Abstract
The once-heretical concept that a misfolded protein is the infectious agent responsible for prion diseases is now widely accepted. Recent exciting research has led not only to the end of the skepticism that proteins can transmit disease but also to expanding the concept that transmissible proteins might be at the root of some of the most prevalent human illnesses. At the same time, the idea that biological information can be transmitted by propagation of protein (mis)folding raises the possibility that heritable protein agents may be operating as epigenetic factors in normal biological functions and participating in evolutionary adaptation.
The discovery that proteins can behave like infectious agents to transmit disease is a significant milestone in biology. The unorthodox prion hypothesis was proposed decades ago to explain the surprising transmission mechanisms of a group of rare diseases known as transmissible spongiform encephalopathies (TSEs), or prion diseases (Griffith, 1967; Prusiner, 1982). The prion hypothesis states that the infectious agent in TSEs is composed exclusively of a misfolded form of the prion protein (PrPSc), which replicates in infected individuals by transforming the normal version of the prion protein (PrPC) into more of the misfolded isoform (Prusiner, 1998). This hypothesis remained controversial for decades, but recent studies have settled all doubts by demonstrating that infectious material can be generated in vitro, in the absence of genetic material, by replication of the protein misfolding process (Legname et al., 2004; Castilla et al., 2005; Deleault et al., 2007; Wang et al., 2010). Despite the obvious differences between prions and conventional infectious micro-organisms (e.g. bacteria or viruses), prions exhibits the typical characteristics of bona-fide infectious agents, namely: exponential multiplication in an appropriate host, transmission between individuals by various routes including food-borne and blood-borne, titration by infectivity bioassays, resistance to biological clearance mechanisms, penetration of biological membrane barriers, “mutation” by structural changes forming diverse strains, and transmission controlled by species barriers. Despite that prions fulfill the Koch's postulates for infectious agents, it remains surprising that a single protein possesses the complexity and flexibility required to act like living micro-organisms that transmit disease.
Prion replication requires exposure to tiny quantities of PrPSc, present in the infectious material, to trigger the auto-catalytic conversion of host PrPC to PrPSc. This process follows a crystallization-like model in which the infectious particle (a small PrPSc aggregate) acts as a nucleus to recruit monomeric PrPC into the growing PrPSc polymer (Lansbury, Jr. and Caughey, 1995). A key step in prion replication is the breakage of large PrPSc aggregates into many smaller seeding-competent polymers that amplify the prion replication process, resulting in the exponential accumulation of PrPSc (Saborio et al., 2001). This seeding-nucleation mechanism of prion propagation has been reproduced in vitro to “cultivate” prions with infectious properties when inoculated into animals (Castilla et al., 2005; Deleault et al., 2007; Wang et al., 2010). Additional research is needed to elucidate the precise mechanisms and cellular factors required for prion replication in vivo as well as the detailed structure of the infectious folding of the prion protein.
The prion principle in other Protein Misfolding Disorders
The transformation of a natively folded protein into a misfolded, toxic form that causes tissue damage and disease is not a mechanism exclusive to prion diseases. Misfolded protein aggregates are implicated in more than 20 human diseases, collectively called protein misfolding disorders (PMDs), including highly prevalent and insidious illnesses such as Alzheimer's disease, Parkinson's disease, and type 2 diabetes (Soto, 2003; Chiti and Dobson, 2006). Although the proteins implicated in each of these pathologies and the clinical manifestations of the diseases differ, the molecular mechanism of protein misfolding is strikingly similar. Unfortunately, despite the extensive knowledge about the molecular basis of these disorders, the factors that trigger protein misfolding and initiate the disease pathologies remain unknown.
The protein misfolding processes in other PMDs share the same replication mechanism and result in the formation of similar intermediates and end-products as the PrPC to PrPSc conversion in prion diseases. The protein conformational changes associated with the pathogenesis of PMDs produce βsheet rich oligomers partially resistant to proteolysis, and with a high tendency to form amyloid-like aggregates (Soto, 2003; Chiti and Dobson, 2006). Interestingly, the available data indicate that misfolding and aggregation processes in PMDs follow a seeding-nucleation mechanism (Harper and Lansbury, Jr., 1997; O'Nuallain et al., 2004; Soto et al., 2006). The process is initiated by the slow interaction between protein monomers to form a stable oligomeric nucleus (or seed) around which a faster phase of polymeric elongation occurs. The limiting step in this process is nuclei formation and the rate of misfolding and aggregation depends upon the number of seeds produced (Harper and Lansbury, Jr., 1997). As described above, the key element that makes PrPSc infectious is its ability to act as a seed to induce the conversion of PrPC to PrPSc (Lansbury, Jr. and Caughey, 1995; Soto et al., 2006). In the same way as PrPSc, extensive evidence indicate that oligomeric structures of the proteins implicated in PMDs are able to seed accelerated misfolding and aggregation of the natively folded monomeric protein (O'Nuallain et al., 2004).
Given the striking similarities between the pathological mechanisms of TSEs and other PMDs, a critical question is whether other PMDs are transmissible and the proteins implicated may also behave as infectious agents. In this article we refer to the proteins able to propagate biological information by the prion principle as “transmissible proteins” to avoid the misunderstanding produced by calling them prions. It is important to emphasize that the concept of transmissible proteins not necessarily implies that the outcome of the transmission of protein misfolding should be a disease, but as described in the sections below, transmissible proteins could also be implicated in normal biological processes or even provide an evolutionary tool for environmental adaptation.
The putative transmissibility of PMDs has not been analyzed in detail, but the lack of epidemiological data supporting disease transmission is often used to rule out an infectious origin for these diseases. However, it is likely that without the fortuitous transmission of sheep scrapie in 1937 (Cullie and Chelle, 1939) or Gadjusek's milestone discovery of kuru transmission by cannibalism (Gajdusek et al., 1966), an infectious origin for TSEs might have never been suspected. Indeed, epidemiological studies of relatives and people in contact with Creutzfeldt-Jakob disease (CJD) patients consistently produce negative results. Epidemiological tracking of an infectious origin for these diseases can be complicated by variable and extended time between exposure to the infectious agent and the onset of clinical symptoms, especially when this interval can be decades, as is typical for human TSEs.
A series of recent studies have shown experimental evidences for prion-like mechanisms of pathological transmission in various common neurodegenerative diseases (Table 1). Alzheimer's disease (AD) is associated with the misfolding and aggregation of two proteins: amyloid-beta (Aβ) accumulation in extra-cellular amyloid plaques, and hyperphosphorylated Tau, which forms neurofibrillary tangles inside neurons. To assess the possibility that AD pathology might be transmissible by a prion-like mechanism, transgenic mice expressing the human amyloid protein were injected intra-cerebrally with diluted brain homogenates derived from AD patients (Kane et al., 2000; Meyer-Luehmann et al., 2006). The results clearly showed accelerated Aβ-deposition in the brain of inoculated animals. Control experiments depleting the material from Aβ aggregates or inactivating their conformation did not produce acceleration of the pathology, indicating that pre-formed Aβ aggregates were required to seed amyloid plaque deposition in vivo (Meyer-Luehmann et al., 2006). Reminiscent to prions, seed capable Aβ aggregates are partially resistant to proteolysis and consists of a continuum of various sizes with the most efficient seeds being smaller Aβ oligomers (Langer et al., 2011). However, unlike prion disease, which can be induced de novo in animals that do not spontaneously develop the pathology, the induction of Aβ deposition observed in these studies only represents an acceleration of few months of the spontaneous process that was set to occur by introduction of the mutant gene. Recent experiments performed in transgenic animals expressing low levels of wild type human amyloid precursor protein found that disease alterations can be induced in animals that without exposure to this material will never develop the pathology during their entire life span (Morales et al., 2011; Rosen et al., 2011), getting much closer to the bona fide prion transmission observed in TSEs. Another important step forward in the similarities between Aβ and prion transmission was the demonstration that AD brain abnormalities could be induced by intra-peritoneal inoculation of transgenic mice with Alzheimer's brain extracts (Eisele et al., 2010). This finding suggests that seeds acquired by peripheral route of exposure may induce disease in the brain. However, since the source of misfolded Aβ used in these experiments is sick brain homogenates, the relevance of these findings for AD transmissibility is uncertain. An important step forward in this direction is the recent observation that AD pathological and behavioral abnormalities could be induced in vivo by blood transfusion (Morales et al., 2012), a medically relevant route that has been shown to transmit prion diseases in animals and humans (Hunter et al., 2002; Peden et al., 2005). These results suggest that seeding-capable Aβ aggregates are circulating in blood, which can get access to the brain in quantities sufficient to promote Aβ misfolding and aggregation and related neuropathological alterations. These findings get much closer the possibility that prion-like transmission of AD may be occurring under “real life” conditions. However, since the experiments were done in transgenic mice that artificially over-express a human mutated version of the amyloid protein, caution must be exercised in the extrapolation of these findings to humans.
Table 1.
Potential candidate disease-associated transmissible proteins.
| Disease | Protein (location) | Experimental Transmission | Natural Transmission | References* |
|---|---|---|---|---|
| Prion diseases | PrPSc (extracellular) | Infectious in diverse animal species by various routes | Infectious in diverse species by various routes | (Prusiner, 1998; Aguzzi and Calella, 2009) |
| Alzheimer's disease | Aβ (extracellular) | Induction of pathology in transgenic mice by intracerebral and intraperiotoneal inoculation | Not shown | (Kane et al., 2000; Meyer-Luehmann et al., 2006; Eisele et al., 2010; Morales et al., 2011) |
| Parkinson's disease | α-synuclein (cytoplasmatic) | Cell-to-cell and host-to-graft spreading in animal models | Host-to-graft spreading in humans | (Desplats et al., 2009; Luk et al., 2009; Volpicelli-Daley et al., 2011; Mougenot et al., 2011; Hansen et al., 2011) |
| Huntington's disease | Huntingtin (nuclear) | Cell-to-cell spreading in culture | Not shown | (Ren et al., 2009) |
| Tauopathies | Tau (cytoplasmatic) | Cell-to-cell spreading in culture and transmission in transgenic mice by intracerebral inoculation | Not shown | (Clavaguera et al., 2009; Frost et al., 2009; Nonaka et al., 2010; Guo and Lee, 2011) |
| Secondary amyloidosis | Amyloid-A (extracellular) | Acceleration of pathology in mice by various routes of administration | Possible transmission to captive cheetah | (Lundmark et al., 2002; Zhang et al., 2008) |
| Mouse senile amyloidosis | Apolipoprotein A (extracellular) | Acceleration of pathology in mice by various routes of administration | Transmission to mice in the same cage by feces consumption | (Xing et al., 2001; Korenaga et al., 2006) |
There are several more references that could have been cited, but for space constraints only the most relevant articles are listed.
Various studies have been also performed to analyze the transmission by seeding of Tau aggregates, the other typical feature of AD, which is also found in other neurodegenerative diseases, collectively called tauopathies (e.g. Fronto-temporal dementia, chronic traumatic encephalopathy, etc). Intra-cerebral injection of brain extract containing Tau aggregates into transgenic mice expressing human wild type Tau that do not form aggregates spontaneously, induced the assembly of native Tau into misfolded aggregates in recipient mice (Clavaguera et al., 2009). Interestingly, the pathology spread over time beyond the site of injection to anatomically connected neighboring brain regions (Clavaguera et al., 2009). Unlike Aβ and PrPSc, Tau aggregates are located in the cytoplasm, suggesting that in this case, protein misfolding was transmitted from outside to inside the cell. This hypothesis is further supported by in vitro studies of cultured cells in which extracellular Tau aggregates were taken up and induced the misfolding and aggregation of intracellular Tau (Frost et al., 2009; Nonaka et al., 2010; Guo and Lee, 2011). These intracellular Tau aggregates also spread among cells to extend the pathology to the entire culture.
Several exciting neuropathological studies in Parkinson's disease (PD) patients additionally supports the hypothesis that prion-like spreading of the pathology is a common mechanisms in PMDs. PD is characterized by the accumulation of intra-cytoplasmic aggregates (termed Lewy bodies) made of α-synuclein and primarily located in the substantia nigra. Autopsies of PD patients who received grafts from healthy embryonic neurons many years before showed that some transplanted neurons contained α-synuclein aggregates (Li et al., 2008; Kordower et al., 2008). Interestingly, studies designed to model the human in vivo observations showed that transgenic mice developing α-synuclein inclusions propagated the pathology to healthy neuronal grafts (Desplats et al., 2009; Hansen et al., 2011). This conclusion is supported by in vitro experiments demonstrating the induction of Lewy body-like aggregates in neurons in culture by exposure to α-synuclein aggregates (Luk et al., 2009; Nonaka et al., 2010; Volpicelli-Daley et al., 2011). Moreover, co-culture experiments showed transmission of misfolded α-synuclein aggregates among cells in culture (Desplats et al., 2009). Finally, intra-cerebral inoculation of transgenic mice expressing human α-synuclein with extracts from old mice showing motor clinical signs due to accumulation of synuclein aggregates triggered an early onset of disease (motor alterations and accumulation of protein aggregates), compared with uninoculated mice that remained healthy (Mougenot et al., 2011). These findings suggest that α-synuclein pathology is spreading in the brain by a prion-like transmission process in which intra-cellular aggregates gain access to the extracellular space either by secretion or by damage of the host cell and get internalized to neighboring cells -most likely through endocytosis- where they bind the normally folded, soluble protein and template the misfolding process. It is also possible that cell-to-cell transmission occurs through direct cellular contact, involving nanotubes, or mediated by exosomes or microvesicles (Aguzzi and Rajendran, 2009). Similar findings have been also reported with other misfolded proteins, such as huntingtin, superoxide dismutase, and TDP-43, associated to Huntington's disease and amyothropic lateral sclerosis (Ren et al., 2009; Munch et al., 2011; Grad et al., 2011; Furukawa et al., 2011).
Transmission of protein misfolding may provide a mechanistic explanation for the well-known, but puzzling observation that pathological changes in various neurodegenerative diseases progress with time in a step wise characteristic anatomical pattern. Neuropathological studies by Braak et al and various other groups have shown that neurofibrillary tangles in AD and Lewy bodies in PD initiate very early in the disease in a circumscribed area of the brain and pathology progresses in a topographically predictable manner through anatomical connections (Braak and Braak, 1991; Braak et al., 1996; Brundin et al., 2008). The defined spatiotemporal progression of the lesions may well be explained by spreading of misfolded proteins between cells and by axonal transport between different brain regions to propagate the pathology by transmission of protein misfolding.
The studies described above have come a long way in demonstrating that protein misfolding may be responsible for the pathogenesis and transmission of many PMDs. Furthermore, these findings strongly suggest that at least some aspects of the prion behavior, such as the molecule-to-molecule transmission of protein misfolding and the cell-to-cell spreading of the pathology, are indeed occurring in other PMDs. In this sense, it is important to consider the possibility that, instead of being exogenously acquired by infection, the first seeds might be generated inside the body by an unrelated biological process, such as transcriptional/translational errors or tissue injury (e.g. brain trauma or subclinical stroke). In the absence of prion-like spreading, a small amount of misfolded proteins generated in this way would be short-lived and have not the long-lasting consequences that the prion transmission process would produce. Another aspect that needs to be considered is that misfolded aggregates composed of one protein may interact and promote the aggregation of another protein by a phenomenon known as cross-seeding. Evidence for this phenomenon has been found for several PMDs, using animal models, in vitro systems, and human epidemiological analysis (see (Morales et al., 2009) and references therein). Thus, exposure to one misfolded protein may lead to development of another PMD, further complicating epidemiological tracking of a putative infectious origin of these diseases.
What makes a misfolded protein become an infectious agent?
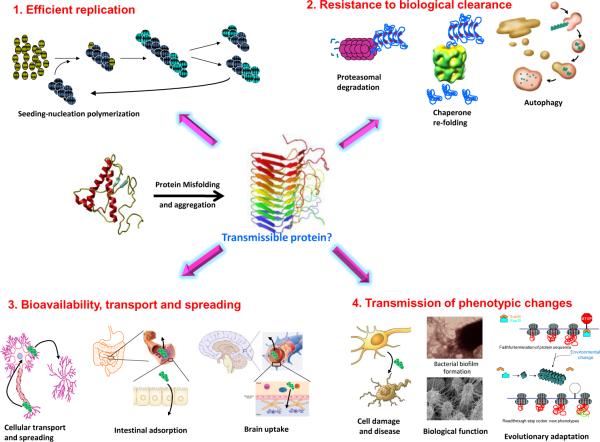
In spite of the substantial advance over the past few years, the central question remains: are other PMDs transmissible beyond the experimental setting and the misfolded proteins bonafide infectious agents? To answer this question, we must understand the requirements for a misfolded protein to become an infectious agent. Based on the knowledge accumulated from studies of mammalian prions, the following properties are likely required (Fig. 1):
The transmissible protein must faithfully and efficiently self-propagate. As discussed above, the seeding-nucleation mechanism of protein misfolding and aggregation allows proteins to replicate their folding characteristics. Notably, the most efficient transmissible proteins might be those that form aggregates stable enough to not spontaneously disassemble yet not so stable to permit frequent fragmentation to multiply seeds.
The transmissible protein must survive biological clearance mechanisms. The net accumulation of misfolded aggregates depends on a delicate equilibrium between new aggregate formation and their elimination via several biological processes including metabolism, excretion, degradation, and correction of protein misfolding. Indeed, several cellular pathways eliminate misfolded, nonfunctional proteins, including the unfolded protein response, the endoplasmic reticulum associated protein degradation pathway, and autophagy. The high resistance of PrPSc to proteases and extreme conditions may be key to the efficiency of prions as infectious agents.
The transmissible protein must reach the site of replication and pathology. For PrPSc to produce TSEs, the agent must enter the brain in quantities sufficient to trigger the slow but progressive prion replication process. Moreover, when prions are acquired by consumption of contaminated food, the transmissible protein must resist degradation in the digestive system and penetrate the intestinal barrier. For misfolded proteins that accumulate intracellularly, the transmissible protein must be transferred from one cell to another, or after cellular lysis, from outside to inside the cell.
The transmissible protein must trigger a phenotypic change, which in the case of disease-associated proteins is a toxic process resulting in cell damage, organ dysfunction, and disease. Although accumulation of misfolded proteins is clearly associated with cytotoxicity, we remain far from understanding the mechanism(s) by which misfolded aggregates produce disease. Several pathways have been proposed including loss of the biological function of the natively folded protein, formation of ionic channels, activation of receptor-mediated signaling cascades, and sequestration of essential cellular factors. For transmissible proteins not associated to a disease, the self-propagating misfolding must result in a functional change, which sometimes may contribute to evolutionary adaptation (see below).
Figure 1. Properties required for misfolded aggregates to behave as transmissible proteins.
Based on the knowledge of the factors controlling prion transmission in vivo, it is likely that the following characteristics are needed for spreading of misfolded proteins: 1) Efficient replication by the seeding-nucleation process. 2) Resistance to biological clearance, which in the figure is illustrated by proteasomal degradation, chaperone re-folding and autophagy, although it is likely that other pathways might be also operating. 3) Ability to reach the target tissue and cellular location, which is represented in the figure by cellular transport and spreading, and penetration across the intestinal and blood-brain barriers. The biological barriers to be surmounted by the transmissible protein depend on the specific disease, sub-cellular location of the misfolded aggregates and routes of exposure. 4) Capability to transmit a biological change. For disease-associated misfolded proteins the change is cellular damage, tissue dysfunction and clinical disease and for functional infectious proteins, the phenotypic change is the modulation of a biological activity or acquisition of a new function. In the figure, functional changes are illustrated with the case of the Curli bacterial amyloid involved in biofilm formation (Chapman et al., 2002) and with the regulation of translational termination by Sup35 in yeasts that may be implicated in evolutionary adaptation to environmental changes (True and Lindquist, 2000).
The recent developments in the field have demonstrated that misfolded proteins associated with various PMDs can initiate the conversion of the normal form of the protein into the misfolded form and propagate these changes to neighboring cells in experimental models. The exciting goal for future research is to determine if misfolded proteins implicated in PMDs are infectious and transmit disease under natural conditions. In other words, we need to carefully assess whether misfolded proteins are transmitted between individuals and propagate within communities as conventional infectious agents. Despite the excitement generated by the recent findings, the strongest evidence for transmissibility in PMDs other than TSEs was generated by earlier studies in secondary reactive amyloidosis, associated with amyloid-A protein aggregation (Lundmark et al., 2002), and mouse senile amyloidosis, which is related to apolipoprotein AII aggregation (Xing et al., 2001) (Table 1). In these diseases, even tiny quantities of misfolded aggregates can be transmitted between individuals and cause disease by diverse routes, including blood transfusion and oral administration. In the case of amyloid-A amyloidosis, evidence also exists for natural transmission in captive cheetah populations (Zhang et al., 2008).
Functional transmissible proteins: a new biological paradigm?
Considering that several diseases might be transmissible by propagation of protein misfolding, an intriguing question arises: why transmissible proteins have not been eliminated during evolution? One possible explanation suggested by Dobson is that these diseases are post-evolutionary disorders, since disease onset usually occurs beyond the reproductive age of patients (Dobson, 2002). An alternative possibility is that transmissibility of protein misfolding may be a common process in biology that plays useful functions in healthy cells. In other words that many transmissible proteins are constantly operating in organisms, silently providing beneficial functions and that the disease outcome produced in some cases is just a degeneration of a normal biological process.
The view that the phenomenon of transmission of biological information by propagation of protein misfolding was exclusive to prion pathologies changed dramatically with the discovery of proteins behaving like prions in yeasts and other fungal organisms (Table 2). In yeast, transmissible proteins do not behave like an infectious agent, killing their host cells to produce disease; they are transmitted from parent cells to progeny acting like epigenetic factors to produce new metabolic phenotypes (Lindquist, 1997; Wickner et al., 2004). However, like mammalian prions, yeast prions are proteins that have acquired an abnormal conformation and propagate by inducing their normal protein counterparts to adopt the same altered conformation. Thus, in both cases proteins act in a manner previously thought to be unique to nucleic acids, in the mammalian case as transmissible agents of disease, and in yeasts as heritable determinants of phenotype.
Table 2.
Functional amyloids and potential candidates for biological transmissible proteins*.
| Protein (organism) | Protein function | Changes produced by misfolded state | Transmissibility | References** |
|---|---|---|---|---|
| Sup35 (Yeasts) | Translation termination | Reduced translational fidelity; Increased survival in fluctuating environments | Transmitted from parent to daughter cells during cell division; Synthetic prions produced in vitro | (Wickner et al., 1995; Derkatch et al., 1996; Patino et al., 1996; Tanaka et al., 2004) |
| Ure2 (Yeasts) | Nitrogen catabolism | Indiscriminate utilization of nitrogen sources | Transmitted from parent to daughter cells during cell division; Synthetic prions produced in vitro | (Wickner, 1994; Masison et al., 1997; Brachmann et al., 2005) |
| Rnq1 (Yeasts) | Unknown | Induction of other prions | Transmitted from parent to daughter cells during cell division | (Sondheimer and Lindquist, 2000; Patel and Liebman, 2007) |
| HET-S (P. anserina) | Heterokaryon incompatibility | Inhibits mycelia fusion | Transmitted from parent to daughter cells during cell division; Synthetic prions produced in vitro | (Coustou et al., 1997; Maddelein et al., 2002) |
| CPEB (Aplysia) | Translational control of synapse-specific mRNAs | Maintains long-term synapse facilitation | Transmitted from parent to daughter cells during cell division in yeast; Synthetic prions produced in vitro | (Si et al., 2003; Si et al., 2010; Heinrich and Lindquist, 2011) |
| MAVS (Humans) | Anti-viral signaling | Binding to the transcription factor IRF3 and activation of innate immunity | Transmission in purified mitochondrial cultures | (Hou et al., 2011) |
| Curli (Bacteria) | Biofilm formation | Biofilm formation, host invasion | Not shown | (Chapman et al., 2002) |
| Chaplins (Bacteria) | Modulation of water surface tension | Aerial hyphae formation | Not shown | (Claessen et al., 2003) |
| Microcin (Bacteria) | Bacteriotoxin | Inhibit bacteriotoxic activity | Not shown | (Bieler et al., 2005) |
| Chorion proteins (Insects and fish) | Formation of eggshell | Protection of eggshell | Not shown | (Iconomidou et al., 2000) |
| Spidroins and other silk proteins (Spiders) | Silk formation | Structural component of spider web | Not shown | (Kenney et al., 2002) |
| Various peptide hormones (Humans) | Hormonal activity | Storage in pituitary secretory granules | Not shown | (Maji et al., 2009) |
| Fragments of prostatic acidic phosphatase | Phosphatase activity | Capture and promote HIV virion attachment to target cells | Not shown | (Munch et al., 2007) |
| Pme17 (Humans) | Pigment formation | Scaffolding and sequestration of toxic products in melanin synthesis | Not shown | (Fowler et al., 2006) |
There are more than 20 other yeast proteins that are not listed here, which have been proposed to act as prions (for a reference, see (Halfmann et al., 2010)
There are several more references that could have been cited, but for space constraints only the most relevant articles are listed.
The discovery of transmissible proteins in yeast expanded the prion concept and showed that transmission of protein misfolding can also participate in normal biological processes in addition to their established roles in disease pathology. Currently, the number of proteins utilizing the prion mechanism to perform their biological functions is unknown, but the regulation of protein function by conversion to alternatively folded forms without the need for genetic changes offers many advantages likely to be conserved. A major goal of future research will be to identify other transmissible proteins that perform useful functions in healthy cells. Identification of new transmissible proteins is a challenging task, especially when the result is not a dramatic disease as in TSEs or a weird genetic property as in yeast prions, but just a slight change in the biological function of a particular protein. A recent genome-wide study using bioinformatic techniques enabled to identify ~200 prion candidates in S. cerevisiae, out of which at least 24 contain a prion-forming domain, able to aggregate into amyloid fibrils and confer prion characteristics to Sup35 (Alberti et al., 2009).
In an interesting study, Kandel's group reported prion-like properties of a neuronal member of the CPEB family (cytoplasmic polyadenylation element binding protein) in aplysia, which regulates mRNA translation (Si et al., 2003; Si et al., 2010). They hypothesize that CPEB conversion to a prion-like state in stimulated synapses helps maintain long-term synaptic changes associated with memory storage. Interestingly, unlike most yeast prions which propagate their phenotypic changes by the loss of function of the normally folded protein, it is the prion form of CPEB that is the active form. A recent study showed that the yeast CPEB homolog in the absence of any neuronal factors can stably adopt either active or inactive states (Heinrich and Lindquist, 2011). Moreover, yeast CPEB can adopt several distinct activity states or “strains” that can remain stable for a long time (Heinrich and Lindquist, 2011). Finally, purified recombinant CPEB aggregated into amyloid fibrils can transform the endogenous CPEB into the functional form in yeast. These findings suggest that CPEB employs a prion mechanism to create stable, finely tuned self-perpetuating biochemical memories.
The most recent addition to the functional prion arsenal was reported by Hou and colleagues (Hou et al., 2011). The mitochondrial protein MAVS, which plays a critical role in antiviral signaling, acquires conformational changes upon viral infection, leading to the formation of large protein aggregates that propagate in cultured cells to induce an efficient innate immune response. Strikingly, recombinant MAVS protein can form fibrillar aggregates in vitro and recruit endogenous MAVS into the functional form in purified mitochondria (Hou et al., 2011). The prion amplification cascade may provide a mechanistic explanation for the high efficiency of this anti-viral pathway, which can be activated with less than 20 molecules of viral RNA (Zeng et al., 2010). The utilization of the prion-like mechanism for this critical signaling pathway is another elegant example of the high potential of functional transmissible proteins in biology.
A common characteristic of all the so far known transmissible proteins is that the misfolded version forms β-sheet-rich aggregates that resemble amyloid fibrils. Protein oligomerization in β-sheet structures is not only a typical characteristic of prions, but the key element that enables them to propagate by a seeding mechanism. Therefore, one way to identify novel “functional transmissible proteins” is to search for proteins that form β-sheet amyloid-like aggregates in their normal cellular functioning. Functional amyloids have been described in various organisms from bacteria to humans (Table 2). Acquisition of the amyloid structure has been shown to participate in a variety of functions including biofilm development, regulation of cytotoxicity, modulation of water surface tension, formation of spider webs, eggshell protection, enhancing HIV viral infection, modulation of melanin biosynthesis and storage of peptide hormones (Fowler et al., 2007). Although the proteins involved in these processes form β-sheet-rich amyloid-like aggregates following a seeding-nucleation model of polymerization, additional research is needed to determine whether these proteins naturally propagate their phenotype using the prion principle.
The notion that protein misfolding and aggregation into amyloid structures is associated with a small family of proteins has dramatically changed in recent years. The pioneering work of Dobson and colleagues has demonstrated that many (if not all) proteins can form β-sheet intermolecular interactions to adopt an amyloid-like conformation under appropriate conditions (Chiti and Dobson, 2006). Even prototype globular and α-helical proteins can form amyloid structures, arguing that amyloid is the primordial conformation of proteins (Dobson, 2002). Strikingly, the structures formed and the underlying mechanism of misfolding and aggregation (following a seeding-nucleation model) are similar to those associated to PMDs. These findings suggest that any protein has the potential to form misfolded aggregates under appropriate conditions, which then could replicate by the prion principle. Taken together, these results form the basis of a general biological principle whereby protein function and activity might be regulated by folding changes that can be rapidly propagated from protein to protein. An exciting future research goal is, therefore, to determine if the prion phenomenon of propagation of biological information is indeed a widespread process in biology.
A role for transmissible proteins in evolution
The rapid and auto-catalytic transformation of a protein to an alternate structure may also be used to adapt to challenging environmental conditions. In this way, living organisms can have the flexibility to change rapidly, providing a mechanism for intra-generational evolution without the need for long term genetic changes. This evolutionary mechanism based on transmissible proteins can be used as a strategy for rapid adaptation and survival of organisms facing new or short-term environmental challenges, prior to more permanent adaptation by natural selection of genetic modifications (Halfmann et al., 2010). A possible example of this evolutionary mechanism can be found in the Sup35 yeast prion (True and Lindquist, 2000). When Sup35 is converted to the alternatively folded and aggregated form, it allows the temporary translation of normally untranslated DNA sequences. This mechanism enables very rapid switches between distinct phenotypes, facilitating environmental adaptation and the development of new evolutionary traits (True and Lindquist, 2000). Interestingly, several other yeast prions have been shown to modulate gene expression (Halfmann et al., 2010). This effect may multiply the biological and adaptive consequences of responses to environmental changes that lead to the formation of the prion state. The selective enrichment of transmissible proteins (at least in yeasts) among proteins that modulate genetic control, suggests that molecular switches based on self-propagating infectious proteins may be very effective in producing biological diversity upon which natural selection and evolution may occur.
Since transmissible proteins depend on the propagation of changes in protein conformation, it is not surprising that fluctuating environments trigger prion mechanisms. Indeed, protein folding is highly responsive to changes in environmental conditions such as temperature, pH, salinity, and metal ion concentration. Thus, alterations in these parameters may lead to protein misfolding and formation of oligomeric seeds with the consequent rapid propagation of the misfolding event by the prion principle. Another way by which the formation of misfolded transmissible proteins may be controlled by environmental changes is through stimulation of cellular protein quality control pathways. The emergence of diverse prions and amyloids is tightly regulated by the cellular signaling pathways involved in protein folding. A particularly important role in this sense is played by molecular chaperones, which usually act to prevent, correct, or eliminate misfolded proteins (Broadley and Hartl, 2009). However, in some cases molecular chaperones may facilitate the propagation of protein misfolding. For example, the Hsp104 chaperone enhances replication of various yeast prions by facilitating fragmentation of large polymers and multiplying the number of infectious nucleating seeds (Wegrzyn et al., 2001).
Thus, environmental changes may directly or indirectly induce protein misfolding events that rapidly propagate by the prion mechanism, resulting in phenotypic changes that may enable an organism to better adapt to new conditions. The phenotypic changes can result from loss of the biological function of the correctly folded protein or from the acquisition of new biological functions after misfolding. Interestingly, both mammalian and yeast prions appear in various forms, often called strains in analogy to genetic strains of traditional infectious agents (Collinge and Clarke, 2007). These prion strains are slightly different conformers of the infectious protein that can either replicate at a different rate or produce a distinct phenotypic outcome. From an evolutionary viewpoint, the possibility that one type of environmental change may lead to a variety of phenotypic manifestations, enhances the chances of beneficial evolutionary adaptation. At present, the idea that propagation of protein misfolding contributes to evolution is mostly speculative and more research is needed to assess this intriguing possibility.
Acknowledgements
I thank my lab members Rodrigo Morales, Dennisse Gonzalez-Romero, Marcelo Barria, Akihiko Urayama, and Rodrigo Diaz-Espinoza for critical reading of the manuscript and inspiring discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aguzzi A, Calella AM. Prions: protein aggregation and infectious diseases. Physiol Rev. 2009;89:1105–1152. doi: 10.1152/physrev.00006.2009. [DOI] [PubMed] [Google Scholar]
- Aguzzi A, Rajendran L. The transcellular spread of cytosolic amyloids, prions, and prionoids. Neuron. 2009;64:783–790. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Alberti S, Halfmann R, King O, Kapila A, Lindquist S. A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell. 2009;137:146–158. doi: 10.1016/j.cell.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieler S, Estrada L, Lagos R, Baeza M, Castilla J, Soto C. Amyloid formation modulates the biological activity of a bacterial protein. J. Biol. Chem. 2005;280:26880–26885. doi: 10.1074/jbc.M502031200. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Yilmazer D, De Vos RA, Jansen EN, Bohl J. Pattern of brain destruction in Parkinson's and Alzheimer's diseases. J. Neural Transm. 1996;103:455–490. doi: 10.1007/BF01276421. [DOI] [PubMed] [Google Scholar]
- Brachmann A, Baxa U, Wickner RB. Prion generation in vitro: amyloid of Ure2p is infectious. EMBO J. 2005;24:3082–3092. doi: 10.1038/sj.emboj.7600772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley SA, Hartl FU. The role of molecular chaperones in human misfolding diseases. FEBS Lett. 2009;583:2647–2653. doi: 10.1016/j.febslet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson's disease pathology spread. Nat. Rev. Neurosci. 2008;9:741–745. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S, Hultgren SJ. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science. 2002;295:851–855. doi: 10.1126/science.1067484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Claessen D, Rink R, de Jong W, Siebring J, de Vreugd P, Boersma FG, Dijkhuizen L, Wosten HA. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 2003;17:1714–1726. doi: 10.1101/gad.264303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat. Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- Coustou V, Deleu C, Saupe S, Begueret J. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9773–9778. doi: 10.1073/pnas.94.18.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullie J, Chelle PL. Experimental transmission of trembling to the goat. Comptes Rendus des Seances de l'Academie des Sciences. 1939;208:1058–1160. [Google Scholar]
- Deleault NR, Harris BT, Rees JR, Supattapone S. From the Cover: Formation of native prions from minimal components in vitro. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 1996;144:1375–1386. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM. Getting out of shape. Nature. 2002;418:729–730. doi: 10.1038/418729a. [DOI] [PubMed] [Google Scholar]
- Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally Applied A{beta}-Containing Inoculates Induce Cerebral {beta}-Amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid--from bacteria to humans. Trends Biochem. Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, ory-Jost C, Marks MS, Balch WE, Kelly JW. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J. Biol. Chem. 2011;286:18664–18672. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek DC, Gibbs CJ, Alpers M. Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature. 1966;209:794–796. doi: 10.1038/209794a0. [DOI] [PubMed] [Google Scholar]
- Grad LI, Guest WC, Yanai A, Pokrishevsky E, O'Neill MA, Gibbs E, Semenchenko V, Yousefi M, Wishart DS, Plotkin SS, et al. Intermolecular transmission of superoxide dismutase 1 misfolding in living cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:16398–16403. doi: 10.1073/pnas.1102645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JS. Self-replication and scrapie. Nature. 1967;215:1043–1044. doi: 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- Guo JL, Lee VM. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 2011;286:15317–15331. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann R, Alberti S, Lindquist S. Prions, protein homeostasis, and phenotypic diversity. Trends Cell Biol. 2010;20:125–133. doi: 10.1016/j.tcb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JD, Lansbury PT., Jr. Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- Heinrich SU, Lindquist S. Protein-only mechanism induces self-perpetuating changes in the activity of neuronal Aplysia cytoplasmic polyadenylation element binding protein (CPEB) Proc. Natl. Acad. Sci. U.S.A. 2011;108:2999–3004. doi: 10.1073/pnas.1019368108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, MacKenzie C, Houston F. Transmission of prion diseases by blood transfusion. J. Gen. Virol. 2002;83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- Iconomidou VA, Vriend G, Hamodrakas SJ. Amyloids protect the silkmoth oocyte and embryo. FEBS Lett. 2000;479:141–145. doi: 10.1016/s0014-5793(00)01888-3. [DOI] [PubMed] [Google Scholar]
- Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, Roher AE, Walker LC. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J. Neurosci. 2000;20:3606–3611. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JM, Knight D, Wise MJ, Vollrath F. Amyloidogenic nature of spider silk. Eur. J. Biochem. 2002;269:4159–4163. doi: 10.1046/j.1432-1033.2002.03112.x. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Korenaga T, Yan J, Sawashita J, Matsushita T, Naiki H, Hosokawa M, Mori M, Higuchi K, Fu X. Transmission of amyloidosis in offspring of mice with AApoAII amyloidosis. Am. J. Pathol. 2006;168:898–906. doi: 10.2353/ajpath.2006.050350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M. Soluble Abeta seeds are potent inducers of cerebral beta-amyloid deposition. J. Neurosci. 2011;31:14488–14495. doi: 10.1523/JNEUROSCI.3088-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbury PT, Jr., Caughey B. The chemistry of scrapie infection: implications of the 'ice 9' metaphor. Chem. Biol. 1995;2:1–5. doi: 10.1016/1074-5521(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Mad cows meet psi-chotic yeast: the expansion of the prion hypothesis. Cell. 1997;89:495–498. doi: 10.1016/s0092-8674(00)80231-7. [DOI] [PubMed] [Google Scholar]
- Luk KC, Song C, O'Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark K, Westermark GT, Nystrom S, Murphy CL, Solomon A, Westermark P. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6979–6984. doi: 10.1073/pnas.092205999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddelein ML, Dos RS, Duvezin-Caubet S, Coulary-Salin B, Saupe SJ. Amyloid aggregates of the HET-s prion protein are infectious. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7402–7407. doi: 10.1073/pnas.072199199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KP, Simon R, Schubert D, et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 2009;325:328–332. doi: 10.1126/science.1173155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masison DC, Maddelein ML, Wickner RB. The prion model for [URE3] of yeast: spontaneous generation and requirements for propagation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12503–12508. doi: 10.1073/pnas.94.23.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Coomaraswamy J, Bolmont T, Kaeser S, Schaefer C, Kilger E, Neuenschwander A, Abramowski D, Frey P, Jaton AL, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- Morales R, Green KM, Soto C. Cross currents in protein misfolding disorders: interactions and therapy. CNS. Neurol. Disord. Drug Targets. 2009;8:363–371. doi: 10.2174/187152709789541998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. De novo induction of Amyloid-beta deposition in vivo. Mol. Psych. 2011 doi: 10.1038/mp.2011.120. Advanced Online Publication October 4, 2011 (DOI 10.1038/mp.2011.120) [DOI] [PubMed] [Google Scholar]
- Morales R, Duran-Aniotz C, Estrada LD, Urayama A, Morales-Scheihing D, Soto C. Induction of Amyloid Deposition and Memory Impairments in Animal Models of Alzheimer's disease by Blood Transfusion. Nature (under second round of review) 2012 [Google Scholar]
- Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchere J, Lakhdar L, Legastelois S, Baron T. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging. 2012 doi: 10.1016/j.neurobiolaging.2011.06.022. In press. [DOI] [PubMed] [Google Scholar]
- Munch C, O'Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, Wildum S, Chinnadurai R, Rajan D, Specht A, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of alpha-synuclein and tau: cellular models of neurodegenerative diseases. J. Biol. Chem. 2010;285:34885–34898. doi: 10.1074/jbc.M110.148460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Nuallain B, Williams AD, Westermark P, Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J. Biol. Chem. 2004;279:17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- Patel BK, Liebman SW. “Prion-proof” for [PIN+]: infection with in vitro-made amyloid aggregates of Rnq1p-(132–405) induces [PIN+] J. Mol. Biol. 2007;365:773–782. doi: 10.1016/j.jmb.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino MM, Liu JJ, Glover JR, Lindquist S. Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science. 1996;273:622–626. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- Peden AH, Ritchie DL, Ironside JW. Risks of transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Folia Neuropathol. 2005;43:271–278. [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Ren PH, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat. Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Fritz JJ, Dooyema J, Cintron AF, Hamaguchi T, Lah JJ, Levine H, III, Jucker M, Walker LC. Exogenous seeding of cerebral beta-amyloid deposition in betaAPP-transgenic rats. J. Neurochem. 2012 doi: 10.1111/j.1471-4159.2011.07551.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Aplysia CPEB can form prion-like multimers in sensory neurons that contribute to long-term facilitation. Cell. 2010;140:421–435. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Si K, Lindquist S, Kandel ER. A neuronal isoform of the aplysia CPEB has prion-like properties. Cell. 2003;115:879–891. doi: 10.1016/s0092-8674(03)01020-1. [DOI] [PubMed] [Google Scholar]
- Sondheimer N, Lindquist S. Rnq1: an epigenetic modifier of protein function in yeast. Mol. Cell. 2000;5:163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- Soto C, Estrada L, Castilla J. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem. Sci. 2006;31:150–155. doi: 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- True HL, Lindquist SL. A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature. 2000;407:477–483. doi: 10.1038/35035005. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang X, Yuan C-G, Ma J. Generating a Prion with Bacterially Expressed Recombinant Prion Protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn RD, Bapat K, Newnam GP, Zink AD, Chernoff YO. Mechanism of prion loss after Hsp104 inactivation in yeast. Mol. Cell Biol. 2001;21:4656–4669. doi: 10.1128/MCB.21.14.4656-4669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Edskes HK, Roberts BT, Baxa U, Pierce MM, Ross ED, Brachmann A. Prions: proteins as genes and infectious entities. Genes Dev. 2004;18:470–485. doi: 10.1101/gad.1177104. [DOI] [PubMed] [Google Scholar]
- Wickner RB, Masison DC, Edskes HK. [PSI] and [URE3] as yeast prions. Yeast. 1995;11:1671–1685. doi: 10.1002/yea.320111609. [DOI] [PubMed] [Google Scholar]
- Xing Y, Nakamura A, Chiba T, Kogishi K, Matsushita T, Li F, Guo Z, Hosokawa M, Mori M, Higuchi K. Transmission of mouse senile amyloidosis. Lab Invest. 2001;81:493–499. doi: 10.1038/labinvest.3780257. [DOI] [PubMed] [Google Scholar]
- Zeng W, Sun L, Jiang X, Chen X, Hou F, Adhikari A, Xu M, Chen ZJ. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell. 2010;141:315–330. doi: 10.1016/j.cell.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Une Y, Fu X, Yan J, Ge F, Yao J, Sawashita J, Mori M, Tomozawa H, Kametani F, et al. Fecal transmission of AA amyloidosis in the cheetah contributes to high incidence of disease. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7263–7268. doi: 10.1073/pnas.0800367105. [DOI] [PMC free article] [PubMed] [Google Scholar]