Abstract
The medial amygdala plays a key role in regulating adult social behavior and undergoes structural changes during puberty that may be driven by gonadal hormone secretion during this developmental period. The current study sought to investigate potential organizational effects of testosterone during puberty, activational effects of testosterone in adulthood, and any interactions on regional volume and neuronal number of the medial amygdala. Male Syrian hamsters either did or did not experience endogenous testosterone during pubertal brain development, and then received either testosterone-filled or blank capsules during adulthood two weeks before tissue collection. The results show that pubertal testosterone has long-term organizational effects on volume of specific subregions of the medial amygdala such that the presence of pubertal testosterone resulted in 1) decreased volume of the anterior ventral amygdala and, to a lesser extent, the anterior dorsal medial amygdala; 2) increased volume of the posterior dorsal medial amygdala. Both effects were independent of the presence of testosterone during adulthood. Pubertal testosterone also decreased neuronal number in the anterior dorsal medial amygdala, suggesting a possible mechanism by which pubertal testosterone decreases volume in this subregion. In addition, there was a significant interaction between pubertal and adult testosterone, such that testosterone in adulthood increased the number of neurons in the posterior ventral medial amygdala only in males that did not experience endogenous pubertal testosterone. In conclusion, pubertal testosterone organizes the medial amygdala in a subregion-specific manner, which may contribute to the maturation of adult-typical social behavior.
Keywords: puberty, medial amygdala, testosterone, regional brain volume, neuronal number
1. Introduction
Many of the physiological and behavioral changes associated with adolescence can be attributed to puberty and the associated increase in gonadal steroid hormones. These hormones influence the adolescent brain and maturation of social behaviors necessary for survival and reproduction in adulthood. Gonadal hormones have two modes of action on social behaviors and their underlying neural circuits: long-lasting organizational influences that typically occur during development, and short-term activational influences that typically occur in adulthood. Activational responses to hormones are often programmed by earlier organizational effects. For example, the perinatal organization of the brain by testicular hormones sets up male-typical behavioral responses to testosterone in adulthood (for review, see Arnold, 2009).
Using the male Syrian hamster as an animal model to understand neuroendocrine mechanisms underlying the maturation of adult social behaviors, we have shown that puberty is an extension of the perinatal period of sensitivity to organizing effects of testicular hormones (Schulz et al., 2004, Schulz and Sisk, 2006, Schulz et al., 2009). Depriving males of testosterone during puberty results in long-lasting deficits in hormone-dependent reproductive and agonistic behavior. Thus, it appears that pubertal testosterone organizes the male brain a second time, perhaps by fine-tuning the perception of socially relevant sensory stimuli (Romeo et al., 2003, Schulz et al., 2004, Schulz et al., 2006, Schulz et al., 2009). In the current study we sought to determine whether there are organizational influences of pubertal testosterone on gross morphological features of the medial amygdala (Me) in light of its key role in the expression of adult social behaviors, namely, the integration of chemosensory social cues with the internal steroid hormone milieu (Wood, 1998, Choi et al., 2005).
The rodent Me is subdivided into four well-defined subregions, or quadrants, based on differing cytoarchitecture, connectivity, steroid hormone receptor expression, and function: anterior ventral (MeAV), anterior dorsal (MeAD), posterior ventral (MePV), and posterior dorsal (MePD) (for review, see Petrulis, 2009). Region-specific structural changes occur in all quadrants during pubertal development, including alterations in volume, cell number, and dendritic morphology (Romeo and Sisk, 2001, Zehr et al., 2006, Cooke et al., 2007, Ahmed et al., 2008, Schulz et al., 2009). For example, in Syrian hamsters, MeAD volume decreases, whereas MePD volume increases, across pubertal development (Romeo and Sisk, 2001, Schulz et al., 2009). It is not clear whether these developmental changes in volume are the result of organizational or activational effects of testicular hormones, or neither. However, generally speaking, Me volume is subject to activational influences of testosterone in adulthood, since castration in adulthood typically reduces volume, and testosterone replacement restores it to pre-castration size (Cooke et al., 1999, Cooke et al., 2002, Cooke et al., 2003, Morris et al., 2008a, Morris et al., 2008b). Furthermore, activational influences of testosterone on Me volume in adulthood may be programmed by organizational influences of testosterone during puberty.
The current study was designed to tease apart organizational and activational influences of testosterone, and any interactions, on gross morphology of Me. We used a 2 by 2 design in which male Syrian hamsters either did or did not experience endogenous circulating testosterone during adolescence (gonadectomy either prepubertally or in adulthood), and either did or did not receive exogenous testosterone replacement in adulthood six weeks after gonadectomy. We focused on regional volume and neuronal number as easily quantified structural features by which to compare potential organizational and activational effects of testosterone across the Me quadrants. This study identified organizational influences of pubertal testosterone on Me regional volume and neuron number that are structural correlates of the organizational influences of pubertal testosterone on male social behaviors.
2. Results
2.1 Testosterone concentrations within each experimental group
Adult males given testosterone-filled capsules (T-treated) that went through pubertal development either without (NoT@P) or with (T@P) endogenous testosterone had circulating testosterone concentrations within adult male physiological range (3.00 ± 0.55 ng/mL and 3.61 ± 0.41 ng/mL, respectively). As expected, NoT@P and T@P males given blank capsules (blank-treated) had nearly undetectable levels of circulating testosterone (0.12 ± 0.05 ng/mL and 0.11 ± 0.07 ng/mL, respectively).
2.2 Effects of pubertal and adult testosterone on Me regional volume
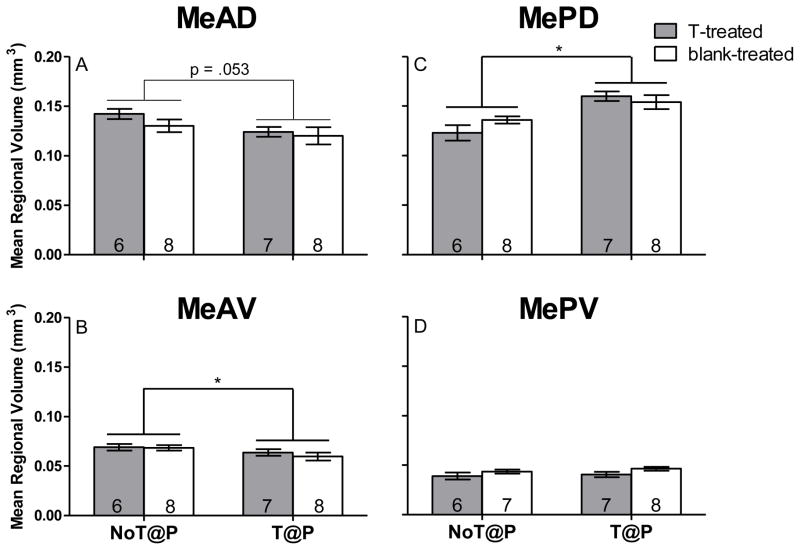
Two-way ANOVA revealed a main effect of pubertal testosterone on MeAV volume, [F(1,25) = 4.25, p = .050; Figure 1B] and a trend towards an effect for the MeAD, [F(1,25) = 4.13, p = .053, Figure 1A], with NoT@P males having larger volumes compared to T@P males in both subregions, independently of hormone treatment in adulthood. A main effect of pubertal testosterone on MePD volume [F(1,25) = 20.91, p < .000; Figure 1C] was found, with NoT@P males having a smaller volume compared to T@P males. There was no effect of pubertal testosterone on MePV volume (Figure 1D). Statistical analysis revealed neither a main effect of adult testosterone nor an interaction between pubertal and adult testosterone on volume of any of the Me quadrants.
Figure 1.
Mean (±SEM) regional volume of specific quadrants of the medial amygdala (Me) is pubertal testosterone-dependent in adult male Syrian hamsters. A: The presence of testosterone during puberty approached significance in decreasing regional volume of the anterior dorsal Me (MeAD). B: The presence of testosterone during puberty significantly decreased regional volume of the anterior ventral Me (MeAV). C: The presence of testosterone during puberty significantly increased regional volume of the posterior dorsal Me (MePD). D: There was no effect of testosterone on regional volume of the posterior ventral Me (MePV). Numbers on bars indicate sample size. * indicates a significant difference between males that did not have testosterone during puberty (NoT@P) and males that did have testosterone during puberty (T@P) with p ≤ 0.05.
2.3 Effects of pubertal and adult testosterone on Me neuronal number
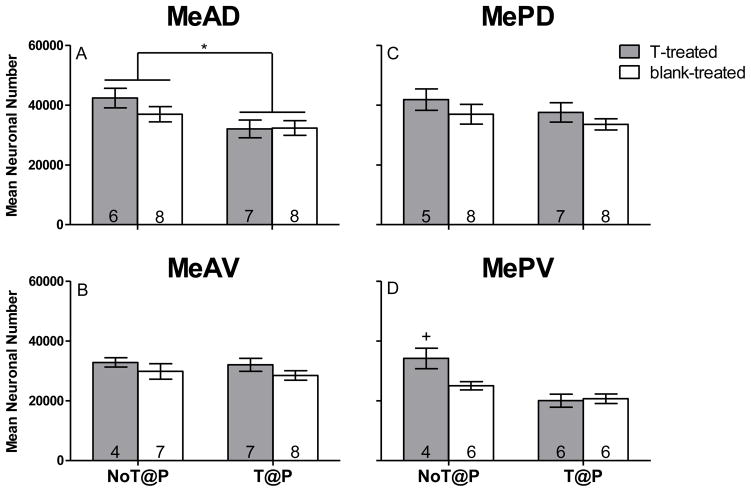
Two-way ANOVA revealed a main effect of pubertal testosterone on neuronal number in MeAD [F(1,25) = 7.10, p = .013; Figure 2A], with NoT@P males having more neurons compared to T@P males, independently of hormone treatment in adulthood. No effect of pubertal testosterone was found for MeAV or MePD neuronal number (Figures 2B and C, respectively). A main effect of pubertal testosterone on neuron number was revealed in MePV [F(1,18) = 19.76, p = .000; Figure 2D], but this was qualified by an interaction between pubertal and adult testosterone [F(1,18) = 5.53, p = 0.030; Figure 2D]. Independent t-tests revealed that T-treated NoT@P males had more MePV neurons than all other groups, which did not differ from each other (blank-treated NoT@P [t(8) = 2.88, p = 0.020], T-treated T@P [t(8) = 3.69, p = 0.006], and blank-treated T@P [t(8) = 4.02, p = 0.004]). Thus, the interaction is that adult testosterone increased MePV neuron number only when testicular hormones were absent during puberty. No main effect of adult testosterone on neuronal number was detected for any of the Me quadrants.
Figure 2.
Mean (±SEM) neuronal number of specific quadrants of the medial amygdala (Me) is testosterone-dependent in adult male Syrian hamsters. A: The presence of testosterone during puberty significantly increased neuronal number in the anterior dorsal Me (MeAD). B: There was no significant effect of testosterone on neuronal number in the anterior ventral Me (MeAV). C: There was no significant effect of testosterone on neuronal number in the posterior dorsal Me (MePD). D: There was an interaction between pubertal and adult testosterone on neuronal number in the posterior ventral Me (MePV), where adult testosterone significantly increased neuron number only in males that experienced the absence of pubertal testosterone (T-treated NoT@P). Numbers on bars indicate sample size. * indicates a significant difference between males that did not have testosterone during puberty (NoT@P) and males that did have testosterone during puberty (T@P) with p ≤ 0.05. +indicates an interaction between pubertal and adult testosterone with p ≤ 0.05.
3. Discussion
This is the first documentation of organizational effects of pubertal testosterone on regional volume and neuronal number within the male Syrian hamster Me. The presence of pubertal testosterone resulted in decreased volume or neuronal number in the anterior Me subregions, independently of the presence or absence of testosterone in adulthood. In contrast, the presence of testosterone during puberty resulted in increased regional volume of MePD. It is unlikely that the decrease in anterior Me volume is a function of the increase in MePD volume because pubertal testosterone led to a decrease in length (rostro-caudal extent) of both MeAV and MeAD, while there was no effect of pubertal testosterone on rostro-caudal extent of MePD (data not shown). These results extend previous reports that anterior Me is larger, while posterior Me is smaller, in prepubertal hamsters compared with adult males (Romeo and Sisk, 2001, Schulz et al., 2009), by demonstrating that these developmental changes in size are the result of organizational, and not activational, effects of testosterone during puberty. Furthermore, the current study shows that a decrease in neuron number is correlated with the pubertal decrease in MeAD volume, suggesting that organizational influences of pubertal testosterone include promotion of cell death within this quadrant. Thus, the primary finding of this study is that pubertal testosterone organizes structural features of the Me, providing a neural substrate for the well-documented organizational influences of pubertal testosterone on male social behaviors.
No activational effects of adult testosterone on Me volume or neuronal number were found in the current study. This result was surprising given several previous reports of activational effects of testosterone on regional Me volume in adult male and female rats and Siberian hamsters, where the presence of testosterone is associated with larger Me regional volumes (Cooke et al., 1999, Cooke et al., 2002, Cooke et al., 2003, Cooke, 2006, Morris et al., 2008a). Although there may be species differences in this regard, it seems more likely that differences in the duration of adult testosterone replacement account for the discrepancy between the current and previous studies. In the prior studies, gonadectomized animals were either compared to intact animals or treated immediately with androgen for at least 28 days before tissue collection. This design is equivalent to a maintenance paradigm, in which there is no washout period before hormone replacement. In contrast, in the current study, hamsters were gonadectomized for six weeks and then treated with testosterone for 14 days before tissue collection. Fourteen days of testosterone replacement is sufficient to reinstate male sexual behavior after castration in rats (McGinnis et al., 1989) and hamsters (Schulz et al., 2006). However, some studies have found that it takes up to 3 weeks for complete recovery of sexual behavior in long-term castrated male hamsters (Arteaga-Silva et al., 2005, Arteaga-Silva et al., 2008). Thus, it may require more than two weeks of testosterone treatment to result in increases in regional volume of the Me, and more prolonged testosterone treatment may reveal activational influences of testosterone on Me volume in Syrian hamsters.
The MePV was the only Me quadrant in which we found evidence for an interaction between pubertal and adult testosterone. In this case, testosterone treatment in adulthood led to a 25% increase in the number of MePV neurons, but only in males that did not experience testosterone during puberty. This is a novel and intriguing finding suggesting that organizational influences of pubertal testosterone reduce sensitivity to activational effects of adult testosterone on MePV neuron number. Although it is intuitive to suppose that the MePV would retain remarkable plasticity in the absence of organizing influences of pubertal testosterone, this finding is nevertheless surprising given the absence of any observed activational effects in the other Me subregions, and it warrants further investigation.
Organizational influences of pubertal testosterone on Me regional volume may reflect a number of hormone-modulated mechanisms, including neuro- or gliogenesis, cell death, synapse formation, and synapse elimination. Here we found that the decrease in MeAD volume due to pubertal testosterone was correlated with a decrease in MeAD neuron number, suggesting that pubertal testosterone facilitates cell death or hinders cell survival in this quadrant. Indeed, this type of mechanism is operating in rat primary visual cortex development, in which the greater number of neurons in adult males is due to an ovarian hormone-dependent reduction in neuron number in females during puberty (Nunez et al., 2002). Separate mechanisms apparently underlie volume changes observed in MeAV and MePD, as parallel changes in neuron number were not observed in these subregions. These other mechanisms could include changes in soma size, spine density, dendritic branching or arborization, as well as the size of glial and vascular compartments. In fact, both testosterone and its metabolite estradiol modulate structural features of Me neurons, including dendrite length, distal dendritic branching, spine density, and soma size (Gomez and Newman, 1991, Lorenzo et al., 1992, Cooke et al., 2003, Zehr et al., 2006). The presence of both androgen and estrogen receptors in the Syrian hamster Me (Wood and Newman, 1995) implies that either testosterone or estradiol could organize Me during puberty via one or more of these mechanisms.
A functional correlate of pubertal organization of the Me may be the adolescent maturation of social behaviors that are crucial for success in adulthood. The Me has long been associated with hormone-dependent expression of sexual and agonistic behaviors in rodents (Harding and McGinnis, 2003; Lehman et al., 1980; Wood and Newman, 1995b). Anterior Me is the primary recipient of olfactory bulb input, and it filters and transmits predatory and social chemosensory information to posterior Me, which then integrates sensory information with the hormonal milieu to moderate behavioral responses to social stimuli (Dielenberg et al., 2001, for review, see Petrulis, 2009, Maras and Petrulis, 2010a, b). Here we observed organizational influences of pubertal testosterone on regional volume in opposite directions in the anterior and posterior regions of Me. Hormone-dependent pruning of cells or synapses in anterior Me during puberty may represent an acquired efficiency in processing socially relevant sensory stimuli. In contrast, a larger and more intricately connected posterior Me may be required for appropriate and context-dependent behavioral responses to social stimuli, and for these responses to be shaped by learning and experience in adulthood.
Disruption of hormone-dependent pubertal organization of the Me may underlie the detrimental effects of the absence of pubertal testosterone on adult social behaviors. For example, NoT@P male Syrian hamsters show long-lasting impairments in both sexual and agonistic behaviors, and they appear to either misinterpret or respond inappropriately to social stimuli (Romeo et al., 2003, Schulz et al., 2004, Schulz et al., 2006). Given that social information processing and determining behavioral responses to social stimuli fall under the purview of the Me, organizational influences of pubertal testosterone on Me volume and neuronal number found in this study likely contribute to the successful maturation of adult male social behavior. Future studies will be aimed at determining what structural changes within Me are related to maturation of adult social behaviors.
3.1 Conclusions
The current study examined both the organizational influences of testosterone during puberty and the activational influences of testosterone in adulthood on volume and neuronal number in the four subregions of Me. The primary finding is that pubertal testosterone organizes Me in a subregion specific manner, resulting in decreased volume and/or neuronal number of the anterior subregions, increased volume of MePD, and no effect on MePV volume. In some cases pubertal testosterone affected regional Me volume and neuronal number independently of one another, demonstrating that hormone-dependent organization of regional volume can involve mechanisms other than parallel changes in neuron number. These results provide structural correlates of hormone-dependent organization of male social behaviors during puberty.
4. Experimental Procedure
4.1 Animals
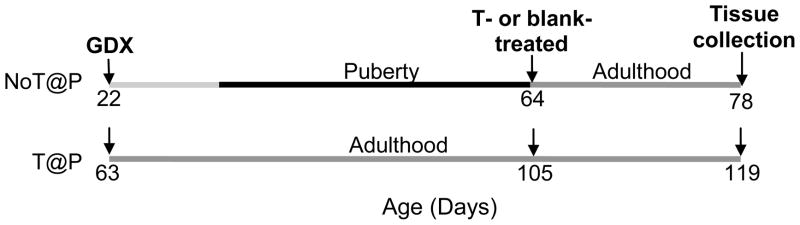
Thirty-two 18 day old (P18) male Syrian hamsters (Mesocricetus auratus) were obtained from Harlan Sprague–Dawley laboratories (Madison, WI) and arrived with their mothers. Males were housed with mothers and littermates until weaning at 21 days of age. Thereafter, they were housed individually in clear polycarbonate cages (12 × 4 × 8 in.) with ad libitum access to food (Telkad Rodent Diet No. 8640, Harlan) and water. Colony rooms were maintained on a 14 h light/10 h dark schedule (lights off at 1200 h EST) and at a temperature of 21 ± 2°C. Pre-pubertal (P22; n = 16) and adult (P63; n = 16) male hamsters were gonadectomized (GDX) to create groups of animals that experienced pubertal development either without (NoT@P) or with (T@P) endogenous testosterone, respectively. Six weeks later, when both NoT@P and T@P males were in young adulthood (P64 and P105, respectively), eight males in each group received either testosterone capsules (7 mm and 15mm length, inner diameter: 1.98mm, outer diameter: 3.18mm; T-treated) or blank capsules (blank-treated) of matched size two weeks before tissue collection (Figure 3). Sham-operated control groups were not included in this study as previous experiments from this lab have not found an effect of sham surgeries at any age on brain or behavioral measurements (Schulz et al., 2006, Schulz et al., 2009). One day before tissue collection, all males were used in a resident-intruder behavioral paradigm for an unrelated study. All animals were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals and protocols were approved by the Michigan State University Institutional Animal Care and Use Committee.
Figure 3.
Experimental design. Pre-pubertal (P22; NoT@P) and adult (P63; T@P) male hamsters were gonadectomized (GDX) such that animals experienced adolescent development either without or with endogenous testosterone, respectively. Six weeks later, eight males in each group either received testosterone-filled (T-treated) or blank (blank-treated) capsules two weeks before tissue collection.
4.2 Tissue Collection
The NoT@P and T@P males on P78 and P119, respectively, were euthanized with an overdose of sodium pentobarbital (130mg/kg ip) and perfused intracardially with 100ml of heparinized buffered saline rinse followed by 150ml of 4% paraformaldehyde. Brains were post fixed in 4% paraformaldehyde for 1 hour and stored in 20% sucrose/phosphate buffered saline solution.
4.3 Plasma testosterone concentration
Plasma testosterone levels were determined from blood collected via cardiac puncture at the time of sacrifice. Duplicate 50-μl samples were analyzed within a single assay using the Coat-A-Count Total testosterone Kit (Diagnostic Products, Los Angeles, CA). The minimum detectable concentration for the assay was 0.1 ng/ml of testosterone. The intra-assay coefficient of variance was 2.3%.
4.4 Histological procedures and analysis
4.4.1 Brain Sectioning
Sections were cut at 40 μm thickness into three series using a cryostat, and one series was thaw mounted onto glass slides. Sections were allowed to dry and then thionin-stained and cover-slipped for Nissl-based identification of the subregions of interest.
4.4.2 Microscopic analysis of regional volume
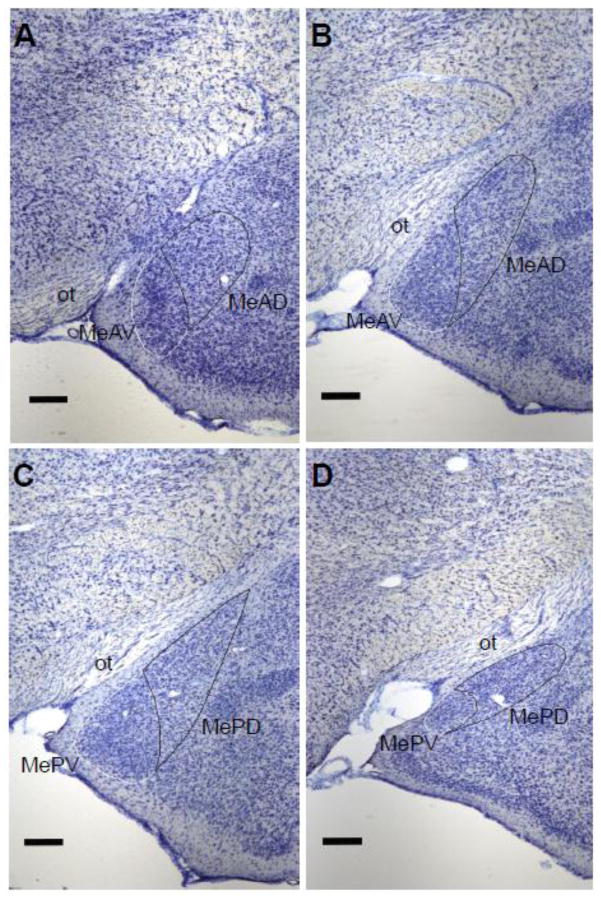
From the Nissl-stained sections, the MeAV, MeAD, MePV, and MePD quadrants were identified and traced bilaterally using Neurolucida (version 7; Microbrightfield, Williston, VT) at 4x objective (Figure 4). All sections in this series that contained the four subregions of Me, as identified in the Morin and Wood (2001) hamster brain atlas, were traced. The beginning of MeAD and MeAV was defined as the section in which cell orientation became more dorsomedial as compared to the more anterior pole of Me, and the cytoarchitecture and orientation of cells in MeAD and MeAV could be distinguished from one another. The beginning of MePD and MePV was defined as the section in which the optic tract extended nearly to the tip of the dorsal Me and the cells at the tip of the dorsal Me were oriented more dorsomedially and formed a pointed tip. The MePV always ends before the MePD; therefore, more sections contained the MePD than the MePV. Regional volumes were calculated by summing the traced cross-sectional areas and multiplying by the distance between sections (120 μm).
Figure 4.
Boundaries of the Me quadrants as determined in Nissl-stained coronal sections. A: The most rostral section containing MeAD and MeAV as defined by the differential orientation of cells extending dorsomedially. B: The most caudal section containing MeAD and MeAV as defined by the optic tract (ot) extending midway to the dorsal tip of the MeAD, while the MeAD still maintains a round shape. C: The most rostral section containing MePD and MePV as defined by the ot extending to the dorsal tip of the MePD, while the MePD forms a point at the most dorsal end. D: The most caudal section containing both MePD and MePV defined as the MePD starting to round out and move more dorsomedial, while the MePV remains a distinct small round group of cells. Scale bar = 250 μM.
4.4.3 Stereological analysis of neuron number
Neurons were counted in all traced sections using an unbiased stereological approache adapted from Morris et al. (2008a). A cell was identified as a neuron if it met at least three out of these four criteria: 1) one distinct nucleolus; 2) distinct cytoplasm; 3) large in size relative to nearby glial cells; 4) visible extension(s) (Figure 5). StereoInvestigator (version 7, Microbrightfield, Williston, VT) optical fractionator method was used to estimate total number of objects (Nobj, here number of neurons) according to the following equation: Nobj = (ΣN)(1/ssf)(1/asf)(1/tsf). Here, ΣN is the sum of objects sampled in a known fraction of the reference space. The section sampling fraction (ssf) is the number of sections sampled divided by the entire number of sections through the region of interest, area sampling fraction (asf) is the total area sampled by the range of counting frames divided by the total area of all sampled sections, and thickness sampling fraction (tsf) is the height of the dissector divided by the average total section thickness.
Figure 5.
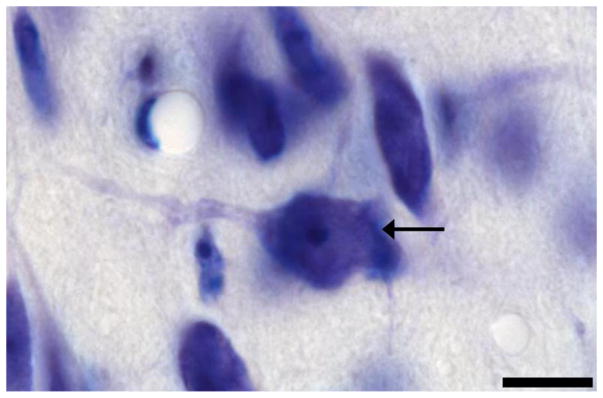
Classification of a neuron. The cells in the Nissl-stained Me quadrants were viewed under 100x objective. Black arrow indicates a neuron that met all four criteria. Scale bar = 10 μM
Neurons were counted using a 100x oil-immersion (1.4 N.A.) objective. Average thickness of each section from each brain was determined by measuring the section thickness every other counting frame, which yielded an average of 20 μm. Parameter settings were as follows: height, 6 μm; guard height, 1.5 μm minimum; counting frame area, 1225 μm2; x-y 250 μm. Sampling parameters were set to allow a coefficient of error (Gundersen et al., 1999, m = 1) to be no more than 0.1 for each subregion within each animal. If the coefficient of error for a specific subregion within an animal was more than 0.1, then it was not used in this data set. As this parameter was not needed to determine regional volume, sample sizes differ between the two analyses (volume and neuronal number).
4.5 Statistics
A repeated measures ANOVA was used to detect laterality between right and left hemispheres within each experimental group. Because no significant differences were found between the two hemispheres for any of the Me quadrants (data not shown), the data were averaged for both hemispheres into one measure per quadrant per animal. Two-way ANOVAs were then conducted for each quadrant with pubertal hormone (NoT@P or T@P) and adult treatment (T-treated or blank-treated) as independent variables and either regional brain volume or number of neurons as the dependent variable.
Highlights.
Pubertal testosterone organizes the medial amygdala in a subregion-specific manner
Adult testosterone had no effect on medial amygdala volume or neuron number
Neuronal number contributes to volume changes in a subregion-specific manner
Volume and neuronal number are independently affected by pubertal testosterone
Acknowledgments
This work was supported by National Institutes of Health grants R01-MH068764 to C.L.S. and T32-MH070343 support of K.C.D. Many thanks to Margaret Bell, Margaret Mohr, Ashley Pratt, Dr. Heather Molenda-Figueira, Dr. Eman Ahmed, Dr. Sarah Meerts, Jane Venier, and Rayson Figueira for their contributions to data collection and comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nature neuroscience. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and behavior. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga-Silva M, Marquez-Villanueva Y, Martinez-Garcia R, Hernandez-Gonzalez M, Bonilla-Jaime H, Retana-Marquez S. Effects of hormonal replacement with androgens and estrogens on male sexual behavior and plasma levels of these steroids in gonadectomized golden hamsters (Mesocricetus auratus) Physiology & behavior. 2005;85:571–580. doi: 10.1016/j.physbeh.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Arteaga-Silva M, Vigueras-Villasenor RM, Retana-Marquez S, Hernandez-Gonzalez M, Chihuahua-Serrano C, Bonilla-Jaime H, Contreras JL, Morali G. Testosterone, androstenedione, and 5alpha-dihydrotestosterone on male sexual behavior and penile spines in the hamster. Physiology & behavior. 2008;94:412–421. doi: 10.1016/j.physbeh.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Cooke BM. Steroid-dependent plasticity in the medial amygdala. Neuroscience. 2006;138:997–1005. doi: 10.1016/j.neuroscience.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Hormones and behavior. 2003;43:336–346. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Hegstrom CD, Breedlove SM. Photoperiod-dependent response to androgen in the medial amygdala of the Siberian hamster, Phodopus sungorus. J Biol Rhythms. 2002;17:147–154. doi: 10.1177/074873002129002438. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Jordan CL, Breedlove SM. Pubertal growth of the medial amygdala delayed by short photoperiods in the Siberian hamster, Phodopus sungorus. Hormones and behavior. 2007;52:283–288. doi: 10.1016/j.yhbeh.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Gomez DM, Newman SW. Medial nucleus of the amygdala in the adult Syrian hamster: a quantitative Golgi analysis of gonadal hormonal regulation of neuronal morphology. The Anatomical record. 1991;231:498–509. doi: 10.1002/ar.1092310412. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. Journal of microscopy. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Lorenzo A, Diaz H, Carrer H, Caceres A. Amygdala neurons in vitro: neurite growth and effects of estradiol. Journal of neuroscience research. 1992;33:418–435. doi: 10.1002/jnr.490330308. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. The European journal of neuroscience. 2010a;32:469–482. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Lesions that functionally disconnect the anterior and posterodorsal sub-regions of the medial amygdala eliminate opposite-sex odor preference in male Syrian hamsters (Mesocricetus auratus) Neuroscience. 2010b;165:1052–1062. doi: 10.1016/j.neuroscience.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY, Mirth MC, Zebrowski AF, Dreifuss RM. Critical exposure time for androgen activation of male sexual behavior in rats. Physiology & behavior. 1989;46:159–165. doi: 10.1016/0031-9384(89)90249-7. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the Golden hamster brain. Academic Press; San Diego, CA: 2001. [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol. 2008a;506:851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Res. 2008b;1190:115–121. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. Journal of neurobiology. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Petrulis A. Neural mechanisms of individual and sexual recognition in Syrian hamsters (Mesocricetus auratus) Behavioural brain research. 2009;200:260–267. doi: 10.1016/j.bbr.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Schulz KM, Nelson AL, Menard TA, Sisk CL. Testosterone, puberty, and the pattern of male aggression in Syrian hamsters. Developmental psychobiology. 2003;43:102–108. doi: 10.1002/dev.10125. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain research. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Hormones and behavior. 2006;50:477–483. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Hormones and behavior. 2004;45:242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Molecular and cellular endocrinology. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Zehr JL, Salas-Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150:3690–3698. doi: 10.1210/en.2008-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI. Integration of chemosensory and hormonal input in the male Syrian hamster brain. Ann N Y Acad Sci. 1998;855:362–372. doi: 10.1111/j.1749-6632.1998.tb10594.x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. Journal of neurobiology. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]