Abstract
Tactile social contact is the most effective form of environmental enrichment for promoting normal behavior in captive primates. For laboratory macaques housed indoors, pair housing is the most common method for socialization. Pairs can be housed either in full contact (FC), or in protected contact (PC). At Washington National Primate Research Center, PC is provided by grooming-contact (GC) cages whereby two partners are housed individually in adjacent cages with access to each other through widely spaced vertical bars. Grooming-contact has been used to accommodate research protocol restrictions and improve the likelihood of compatibility for various pairings, in part by enabling male-female pairs. This study compares the benefits between the two housing types by video recording 14 pairs of adult female Macaca fascicularis in four sequential housing phases following an ABBA design: baseline grooming-contact, full contact shortly after introduction, 1-month-later full contact, and after reversion to grooming-contact. Prior to this study, pairs had been housed compatibly in GC. Twelve of the 14 long-term pairs transitioned successfully to full contact and data presented exclude the two failed pairs. Allogrooming increased significantly when pairs first switched from GC to FC (P = 0.018), but the effect did not last through the on 1-month-later FC phase suggesting that the initial improvement in affiliative behavior was a transitory novelty response that did not persist. Self-grooming significantly decreased between the first GC and first FC phases (P = 0.016), likely due to redirected allogrooming. Non-contact affiliative behavior towards partner or other conspecifics in the room did not differ, nor did agonism towards partner or others in the room. Occurrence of abnormal, tension, manipulation, miscellaneous active, and inactive behaviors did not differ significantly across housing phases. Proximity measurements indicated that pairs were significantly out of arm’s reach more often in protected contact than when in full contact (P ≤ 0.02). Proportion of time spent in physical contact significantly increased between the first GC and first FC phases (P = 0.002), but subsequently declined. For both FC phases, partners chose to spend about 50% of their time in the same cage. Few behavioral improvements were seen after pairs switched to full contact and no negative effects came of reversion to grooming contact. This study suggests that tactile contact provided through widely spaced bars (grooming-contact) is a viable alternative to full contact housing for adult female longtailed macaques. It provides a degree of social housing while allowing both partners choice and control, key concepts in contemporary animal welfare guidelines.
Keywords: Pair-housing, social housing, abnormal behavior, environmental enrichment, psychological well-being, laboratory macaques
1. INTRODUCTION
USA law dictates that facilities holding nonhuman primates (NHP) in captivity must implement environmental enhancement plans to promote the NHPs’ psychological well-being (U. S. Department of Agriculture, 1991). The plan “must include specific provisions to address the social needs of nonhuman primates of species known to exist in social groups in nature.” An amendment to the European Convention will no longer allow single housing of primates barring veterinary reasons, or in order to ensure good science (European Parliament, 2009; Eversheds, 2010). Recent changes to U.S. guidelines stress that single housing should be the exception and, in cases where it is necessary to house animals singly, it should be for the shortest duration possible (National Research Council, ILAR, 2011).
Tactile social contact has been identified as the most effective form of environmental enrichment for nonhuman primates kept in captivity (Lutz and Novak, 2005). Unlike manipulanda or other types of passive enrichment, social contact is a dynamic form of enrichment resistant to habituation with the potential for long-lasting beneficial results (Lutz and Novak, 2005). Moreover, early socialization of laboratory monkeys reduces the development of abnormal behaviors in adulthood and social deprivation at a young age can result in serious long-term emotional and behavioral problems (Bellanca and Crockett, 2002; Novak et al., 2006; Rommeck et al., 2009). Inadequate socialization not only creates poor welfare for the monkey, but can also have detrimental consequences for laboratory research (Jennings and Prescott, 2009).
Orchestrating social housing in a large research facility is a difficult and time-consuming process requiring constant cooperation among dedicated behavioral management, husbandry, veterinary, and research staffs. A survey of 22 primate research institutions in the USA reported that whereas 73% of nonhuman primates were socially housed overall, only 41% of indoor-housed primates were socialized (Baker et al., 2007); the predominant method for providing tactile social contact indoors for macaques was pair housing (unpublished survey data). Of the reasons given for single housing, incompatibility between animals was given by almost as many respondents (73%) as research protocol constraints (77%) (Baker et al., 2007).
There are two main social housing options for monkeys that are housed as pairs in indoor cages (Baker et al., 2007): full contact (FC), and grooming contact (GC) (also called protected contact, PC). At Washington National Primate Research Center (WaNPRC) at the University of Washington, Seattle, USA, protected contact is provided in specially designed grooming-contact cages (Crockett et al., 1997). In full-contact housing, partners in adjoining cages share the space of both cages and have full access to each other. In grooming-contact housing at WaNPRC, animals live in adjacent individual cages equipped with a panel of widely spaced vertical bars that permit partners to interact physically while preventing aggressive pursuit between cages and resource-monopolization by a dominant animal. Both partners must choose to interact; one cannot dominate the other one if it chooses to stay away. Another key difference between the two housing types is that grooming-contact enables the formation of male-female pairs while preventing pregnancy (Crockett et al., 1997). This is especially important for projects in which other birth control methods interfere with ongoing research objectives. We have found male-female pairs of several species to be more successful than same-sex pairings (Crockett, et al., 2006; Lee, et al., 2005) (Table 1). The percentage of indoor-housed monkeys provided tactile social contact at WANPRC tripled between 2002 and 2008 (18% to 55%) and grooming-contact was widely employed to achieve this increase.
Table 1.
Percentage of adult pairs of laboratory monkeys found to be fully compatible in grooming-contact (F=female; M = male). Based on 422 adult pairs introduced between 1997 and 2005 (Crockett et al., 2006).
| Species/Sex | FF | MF | MM | Overall | n of pairs |
|---|---|---|---|---|---|
| Papio cynocephalus | 50.0 | 86.7 | No pairings | 64.1 | 39 |
| Macaca fascicularis | 60.9 | 79.4 | 38.9 | 66.5 | 155 |
| M. nemestrina | 44.4 | 70.4 | 41.2 | 51.0 | 147 |
| M. mulatta | 5.0 | 22.9 | 15.4 | 16.0 | 81 |
| Overall | 47.0 | 65.4 | 32.1 | ||
| n of pairs | 185 | 159 | 78 | 422 | |
One study (Baker et al., 2008) suggested that protected contact does not provide the same benefits as full contact pairing for adult female rhesus macaques. However, little published research directly compares the two housing methods and we believe that it is premature to dismiss this tool for improving the welfare of laboratory-housed primates. This inspired us to compare the relative benefits of grooming-contact and full contact pairing for adult female longtailed macaques. A subset of our data (selected behavioral categories from two of four phases, GC1 and FC2) is combined with comparably sampled behaviors of rhesus at Tulane NPRC (Baker et al., in press).
The present study investigates whether a particular housing type is more beneficial to adult female longtailed macaques, Macaca fascicularis, by looking for a reduction in abnormal behaviors or an increase in species-typical behaviors such as social grooming. Our project involved adult females that were already compatibly housed in grooming-contact cages at the onset of data collection. The study design allowed a comparison of behavior in GC with the pairs’ initial responses to full contact (FC) and their behavior after 1 month in full contact. Subsequently, the pairs were returned to grooming contact. Significant changes that remained after 1 month in full contact can be considered to reflect long-term changes for evaluating relative benefits.
2. METHODS
2.1 Subjects and Housing
Twenty-eight adult female Macaca fascicularis in 14 pre-established social grooming-contact pairs were selected. At the beginning of study participation, each social pair had been successfully introduced in grooming-contact with the partner 0.63–4.72 years previously (mean = 2.1, SD = 1.6), with periodic separations required by a former research protocol. During the separations, tactile contact was disallowed, but partners remained next to each other in visual contact. Subjects were between 8.9–15.2 years old (mean = 11.4) and weighed between 2.5–5.1 kg (mean = 3.4). All were mother-reared in Indonesia and had been at WaNPRC for about 6 years prior to the start of this study. During the years they had been at WaNPRC, the 28 subjects had experienced 54 different grooming-contact pairings, of which 65% overall were successful (i.e., passed initial compatibility screening and were not separated later owing to excessive aggression). This rate of success in GC is similar to the overall rate of 61% found for female M. fascicularis at our facility (Crockett et al., 2006; Table 1). Nine of the 28 subjects had a prior history of abnormal behaviors, three exhibiting overgrooming, three self-biting, and three locomotor stereotypy.
Owing to space, equipment, and subject availability, data were first collected on eight pairs beginning June 2007 (Group 1 subjects). Beginning March 2008, simplified procedures were replicated with six additional pairs (Group 2 subjects). All subjects were housed at the Washington National Primate Research Center’s (WaNPRC) Biosafety Level 2–3 facility and had completed studies that did not involve permanent biologic changes (e.g., had not been inoculated with pathogens). The subjects were not participating in any other research project during the time of data collection. The two subject groups were housed in different animal rooms, but room demographics were similar; only female M. fascicularis were housed in the rooms. Animal rooms were maintained on a 12:12 h light:dark cycle. The ambient temperature was 22.2° to 25.6° C with a relative humidity of 30% to 50%. Each animal was provided with a portable toy and a foraging device hung externally on the cage, and received fresh produce or foraging opportunities seven days a week. Commercial monkey biscuits were provided twice daily – once before 9:00 h and once after 14:00 h, and water was provided ad libitum from water spigots (lixits).
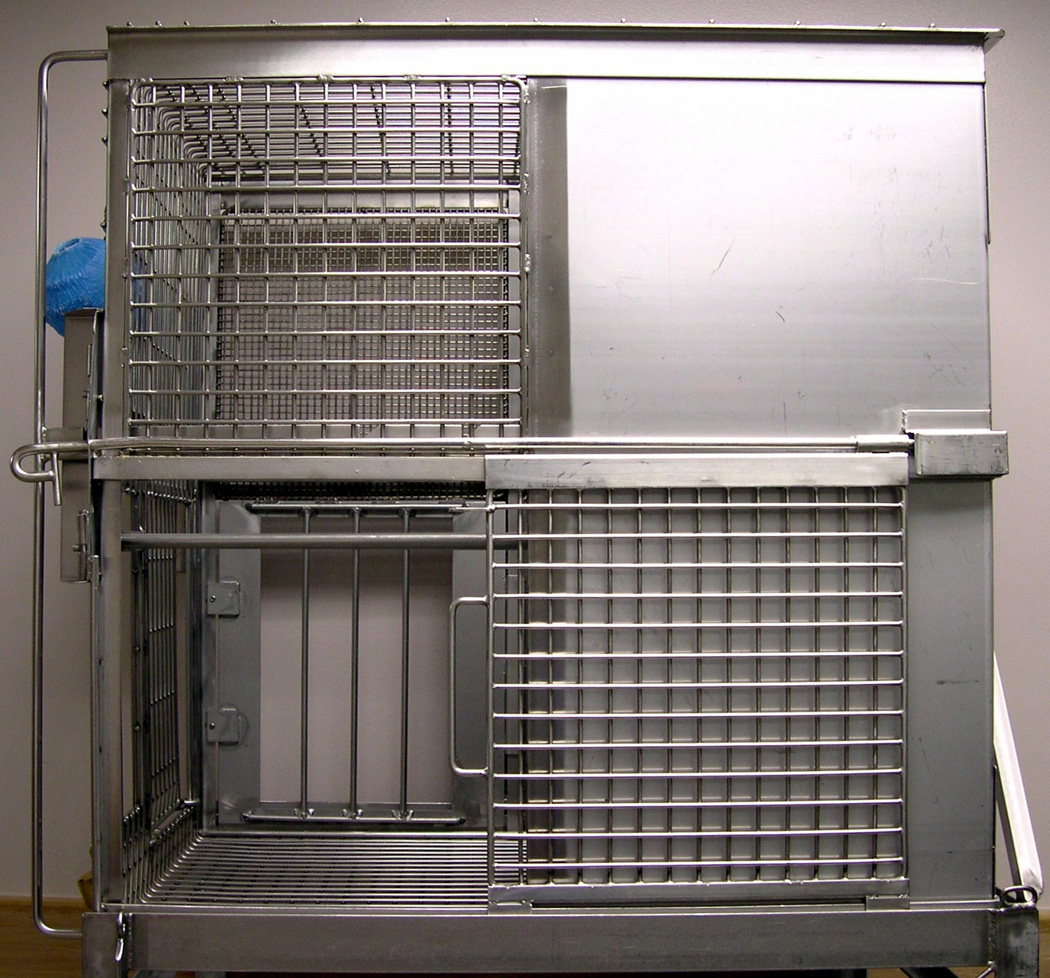
Subjects were housed in two-tiered stainless steel cages with 1.31 m2 of floor space and 76.2 cm of height, consistent with Animal Welfare Act USDA standards for NHPs weighing 3–10 kg (USDA, 1991). Each cage was furnished with a perch made of three stainless steel rods, positioned front to back. The back half of each cage had solid panels on each side to provide privacy. The front half of each cage included two different sizes of mesh (fine 0.64 cm mesh on the left side, 2.54 cm × 2.54 cm square mesh on the right side), and the bottom 39.37 cm of each side consisted of sliding mesh side-gates, an original “Seattle style” design (Bielitzki et al., 1990). Opening the mesh side-gates revealed three vertical GC bars providing four openings 5.16 cm × 38.1 cm of space (see Fig. 2 and 3 in Crockett et al., 1997, and Fig. 1). The same cages could be arranged to provide full contact by reversing left-right positions so that opening the side-gates revealed an unobstructed opening 34.29 cm × 38.74 cm tall.
Fig. 2.
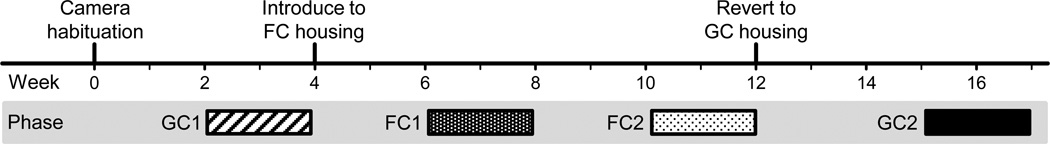
Sequence of events. Prior to week 0 subjects had been socially housed in grooming-contact for a mean of 2.1 years.
Fig. 3.
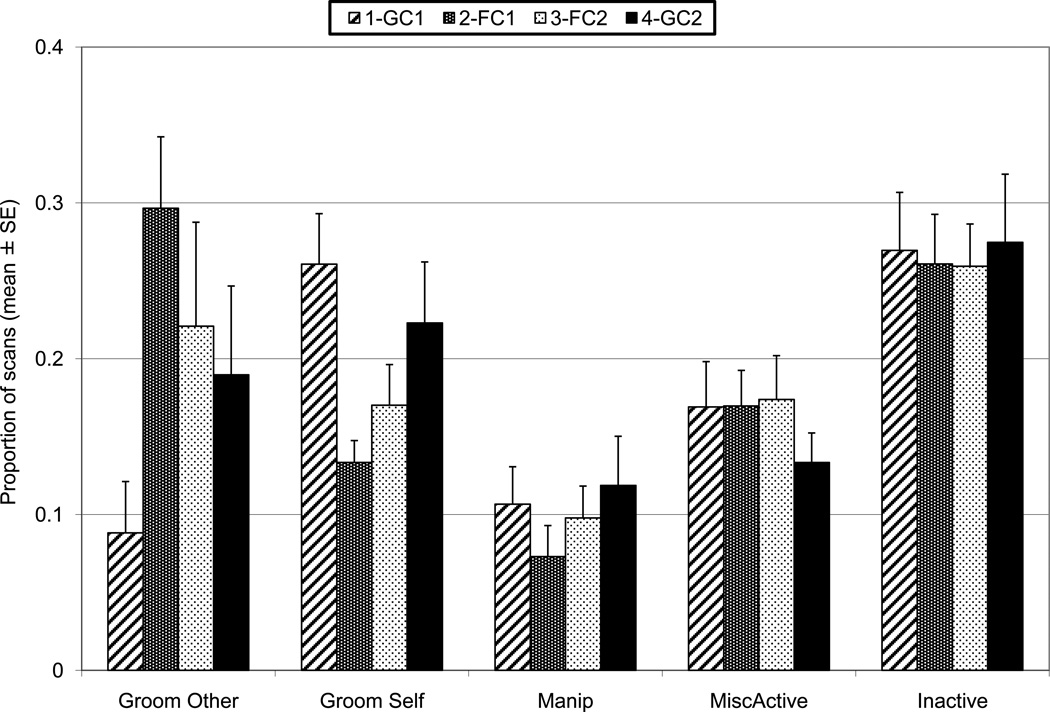
The five most predominant behavior categories by phase, each averaged across 12 pairs. The error bars represent standard error.
Fig. 1.
Seattle-style caging of the type used in this study (upper cage of two-tiered unit). Mesh side gates open to reveal grooming-contact bars on one side (rear of photo) and unobstructed opening on the other (front of photo). When two mirror image cages are connected with openings facing each other, the cage provides full contact (FC). When the cages are connected with grooming-contact bars facing, they provide GC.
2.2 Experimental Design/Procedures
Data were collected during four sequential social housing phases following an ABBA design (Fig. 2): baseline grooming-contact (GC1), full-contact housing (FC1), 1-month-later full-contact housing (FC2), and reversion to grooming-contact (GC2). The length of the housing phases was based on 2-week increments to coincide with the regular cage change-out schedule, which is when alterations to room arrangements are usually made.
During a 2-week period between the first two phases, GC1 and FC1, pairs transitioned from grooming-contact to full-contact housing through a progressive introduction process involving one to four, 30-min observation periods. Each pair experienced the same compatibility screening process that they underwent when they were initially given grooming-contact an average of 2 years prior to the start of this study. By opening one and then both mesh side-gates, pairs were gradually given greater degrees of tactile contact during the 30-min introduction period. Pairs were considered compatible if affiliative behaviors were observed in the absence of contact aggression. Two pairs that did not appear compatible by the fourth introduction attempt to FC did not receive the FC condition and their data were not analyzed.
After completion of the second GC phase (GC2), the 12 pairs that had successfully transitioned to FC were returned to FC. Informal notes on compatibility, but no formal data, were taken.
2.3 Data collection and scoring
In the 2 weeks prior to start of GC1, monkeys were habituated to the presence of video cameras (Iredale et. al., 2010) and setup procedure. During each housing phase, video collection was done three times over 2 weeks, spaced 1–3 days apart. Video collection was always done on a weekday between 12:00 h and 14:00 h. Multiple Sony HDD digital video camera recorders (Model No. DCR-SR40, made in Japan) were used simultaneously, one for each pair. Recording did not begin until after all cameras were set up. No personnel were present in the room after recording began. A minimum of 52 min was recorded each session using the LP setting.
For Group 1 subjects, three, 10-min segments (30 min total) were coded for each of the 3 days in four phases (GC1, FC1, FC2, GC2). The first coded segment began at 5 min after the start of the video to allow monkeys time to settle into normal routines. Subsequent coded segments were separated by 5 min to capture a more accurate representation of the 52-min recording session. After inspection of Group 1 data, for Group 2 subjects only two, 10-min segments (20 min total) were coded on each of the 3 days instead of three segments due to limitations on staff time. The first segment started at the same 5-min interval after start of video as for Group 1. The second started at the same time of the third interval in Group 1. All data were converted to proportions (see below) so that analyzed data were equivalent across pairs and sampled the same number of days.
Two individuals coded the videos using instantaneous scan sampling at 15-s intervals (Crockett and Ha, 2010). To minimize observer drift across housing conditions, the same person coded all data for all phases for a given pair before coding the next pair. On each scan, behaviors of interest (Table 2A) were recorded as being seen in 0, 1, or both pair members. For social grooming, both members of the pair were scored as grooming whether actor or recipient, so a score could be 0 or 2 for each 15-s scan. For other behaviors, e.g., self-grooming, 0, 1, or both pair members might be grooming on a scan. Proximity and cage location were also scored on a per-pair basis on each scan to see whether animals’ proximity to each other was influenced by the GC bars. Proximity codes were: in physical contact, within an arm’s reach, and beyond an arm’s reach. For FC conditions, it was recorded whether the animals were in the same or different cages. An overall compatibility rating was assigned at the end of each 10-min coded segment (Table 2B). Because we were especially interested in changes in abnormal behavior and did not want to miss those between scans, abnormal was also recorded on a 1-0 basis, and given a score of 0, 1, or 2 depending on whether no, one, or both partners exhibited abnormal behavior during the 15-s interval.
Table 2.
Definitions of behavioral and compatibility categories
| A. Behavioral Categories | ||
|---|---|---|
| Social affiliation | Groom other | Picking, stroking, or licking partner’s fur. |
| Affiliative to partner | Look/approach in a nonaggressive manner; showing “social interest.” Overt affiliative gestures: lipsmack, present side, chest, or other body part for grooming. | |
| Affiliative to other | Overt affiliative gestures only; lipsmack, presenting body part for grooming. | |
| Social Agonism | Agonism to partner | Aggressive and submissive behavior: lunge, hit cage, open mouth threat, chase, grimace, crouch, screech vocalization, persistent retreat. |
| Agonism to other | Lunge, hit cage, open mouth threat, finger-fighting over top/around front/through sides of cages. | |
| Contact Aggression | Hitting, grabbing, hair pulling, biting directed to partner. | |
| Resource monopolization | Take item (food or toy) away when it is not offered. Horde item or prevent other animal from approaching it. When an animal takes a biscuit from another animal’s cage (applies to GC housing only). | |
| Nonsocial | Groom self | Picking, stroking, or licking own fur. |
| Abnormal | Self-abusive behaviors, floating limb activities, overgrooming, locomotor stereotypy, miscellaneous self-stimulation stereotypies. | |
| Tension | Cage shake, scratch, yawn, tooth grind or chomp. | |
| Manipulate | Manipulate toy, enrichment device, cage part, or bedding. | |
| Miscellaneous active | Locomotion: walk, climb, jump, scoot, travel at least one “butt” profile distance. Eat/Drink: Pick up food item, handle food, put food item in mouth or dislodge from cheek pouch. Drink from lixit. Other Active: Miscellaneous active movements not included in other codes. | |
| Inactive | Sleep, sit, stand, lie, passive visual exploration, chewing in the absence of active food handling or pushing food from check pouch. | |
| B. Compatibility categories | ||
|---|---|---|
| Very compatible | ++ | Animals are given this score if they engage in social grooming at least once during the interval and no agonistic behaviors are observed. |
| Somewhat compatible | + | One or both display affiliative behaviors without agonistic behaviors but do not groom. Or if they display more affiliative than agonistic behaviors (no contact aggression) towards each other, regardless of grooming. |
| Neutral | ig | Neither display affiliative or agonistic behaviors toward each other, or one shows only affiliative social interest and the other ignores. |
| Somewhat incompatible | - | Animals display more agonistic behaviors (no contact aggression) than affiliative behaviors. |
| Incompatible | -- | Animals display many agonistic behaviors or engage in contact fighting. Partners that groom or display some affiliative behaviors are rated as incompatible if any contact aggression occurs. |
2.4 Statistical Analyses
To insure independence of data points, in statistical analyses we used the proportion of scans averaged across the individuals in a pair. For each coded behavior category, we computed the proportion of scans scored for each of the four phases (GC1, FC1, FC2, GC2), calculated as the per-individual average within each pair. Summary statistics were run on all the coded categories and checked for skewness, kurtosis, and variance. Categories that met normality and homogeneity of variance assumptions were tested with GLM ANOVA or MANOVA (Velleman, Data Desk, 1997). Although the data were proportions, arcsine transformation was not necessary. Those categories that did not meet the assumptions, even when transformed, were tested with Friedman ANOVA (Lehner, 1996, p. 434). Correlations between the behavioral variables and years in grooming contact before the onset of the study were computed as described in the results.
3. RESULTS
Twelve of the 14 long-term pairs successfully switched from GC to FC housing. The data presented are based on the 12 successful pairs.
3.1 Behaviors
Over 90% of the time budget comprised inactive, grooming other (partner), grooming self, manipulation, other active, locomotion, and eat/drink. The categories locomotion and eat/drink were not normally distributed and were combined with other active to form a miscellaneous active category. The behaviors (Fig. 3) inactive, grooming other, grooming self, other active, manipulation, and miscellaneous active were dependent variables in a GLM MANOVA with factors Phase (GC1, FC1, FC2, GC2) and Pair (repeated measures). Individual differences (Pair) were significant overall (Wilks Lambda = 0.0019, F=7.23, df = 55, 138, P < 0.01) and for all individual behaviors (P < 0.05 or less). Phase was significant overall (Wilks Lambda = 0.3634, F = 2.38, df = 15, 80, P = 0.007). Groom Other differed significantly by phase (F = 3.98, df = 3, 33, P = 0.016). Scheffe’s Post Hoc tests found GC1 to differ significantly from FC1 (more grooming other in FC1, P = 0.018); no other phase comparisons were significant. Groom Self differed significantly by phase (F = 4.66, df = 3, 33, P = 0.008). Scheffe’s Post Hoc tests found GC1 to differ significantly from FC1 (more grooming self in GC1, P = 0.016); no other phase comparisons were significant. Three behavioral categories did not differ significantly by phase: Manip (P = 0.10), Misc. Active (P = 0.28), and Inactive (P = 0.96).
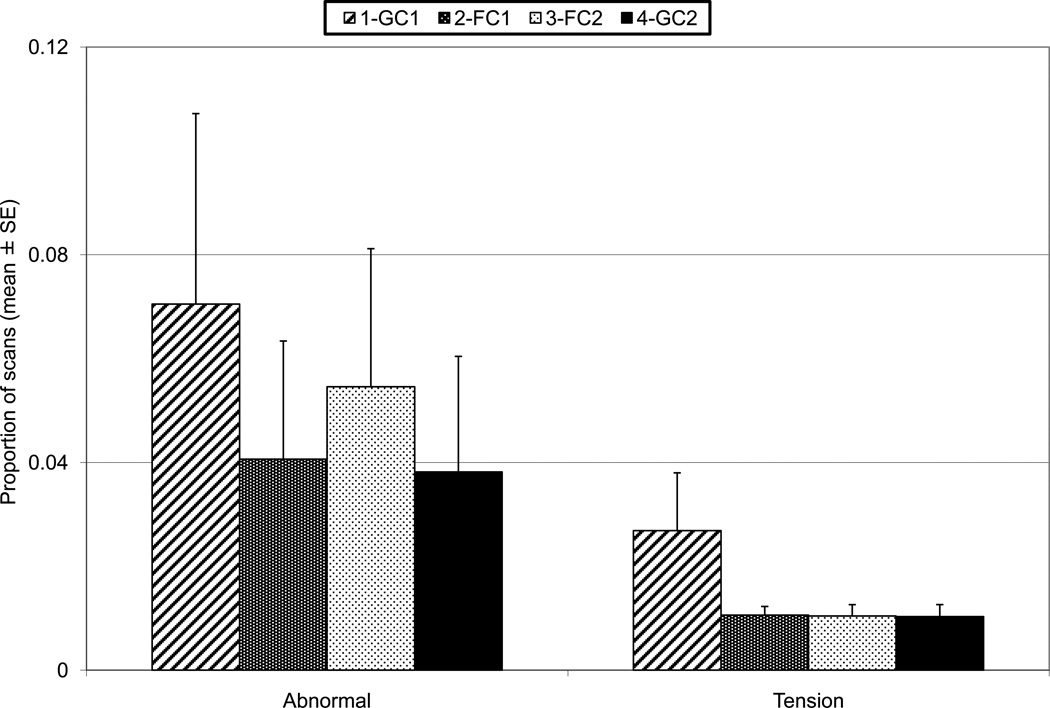
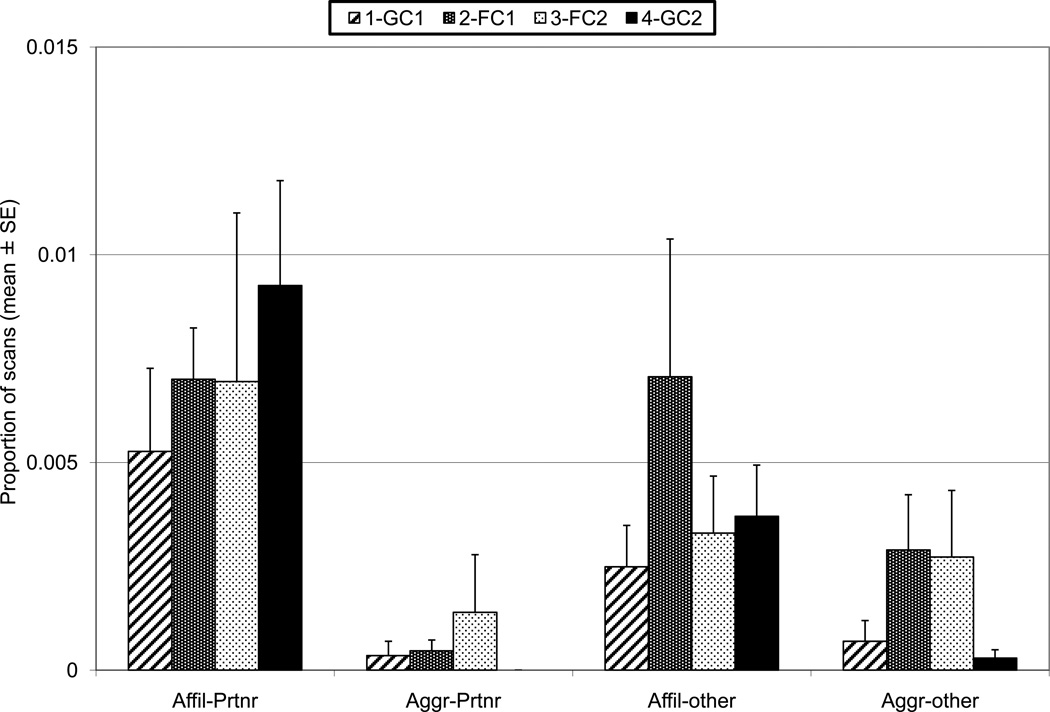
Abnormal behavior was scored on 5% of scans and during 6% of 1/0 samples; 92% of abnormal behavior scored was locomotor stereotypy (e.g., pacing). Total Abnormal (scan), Tension, Affiliative to Partner, Affiliative to Other, and Aggressive to Other were tested with Friedman ANOVAs, and no significant phase differences were found (Fig. 4 and 5). Total Abnormal (1/0) was also not significant, and was very similar to the scan measure. Aggressive to Partner was very infrequent and was not tested. Contact Aggression never occurred on the scan samples. Resource monopolization was observed so rarely that it was not analyzed.
Fig. 4.
Total Abnormal and Tension by phase, each averaged across 12 pairs. The error bars represent standard error.
Fig. 5.
Affiliative and Aggressive behaviors directed towards Partner or towards Other animals by phase, each averaged across 12 pairs. The error bars represent standard error.
To evaluate whether the time since initial GC introduction of the pair before the onset of the study was related to any of the behavioral outcomes, we ran correlations with the behavioral variables for each phase. Of the five time budget categories (Fig. 3), only grooming partner in GC1 was positively correlated with time since initial GC introduction (r = + 0.627, P < 0.05). This pattern disappeared in the subsequent phases. In the FC2 phase, there were significant (P < 0.05) correlations with affiliative toward partner (rs = + 0.552) and with tension (rs = − 0.559). No other correlations with time since initial GC introduction were significant.
3.2 Compatibility Ratings
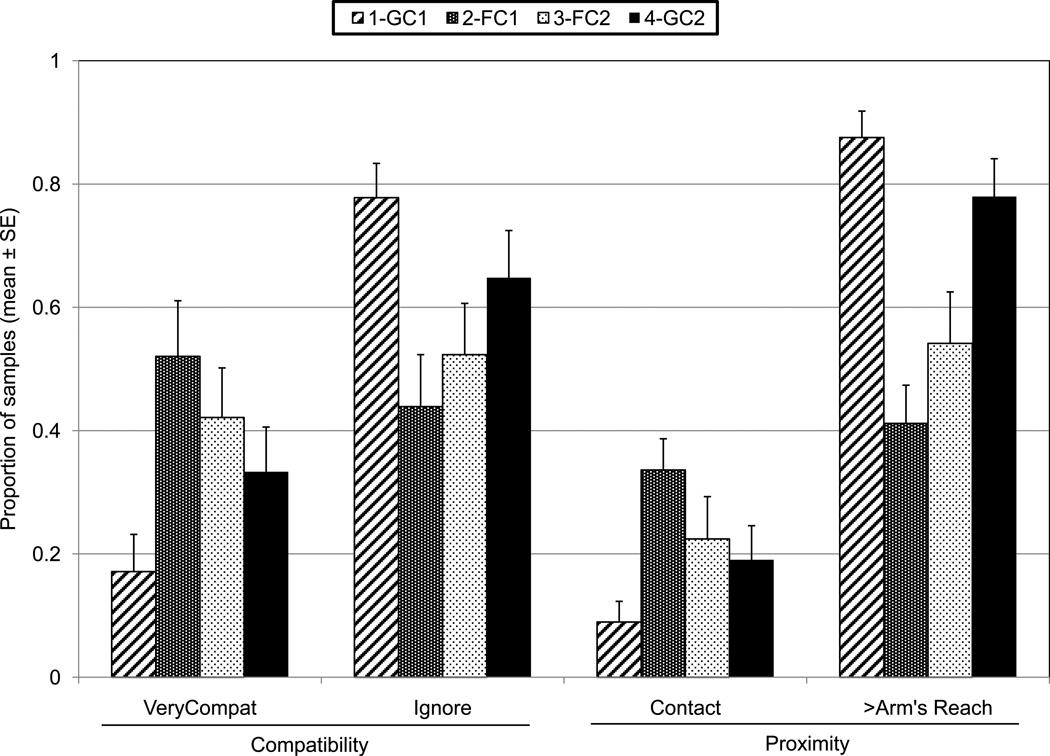
Compatibility ratings were based on the occurrence of aggressive and affiliative interactions during each 10-min coded segment, not just those occurring on the 15-s scan samples. Negative compatibility ratings (-- and -) were nearly absent, and compatible (+) was rarely scored and not normally distributed. Ignore and Very Compatible (++) were dependent variables in a GLM MANOVA with Phase and Pair as factors. Pair was significant overall (Wilks Lambda = 0.2748, F= 2.64, df = 22, 64, P = 0.0014) and for both compatibility ratings (P < 0.001). Phase was significant overall (Wilks Lambda = 0.55, F = 3.72, df = 6, 64, P = 0.003). Ignore was significant overall and occurred at significantly higher proportions in GC1 than in FC1 or FC2 (F = 7.86, df = 3, 33, P = 0.0004; Scheffe’s Post hoc tests: GC1 vs FC1, P = 0.001; GC1 vs FC2, P = 0.018). Very Compatible was significant overall and occurred at significantly lower proportions in GC1 than in FC1 or FC2 (F = 7.44, df = 3, 33, P = 0.0006; Scheffe’s Post hoc tests: GC1 vs FC1, P = 0.001; GC1 vs FC2, P = 0.025) (Fig. 6). Compatibility during GC2 did not differ from GC1 or either FC phase.
Fig. 6.
Compatibility and Proximity by phase, each averaged across 12 pairs. The error bars represent standard error.
3.3 Proximity
The proximity categories Contact and Greater Than Arm’s Length were dependent variables in a GLM MANOVA with Phase and Pair as factors. Pair was significant overall (Wilks Lambda = 0.35, F=2.01, df = 22, 64, P = 0.016) and for both proximity categories (P < 0.01). Phase was significant overall (Wilks Lambda = 0.2398, F = 11.1, df = 6, 64, P < 0.01). Contact was significantly higher in FC1 than GC1 (Scheffe Post hoc test, P = 0.002). Pairs were at greater than arm’s length during both GC phases than during the FC phases (Scheffe Post hoc tests: GC1 vs FC1, P = 0.000005; GC1 vs FC2, P = 0.0007; GC2 vs FC1, P = 0.00020; GC2 vs FC2, P = 0.02) (Fig. 6).
3.4 Same Cage during Run-Through Phases
GLM ANOVA was used to test whether the proportion of scans in the same cage differed between FC1 and FC2, with Phase and Pair as factors. Although the proportion declined between the two phases, the difference was not significant (FC1 mean = 0.58 ± 0.07 SE; FC2 mean = 0.51 ± 0.07 SE; F = 0.785, df = 1, 11, P = 0.395).
3.5. Return to Full Contact after Completion of Data Collection
All 12 pairs that had successfully transitioned to full contact in FC1 were returned to FC after the end of phase GC2. They remained compatible.
4. DISCUSSION
The data across four study phases found no significant lasting behavioral benefits to adult female Macaca fascicularis when housed in full contact rather than protected grooming-contact. Correlations between a pair’s prior time in grooming contact and the behavioral variables in the study phases were minimal. There were no significant differences in rates of abnormal behavior across all phases, and activity levels did not differ. We had anticipated confounding variables in the full contact phases due to each animal doubling its floor space while also gaining more options of where to be, objects to manipulate, and additional neighbors to interact with. However, there were no significant differences in rates of locomotion, manipulation, interaction with neighbors, or miscellaneous active behaviors between the housing types. This is consistent with past findings in M. fascicularis and M. nemestrina that a moderate increase in cage space for a single animal does not significantly enhance well-being assessed by behavior and urinary cortisol (Crockett et al., 1993, 1995, 2000). Moreover, when pairs reverted from full contact to GC housing, there was no increase in undesirable measures (rebound effect), e.g., abnormal behavior that is sometimes associated with the removal of enrichment (Bayne, 1992).
After switching to full contact, there was a spike in allogrooming, but this did not persist through the one-month later FC2 phase, suggesting a novelty effect rather than long-lasting improvement. If allogrooming is interpreted as a displacement behavior to relieve social tensions in a crowded or stressful situation (Judge, 2000) the short-lived allogrooming increase between phases GC1 and FC1 might suggest that the switch to FC was stressful. But as allogrooming increased, Groom Self decreased so the change could be attributed to a retargeting of self-grooming activities, rather than indicating that the switch to FC was stressful. The average levels of social- and self-grooming combined, 38% in grooming contact and 41% in full contact, are very similar to the 37% of the time budget spent in self-grooming reported for female longtailed macaques when singly housed (Crockett et al., 1995). The 37% self-grooming in the 1995 study was based on considerably more hours of data collection and was scored using a continuous sampling method rather than scan sampling.
Close proximity was associated with “very compatible” patterns across all housing phases and partners spent about half their time in the same cage during both full contact phases. A follow up to this study might examine behavior at night as proximity has been shown to vary between day and night: paired rhesus females spent 80% of time at night and 40% of the day in either physical contact or close proximity (Eaton et al., 1994).
Long-term behavioral profiles of compatible pairs should not be the only measure of interest when comparing the two forms of pair housing. The immediate risks and relative success rates between the two should be considered, a point also made by Baker et al. (in press). Our study found that the degree of tactile social contact provided can render a social pair compatible or incompatible. Two of 14 long-term grooming-contact pairs failed to complete the study owing to incompatibility upon introduction to full-contact housing. When screening potential female rhesus study subjects Baker et al. (in press) reported a protected-contact-to-full-contact failure rate of 36% compared to the 14% failure rate in this study of longtailed macaques. An interesting anecdote emerged when one of our failed pairs was deemed compatible when given a second chance at full contact pair-housing in larger cages. Two connected activity cages totaling 3.66 m2 of floor space provided 2.8 times more floor space and about twice the height than pairs were given during FC phases of this study. After 1 month in this housing situation the pair was switched to full contact in two standard-sized cages of the type used in the study but failed again due to aggression. Although just a single case, it illustrates difficulty in finding fully compatible pairs in situations where animals have complete access to each other, but are kept in a relatively limited space. In previous studies of adult M. fascicularis at WaNPRC, only 40% of male-male full-contact pairings were compatible after 2 weeks (Crockett et al., 1994); however, 89% of male-male pairings involving the same males were still compatible after 2 weeks in grooming-contact cages (Crockett et al., 1997). Individual housing with widely spaced bars between adjacent cages may improve compatibility for certain pairs as well as increasing a facility’s socialization success rate overall by allowing male-female pairings which we have found to be more successful than isosexual pairings (Crockett et. al., 2006; Lee et. al., 2005; Table 1).
In the laboratory setting, grooming-contact can accommodate partners with varied feeding schedules or diets, and allows monitoring of individual food intake/metabolic output for both health and research objectives whereas continuous full contact does not. GC also makes it easier for staff to identify and access individuals on a frequent basis, although convenience alone is no justification to preclude full contact housing for compatible pairs. It is important to consider the benefits of GC to monkeys as well. The presence of GC bars limits aggression and food monopolization, and prevents pursuit by a dominant animal. A study exploring the dominance effect in female longtailed macaques (Schaub, 1995) showed that dominant females could suppress competitive behavior of subordinate females only at shorter interindividual distances and that if the dominant was prevented from approaching closely then the effect was lost.
The inability of subordinate animals to physically avoid dominant individuals is associated with stress (Sapolsky, 2005). Studies have found social subordination in macaque groups to be a chronic psychosocial stressor as measured by effects on behavior (Shively et al., 1998), gonadal steroid modulation (Wilson et al., 2005), feeding habits, and reduced glucocorticoid negative feedback (Wilson et al., 2008). In addition to protecting subordinate animals from social stress, GC allows each individual more control to choose when they want to interact. Crockett et al. (1997) trained longtailed macaques to open a solid panel to gain access to grooming-contact panels. Both partners had to open their panels to choose to have tactile contact. Both members of female-female pairs opened their panels 93% of the time, male-female pairs 91% of the time, but male-male pairs only 58% of the time for access to unfamiliar males and 49% for familiar males. While recent changes to guidelines mandate higher degrees of tactile social contact, the guidelines also specify that animals should be given opportunities for control (European Parliament, 2009; National Research Council (ILAR), 2011; IPS, 2007).
It must be noted that partitions or panels providing limited tactile contact vary across centers and manufacturers. The terms “grooming-contact” or “protected contact” are applied to many configurations: holes drilled into sheets of metal or plastic (Baker et al., 2008; Watson, 2010), square or rectangular mesh of varied sizing, a grid of vertical 5.08 by 2.54 cm openings (Baker et al., in press). The grooming-contact bars developed at WaNPRC (Crockett et al., 1997) are spaced to provide a minimum of 5.16 cm-wide opening with increased spacing for larger cages and animals. The basic design criterion is to make the space as wide as possible, as long as it is narrower than the inhabitant’s head, so as to prevent entrapment. Bar placement is vertical because that orientation best accommodates grooming actions and the animal can extend most of its arm into the neighbor’s cage. Other studies reporting results for protected contact must be reviewed carefully to see how much tactile contact was actually provided; studies that allowed only minimal access between partners may not be directly comparable to the GC bars at WaNPRC which have always provided enough spacing for partners to embrace and play, thus promoting more species-typical behaviors than just grooming.
In a research environment, successful social pairings are frequently ended due to study needs such as one or both partners being assigned to different investigators, projects, or experimental groups. It is more likely for animals in GC to remain paired even if individuals are assigned to different projects. When pair turnover rates are high it may be a better behavioral management strategy to get as many individuals in stable GC pairs rather than expend limited time finding pairs that are compatible in full contact. Moreover, the process of social introduction can be a stressful event even in the absence of serious wounding, as evidenced by increased cortisol levels, behavioral changes, and cellular immune responses (Crockett et. al., 1994; Clarke et al., 1996; Clarke et. al., 1995; Gust et al., 1991; Line et al., 1996) suggesting it may not be in an animal’s best interest to be constantly introduced to unfamiliar individuals.
We found that GC (with widely spaced bars) was roughly equivalent to FC for social housing of adult female longtailed macaques and has been a useful tool for behavioral management staff to increase social housing in a research facility. Although FC did not show significant lasting benefits to GC for adult female M. fascicularis, full contact may be significantly more beneficial to juvenile or sub-adult monkeys who are more active and devote a greater amount of their social time to playing than adults. Social play is very important to behavioral development (Bekoff, 2001) so it is likely that young animals stand to benefit more from full contact.
There appear to be species differences in the relative benefits: Baker et al. (in press) found that female rhesus showed some behavioral benefits in FC over protected contact. Also, we have consistently found rhesus grooming-contact introductions to have significantly lower success rates than for M. fascicularis, M. nemestrina, and Papio (Crockett et al., 2006; Lee et al., 2005; Table 1). Our GC compatibilities for rhesus are considerably lower than some success rates published for rhesus in FC (Neu et al., 2007). It may be that rhesus macaque pairings are more likely to be compatible in full-contact housing that provides for unequivocal establishment of dominance. However, protected contact phases have been used during the establishment of compatible full-contact rhesus pairs (Doyle et al., 2008).
5. CONCLUSION
It is widely accepted that some tactile social contact is better than none. The female monkeys in this study averaged 14% of the time in social grooming while in grooming-contact, whereas none would be possible in single caging. Although the current wording of regulations mandating social contact for laboratory primates does not specifically address GC, it can be inferred that full contact is the stipulation. Still, GC with widely spaced bars is not truly single housing of the past and we found it to have no detrimental effects to adult female longtailed macaques when compared with full contact. In light of these results we believe it should be considered a viable option for social housing of adults when full contact is contraindicated (also see Coleman et al., in press). Grooming-contact provides a degree of social housing while allowing both partners choice and control, key concepts in contemporary animal welfare guidelines.
ACKNOWLEDGEMENTS
This study was conducted in compliance with USA animal care regulations and applicable national laws. The University of Washington is accredited by AAALACi. This research was supported by NIH RR00166. We would like to thank Rita U. Bellanca and two anonymous reviewers for their helpful comments. Thanks also to Kate Baker and Brooke Oettinger for collaboration and sharing their rhesus ethogram.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Grace H. Lee, Email: gracfish@wanprc.org, Washington National Primate Research Center.
Jinhee P. Thom, Email: jinheet@wanprc.org, Washington National Primate Research Center.
Katherine L. Chu, Email: katielchu@gmail.com, Washington National Primate Research Center.
Carolyn M. Crockett, Email: crockett@uw.edu, Washington National Primate Research Center.
REFERENCES
- Baker KC, Crockett CM, Lee GH, Oettinger BC, Schoof V, Thom JP. Pair housing for female longtailed and rhesus macaques in the laboratory: Behavior in protected contact versus full contact housing. J. Appl. Anim. Welf. Sci. doi: 10.1080/10888705.2012.658330. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Weed JL, Crockett CM, Bloomsmith MA. Survey of environmental enhancement programs for laboratory primates. Am. J. Primatol. 2007;69:377–394. doi: 10.1002/ajp.20347. [DOI] [PubMed] [Google Scholar]
- Baker K, Bloomsmith M, Neu K, Griffis C, Oettinger B, Schoof V, Clay A, Maloney M. Benefits of isosexual pairing of rhesus macaques (Macaca mulatta) vary with sex and are limited by protected contact but not by frequent separation. Am. J. Primatol. 2008;70(Suppl 1):44. [Google Scholar]
- Bayne K, Dexter S. Removing an environmental enrichment device can result in a rebound of abnormal behavior in rhesus monkeys (Macaca mulatta) Am. J. Primatol. 1992;27(1):15. [Google Scholar]
- Bekoff M. Social play behavior. J. Consciousness Studies. 2001;8:81–90. [Google Scholar]
- Bielitzki J, Susor TG, Elias K, Bowden DM. Improved cage design for single housing of social nonhuman primates. Lab. Anim. Sci. 1990;40(4):428–431. [PubMed] [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. Am. J. Primatol. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Czekala NM, Lindurg DG. Behavioral and adrenocortical responses of male cynomolgus and lion-tailed macaques to social stimulation and group formation. Primates. 1995;36(1):41–56. [Google Scholar]
- Clarke MR, Harrison RM, Didier ES. Behavioral, immunological, and hormonal responses associated with social change in rhesus monkeys (Macaca mulatta) Am. J. Primatol. 1996;39(4):223–233. doi: 10.1002/(SICI)1098-2345(1996)39:4<223::AID-AJP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Coleman K, Bloomsmith MA, Crockett CM, Weed JL, Schapiro S. Behavioral management, enrichment and psychological well-being of laboratory nonhuman primates. In: Abee CR, Mansfield K, Tardif SD, Morris T, editors. Nonhuman Primates in Biomedical Disease. 2nd ed. San Diego: Elsevier; In press. [Google Scholar]
- Crockett CM, Bowers CL, Sackett GP, Bowden DM. Urinary cortisol responses of longtailed macaques to five cage sizes, tethering, sedation, and room change. Am. J. Primatol. 1993;30(1):55–74. doi: 10.1002/ajp.1350300105. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Bowers CL, Bowden DM, Sackett GP. Sex differences in compatibility of pair-housed adult longtailed macaques. Am. J. Primatol. 1994;32(2):73–94. doi: 10.1002/ajp.1350320202. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Bowers CL, Shimoji M, Leu M, Bowden DM, Sackett GP. Behavioral responses of longtailed macaques to different cage sizes and common laboratory experiences. J. Comp. Psychol. 1995;109(4):368–383. doi: 10.1037/0735-7036.109.4.368. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Bellanca RU, Bowers CL, Bowden DM. Grooming-contact bars provide social contact for individually caged laboratory macaques. Contemp. Top. Lab. Anim. Sci. 1997;36(6):53–60. [PubMed] [Google Scholar]
- Crockett CM, Shimoji M, Bowden DM. Behavior, appetite, and urinary cortisol responses by adult female pigtailed macaques to cage size, cage level, room change, and ketamine sedation. Am. J. Primatol. 2000;52(2):63–80. doi: 10.1002/1098-2345(200010)52:2<63::AID-AJP1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Lee GH, Thom JP. Sex and age predictors of compatibility in grooming-contact caging vary by species of laboratory monkey. Int. J. Primatol. 2006;27(Suppl 1) Abst #417. [Google Scholar]
- Crockett CM, Ha RR. Data collection in the zoo setting, emphasizing behavior. In: Kleiman DG, Thompson KV, Baer CK, editors. Wild Mammals in Captivity: Principles and Techniques for Zoo Management. 2nd ed. Chicago: University of Chicago Press; 2010. pp. 386–405. [Google Scholar]
- Eaton G, Kelley S, Axthelm M, Iliff-Sizemore S, Shiigi S. Psychological well-being in paired adult female rhesus (Macaca mulatta) Am. J. Primatol. 1994;33(2):89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- Eversheds LLP. [Retrieved 5/12/11];Revised protection for animals used for scientific purposes. 2010 from www.eversheds.com/uk/home/articles/index1.page?ArticleID=templatedata\Eversheds\articles\data\en\Healthcare\Revised_protection_for_animals_used_for_scientific_purposes.
- European Parliament. European Directive 86/609/EEC, Amendments approved in the First Reading at the European Parliament in May 2009. [Retrieved 4/15/11];European Parliament web page. 2009 from http://www.europarl.europa.eu/sides/getDoc.do?type=TA&reference=P6-TA-2009-0343&language=EN&ring=A6-2009-0240.
- Doyle L, Baker K, Cox L. Physiological and behavioral effects of social introduction on adult male rhesus macaques. Am. J. Primatol. 2008;70(6):542–550. doi: 10.1002/ajp.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav. Immun. 1991;5(3):296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- International Primatological Society. IPS International Guidelines for the Acquisition, Care and Breeding of Nonhuman Primates. 2007. p. 76. [Google Scholar]
- Iredale SK, Nevill CH, Lutz CK. The influence of observer presence on baboon (Papio spp.) and rhesus macaque (Macaca mulatta) behavior. Appl. Anim. Behav. Sci. 2010;122:53–57. doi: 10.1016/j.applanim.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M, Prescott MJ. Refinements in husbandry, care and common procedures for non-human primates: Ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab Anim. 2009;43(Suppl. 1):1–47. doi: 10.1258/la.2008.007143. [DOI] [PubMed] [Google Scholar]
- Judge P. Coping with crowded conditions. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. Berkeley: Univ California Press; 2000. pp. 129–154. [Google Scholar]
- Lee GH, Thom JP, Crockett CM. Factors predicting compatible grooming-contact pairings in four species of laboratory monkey. Am. J. Primatol. 2005;66(suppl 1):83–84. [Google Scholar]
- Lehner P. Handbook of Ethological Methods. 2nd ed. New York: Cambridge Univ Press; 1996. p. 434. [Google Scholar]
- Line S, Kaplan J, Heise E, Hilliard J, Cohen S, Rabin B, Manuck S. Effects of social reorganization on cellular immunity in male cynomolgus monkeys. Am. J. Primatol. 1996;39(4):235–249. doi: 10.1002/(SICI)1098-2345(1996)39:4<235::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Novak MA. Environmental enrichment for nonhuman primates: theory and application. ILAR J. 2005;46(2):178–191. doi: 10.1093/ilar.46.2.178. [DOI] [PubMed] [Google Scholar]
- National Research Council (Institute of Laboratory Animal Resources) Guide for the Care and Use of Laboratory Animals. Washington D. C.: National Academy Press; 2011. [Google Scholar]
- Neu KA, Bloomsmith MA, Baker KC, Griffis C, Oettinger BC. Is pre-introduction behavior associated with the outcome of social introductions in rhesus macaques (Macaca mulatta)? Am. J. Primatol. 2007;69(Suppl. 1):52. [Google Scholar]
- Novak MA, Meyer JS, Lutz C, Tiefenbacher S. Deprived environments: Developmental insights from primatology. In: Mason G, Rushen J, editors. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. 2nd Edition. Wallingford, UK: CABI; 2006. pp. 153–189. [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, McCowan B. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. J. Appl. Welf. Sci. 2009;12:61–72. doi: 10.1080/10888700802536798. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schaub H. Dominance fades with distance: an experiment on food competition in long-tailed macaques (Macaca fascicularis) J. Comp. Psychol. 1995;109(2):96–202. doi: 10.1037/0735-7036.109.2.196. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior and central monoaminergic function in female cynomolgus monkeys. Biol. Psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- U. S. Department of Agriculture. Animal Welfare, Standards, Final Rule (Part 3, Subpart D: Specifications for the humane handling, care, treatment, and transportation of nonhuman primates) Fed Register. 1991;56(32):6495–6505. [PubMed] [Google Scholar]
- Velleman PF. Learning Data Analysis with Datadesk for Windows 6.0. San Francisco: Addison Wesley Benjamin-Cummings; 1997. [Google Scholar]
- Wilson ME, Legendre A, Pazol K, Chikazawa K. Gonadal steroid modulation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: Social status effects on caloric consumption. Physiol. Behav. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]