expression of genes essential for metanephric mesenchyme formation and kidney development.
In order to bypass the lethality of COUP-TFII mutant embryo at E11, we injected Tam at E7.5 to delete COUP-TFII and collected the embryos at E10.5. We then dissected the metanephric mesenchyme tissue with the Wolffian duct and cultured it for 36 hours. The results obtained from organ cultured of three litters of mice indicate that the ureteric bud was induced to outgrow from the Wolffian duct and started to branch from all ten COUP-TFIIf/f controls (Fig. 2K). By contrast, we found that majority of COUP-TFIId/d mutants (six out of eight) did not display any ureteric bud outgrowth (Fig. 2L). Therefore, the inability of the mutant metanephric mesenchyme to induce ureteric bud outgrowth and branching suggests that the mature kidney will not be able to form in the COUP-TFII mutants at later stages.
During this stage, the early mesonephros structure also formed. The mesonephros develops when the Wolffian duct reaches the prospective mesonephric mesenchyme and induces adjacent mesenchymal cells to condense and form mesonephric tubules (Saxén, 1987). First, we investigated whether COUP-TFII is also expressed in the mesonephros (also called the mesonephric tubule). COUP-TFII staining in E10 embryos indicated that COUP-TFII is expressed in the mesonephros but not in the Wolffian duct (supplementary material Fig. S2A). Pax2 is expressed in the Wolffian duct and mesonephros. By using Pax2 as a mesonephric tubule marker, we found COUP-TFII colocalizes with Pax2 in the mesonephros but not in the Wolffian duct (supplementary material Fig. S2B). We examined the E9.5 embryos and found that the mesonephros is still intact in the COUP-TFII–/– mutants in which COUP-TFII is deleted very early in the germ cells, compared with COUP-TFII+/+ controls (supplementary material Fig. S2C,D). Therefore, even though COUP-TFII is expressed in the mesonephros, it is not essential for mesonephros formation.
COUP-TFII specifically regulates Eya1, Wt1 and Six2 in the metanephric mesenchyme
Although the metanephric mesenchyme markers, such as Pax2 or Six2 expression, are not detected in the COUP-TFIId/d mutant embryos (Fig. 3F,H), the presence of lacZ-positive cells indicates that it is still possible that the lack of marker gene expression could be due to the loss of metanephric mesenchyme cells. To demonstrate that COUP-TFII does indeed regulate metanephric mesenchyme marker gene expression, we deleted COUP-TFII one day later by injecting Tam at E8.5 and collecting embryos at E10.5. We reasoned that by deleting COUP-TFII one day later, we would have metanephric mesenchyme formation, but in some cells COUP-TFII would be completely deleted, in some one allele would be deleted, and in some it would not be deleted at all. In this scenario, a decrease in overall expression of these marker genes per cell would be observed in the formed metanephric mesenchyme. In addition, those cells with COUP-TFII deletion would have lower levels of target gene expression and those cells with no COUP-TFII deletion would have higher levels of target gene expression. As expected, we found that the metanephric mesenchyme was formed in both COUP-TFIIf/f control (Fig. 3A) and COUP-TFIId/d mutant (Fig. 3B) embryos, even though the mutant metanephric mesenchyme is smaller and less condensed compared with the control. Using in situ hybridization to examine Eya1 expression, we found that both the number of cells expressing Eya1 and the expression level per cell are decreased in the COUP-TFIId/d mutant (Fig. 3C,D). Similarly, Wt1 and Six2 expression levels in the metanephric mesenchyme are significantly decreased in the COUP-TFIId/d mutant (Fig. 3F,H) compared with the controls (Fig. 3E,G). To define further whether COUP-TFII specifically regulated Six2 expression in the metanephric mesenchyme, we double labeled Six2 with lacZ and found that Six2 colocalizes with lacZ expression in the metanephric mesenchyme of COUP-TFIId/d mutant (Fig. 3I). Under high magnification, we examined the Six2-lacZ-positive cells (Fig. 3J) and found that those cells with a higher lacZ signal (i.e. in which COUP-TFII is deleted) have lower Six2 expression (Fig. 3K,L, arrowheads). By contrast, if lacZ expression is low (i.e. COUP-TFII is not deleted), Six2 expression is higher (Fig. 3K,L, arrows).
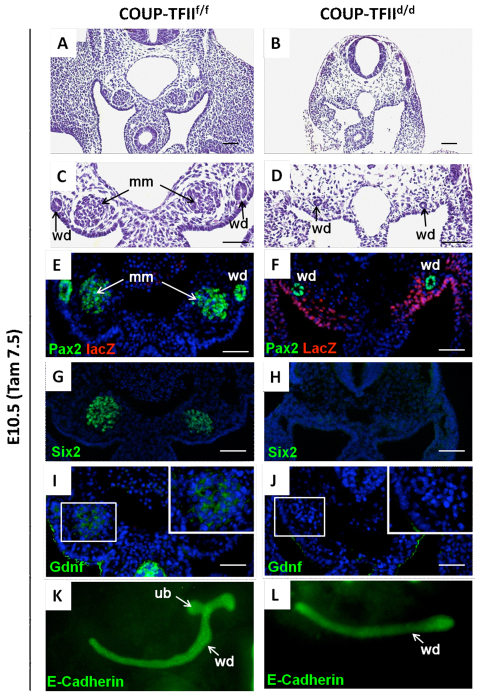
Fig. 3.
COUP-TFII specifically regulates the metanephric mesenchyme gene expression. Tamoxifen was administered at E8.5 to delete COUP-TFII. Transverse sections of the metanephric mesenchyme region from the embryos were stained with Hematoxylin and Eosin at E10.5. (A,B) The condensed metanephric mesenchyme (mm) was seen in both the COUP-TFIIf/f control (A) and the COUP-TFIId/d mutant (B) embryos. (C-H) At E10.5, the metanephric mesenchyme of COUP-TFIId/d mutant embryos expresses Eya1 (D), Wt1 (F) and Six2 (H); however, the expression levels per cell of all of these three factors are lower than control (C,E,G). Eya1 expression level was measured by in situ hybridization (C,D). The DAPI counterstain for nuclei (blue) was applied in E-H. (I-L) lacZ staining indicates that COUP-TFII is deleted in the mesenchyme cells and is specifically colocalized with Six2 in that area of COUP-TFIId/d mutant (I,J). J shows a high magnification of the dashed box in I. Arrows indicate that cells that express higher Six2 levels have lower lacZ expression (L) and arrowheads indicate that cells that express higher lacZ have lower Six2 expression (K). (M) The Six2 expression level of Six2-positive cells was measured by the fluorescence density divided by area (densitometric mean) from COUP-TFIIf/f control and COUP-TFIId/d mutant embryos. Four metanephric mesenchyme samples were measured and the results indicate that COUP-TFII deletion in the metanephric mesenchyme significantly reduces Six2 expression. **P<0.005. ur, urogenital ridge; wd, Wolffian duct. Scale bars: 50 μm.
In order to quantify the results, we measured the fluorescence intensity of Six2-positive cells (metanephric mesenchyme cells) in the COUP-TFIIf/f controls (n=131 cells) and COUP-TFIId/d mutants (n=65 cells) shown in Fig. 3G,H and divided by the cell area to obtain densitometric levels of Six2 expression. The result shows that deletion of COUP-TFII significantly decreases Six2 expression in the COUP-TFIId/d mutant (Fig. 3M). These data clearly indicate that COUP-TFII does indeed regulate the expression of the metanephric mesenchyme markers Six2, Eya1 and Wt1.
Deletion of COUP-TFII abolishes Wt1 expression and increases metanephric mesenchyme cell apoptosis
We observed a reduction in cell number (Fig. 4A,B) in the nephrogenic mesenchyme region of the COUP-TFIId/d mutant embryos compared with controls. We investigated whether the loss of mesenchymal cells in this region is due to decreased cell proliferation or increased apoptosis. Embryo-wide comparison of Ki67 staining revealed no obvious differences in overall proliferation in controls versus COUP-TFIId/d mutants (Fig. 4A,B). By contrast, the COUP-TFIId/d mutant metanephric mesenchyme region showed a significant decrease in Ki67-positive cell numbers (Fig. 4C,D, red circled regions), indicating a specific decrease in proliferation of the metanephric mesenchyme. Furthermore, we observed an increase in the number of apoptotic cells in the metanephric mesenchyme of mutant mice as detected by both cleaved Caspase 3 and tunnel assays (Fig. 4E-H).
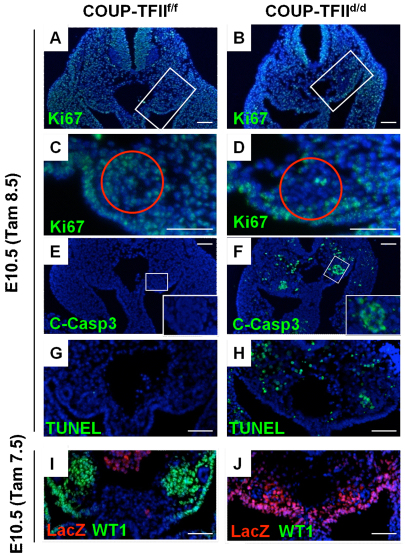
Fig. 4.
COUP-TFII deletion abolishes Wt1 expression and increases apoptosis of metanephric mesenchyme cells. (A-F) Tam was administered at E8.5 and embryos were examined at E10.5. Proliferation was determined by Ki67 immunostaining which appeared to show similar Ki67-positive cells for both mutant (B) and control (A). However, upon close examination, the metanephric mesenchyme (C,D red circles) had fewer Ki-67-positive cells in the mutant. Apoptosis analysis results indicated that there are many more cleaved Caspase 3 (C-Casp3)-positive cells located in the metanephric mesenchyme region of the COUP-TFIId/d mutant embryos compared with control embryo (E,F). (G-J) Tam was administered one day earlier at E7.5 and embryos were examined at E10.5. TUNEL assay shows a significantly increase in apoptosis in the metanephric mesenchyme region of the COUP-TFII knockout embryo (G,H). Wt1 is expressed in the urogenital ridge and metanephric mesenchyme in the COUP-TFIIf/f control embryo (I) and the expression is lost in the COUP-TFIId/d embryo (J). lacZ staining indicates that COUP-TFII-deleted cells are still present. The slides are counterstained with DAPI for nuclei (blue). Scale bar: 50 μm.
The Wilms tumor suppressor gene (Wt1) is expressed in the metanephric mesenchyme and has been shown to be essential for the survival of metanephric mesenchymal cells (Kreidberg et al., 1993; Kuure et al., 2000; Moore et al., 1999). Therefore, we investigated whether Wt1 expression is compromised in COUP-TFIId/d mutants. First, we determined whether COUP-TFII and Wt1 colocalize with each other. As shown in supplementary material Fig. S3A-C, we found that COUP-TFII colocalizes with Wt1 in the metanephric mesenchyme and in the urogenital ridge. Next, we investigated whether Wt1 expression level is altered with COUP-TFII deletion. Indeed, we found Wt1 expression to be significantly decreased in the COUP-TFII mutant urogenital ridge and metanephric mesenchyme (deletion cells shown as the lacZ positive cells) (Fig. 3E,F and Fig. 4I,J). These results indicate Wt1 lies downstream of COUP-TFII and is regulated by COUP-TFII. Owing to the importance of Wt1 in metanephric mesenchyme cell survival, our findings suggested that loss of Wt1 expression due to COUP-TFII deletion is the likely reason for the increased metanephric mesenchyme apoptosis in COUP-TFIId/d mutants.
COUP-TFs regulate the expression of Eya1, Six2, Wt1 and Gdnf in metanephric mesenchyme cells
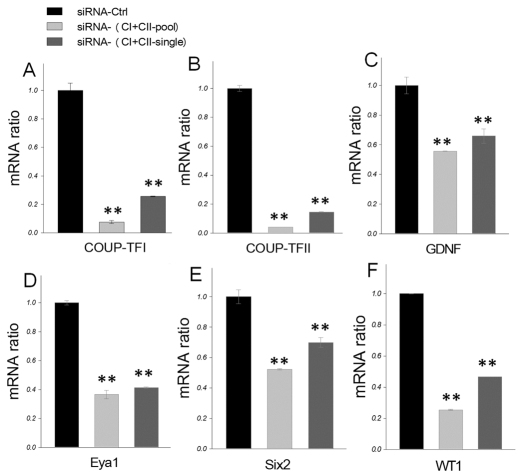
In order to study the detailed molecular mechanism by which COUP-TFII controls regulatory genes in the metanephric mesenchyme, we employed the conditionally immortalized rat inducible metanephric mesenchyme cell line (RIMM-18). RIMM-18 cells were generated from rat mesenchymal cells by transfection with a vector encoding an estradiol-dependent E1A-ER fusion protein and these cells maintain its metanephric mesenchyme characteristics (Levashova et al., 2003). Using RT-PCR, we found that the expression levels of both COUP-TFI and COUP-TFII are high in RIMM-18 cells. Despite this, in the E10.5 mouse embryos, only COUP-TFII is expressed in the metanephric mesenchyme, whereas COUP-TFI is located in the stroma mesenchyme. Our laboratory has reported that COUP-TFI and COUP-TFII are highly homologous and also functionally redundant (Tsai and Tsai, 1997; Wu et al., 2010). Therefore, during in vitro experiments, we used siRNAs to knock down both COUP-TFI and COUP-TFII to study the underlying mechanism of COUP-TF regulation of transcription factors important for kidney development. Knockdown of COUP-TFI and COUP-TFII significantly decreases Gdnf, Eya1, Six2 and Wt1 (Fig. 5C-F) expression. In order to confirm whether COUP-TFII alone can regulate these transcription factors in RIMM-18 cells, we also used siRNA to knock down COUP-TFII specifically. We found that knockdown of COUP-TFII alone is enough to significantly decrease Eya1 and Wt1 (supplementary material Fig. S4B,C) expression. These results indicate that COUP-TFs can indeed positively regulate genes expressed early in metanephric mesenchyme development.
Fig. 5.
COUP-TFs regulate Gdnf, Eya1, Six2 and Wt1 expression in the metanephric mesenchyme in vitro. (A-F) Quantitative RT-PCR analysis of COUP-TFI (A), COUP-TFII (B), Gdnf (C), Eya1 (D), Six2 (E) and Wt1 (F) in rat metanephric mesenchyme-derived cells (RIMM-18), after knockdown of both COUP-TFI and COUP-TFII using specific siRNAs. All mRNA measurements were normalized to 18S rRNA. Error bars indicate s.d. **P<0.005.
COUP-TFII signaling is independent of Osr1 in the nephrogenic mesenchyme
Similar to COUP-TFII, Odd-skipped related 1 (Osr1) is also required for the formation of the metanephric mesenchyme and is upstream of Eya1. In order to define the relationship between Osr1 and COUP-TFII in the regulatory cascade, we first investigated whether COUP-TFII is upstream of Osr1 by examining the expression of Osr1 in COUP-TFII mutants by in situ hybridization. Osr1 is broadly expressed in the nephrogenic mesenchyme regions in the control mice (supplementary material Fig. S5A). Despite the fact that the COUP-TFIId/d mutant has fewer mesenchyme cells, the Osr1 expression level in the mesenchyme is equivalent to that of the control (supplementary material Fig. S5B). Next, we examined COUP-TFII expression in Osr1 knockout mice to determine whether COUP-TFII is downstream of Osr1. The COUP-TFII expression level is similar in the urogenital ridge of Osr1 knockout and wild-type mice (supplementary material Fig. S5C-F). Although Osr1 knockouts lack the metanephric mesenchyme, COUP-TFII expression in the nephrogenic mesenchyme surrounding the Wolffian duct is similar compared with wild-type controls (supplementary material Fig. S5C-F). These results indicate that Osr1 and COUP-TFII act in parallel with one another and are both required for Eya1 activation to control metanephric mesenchyme differentiation and nephrogenesis. To support this conclusion further, we employed RIMM-18 cell culture experiments and found that simultaneous knockdown of COUP-TFI and COUP-TFII by siRNA does not affect expression of Osr1 (supplementary material Fig. S5G-I). Similarly, knockdown of Osr1 had no significant effect on COUP-TFI or COUP-TFII expression (supplementary material Fig. S5J-L). These results support the conclusion that COUP-TFII and Osr1 act in parallel to regulate Eya1 expression.
COUP-TFII directly regulates Eya1 gene expression by interacting with Sp1
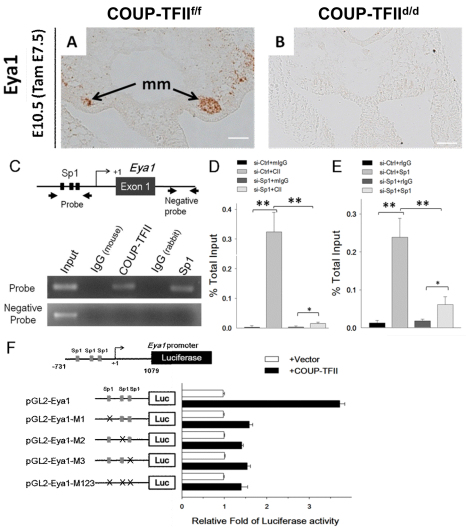
Eya1 has been shown to specify the metanephric mesenchyme fate and is indispensable for metanephric mesenchyme formation. In order to determine whether Eya1 expression is regulated by COUP-TFII at the transcriptional level, we carried out in situ hybridization to see whether Eya1 is regulated by COUP-TFII at the mRNA level. Indeed, Eya1 mRNA is detected in the metanephric mesenchyme of COUP-TFIIf/f controls but is absent in COUP-TFIId/d mutants (Fig. 6A,B). This indicates that Eya1 is a COUP-TFII target and is likely to be regulated by COUP-TFII at the transcriptional level.
Fig. 6.
COUP-TFII directly regulates Eya1 gene transcription by tethering to Sp1 at Sp1-binding sites. (A,B) Tam was administered at E7.5 and embryos were examined at E10.5 for Eya1 expression in the metanephric mesenchyme by in situ hybridization. Eya1 RNA is detected in the metanephric mesenchyme (mm) of the COUP-TFIIf/f control embryo (A), but is absent in the COUP-TFIId/d mutant embryo (B). Scale bars: 50 μm. (C) ChIP analysis showed that COUP-TFII and Sp1 are recruited to the conserved Sp1-binding sites of the Eya1 promoter in RIMM-18 cells (C). (D,E) RT-PCR analysis of ChIP assay of COUP-TFII (CII; D) and Sp1 binding (E) to the conserved Sp1-binding sites of the rat Eya1 promoter control siRNA- (siCtrl, 25 nM) or Sp1- (siSp1, 25 nM) specific siRNA-treated RIMM-18 cells. All values were normalized to the total input and error bars indicate s.d. *P<0.05, **P<0.005. (F) Relative luciferase activities of Eya1 promoter-driven reporter pGL2-Eya1 and the Sp1-binding site mutant reporters pGL2-Eya1-M1, pGL2-Eya1-M2, pGL2-Eya1-M3 and pGL2-Eya1-M123 (250 ng each) were measured after co-transfection with or without COUP-TFII expression plasmid (50 ng) into HEK 293 cells.
To support this conclusion further, we carried out chromatin immunoprecipitation (ChIP) assays to determine whether COUP-TFII is recruited to the promoter/enhancer region of the Eya1 gene locus. Our laboratories have previously demonstrated that COUP-TFII acts as a positive regulator, enhancing target gene expression by interacting with Sp1 at Sp1-binding sites (Kim et al., 2009; Lin et al., 2010; Pipaon et al., 1999; Qin et al., 2010). To assess whether COUP-TFII regulates Eya1 expression through its interaction with Sp1, we searched for the evolutionarily conserved Sp1-binding sites in human, mouse and rat sequences on and surrounding the Eya1 gene and found three conserved sites upstream of the transcription start site (Fig. 6C, black boxes). We then performed ChIP assays to evaluate whether endogenous COUP-TFII is recruited to the Sp1 sites of the Eya1 promoter. Indeed, we found COUP-TFII is preferentially recruited to the promoter regions containing Sp1-binding sites but not recruited to the region lacking Sp1-binding sites (Fig. 6C, Sp1). In parallel, Sp1 was also specifically recruited to the same region as COUP-TFII, but not to the region without Sp1-binding sites (Fig. 6C). To substantiate that COUP-TFII was recruited by Sp1 to the Eya1 promoter, we knocked down endogenous Sp1 expression with Sp1-specific siRNA (si-Sp1). Recruitment of COUP-TFII and Sp1 to the Eya1 promoter was, in fact, significantly reduced in Sp1-knockdown cells compared with controls (Fig. 6D,E).
To test further whether COUP-TFII binding to the Eya1 promoter leads to activation of transcription, we performed luciferase reporter assays in HEK293 cells using a 1.8-kb (–731 bp to 1079 bp) human EYA1 promoter fragment that included the three conserved Sp1-binding sites. Luciferase reporter activity was significantly increased when COUP-TFII was expressed compared with control cells (Fig. 5F). Next, we mutated these three conserved Sp1-binding sites as depicted in supplementary material Fig. S4A. We generated reporters with three single Sp1-binding sites or with all three sites mutated (supplementary material Fig. S6A; pGL2-Eya1-M1, pGL2-Eya1-M2, pGL2-M3 and the triple-mutation pGL2-Eya1-M123). In the presence of COUP-TFII expression plasmid, activation of all four mutant luciferase reporters was significantly diminished versus the intact reporter (Fig. 6F). This result indicates that COUP-TFII works through Sp1 on the Sp1-binding sites to activate Eya1 expression. Collectively, these results substantiate a model in which COUP-TFII is recruited to the Eya1 promoter in an Sp1-dependent manner to directly activate Eya1 transcription.
COUP-TFII interacts synergistically with Sp1 to regulate Wt1 expression directly
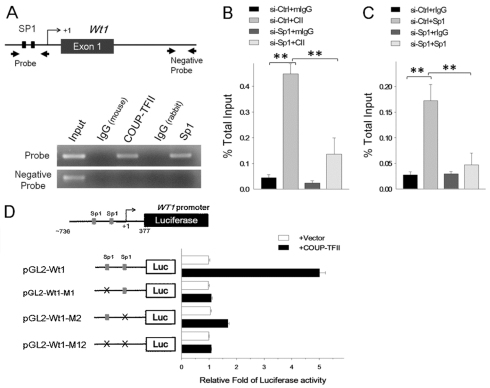
Next, we investigated whether COUP-TFII also directly regulates the transcription of Wt1 through its interactions with Sp1. We identified two evolutionarily conserved Sp1-binding sites surrounding the Wt1 promoter (Fig. 7A, black boxes). ChIP analysis showed that COUP-TFII is indeed preferentially recruited to Sp1-binding sites at the Wt1 promoter but is not recruited to the region lacking Sp1-binding sites (Fig. 7A). Sp1 was recruited to the same regions as COUP-TFII. To determine whether recruitment of COUP-TFII to the Wt1 promoter is Sp1-dependent, we knocked down endogenous Sp1 by siRNA. With knockdown, COUP-TFII recruitment to the Wt1 promoter was significantly reduced (Fig. 7B,C).
Fig. 7.
COUP-TFII synergistically interacts with Sp1 to regulate Wt1 expression. (A) ChIP analysis showed that COUP-TFII and Sp1 are recruited to the conserved Sp1-binding sites of the Wt1 promoter in RIMM-18 cells. (B,C) RT-PCR analysis of ChIP assay of COUP-TFII (CII; B) and Sp1 (C) binding to the conserved Sp1-binding sites of the rat Wt1 promoter in control siRNA- (si-Ctrl, 25 nM) or Sp1- (si-Sp1, 25 nM) specific siRNA-treated RIMM-18 cells. All values were normalized to the total input and error bars indicate s.d. **P<0.005. (D) Relative luciferase activity of wild-type promoter-driven reporter (pGL2-Wt1, 250 ng) and Sp1-binding site mutant reporters (pGL2-Wt1-M1, pGL2-Wt1-M2 and pGL2-Wt1-M12, 250 ng each) were measured after co-transfection with or without COUP-TFII expression plasmid (50 ng) in HEK 293 cells.
To confirm whether COUP-TFII binding to the Wt1 promoter leads to transcription activation, we performed luciferase reporter assays using a 1.1-kb (–736 bp to 377 bp) human WT1 promoter fragment that contains the two conserved Sp1-binding sites. As shown in Fig. 7D, Wt1-luciferase reporter (pGL2-Wt1) activity was significantly increased by COUP-TFII. In addition, when Sp1-binding sites were mutated in the Wt1-luciferase reporter (supplementary material Fig. S6B; pGL2-Wt1-M1, pGL2-Wt1-M2 and the double-mutation pGL2-Wt1-M12), COUP-TFII-dependent luciferase activity decreased dramatically (Fig. 7D). Taken together, these results strengthen our proposed model that recruitment of COUP-TFII to the Wt1 promoter is Sp1-dependent, and that COUP-TFII directly activates Wt1 transcription.
DISCUSSION
COUP-TFII directly regulates transcription of Eya1 to modulate Gdnf expression during metanephric induction
COUP-TFII is expressed in the mesenchyme of developing organs and has been shown to play a key role in their organogenesis. In this study, we found that COUP-TFII is expressed in the kidney precursor intermediate mesoderm and later expressed in the metanephric mesenchyme. Knockout of COUP-TFII at an early developmental stage (E7.5) results in the loss of metanephric mesenchyme in the mutant embryo at E10.5. In addition, ablation of COUP-TFII results in the loss of expression of many key factors, including Six2, Pax2, Wt1, Eya1 and Gdnf, which are essential for metanephric mesenchyme formation. These results place COUP-TFII as an upstream regulator of these important regulatory factors.
Our in situ hybridization data showed that Eya1 expression is significantly decreased in the COUP-TFII mutant (Fig. 3C,D), suggesting that COUP-TFII directly regulates Eya1 expression in the metanephric mesenchyme. Indeed, ChIP assays showed that COUP-TFII is recruited to the conserved Sp1-binding site of the Eya1 promoter by tethering to Sp1 for direct regulation of Eya1 expression. Furthermore, expression of COUP-TFII can enhance Eya1 promoter-driven reporter activity. Collectively, these results clearly indicate that COUP-TFII promotes Gdnf signaling cascade by direct regulation of Eya1 expression and, thus, induces metanephric mesenchyme fate in kidney progenitor cells. It should be noted that in Eya1 knockout mice, ureteric buds fail to grow out into the metanephric mesenchyme (Sajithlal et al., 2005). Similar to Eya1, we observed no ureteric bud outgrowth in six out of eight COUP-TFII mutants using the kidney organ cultures (Fig. 2L). It is not clear, however, whether the remaining two COUP-TFII mutants have ureteric bud outgrowth (supplementary material S7A,B, arrowheads). In any event, if they indeed have ureteric bud outgrowth, it is possible that COUP-TFII was not deleted early enough in these two particular mutants, resulting in partially committed metanephric mesenchyme and subsequent induction of ureteric bud outgrowth. As the majority of mutants do not have ureteric buds outgrowth, it indicates that COUP-TFII is required for the proper formation of metanephric mesenchyme and subsequent induction of ureteric bud outgrowth.
COUP-TFII acts in parallel with Osr1 to regulate Eya1 expression during metanephric induction
Osr1 is known to be the earliest marker for kidney development in the intermediate mesoderm. Mice lacking Osr1 do not form metanephric mesenchyme and do not express many factors essential for metanephric mesenchyme formation (James et al., 2006; Wang et al., 2005). These phenotypes bear strong resemblance to that of the COUP-TFII knockout mice. To address whether these two genes work in the same pathway, we examined whether Osr1 expression is affected in the COUP-TFII knockout. Our results clearly show that Osr1 expression in the nephrogenic mesenchyme region is not altered in the COUP-TFII mutant. Similarly, COUP-TFII expression in the metanephric mesenchyme region remains the same in the Osr1 knockout embryo. Together, these results indicate that COUP-TFII and Osr1 act independently to regulate kidney morphogenesis. This notion is further supported by in vitro cell culture experiments, in which we showed that COUP-TFII and Osr1 do not regulate each other’s expression. Interestingly, whereas Wt1 is expressed in the metanephric mesenchyme of Osr1 null mice (James et al., 2006), Wt1 expression in the COUP-TFII knockout mutant is totally lost. This result indicates that although both COUP-TFII and Osr1 are expressed in the intermediate mesoderm and both regulate Eya1, Pax2 and Six2 expression, they do not regulate each other to control the expression of those key factors important for metanephric mesenchyme formation. Based on all these results, our working model is that COUP-TFII and Osr1 act in parallel to regulate Eya1 and its downstream target Gdnf to specify metanephric mesenchyme (Fig. 8).
Fig. 8.
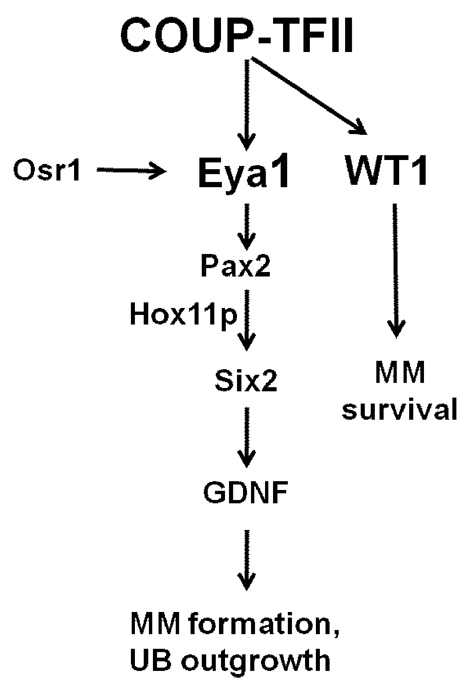
COUP-TFII directly regulates Eya1 and Wt1 expression to specify metanephric mesenchyme cell fate and maintain metanephric mesenchyme precursor cell survival. COUP-TFII acts in parallel with Osr1 to regulate Eya1 transcription. COUP-TFII in the intermediate mesoderm kidney precursor cells specifies metanephric mesenchyme fate by directly regulating Eya1 expression. Eya1 regulates Pax2 expression, forms a complex with Pax2 and Hox11 paralogous proteins (including Hoxa11, Hoxc11 and Hoxd11) and binds directly to the Six2 enhancer to regulate the expression of Six2 and its downstream target Gdnf. Gdnf, a crucial factor for the specification of the metanephric mesenchyme, will then induce ureteric bud outgrowth from the Wolffian duct and initiate kidney organogenesis. In addition, COUP-TFII is essential for the survival of the metanephric mesenchyme precursor cells through its direct regulation of Wt1 gene expression. Wt1 plays an anti-apoptotic role to maintain metanephric mesenchyme cell survival. MM, metanephric mesenchyme; UB, ureteric bud.
COUP-TFII is essential for survival of the metanephric mesenchyme and for nephron differentiation
Wt1 mutant mesenchyme cannot be induced to form nephric tubules (Kreidberg et al., 1993), suggesting that Wt1 is cell-autonomously required for nephron differentiation. Subsequently, it was shown that kidney precursor cells undergo apoptosis in Wt1-deficient mutant mice, consistent with the notion that Wt1 is essential for the early stage of kidney development (Davies et al., 2004; Kreidberg et al., 1993). The colocalization of COUP-TFII and Wt1 in the metanephric mesenchyme and urogenital ridge and the similar phenotypes exhibited by COUP-TFII and Wt1 mutants in terms of decreased cell numbers in the metanephric mesenchyme strongly implicate that these two factors function in the same pathway. As the expression of Wt1 is drastically decreased in COUP-TFII mutant mice in the metanephric mesenchyme and urogenital ridge (Fig. 3E,F and Fig. 4I,J), it suggests that COUP-TFII is upstream of Wt1 in the signaling cascade. This notion is supported by ChIP analysis, which showed that COUP-TFII is recruited by Sp1 to the conserved Sp1-binding site in the Wt1 promoter to regulate Wt1 transcription directly. Therefore, Wt1 mediates COUP-TFII function to maintain metanephric mesenchyme cell differentiation and survival (Fig. 8).
In summary, we have shown that COUP-TFII has two major roles during metanephric mesenchyme formation. First, COUP-TFII directly regulates Eya1 transcription to specify the kidney precursor cell differentiation into the mature metanephric mesenchyme. Second, COUP-TFII directly regulates Wt1 expression to maintain metanephric mesenchyme survival and differentiation into the mature nephron.
Supplementary Material
Acknowledgments
We thank Dr Thomas Ludwig for providing the ROSA26CRE-ERT2 mice, Dr Thomas M. Schultheiss for the mouse Osr1 probe, Dr Pin-Xian Xu for the Eya1 mouse probe and Dr Alan O. Perantoni for sharing RIMM-18 cells. We appreciate technical help from Ms Wei Qian, Xuefei Tong and Grace Wen Chen. We would like to express gratitude to Eric Buras for the English editing.
Footnotes
Funding
This work was supported by grants from the National Institutes of Health [DK62434 and DK59820 to S.Y.T. and M.-J.T., HL76448 to S.Y.T., DK45641 and HD17379 to M.-J.T. and the Diabetes Endocrinology Research Center P30 DK079638 to M.-J.T.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.076299/-/DC1
References
- Bouchard M., Pfeffer P., Busslinger M. (2000). Functional equivalence of the transcription factors Pax2 and Pax5 in mouse development. Development 127, 3703–3713 [DOI] [PubMed] [Google Scholar]
- Bramblett D. E., Pennesi M. E., Wu S. M., Tsai M. J. (2004). The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron 43, 779–793 [DOI] [PubMed] [Google Scholar]
- Brodbeck S., Besenbeck B., Englert C. (2004). The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech. Dev. 121, 1211–1222 [DOI] [PubMed] [Google Scholar]
- Costantini F., Shakya R. (2006). GDNF/Ret signaling and the development of the kidney. BioEssays 28, 117–127 [DOI] [PubMed] [Google Scholar]
- Davies J. A., Ladomery M., Hohenstein P., Michael L., Shafe A., Spraggon L., Hastie N. (2004). Development of an siRNA-based method for repressing specific genes in renal organ culture and its use to show that the Wt1 tumour suppressor is required for nephron differentiation. Hum. Mol. Genet. 13, 235–246 [DOI] [PubMed] [Google Scholar]
- de Luca C., Kowalski T. J., Zhang Y., Elmquist J. K., Lee C., Kilimann M. W., Ludwig T., Liu S. M., Chua S. C., Jr (2005). Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J. Clin. Invest. 115, 3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler G. R. (2006). The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 22, 509–529 [DOI] [PubMed] [Google Scholar]
- Durbec P., Marcos-Gutierrez C. V., Kilkenny C., Grigoriou M., Wartiowaara K., Suvanto P., Smith D., Ponder B., Costantini F., Saarma M., et al. (1996). GDNF signalling through the Ret receptor tyrosine kinase. Nature 381, 789–793 [DOI] [PubMed] [Google Scholar]
- Gong K. Q., Yallowitz A. R., Sun H., Dressler G. R., Wellik D. M. (2007). A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol. Cell. Biol. 27, 7661–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. G., Kamei C. N., Wang Q., Jiang R., Schultheiss T. M. (2006). Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133, 2995–3004 [DOI] [PubMed] [Google Scholar]
- Kim B. J., Takamoto N., Yan J., Tsai S. Y., Tsai M. J. (2009). Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev. Biol. 326, 378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. (1993). WT-1 is required for early kidney development. Cell 74, 679–691 [DOI] [PubMed] [Google Scholar]
- Kurihara I., Lee D. K., Petit F. G., Jeong J., Lee K., Lydon J. P., DeMayo F. J., Tsai M. J., Tsai S. Y. (2007). COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 3, e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuure S., Vuolteenaho R., Vainio S. (2000). Kidney morphogenesis: cellular and molecular regulation. Mech. Dev. 92, 31–45 [DOI] [PubMed] [Google Scholar]
- Levashova Z. B., Plisov S. Y., Perantoni A. O. (2003). Conditionally immortalized cell line of inducible metanephric mesenchyme. Kidney Int. 63, 2075–2087 [DOI] [PubMed] [Google Scholar]
- Li L., Xie X., Qin J., Jeha G. S., Saha P. K., Yan J., Haueter C. M., Chan L., Tsai S. Y., Tsai M. J. (2009). The nuclear orphan receptor COUP-TFII plays an essential role in adipogenesis, glucose homeostasis, and energy metabolism. Cell Metab. 9, 77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. J., Chen X., Qin J., Hong Y. K., Tsai M. J., Tsai S. Y. (2010). Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J. Clin. Invest. 120, 1694–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. W., McInnes L., Kreidberg J., Hastie N. D., Schedl A. (1999). YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126, 1845–1857 [DOI] [PubMed] [Google Scholar]
- Moore M. W., Klein R. D., Farinas I., Sauer H., Armanini M., Phillips H., Reichardt L. F., Ryan A. M., Carver-Moore K., Rosenthal A. (1996). Renal and neuronal abnormalities in mice lacking GDNF. Nature 382, 76–79 [DOI] [PubMed] [Google Scholar]
- Ohto H., Kamada S., Tago K., Tominaga S. I., Ozaki H., Sato S., Kawakami K. (1999). Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol. Cell. Biol. 19, 6815–6824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipaon C., Tsai S. Y., Tsai M. J. (1999). COUP-TF upregulates NGFI-A gene expression through an Sp1 binding site. Mol. Cell. Biol. 19, 2734–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Tsai M. J., Tsai S. Y. (2008). Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS ONE 3, e3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Chen X., Xie X., Tsai M. J., Tsai S. Y. (2010). COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc. Natl. Acad. Sci. USA 107, 3687–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Cooney A. J., Kuratani S., DeMayo F. J., Tsai S. Y., Tsai M. J. (1994). Spatiotemporal expression patterns of chicken ovalbumin upstream promoter-transcription factors in the developing mouse central nervous system: evidence for a role in segmental patterning of the diencephalon. Proc. Natl. Acad. Sci. USA 91, 4451–4455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainio K., Suvanto P., Davies J., Wartiovaara J., Wartiovaara K., Saarma M., Arumae U., Meng X., Lindahl M., Pachnis V., et al. (1997). Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124, 4077–4087 [DOI] [PubMed] [Google Scholar]
- Sajithlal G., Zou D., Silvius D., Xu P. X. (2005). Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev. Biol. 284, 323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén L. (1987). Organogenesis Of The Kidney. London: Cambridge University Press; [Google Scholar]
- Self M., Lagutin O. V., Bowling B., Hendrix J., Cai Y., Dressler G. R., Oliver G. (2006). Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 25, 5214–5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya R., Watanabe T., Costantini F. (2005). The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev. Cell 8, 65–74 [DOI] [PubMed] [Google Scholar]
- Suh J. M., Yu C. T., Tang K., Tanaka T., Kodama T., Tsai M. J., Tsai S. Y. (2006). The expression profiles of nuclear receptors in the developing and adult kidney. Mol. Endocrinol. 20, 3412–3420 [DOI] [PubMed] [Google Scholar]
- Takamoto N., You L. R., Moses K., Chiang C., Zimmer W. E., Schwartz R. J., DeMayo F. J., Tsai M. J., Tsai S. Y. (2005). COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development 132, 2179–2189 [DOI] [PubMed] [Google Scholar]
- Tang K., Xie X., Park J. I., Jamrich M., Tsai S., Tsai M. J. (2010). COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development 137, 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M., Gomez-Pardo E., Dressler G. R., Gruss P. (1995). Pax-2 controls multiple steps of urogenital development. Development 121, 4057–4065 [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J. (1997). Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr. Rev. 18, 229–240 [DOI] [PubMed] [Google Scholar]
- Vainio S., Lin Y. (2002). Coordinating early kidney development: lessons from gene targeting. Nat. Rev. Genet. 3, 533–543 [DOI] [PubMed] [Google Scholar]
- Wang Q., Lan Y., Cho E. S., Maltby K. M., Jiang R. (2005). Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev. Biol. 288, 582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellik D. M., Hawkes P. J., Capecchi M. R. (2002). Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 16, 1423–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. P., Lee D. K., Demayo F. J., Tsai S. Y., Tsai M. J. (2010). Generation of ES cells for conditional expression of nuclear receptors and coregulators in vivo. Mol Endocrinol. 6, 1297–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Qin J., Lin S. H., Tsai S. Y., Tsai M. J. (2011). Nuclear receptor chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) modulates mesenchymal cell commitment and differentiation. Proc. Natl. Acad. Sci. USA 108, 14843–14848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P. X., Adams J., Peters H., Brown M. C., Heaney S., Maas R. (1999). Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 23, 113–117 [DOI] [PubMed] [Google Scholar]
- Xu P. X., Zheng W., Huang L., Maire P., Laclef C., Silvius D. (2003). Six1 is required for the early organogenesis of mammalian kidney. Development 130, 3085–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L. R., Lin F. J., Lee C. T., DeMayo F. J., Tsai M. J., Tsai S. Y. (2005a). Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435, 98–104 [DOI] [PubMed] [Google Scholar]
- You L. R., Takamoto N., Yu C. T., Tanaka T., Kodama T., Demayo F. J., Tsai S. Y., Tsai M. J. (2005b). Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc. Natl. Acad. Sci. USA 102, 16351–16356 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.