Abstract
Polymethoxyflavones (PMFs) extracted from citrus peel exhibit potent anti-cancer activity, but are highly hydrophobic molecules with poor solubility in both water and oil at ambient and body temperature, which limits their bioavailability. The possibility of encapsulating PMFs within nanoemulsion-based delivery systems to facilitate their application in nutraceutical and pharmaceutical products was investigated. The influence of oil type (corn oil, MCT, orange oil), emulsifier type (β-lactoglobulin, lyso-lecithin, Tween, and DTAB), and neutral cosolvents (glycerol and ethanol) on the formation and stability of PMF-loaded nanoemulsions was examined. Nanoemulsions (r < 100 nm) could be formed using high pressure homogenization for all emulsifier types, except DTAB. Lipid droplet charge could be altered from highly cationic (DTAB), to near neutral (Tween), to highly anionic (β-lactoglobulin, lyso-lecithin) by varying emulsifier type. PMF crystals formed in all nanoemulsions after preparation, which had a tendency to sediment during storage. The size, morphology, and aggregation of PMF crystals depended on preparation method, emulsifier type, oil type, and cosolvent addition. These results have important implications for the development of delivery systems for bioactive components that have poor oil and water solubility at application temperatures.
Keywords: Polymethoxyflavones, PMF, poorly water-soluble drugs, Delivery systems, Nanoemulsion, Crystallization, Bioavailability, Functional foods, Nutraceuticals, Emulsion
1. Introduction
Many bioactive agents intended for oral ingestion (e.g., nutraceuticals and pharmaceuticals) are highly hydrophobic compounds with low water-solubility and poor bioavailability (Bevernage, et al., 2010; Brouwers, Brewster, & Augustijns, 2009; Kleberg, Jacobsen, & Mullertz, 2010). The bioavailability of these compounds can often be increased by incorporating them within lipid-based delivery systems (Kohli, Chopra, Dhar, Arora, & Khar, 2010; Müllertz, Ogbonna, Ren, & Rades, 2010; Neslihan Gursoy & Benita, 2004; Pouton & Porter, 2008). A number of lipid-based delivery systems have been developed for this purpose, which vary in their compositions and structures: simple oil solutions (Burcham, Maurin, Hausner, & Huang, 1997); surfactant dispersions (Ali, Williams, & Rawlinson, 2010; Sinha, Baboota, Ali, Kumar, & Ali, 2009), microemulsions (He, He, & Gao, 2010; Rhee & Mansour, 2011); emulsions (Araya, Tomita, & Hayashi, 2005; Kawakami, et al., 2002; Poullain-Termeau, et al., 2008); self-emulsifying formulations (Barakat, 2010; Gao, et al., 2003; Neslihan Gursoy, et al., 2004; Setthacheewakul, Mahattanadul, Phadoongsombut, Pichayakorn, & Wiwattanapatapee, 2010; Yoo, et al., 2010); solid lipid nanoparticles (Muller, Mader, & Gohla, 2000; Wissing, Kayser, & Muller, 2004).
Emulsion-based delivery systems are a particularly convenient means of encapsulating, protecting, and delivering poorly water-soluble nutraceuticals and drugs via the oral route for both functional food and pharmaceutical applications (Chakraborty, Shukla, Mishra, & Singh, 2009; McClements & Li, 2010). Emulsions with different compositions, structures, and functional performances can be prepared from commercially available ingredients (such as lipids, emulsifiers, and water) using simple unit operations (such as mixing and homogenization). Studies suggest that there is an inverse relationship between the uptake of poorly water-soluble bioactive components and the size of the particles that contain them (Acosta, 2009; Hageman, 2010; Sjöström, Bergenståhl, & Kronberg, 1993; Sjöstrom, Kronberg, & Carlfors, 1993). Consequently, nanoemulsion-based delivery systems, which contain relatively small particles (r < 100 nm), should be particularly convenient for increasing the uptake of highly hydrophobic bioactive agents. In this study, we examined the potential for nanoemulsion-based delivery systems to encapsulate a highly hydrophobic phytochemical that has been shown to have strong bioactivity.
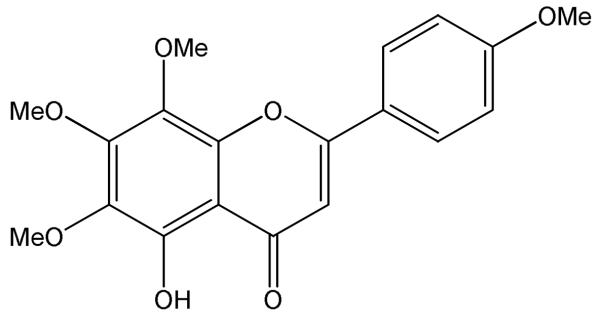
The phytochemical used, 5-hydroxy-6,7,8,4′-tetramethoxyflavone (Figure 1), belongs to a class of compounds known as hydroxyl-polymethoxyflavones (OH-PMFs). PMFs and OH-PMFs are phytochemicals that have been isolated from sweet orange peels (Hirata, et al., 2009; Li, Lo, & Ho, 2006), tangerine peels (Wang, Wang, Huang, Tu, & Ni, 2007) and other citrus plants. OH-PMFs can also be chemically synthesized by direct demethylation or hydroxylation of PMFs (Li, et al., 2007) or from basic building blocks such as methoxylated acetophenones and substituted benzoaldehydes (Bovicelli, et al., 2007; Li, et al., 2007; Li, Wang, Sang, Huang, & Ho, 2006; Tsukayama, Kusunoki, Hossain, Kawamura, & Hayashi, 2007). OH-PMFs may also be produced using a microbial-catalyzed de-methylation reaction (Okuno & Miyazawa, 2004). So far, more than 20 PMFs/OH-PMFs have been isolated and identified from different tissues of citrus plants. For convenience, we use the term “PMFs” to refer to both PMFs and hydroxyl-PMFs in general, and we use the term “PMF” to refer to the specific compound used in this study (5-hydroxy-6,7,8,4′-tetramethoxyflavone), though it should be recognized that it is really a particular kind of hydroxyl-PMF.
Figure 1.
Chemical structure of 5-hydroxy-6,7,8,4′-tetramethoxyflavone, referred to as “PMF “throughout this study.
A number of studies have shown that PMFs have strong biological activity, including anti-inflammatory, anti-carcinogenic, anti-viral, anti-oxidant, anti-thrombogenic, and anti-atherogenic properties (Lai, et al., 2008; Lai, et al., 2007; Li, et al., 2009; Sergeev, Ho, Li, Colby, & Dushenkov, 2007; Sergeev, Li, Colby, Ho, & Dushenkov, 2006; Xiao, et al., 2009). Animal feeding studies using rats showed that consumption of between 2 to 9 mg of a PMF (nobiletin) per day (for a ≈ 71 kcal per day diet) was sufficient to have a significant anti-carcinogenic effect (Kohno, et al., 2001). This value corresponds to about 50 to 250 mg PMF per day for humans (based on a 2000 kcal per day diet). One would therefore aim to fortify functional food products with these levels of PMF in order to achieve the desired biological response. Typically, food manufactures fortify foods with about 10% of the daily recommended dose of bioactive compounds. Nevertheless, the application of PMFs as nutraceuticals in many functional foods is currently limited because they are highly hydrophobic compounds that have high melting points (> 150 °C) (Kinoshita & Firman, 1996), poor water-solubilities (< 100 <g/mL) and low bioavailabilities (Li, et al., 2009). There is therefore a need to develop effective delivery systems to incorporate this kind of hydrophobic bioactive compound into aqueous-based functional food products, such as beverages, sauces, dips, dressings and deserts.
The purpose of the current study was to determine whether PMF (5-hydroxy-6,7,8,4′-tetramethoxyflavone) could be successfully incorporated into oil-in-water nanoemulsions. PMF-loaded nanoemulsions could then be utilized as delivery systems to conveniently incorporate this highly hydrophobic compound into functional foods, dietary supplements, or pharmaceutical products. In particular, we examined the influence of formulation characteristics such as preparation method, emulsifier type, carrier oil type, and neutral cosolvents on the formation and stability of PMF-loaded nanoemulsions. The knowledge gained from this study should facilitate the rational design of lipid-based delivery systems for highly hydrophobic nutraceuticals and pharmaceuticals.
2. Materials & Methods
2.1. Materials
PMF powder was provided by Dr. Hang Xiao’s laboratory at the University of Massachusetts (Dong, et al., 2010). Powdered β-lactoglobulin (BLG) was obtained from Davisco Foods International (Le Sueur, MN). Tween 20 (T20) was purchased from MP Biomedicals LLC (Solon, OH). Lyso-lecithin (Lyso-Lec) was a de-oiled enzyme-modified soy lecithin obtained from the Solae Company (St Louis, MO). Dodecyl trimethyl ammonium bromide (DTAB), glycerol (99%) and ethanol (95%) were purchased from Acros Organics (NJ, USA). Tween 85 (T85) was purchased from Sigma-Aldrich (St Louis, MO). Medium chain triglyceride (MIGLYOL 812N) was purchased from Sasol Germany Gmbh (Witten, Germany). Corn oil was purchased from a local supermarket and used without further purification. Orange oil was provided by Givaudan (East Hanover, NJ). Analytical grade sodium monophosphate, sodium diphosphate, hydrochloric acid (HCl) and sodium hydroxide (NaOH) were purchased from Sigma. Purified water from a Nanopure water system (Nanopure Infinity, Barnstead International, Dubuque, IA) was used for the preparation of all solutions.
2.2. Emulsion Preparation: Emulsifier in Aqueous Phase Method
Oil Phase Preparation
PMF powder (1 wt%) was added into a heated carrier oil (~90 °C) and the system was continuously stirred until the PMF was completely dissolved. Three kinds of carrier oil were used in this study: corn oil; MCT; and, orange oil.
Aqueous Phase Preparation
Emulsifier solutions were prepared by dispersing 1.0 wt% emulsifier (BLG, Tween 20, Lyso-Lecithin, or DTAB) into 5 mM phosphate buffer solution and stirring for at least 2 h to ensure complete dissolution. The protein solutions were kept overnight at 4 °C to ensure complete hydration. All emulsifier solutions were warmed prior to use to avoid PMF crystallization when the oil and aqueous phases were brought into contact.
Emulsion Preparation
A stock emulsion was prepared by homogenizing 5 wt% oil phase (0 or 1 wt% PMF in carrier oil) with 95 wt% aqueous phase (1 wt% emulsifier in buffer solution, pH 7.0) with a high-speed blender for 2 min (M133/1281-0, Biospec Products, Inc., ESGC, Switzerland) followed by five passes at 12K psi through a high pressure homogenizer (Microfluidics M-110Y, F20Y 75 μm interaction chamber, Newton, MA). The oil and aqueous phases were kept warm (> 50 °C) during mixing and homogenization, otherwise the PMF would crystallize and form a gel in the oil phase.
2.3. Emulsion Preparation: Emulsifier in Oil Phase Method
Oil Phase Preparation
PMF powder (1 wt%) was added into a heated carrier oil (~90 °C) and the system was continuously stirred until the PMF was completely dissolved. Then emulsifier (BLG, Tween 20, Lyso-Lecithin, or Tween 20/Tween 85) was added. Three kinds of carrier oil were used in this study: corn oil; MCT; and, orange oil. The surfactants were able to dissolve in the oil phase and form a clear solution, whereas the protein formed an opaque colloidal dispersion in the oil phase. For some experiments we mixed either glycerol (0 to 60 wt%) or ethanol (0 to 10 wt%) with the oil phase.
Aqueous Phase Preparation
Aqueous buffer solutions (5 mM phosphate buffer solution) were prepared and then warmed prior to use to avoid PMF crystallization when the oil and aqueous phases were brought into contact.
Emulsion Preparation
Emulsions were prepared by homogenizing the oil and aqueous phase together using the high-speed blender and high pressure homogenizer under the same conditions as for the previous method. The final composition of the emulsions was the same for both emulsion preparation methods.
2.4. Particle Size and ζ-potential Measurements
The particle size and ζ-potential of the particles in the emulsions were determined using a commercial dynamic light scattering and micro-electrophoresis device (Nano-ZS, Malvern Instruments, Worcestershire, UK). The samples were diluted 100 times in buffer solution (5 mM phosphate buffer, pH 7) at room temperature before measurement. The particle size data are reported as the intensity-weighted (“Z-average”) mean particle diameter, while the particle charge data are reported as the ζ-potential (Malvern Instruments, Worcestershire, UK).
2.5. Emulsion Microstructure & Visual Observations
The microstructures of selected emulsions were observed using an optical microscope (Nikon Eclipse E400, Nikon Corp., Japan). Emulsion samples were thoroughly mixed in a glass test tube before analysis, except when images of the sediment were acquired. A drop of emulsion was placed on a microscope slide, and covered by a cover slip. An image of the sample was acquired using digital image processing software (Micro Video Instruments Inc., Avon, MA) and stored on a personal computer. Emulsions were characterized by both bright field and polarized light microscopy. The general appearance of the emulsions was recorded by taking images using a digital camera (PowerShot SD1300IS, Canon).
2.6. Determination of PMF saturation concentrations
The saturation concentrations of PMF in oil (CO,Sat) and water (CW,Sat) phases were measured by spectrophotometry.
Oil Phases
A weighed amount of PMF powder was added to 10 ml oil and then stirred for 1 hour at 90 to 100 °C to fully dissolve the PMF crystals, which resulted in a clear yellow PMF/oil solution. These solutions were then cooled to 25 °C and stored for 72 hours, which led to crystal formation in samples containing sufficiently high PMF levels. After 72 h storage, the samples were centrifuged (3500 rpm, 15 min) and then the 10 <l of supernatant was collected and mixed with 9.99 mL of chloroform. The absorbance was then measured at 327 nm using a UV-visible spectrophotometer and a previously established calibration curve.
Water Phases
Supersaturated PMF solutions were created by dissolving PMF powder into DMSO. The PMF-DMSO solution was then added to a phosphate buffer solution and mixed thoroughly for 5 seconds using a Vortex, which led to the formation of crystals. The sample was then centrifuged to remove any non-dissolved PMF. The supernatant was collected and the PMF concentration was determined using a UV-visible spectrophotometer and a previously established calibration curve.
2.7. Data Analysis
All experiments were performed at least twice on freshly prepared samples. The results were then reported as averages and standard deviations of these measurements.
3. Results & Discussion
3.1. Thermal behavior of PMF in bulk carrier oils
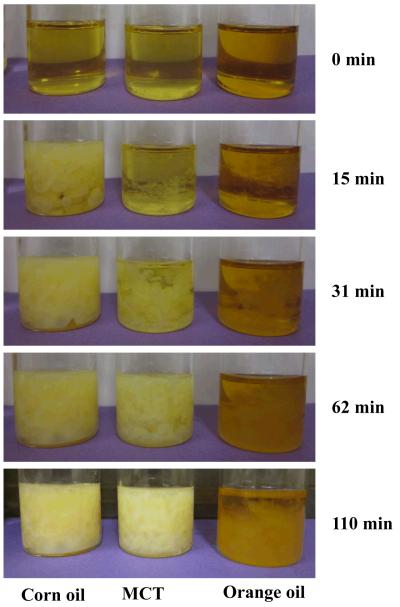
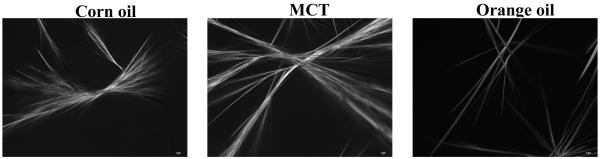
It is difficult to prepare oil-in-water nanoemulsions from lipid phases containing crystalline material because crystals tend to block the narrow chambers in high pressure homogenizers. PMF is crystalline at room temperature, and so it was necessary to dissolve it within carrier oil prior to homogenization. This was achieved by weighing PMF crystals into carrier oils (corn oil, MCT, or orange oil) at ambient temperature, and then heating the resulting mixtures to ≈ 90 °C to fully dissolve the crystals and form a transparent lipid phase (Figure 2a). (In later sections, this heated lipid phase was used to prepare the nanoemulsions). Crystal formation was observed within the bulk lipid phases when they were allowed to stand in beakers (Figure 2a) or on microscope slides (Figure 2b) at ambient temperature, with the rate and extent of crystallization depending on the nature of the carrier oil. The first crystals became visible within the bulk lipid phase (beakers) after about 7 minutes for corn oil, about 9 minutes for MCT, and about 12 minutes for orange oil. After 62 minutes storage at ambient temperature there appeared to be far more crystals in the corn oil and MCT samples, than in the orange oil samples (Figure 2a). Polarized light microscopy indicated that the crystals formed had a needle-like shape that tended to form clusters (Figure 2b). The microscopy study also confirmed that PMF crystals formedmore rapidly in corn oil and MCT than in orange oil. Crystallization occurred faster when lipids were placed on a microscope slide (< 1 minute) than when they were left in a beaker (> 5 minutes), which can be attributed to the faster cooling rate of the thin lipid layer on the microscope slide. These experiments indicated that PMF tends to crystallize in bulk lipids when cooled to ambient temperatures, and form large needle-like crystals. There was a considerable variation in the dimensions of the crystals formed, but many of them were over 10 <m in length (Figure 2a). In the following experiments we aimed to determine whether PMF crystallization would also occur in nanoemulsions, and what effect system composition had on this process.
Figure 2a.
Photographs of 1% PMF in carrier oil solutions after cooling to ambient temperatures. The samples were prepared by mixing the PMF with oils, heating to about 90 °C to fully dissolve the PMF, and then cooling to ambient temperature.
Figure 2b.
Photographs of 1% PMF in carrier oil solutions after cooling to ambient temperatures taken with an polarized optical microscopy (after about 4 minutes). The samples were prepared by mixing the PMF with oils, heating to about 90 °C to fully dissolve, then cooling. The images represent an area of 187 mm × 250 mm.
3.2. Physical stability and PMF crystallization in nanoemulsions
One of the main objectives of this study was to prepare physically stable oil-in-water nanoemulsions that could be used as delivery systems for PMF. Initially, we therefore prepared an oil-in-water nanoemulsion by homogenizing an oil phase (1 wt% PMF in corn oil) and an aqueous phase (1 wt% Tween 20 in buffer solution) together. The system had to be kept warm (50-60 °C) during homogenization to prevent PMF crystallization, otherwise it would not have been possible to pass it through the narrow channels of the homogenizer. After preparation the samples were stored at either ambient temperature (25 °C) or in a refrigerator (4 °C). We found that the storage temperature had little influence on the properties or stability of the emulsions (Table 1a), and so we only consider the data at ambient temperature in the remainder of this study.
Table 1.
Mean particle diameter and ζ-potential of oil-in-water emulsions in the presence and absence of PMF (1 wt% of the lipid phase). The lipid carrier was (a) corn oil; (b) MCT; or, (c) orange oil. Samples were stored at either room temperature or in a refrigerator for 24 hours prior to analysis. Emulsions were prepared by homogenizing an oil phase (carrier oil and 0 or 1% PMF) with an aqueous phase (emulsifier and water).
| (a). Corn oil | Without PMF | With PMF | Without PMF | With PMF | ||||
|---|---|---|---|---|---|---|---|---|
| Emulsifiers | Room temperature (25 °C) | Refrigerator (4 °C) | ||||||
| d (nm) | ζ (mV) | d (nm) | ζ (mV) | d (nm) | ζ (mV) | d (nm) | ζ (mV) | |
| BLG | 163 ± 2 | −61± 2 | 174 ± 3* | −56 ± 2 | 170 ± 3 | −60 ± 1 | 175 ± 5* | −57 ± 2 |
| Lyso-Lec | 216 ± 7 | −73 ± 2 | 167 ± 3** | −73 ± 3 | 210 ± 7 | −70.1 ± 2 | 169 ± 4** | −70 ± 2 |
| T20 | 155 ± 4 | −11.4 ± 0.4 | 146 ± 1*** | −12.6 ± 0.6 | 156 ± 2 | −11.6 ± 0.7 | 149 ± 2*** | −12.4 ± 0.4 |
| DTAB | 516 ± 33 | +39 ± 1 | 356 ± 24*** | +37 ± 1 | 291 ± 5 | +41 ± 1 | 726 ± 26*** | +27 ± 2 |
| (b). MCT | Without PMF | With PMF | Without PMF | With PMF | ||||
|---|---|---|---|---|---|---|---|---|
| Emulsifiers | Room temperature (25 °C) | Refrigerator (4 °C) | ||||||
| d (nm) | ζ (mV) | d (nm) | ζ (mV) | d (nm) | ζ (mV) | d (nm) | ζ (mV) | |
| BLG | 159 ± 5 | −53 ± 1 | 166 ± 4* | −52 ± 3 | 165 ± 3 | −55 ± 2 | 166 ± 4* | −52 ± 2 |
| Lyso Lec | 183 ± 5 | −72 ± 1 | 162 ± 2* | −74 ± 3 | 186 ± 5 | −74 ± 2 | 164 ± 2* | −70 ± 2 |
| T20 | 146 ± 3 | −8.9 ± 0.9 | 135 ± 2* | −10 ± 1 | 147 ± 3 | −9 ± 1 | 133 ± 3* | −10 ± 1 |
| DTAB | 4000 ± 500 | +36 ± 2 | 1590 ± 100* | +33 ± 1 | 6600 ± 2100 | +37 ± 1 | 1941 ± 270* | +32 ± 2 |
| (c) Orange oil | Without PMF | With PMF | Without PMF | With PMF | ||||
|---|---|---|---|---|---|---|---|---|
| Emulsifiers | Room temperature (25 °C) | Refrigerator (4 °C) | ||||||
| d (nm) | ζ (mV) | d (nm) | ζ (mV) | d (nm) | ζ (mV) | d (nm) | ζ (mV) | |
| BLG | 151 ± 6 | −53 ± 1 | 155 ± 2* | −54 ± 1 | 153 ± 3 | −54 ± 1 | 155 ± 1* | −52 ± 2 |
| Lyso Lec | 156 ± 8 | −66 ± 4 | 145 ± 3* | −69 ± 3 | 163 ± 10 | −65 ± 3 | 150 ± 5* | −72 ± 1 |
| T20 | 120 ± 0.5 | −20 ± 1 | 117 ± 3* | −18 ± 1 | 120 ± 35 | −23 ± 5 | 118 ± 2* | −18 ± 2 |
| DTAB | 6140 ± 940 | −8 ± 2 | 6100 ± 1200* | +1.2 ± 0.5 | 6200 ± 500 | −8.4 ± 0.4 | 6200 ± 900* | +4 ± 2 |
Key: = Crystals observed by optical microscopy, but no yellow sediment observed after 24 h storage;
= Few crystals observed by optical microscopy, but yellow sediment observed after 24 h storage;
= Crystals observed by optical microscopy and yellow sediment observed. Samples with no stars appeared stable to crystal formation and sedimentation.
We found that nanoemulsions (r < 100 nm; d < 200 nm) were formed after homogenization both in the presence and absence of PMF (Table 1a). Measurements of the particle size distributions of these nanoemulsions indicated that they had monomodal distributions with a peak around a diameter of 140 nm (Figure 3). The polydispersity indices (PDI) of these nanoemulsions were between 0.1 and 0.3, which indicated that the distributions were relatively narrow. The droplets in the nanoemulsions had a small negative charge (≈ -12 mV), even though they were stabilized by a non-ionic surfactant. The most likely origin of this negative charge is the presence of anionic impurities in the surfactant and/or oil phase, e.g., free fatty acids (McClements, 2005). The presence of PMF in the nanoemulsions appeared to have little influence on the initial particle size or charge, which might be expected since it is a relatively small non-polar neutral molecule (Figure 1) that should not have a major influence on oil phase viscosity or interfacial tension at the levels (1%) used.
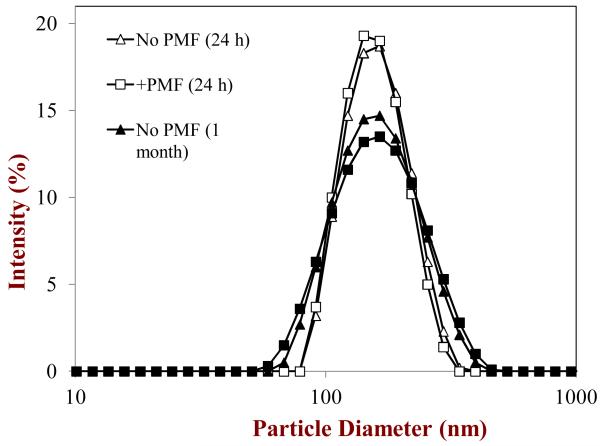
Figure 3.
Particle size distributions of corn oil-in-water emulsions stabilized by Tween 20 in the absence and presence of PMF measured after 1 day and 1 month storage at ambient temperature.
After 24 hours or 1 month storage, we did not observe any evidence of droplet creaming, i.e., the emulsions had a uniform opaque appearance throughout their volume. The good stability of the lipid droplets to gravitational separation can be attributed to their relatively small size (McClements, 2011; McClements & Rao, 2011). On the other hand, we did observe the presence of a thin yellow layer at the bottom of the nanoemulsions after storage (Figure 4). The color of this layer was similar to that of the initial PMF crystals used to prepare the nanoemulsions, and therefore we hypothesized that it was comprised of sedimented PMF crystals. To test this hypothesis we separated the emulsions into a white upper layer and a yellowish lower layer using a syringe and then analyzed each layer. The particle size distribution of the droplets in the white upper layer was similar to that of the droplets in the initial emulsions after 24 hours or 1 month storage (Figure 3), suggesting that the presence of the phase separated PMF crystals had not influenced the physical stability of the lipid droplets. The microstructure of the yellow lower layer was characterized using polarized light microscopy, which clearly showed that there were needle-shaped crystals distributed throughout the sediment (Figure 4). After one month storage a more distinct yellow layer was observed at the bottom of the test tubes, and there was evidence of large clusters of aggregated needle-like crystals in the sediment (Figure 4).
Figure 4.
Overall appearance and polarized microscopy images of corn oil-in-water emulsions stabilized by Tween 20 in the presence of PMF measured after 1 day storage.
3.3. Potential Mechanism of Crystal Formation in Nanoemulsions
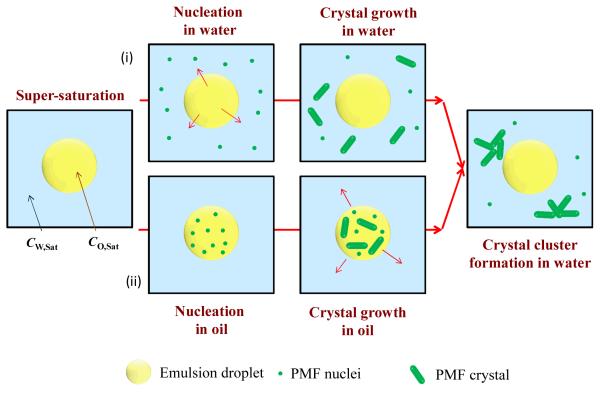
The nanoemulsions were prepared at an elevated temperature and so the PMF would initially have been fully dissolved because its concentration in both the oil and water phases would be below the saturation level. After homogenization at elevated temperatures, we would expect the PMF molecules to be distributed between the oil and aqueous phases according to their equilibrium partition coefficient (KOW). When the nanoemulsion was cooled the PMF would have become supersaturated in the oil and/or aqueous phases, which would favor nucleation and crystal growth (Brouwers, et al., 2009; Lindfors, Forssen, Westergren, & Olsson, 2008; Warren, Benameur, Porter, & Pouton, 2010). Nevertheless, the system could persist in this metastable state for some time, depending on system composition and temperature, due to the presence of activation energies associated with nuclei formation. Nucleation and crystal growth may have occurred in the oil phase, the aqueous phase, and/or the oil-water interface (Figure 5). Our optical microscopy measurements indicated that relatively large crystal clusters were eventually formed that appeared to be present within the aqueous phase (Figure 4). This result suggests that either: (i) nucleation and crystal growth occurred directly in the aqueous phase; or, (ii) nucleation and crystal growth occurred in the oil phase or interfacial region and then the crystals moved into the aqueous phase. We intend to examine this phenomenon in more detail in future studies. Mathematical models have been developed to describe the nucleation and crystallization processes in emulsions, and these models show that monomers may move from an oil droplet into the aqueous phase where they form crystals (Feltham & Garside, 2005).
Figure 5.
Schematic representation of possible mechanisms for formation of PMF crystals in nanoemulsions: (i) some of the PMF diffuses into the water phase until the concentration exceeds the water-solubility, leading to crystal formation; (ii) PMF crystals form in the oil droplets, then move into the aqueous phase.
Our initial experiments indicated that oil-in-water nanoemulsions containing PMF could be formed, but that the PMF crystallized over time and sedimented to the bottom of the samples, especially after prolonged storage. This effect would be expected to have a major impact on the ability of nanoemulsion-based delivery systems to encapsulate and release PMF. We therefore carried out a series of experiments to identify possible strategies to inhibit the crystallization and sedimentation of PMF in the nanoemulsions.
3.4. Influence of emulsifier type on PMF-nanoemulsion properties
In this series of experiments we examined the influence of emulsifier type on the formation, properties, and stability of nanoemulsions containing PMF. Different types of emulsifiers are commonly used to prepare nanoemulsions in food and pharmaceutical applications, including natural and synthetic surfactants and amphiphilic polymers (McClements, 2005; Pouton, 2006). The nature of the emulsifier used to fabricate a nanoemulsion may impact its properties in a variety of ways: the initial droplet size produced by homogenization; emulsion stability during storage; droplet interactions with other components (McClements, 2010, 2011). Emulsifier type may also impact the formation, growth rate, and location of crystals present within an oil-water system by interacting with nuclei or crystal surfaces (Fredrick, Walstra, & Dewettinck, 2010; Rousseau, 2000; Sato, 1999).
Emulsifiers may therefore play multiple roles in the formation, stability, and performance of nanoemulsions containing lipophilic components that may crystallize. For this reason, we tested the effects of a series of emulsifiers with different molecular characteristics on nanoemulsion properties: an anionic surfactant (lyso-lecithin); a non-ionic surfactant (Tween 20); a cationic surfactant (DTAB); and, an amphoteric globular protein (BLG). Initially, oil-in-water nanoemulsions were prepared by homogenizing a lipid phase (0 or 1% PMF in corn oil) and an aqueous phase (1% emulsifier in buffer solution) together, and then they were stored at either room (≈ 25 °C) or refrigerator (≈ 4 °C) temperature prior to analysis. The particle size, particle charge, microstructure and overall appearance of the resulting nanoemulsions was then determined.
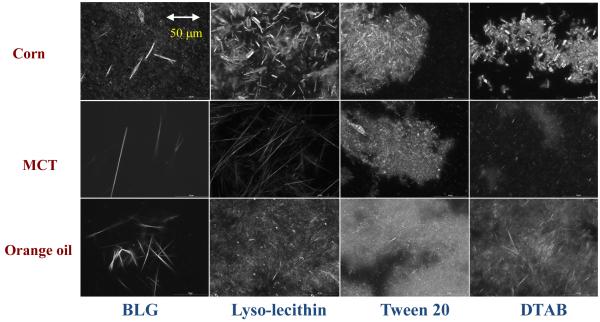
The electrical charge on the lipid droplets depended on emulsifier type, being highly negative for BLG and lyso-lecithin, slightly negative for Tween 20, and highly positive for DTAB (Table 1a). The presence of PMF in the emulsions had no appreciable effect on the electrical characteristics of the lipid droplets, which might be expected since PMF is a neutral molecule (Figure 1). The systems containing Tween 20, BLG and lyso-lecithin as emulsifiers were all able to form corn oil-in-water emulsions containing relatively small droplets, i.e., d < 220 nm (Table 1a). The majority of these systems could therefore be considered to be nanoemulsions (i.e., d < 200 nm). On the other hand, the emulsions prepared using DTAB contained relatively large droplets, i.e., d > 250 nm (Table 1a). After 24 hours storage the emulsions appeared to have a relatively uniform white appearance throughout most of their volume, which indicated that the lipid droplets were stable to gravitational separation. Nevertheless, we again observed a thin yellow layer at the bottom of a number of the emulsions after 24 hours storage, i.e., those containing Tween 20, lyso-lecithin, and DTAB. The only sample where a colored sediment was not clearly visible after 24 hours storage was the one containing lipid droplets stabilized by BLG. Microstructural observations of the samples using conventional optical microscopy and cross-polarizers indicated that all the emulsions contained some crystalline material distributed within the aqueous phase after 24 hours storage (data not shown). The presence of the PMF crystals could be clearly seen by analyzing the sediment formed in the nanoemulsions after one month storage (Figure 6). There were some visible differences in the size, morphology and aggregation of the PMF crystals observed in the emulsions containing different types of emulsifiers. There were large clusters of needle-like crystals in the lyso-lecithin, DTAB-, and Tween-stabilized nanoemulsions; where there were more isolated needle-like crystals in the BLG-stabilized nanoemulsions. These differences may have altered the tendency for crystal sedimentation to occur in the emulsions. One would expect that smaller crystals would be more stable to sedimentation than larger ones, which may account for the fact that a yellow sediment layer was not visible in the BLG-stabilized emulsions after 24 hours storage, even though crystals were observed by microscopy (Table 1a).
Figure 6.
Polarized light microscopy images of the sediments formed in PMF-fortified oil-in-water emulsions after 1 month storage. Each image represents an area of 187 mm × 250 mm. Crystal morphology appeared to depend strongly on oil type and surfactant type. These emulsions were prepared by mixing the emulsifiers with the aqueous phase first.
The size and morphology of the crystals formed in the emulsions may have been influenced by a number of factors related to nucleation and crystal growth rates (Feltham, et al., 2005; Lindfors, et al., 2008). If the nucleation rate was faster than the crystal growth rate, then many small crystals should have been formed. Conversely, if the crystal growth rate was faster than the nucleation rate, then few large crystals should have been formed. The relative rate of these two processes may have been influenced by interactions between PMF and the emulsifiers, e.g., solubilization within surfactant micelles, binding to hydrophobic sites on BLG molecules, adsorption of emulsifiers to nuclei or crystal surfaces, and inhibition/promotion of crystal growth. Further work is clearly needed to better understand the influence of these factors on the formation and morphology of PMF crystals in nanoemulsions.
The general tendency for PMF crystals to form in the emulsions after 24 hours storage was categorized based on their microstructure and physical appearance (Table 1a): no-stars = no evidence of crystal formation or sedimentation; * = Crystals observed by optical microscopy, but no yellow sediment observed after 24 h storage; ** = Few crystals observed by optical microscopy, but yellow sediment observed after 24 h storage; *** = Crystals observed by optical microscopy and yellow sediment observed. For all the emulsifiers studied we found that crystal formation occurred in all of the emulsions, so there were no emulsions in the no-stars category. In addition, after one month storage a yellow sediment was visible for all of the nanoemulsions, and PMF crystals could be observed by polarized light microscopy. In future studies we intend to measure the concentration of crystalline and dissolved PMF in the systems, as this will provide more detailed information about the distribution of this bioactive component within the systems.
3.5. Influence of oil type on PMF-nanoemulsion properties
In this series of experiments we examined the influence of oil type on the formation, properties, and stability of nanoemulsions containing PMF. Different types of oils are commonly used to prepare nanoemulsions in functional food and pharmaceutical applications, including natural and synthetic oils that may be either digestible or non-digestible (McClements, 2005; Pouton, 2006). The nature of the oil used to fabricate a nanoemulsion may impact its properties in a variety of different ways. The solubility of PMF in the lipid phase and its oil-water partition coefficient will depend on the molecular characteristics of the oil used, e.g., its molecular weight and polarity (Brouwers, et al., 2009). The initial size of the droplets produced during homogenization depends on the viscosity and interfacial tension of the oil phase, since these parameters impact the ease of droplet disruption within a homogenizer (S. Jafari, He, & Bhandari, 2007; S. M. Jafari, Assadpoor, He, & Bhandari, 2008). The stability of nanoemulsions to droplet coalescence and Ostwald ripening often depends on the chemical composition of the oil phase (Kabalnov, 2001; McClements, 2005). For these reasons, we examined the influence of oil type on the formation, properties, and stability of nanoemulsions containing PMF. Three different oils were selected based on differences in their molecular characteristics: long chain triglycerides (corn oil); medium chain triglycerides (MCT); and, orange oil. Emulsions were prepared using the same homogenization protocol as outlined in the previous section with Tween 20, lyso-lecthin, DTAB, or BLG as the emulsifier.
The droplet size, droplet charge, emulsion microstructure, and overall appearance of nanoemulsions prepared using different oil types were measured. Overall, the influence of emulsifier type on droplet size and charge was similar for MCT and orange oil (Tables 1b and 1c) as for corn oil (Table 1a); small droplets could be formed using Tween 20, lyso-lecithin and BLG, but not with DTAB; lyso-lecithin and BLG gave highly anionic droplets, Tween 20 gave slightly anionic droplets, and DTAB gave highly cationic droplets. The nature of the oil used to fabricate the emulsions did have an appreciable effect on the size of the droplets: the mean droplet diameter decreased in the following order: corn oil > MCT > orange oil for the same emulsifier (Table 1). This effect can be attributed to the effect of lipid phase properties on the formation of small droplets within the homogenizer. Droplet size tends to decrease as the viscosity and interfacial tension of the lipid phase decreases since this facilitates droplet disruption during homogenization (Qian & McClements, 2010; Wooster, Golding, & Sanguansri, 2008). The viscosity and interfacial tension of orange oil is less than that for triglyceride oils (Kourniatis, Spinelli, Piombini, & Mansur, 2010), which would account for the smaller droplet sizes.
Interestingly, the emulsions prepared using MCT or orange oil appeared to have better stability to crystal sedimentation than the ones prepared using corn oil after 24 hours storage (Table 1). There was no visible evidence of the formation of a yellow sediment at the bottom of the emulsions containing MCT or orange oil after 24 hours storage. Nevertheless, PMF crystals were still observed in these emulsions using optical microscopy, but these crystals appeared to be smaller than those formed in the corn oil emulsions (data not shown). The relatively good stability of the orange oil and MCT nanoemulsions to crystal sedimentation may therefore have been due to the relatively small size of the PMF crystals formed in these systems. Even so, after one month storage all of the emulsions exhibited a visible yellow sediment layer at the bottom that contained PMF crystals (Figure 6). The nature of the crystals present in the sediment depended on oil and surfactant type. For all carrier oil types, BLG appeared to form a relatively small number of large isolated needle-like PMF crystals. For the orange oil, there appeared to be a relatively large number of small clustered needle-like crystals in the emulsions containing surfactants (lyso-lecithin, Tween 20 or DTAB). For the MCT, there appeared to be larger isolated needle-like crystals for lyso-lecithin, but small clustered needle-like crystals in the emulsions containing Tween 20 or DTAB.
To further understand the influence of oil type on crystal formation in the emulsions we measured the saturation concentrations of PMF in the different oils and in water at 25 °C: 3.3 ± 0.4 mM, 2.8 ± 0.1 mM, 6.2 ± 0.6 mM, 0.93 ± 0.02 <M in orange oil, corn oil, MCT, and water respectively. The oil-water partition coefficient (KOW) can be calculated from the saturation concentrations of PMF in oil (CO,Sat) and water (CW,Sat): KOW = CO,Sat / CW,Sat. The PMF partition coefficients calculated from this data for the three oils used in this study were (25 °C): KOW = 3.55 × 103, 3.01 × 103 and 6.67 × 103 (corresponding to LogP = 3.55, 3.48 and 3.82) for orange oil, corn oil, and MCT respectively. PMF had the lowest CO,Sat and KOW values for corn oil, and also formed the largest crystals in the corn oil-in-water nanoemulsion. One would therefore expect that the PMF concentration in the water phase would have been somewhat higher for the corn oil nanoemulsion, which may have led to a greater driving force for nucleation in this phase. However, other factors may also have contributed to the final size of the crystals formed in the nanoemulsions. For example, the nanoemulsions containing corn oil had the largest initial droplet sizes, which may have influenced the final size of the crystals formed. In addition, corn oil has the highest viscosity and interfacial tension of the three oils used, which may also have affected the kinetics and thermodynamics of nucleation and crystal growth. Clearly further work is required to establish the relationship between final crystal characteristics and emulsion composition, properties, and structure.
One approach to avoid formation of crystals in the nanoemulsions would be to ensure that the PMF concentration always remained below the saturation limit, i.e., CO < CO,Sat. Nevertheless, this will limit the maximum amount of PMF that can be incorporated into a system. For a 5 wt% oil-in-water nanoemulsion, the maximum amount of PMF that can be incorporated per 100 g of nanoemulsion can be calculated from the CO,Sat values reported above: 4.6, 3.9 and 8.7 mg for orange oil, corn oil, and MCT systems. This value is considerably below the 50 to 250 mg PMF per day estimated for a beneficial effect in humans (Introduction). However, functional foods are usually fortified at about 10% of the daily recommended amount. The amount of soluble PMF delivered could also be increased by increasing the total oil concentration in the delivery system, or increasing the total amount of delivery system consumed per serving.
3.6. Influence of preparation method on PMF-nanoemulsion properties
We postulated that it may be possible to improve the stability of the nanoemulsions to crystal formation and sedimentation by altering the preparation method used. In the previous sections, we prepared nanoemulsions by homogenizing an oil phase (PMF in carrier oil) with an aqueous phase (emulsifier in buffer solution), i.e., the emulsifier was initially located in the aqueous phase. In this section, we examined the effect of incorporating the emulsifier in the oil phase (rather than in the aqueous phase) prior to homogenization. Thus, nanoemulsions were prepared by homogenizing an oil phase (PMF, carrier oil, and emulsifier) and an aqueous phase (buffer solution) using a high pressure homogenizer. A potential advantage of this latter approach is the ability of some emulsifiers to increase the solubility of highly hydrophobic molecules in oil phases (Pouton, et al., 2008). In these studies we again examined the influence of oil type (corn oil, MCT, and orange oil) and emulsifier type (Tween 20, lyso-lecithin, BLG). However, we did not include DTAB in this study, because it could not be used to form nanoemulsions in either the presence or absence of PMF (Table 1).
We found that the small molecule surfactants (Tween 20 and lyso-lecithin) could easily be dissolved within all three lipid phases leading to a transparent oily solution, but that the globular protein (BLG) could only be dispersed in a particulate form leading to a cloudy oily suspension. Nevertheless, we were still able to form nanoemulsions (d < 200 nm) containing PMF using all three types of emulsifier (Table 2). Indeed, the mean diameter of many of the nanoemulsions was less when the emulsifiers were initially located in the oil phase (Table 2) than when they were initially located in the aqueous phase (Table 1). Interestingly, it was not possible to form a stable emulsion with orange oil and lyso-lecithin using this emulsion preparation approach – instead a highly viscous foamy system was formed after high-speed blending. The physicochemical origin of this effect is currently unknown, but it may be because lyso-lecithin can act as an effective foaming agent. In addition, lyso-lecithin may have been more soluble in the orange oil than in the aqueous phase, which meant that the emulsions formed were unstable.
Table 2.
Mean particle diameter and ζ-potential of oil-in-water emulsions containing 1 wt% PMF dissolved in lipid carrier (corn oil, MCT, or orange oil). Emulsions were prepared by homogenizing the oil phase (carrier oil, PMF and emulsifier) with an aqueous phase (water). Samples were stored at room temperature for 24 hours prior to analysis.
| Corn Oil | MCT | Orange Oil | ||||
|---|---|---|---|---|---|---|
| Composition | d (nm) | ζ (mV) | d (nm) | ζ (mV) | d (nm) | ζ (mV) |
| BLG | 166 ± 3*** | −51 ± 2 | 155 ± 3 *** | −49 ± 3 | 149 ± 2 *** | −45 ± 7 |
| Lyso-Lec | 165 ± 2 * | −75 ± 2 | 150 ± 2 * | −68 ± 3 | N | N |
| T20 | 152 ± 2*** | −10 ± 1 | 137 ± 1 *** | −9 ± 1 | 121 ± 1 *** | −18 ± 2 |
| T20&T85 | 147 ± 3 * | −14 ± 1 | 136 ± 1 * | −11 ± 2 | 118 ± 2 *** | −16 ± 1 |
- N - Emulsions were highly unstable and could not be analyzed.
The general trends in the lipid droplet charge were similar for both preparation methods, and were mainly determined by the nature of the emulsifier used: highly anionic droplets were formed with BLG and lyso-lecithin, while slightly anionic droplets were formed with Tween 20. Visual observation and optical microscopy images again indicated that all the nanoemulsions prepared were unstable to crystal formation and sedimentation (Table 2). After one month storage, the nature of the PMF crystals present within the yellow sediment collected from the emulsions depended on oil and emulsifier type (Figure 7). In addition, adding the emulsifiers to the oil phase prior to homogenization (Figure 7) led to a different crystal morphology than adding the emulsifier to the aqueous phase (Figure 6). This effect was most dramatic for the BLG-stabilized nanoemulsions, where a larger number of densely packed crystals were observed when the emulsifier was added to the oil phase (Figure 7), than when it was added to the aqueous phase (Figure 6).
Figure 7.
Polarized light microscopy images of the sediments formed in PMF-fortified oil-in-water emulsions after 1 month storage. Each image represents an area of 187 mm × 250 mm. Crystal morphology appeared to depend strongly on oil type and surfactant type. These emulsions were prepared by mixing the emulsifiers with the oil phase first.
3.7. Influence of mixed surfactant systems on PMF-nanoemulsion properties
A number of studies have shown that the solubility of highly hydrophobic compounds in oil phases can be increased by using mixtures of hydrophilic and lipophilic surfactants (Pouton, et al., 2008). We therefore compared the properties of nanoemulsions prepared using Tween 20 as the sole emulsifier, with those prepared using a combination of Tween 20 (hydrophilic) and Tween 85 (lipophilic). In this case, the emulsions were prepared by mixing the PMF, surfactants, and carrier oil together first, and then homogenizing with an aqueous phase. Nanoemulsions prepared using the Tween 20/Tween 85 mixture had similar mean particle diameters and charges as those prepared using Tween 20 alone (Table 2). The mean particle diameter again depended on carrier oil type (orange oil < MCT < corn oil), which can be accounted for by the influence of oil phase viscosity and interfacial tension on droplet disruption within the homogenizer (see earlier discussion). There appeared to be some improvement in the stability of the emulsions to crystal sedimentation in the emulsions containing mixed surfactants after 24 hours storage, but PMF crystals were still observed in the optical microscopy images. After one month storage, all of the samples had a visible yellow layer at the bottom that contained crystals (Figure 8).
Figure 8.
Polarized light microscopy images of the sediments formed in PMF-fortified oil-in-water emulsions after 1 month storage. Each image represents an area of 187 mm × 250 mm. Crystal morphology appeared to depend strongly on oil type and surfactant type. These emulsions were prepared by mixing the emulsifiers with the oil phase first.
3.8. Influence of co-solvent addition on PMF-nanoemulsion properties
The results so far indicate that emulsifier type, oil type, or preparation method could not inhibit crystal formation in the nanoemulsions, but that they could inhibit crystal sedimentation. Finally, we therefore examined the influence of adding neutral hydrophilic co-solvents (glycerol or ethanol) to the aqueous phase of the emulsions prior to homogenization. Previous studies suggest that addition of neutral co-solvents may be able to improve the encapsulation of highly hydrophobic substances in emulsions (Warren, et al., 2010). Emulsions were prepared by homogenizing an oil phase (carrier oil, PMF, emulsifier) with an aqueous phase (co-solvent and buffer solution) together. In these experiments, we compared the influence of selected oil types (corn oil and MCT) and emulsifier types (lyso-lecithin) on system properties. We found that nanoemulsions (d < 200 nm) could be formed at all cosolvent levels (0 to 60% glycerol; 0 to 10% ethanol) studied, but that crystallization still occurred in all of the emulsions (Table 3). After one month storage, all of the samples had a visible yellow layer at the bottom that contained a network of aggregated needle-like crystals when observed by polarized optical microscopy (data not shown). These experiments indicate that addition of neutral co-solvents to the aqueous phase was also ineffective at preventing PMF crystallization.
Table 3.
Influence of neutral hydrophilic cosolvents (glycerol or ethanol) on the mean particle diameter and charge of PMF-loaded nanoemulsions fabricated using different types of oils and emulsifiers.
| Corn Oil | MCT | ||||
|---|---|---|---|---|---|
| Emulsifier | Glycerol | d (nm) | ζ (mV) | d (nm) | ζ (mV) |
| T20&T85 | 0% | 147 ± 3 * | −14 ± 1 | 136 ± 1 * | −11 ± 2 |
| 10% | 135 ± 2*** | −15 ± 1 | 128 ± 2 * | −13 ± 1 | |
| 30% | 136 ± 3 * | −12 ± 1 | 127 ± 4*** | −11 ± 1 | |
| 60% | 133 ± 2 * | −14 ± 1 | 136 ± 3 * | −11 ± 1 | |
| Ethanol | |||||
| 10% | 138 ± 1*** | −15 ± 2 | 120 ± 0.5*** | −14 ± 1 | |
| Lyso Lec | 0% | 165 ± 2 * | −75 ± 2 | 150 ± 2 * | −68 ± 3 |
| 10% | 181 ± 12 * | −83 ± 2 | 152 ± 3 * | −68 ± 1 | |
| 30% | 151 ± 3 * | −71 ± 1 | 135 ± 2*** | −66 ± 3 | |
| 60% | 130 ± 2*** | −65 ± 3 | 114 ± 1 * | −56 ± 2 | |
| Ethanol | |||||
| 10% | 148 ± 3*** | −71 ± 2 | 130 ± 1.4*** | −56 ± 3 | |
4. Conclusions
The purpose of this study was to determine whether oil-in-water nanoemulsions could be developed as delivery systems for PMF, which is a highly hydrophobic bioactive component. PMF has a low solubility in both water and oil phases at ambient temperatures, and so it tends to form crystals in nanoemulsions that can sediment to the bottom of the samples. This phenomenon has important consequences for the practical application of PMF-loaded nanoemulsions as delivery systems. If PMF formed crystals that sedimented to the bottom of a functional food product, then they may not be consumed and the beneficial attributes of this bioactive component would not be realized. On the other hand, even if PMF crystals were consumed, it is not currently clear whether they would be adsorbed by the body or how specific crystal characteristics (e.g., size and morphology) would impact their bioavailability. For this reason, we examined a number of factors that might impact the formation and stability of PMF-loaded nanoemulsions, including emulsifier type, carrier oil type, preparation method, and inclusion of neutral cosolvents. In all cases, we could not prevent PMF crystals from forming, but we could influence the size, morphology and aggregation of the PMF crystals formed, which affected their tendency to sediment to the bottom of the nanoemulsions. As would be expected, small PMF crystals appeared to be more stable to sedimentation than larger ones (after 24 hours storage). Further studies are required to understand the fundamental physicochemical mechanisms involved in nucleation and crystal growth within multiphase emulsion systems, and to establish effective strategies to control these processes.
The results of this study may have important implications for the design and fabrication of nanoemulsion-based delivery systems for PMF. Stable nanoemulsion delivery systems could be formulated by ensuring that the PMF concentration remained below the saturation level in the oil phase (i.e., C < CS), which was about 3 – 6 mM PMF in the oils used in this study. These values correspond to about 4 to 9 mg PMF per 100 g of a 5 wt% oil-in-water nanoemulsion, which is about 10 times less than the PMF amounts reported to have a biological effect. These levels could be increased by increasing the oil concentration in the delivery system or the amount of delivery system consumed. Since PMF has a higher saturation concentration in oil than in water it may also be possible to incorporate it into oil-based products (such as cooking oils, salad oils, margarines or spreads) rather than aqueous-based products (such as beverages, dressings, sauces, and dips). Alternatively, a delivery system could be developed that contained a mixture of PMF-saturated oil droplets and PMF crystals. Previous studies have shown that digestible carrier oils (such as medium and long chain triglycerides) can increase the bioavailability of highly lipophilic substances by generating surface active products (monoacylglycerols and free fatty acids) during lipid digestion (Porter, Trevaskis, & Charman, 2007). These surface active digestion products participate in the formation of mixed micelles with bile salts and phospholipids secreted in the human GI tract, which can increase the solubilization capacity of small intestinal juices for highly hydrophobic substances. Thus, the presence of digestible lipid droplets within a nanoemulsion containing PMF crystals might improve the bioavailability of the PMF. Nevertheless, further studies are required to determine whether PMF can be incorporated into mixed micelles, and whether PMF crystals can be directly adsorbed by the body.
Acknowledgements
This work was supported in part by an EPA-NSF-NIFA (AFRI) joint grant (2010-05266) program, NIH grant CA139174, and a CVIP grant from the University of Massachusetts Amherst. This material was also based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, the Massachusetts Agricultural Experiment Station, and the Department of Food Science. The authors have declared no conflict of interest.
References
- Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Current Opinion in Colloid & Interface Science. 2009;14(1):3–15. [Google Scholar]
- Ali W, Williams AC, Rawlinson CF. Stochiometrically governed molecular interactions in drug: Poloxamer solid dispersions. International Journal of Pharmaceutics. 2010;391(1-2):162–168. doi: 10.1016/j.ijpharm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Araya H, Tomita M, Hayashi M. The novel formulation design of O/W microemulsion for improving the gastrointestinal absorption of poorly water soluble compounds. International Journal of Pharmaceutics. 2005;305(1-2):61–74. doi: 10.1016/j.ijpharm.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Barakat NS. Self-emulsifying system for improving drug dissolution and bioavailability: in vitro/in vivo evaluation. Drug Development Research. 2010;71(2):149–158. [Google Scholar]
- Bevernage J, Brouwers J, Clarysse S, Vertzoni M, Tack J, Annaert P, Augustijns P. Drug Supersaturation in Simulated and Human Intestinal Fluids Representing Different Nutritional States. Journal of Pharmaceutical Sciences. 2010;99(11):4525–4534. doi: 10.1002/jps.22154. [DOI] [PubMed] [Google Scholar]
- Bovicelli P, D’Angelo V, Collalto D, Verzina A, D’Antona N, Lambusta D. Efficient synthesis of polyoxygenated flavones from naturally occurring flavanones. Journal of Pharmacy and Pharmacology. 2007;59(12):1697–1701. doi: 10.1211/jpp.59.12.0012. [DOI] [PubMed] [Google Scholar]
- Brouwers J, Brewster ME, Augustijns P. Supersaturating Drug Delivery Systems: The Answer to Solubility-Limited Oral Bioavailability? Journal of Pharmaceutical Sciences. 2009;98(8):2549–2572. doi: 10.1002/jps.21650. [DOI] [PubMed] [Google Scholar]
- Burcham DL, Maurin MB, Hausner EA, Huang SM. Improved oral bioavailability of the hypocholesterolemic DMP 565 in dogs following oral dosing in oil and glycol solutions. Biopharmaceutics & Drug Disposition. 1997;18(8):737–742. doi: 10.1002/(sici)1099-081x(199711)18:8<737::aid-bdd59>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Shukla D, Mishra B, Singh S. Lipid - An emerging platform for oral delivery of drugs with poor bioavailability. European Journal of Pharmaceutics and Biopharmaceutics. 2009;73(1):1–15. doi: 10.1016/j.ejpb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Dong P, Qiu P, Zhu Y, Li S, Ho CT, McClements DJ, Xiao H. Simultaneous determination of four 5-hydroxy polymethoxyflavones by reversed-phase high performance liquid chromatography with electrochemical detection. Journal of Chromatography A. 2010;1217(5):642–647. doi: 10.1016/j.chroma.2009.11.097. [DOI] [PubMed] [Google Scholar]
- Feltham DL, Garside J.s. A mathematical model of crystallization in an emulsion. Journal of Chemical Physics. 2005;122(17) doi: 10.1063/1.1886705. [DOI] [PubMed] [Google Scholar]
- Fredrick E, Walstra P, Dewettinck K. Factors governing partial coalescence in oil-in-water emulsions. Advances in Colloid and Interface Science. 2010;153(1-2):30–42. doi: 10.1016/j.cis.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gao P, Rush BD, Pfund WP, Huang T, Bauer JM, Morozowich W, Kuo M-S, Hageman MJ. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. Journal of Pharmaceutical Sciences. 2003;92(12):2386–2398. doi: 10.1002/jps.10511. [DOI] [PubMed] [Google Scholar]
- Hageman MJ. Preformulation Designed to Enable Discovery and Assess Developability. Combinatorial Chemistry & High Throughput Screening. 2010;13(2):90–100. doi: 10.2174/138620710790596781. [DOI] [PubMed] [Google Scholar]
- He CX, He ZG, Gao JQ. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opinion on Drug Delivery. 2010;7(4):445–460. doi: 10.1517/17425241003596337. [DOI] [PubMed] [Google Scholar]
- Hirata T, Fujii M, Akita K, Yanaka N, Ogawa K, Kuroyanagi M, Hongo D. Identification and physiological evaluation of the components from Citrus fruits as potential drugs for anti-corpulence and anticancer. Bioorganic & Medicinal Chemistry. 2009;17(1):25–28. doi: 10.1016/j.bmc.2008.11.039. [DOI] [PubMed] [Google Scholar]
- Jafari S, He Y, Bhandari B. Optimization of nano-emulsions production by microfluidization. European Food Research and Technology. 2007;225(5):733–741. [Google Scholar]
- Jafari SM, Assadpoor E, He YH, Bhandari B. Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocolloids. 2008;22(7):1191–1202. [Google Scholar]
- Kabalnov A. Ostwald ripening and related phenomena. Journal of Dispersion Science and Technology. 2001;22(1):1–12. [Google Scholar]
- Kawakami K, Yoshikawa T, Moroto Y, Kanaoka E, Takahashi K, Nishihara Y, Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs: I. Prescription design. Journal of Controlled Release. 2002;81(1-2):65–74. doi: 10.1016/s0168-3659(02)00049-4. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Firman K. Highly oxygenated flavonoids from Murraya paniculata. Phytochemistry. 1996;42(4):1207–1210. [Google Scholar]
- Kleberg K, Jacobsen J, Mullertz A. Characterising the behaviour of poorly water soluble drugs in the intestine: application of biorelevant media for solubility, dissolution and transport studies. Journal of Pharmacy and Pharmacology. 2010;62(11):1656–1668. doi: 10.1111/j.2042-7158.2010.01023.x. [DOI] [PubMed] [Google Scholar]
- Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discovery Today. 2010;15(21-22):958–965. doi: 10.1016/j.drudis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Kohno H, Yoshitani S, Tsukio Y, Murakami A, Koshimizu K, Yano M, Tokuda H, Nishino H, Ohigashi H, Tanaka T. Dietary administration of citrus nobiletin inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Life Sciences. 2001;69(8):901–913. doi: 10.1016/s0024-3205(01)01169-9. [DOI] [PubMed] [Google Scholar]
- Kourniatis LR, Spinelli LS, Piombini CR, Mansur CRE. Formation of orange oil-in-water nanoemullsions using nonionic surfactant mixtures by high pressure homogenizer. Colloid Journal. 2010;72(3):396–402. [Google Scholar]
- Lai C-S, Li S, Chai C-Y, Lo C-Y, Dushenkov S, Ho C-T, Pan M-H, Wang Y-J. Anti-inflammatory and antitumor promotional effects of a novel urinary metabolite, 3 ′ ,4 ′ - didemethylnobiletin, derived from nobiletin. Carcinogenesis. 2008;29(12):2415–2424. doi: 10.1093/carcin/bgn222. [DOI] [PubMed] [Google Scholar]
- Lai C-S, Li S, Chai C-Y, Lo C-Y, Ho C-T, Wang Y-J, Pan M-H. Inhibitory effect of citrus 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mice. Carcinogenesis. 2007;28(12):2581–2588. doi: 10.1093/carcin/bgm231. [DOI] [PubMed] [Google Scholar]
- Li S, Lo C-Y, Ho C-T. Hydroxylated Polymethoxyflavones and Methylated Flavonoids in Sweet Orange (Citrus sinensis) Peel. Journal of Agricultural and Food Chemistry. 2006;54(12):4176–4185. doi: 10.1021/jf060234n. [DOI] [PubMed] [Google Scholar]
- Li S, Pan M-H, Lai C-S, Lo C-Y, Dushenkov S, Ho C-T. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorganic & Medicinal Chemistry. 2007;15(10):3381–3389. doi: 10.1016/j.bmc.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Li S, Pan M-H, Lo C-Y, Tan D, Wang Y, Shahidi F, Ho C-T. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. Journal of Functional Foods. 2009;1(1):2–12. [Google Scholar]
- Li S, Wang Z, Sang S, Huang M-T, Ho C-T. Identification of nobiletin metabolites in mouse urine. Molecular Nutrition & Food Research. 2006;50(3):291–299. doi: 10.1002/mnfr.200500214. [DOI] [PubMed] [Google Scholar]
- Lindfors L, Forssen S, Westergren J, Olsson U. Nucleation and crystal growth in supersaturated solutions of a model drug. Journal of Colloid and Interface Science. 2008;325(2):404–413. doi: 10.1016/j.jcis.2008.05.034. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Food Emulsions: Principles, Practice, and Techniques. 2nd ed CRC Press; Boca Raton: 2005. [Google Scholar]
- McClements DJ. Emulsion Design to Improve the Delivery of Functional Lipophilic Components. Annual Review of Food Science and Technology, Vol 1. 2010;Vol. 1:241–269. doi: 10.1146/annurev.food.080708.100722. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter. 2011 [Google Scholar]
- McClements DJ, Li Y. Structured emulsion-based delivery systems: Controlling the digestion and release of lipophilic food components. Advances in Colloid and Interface Science. 2010;159(2):213–228. doi: 10.1016/j.cis.2010.06.010. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Rao J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Critical Reviews in Food Science and Nutrition. 2011;51(4):285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50(1):161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Müllertz A, Ogbonna A, Ren S, Rades T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. Journal of Pharmacy and Pharmacology. 2010;62(11):1622–1636. doi: 10.1111/j.2042-7158.2010.01107.x. [DOI] [PubMed] [Google Scholar]
- Neslihan Gursoy R, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomedecine & Pharmacotherapy. 2004;58(3):173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Okuno Y, Miyazawa M. Biotransformation of Nobiletin by Aspergillus niger and the Antimutagenic Activity of a Metabolite, 4′-Hydroxy-5,6,7,8,3′-pentamethoxyflavone. Journal of Natural Products. 2004;67(11):1876–1878. doi: 10.1021/np034007g. [DOI] [PubMed] [Google Scholar]
- Porter CJH, Trevaskis NL, Charman WN. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nature Reviews Drug Discovery. 2007;6(3):231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- Poullain-Termeau S, Crauste-Manciet S, Brossard D, Muhamed S, Nicolaos G, Farinotti R, Barthelemy C, Robert H, Odou P. Effect of Oil-in-Water Submicron Emulsion Surface Charge on Oral Absorption of a Poorly Water-Soluble Drug in Rats. Drug Delivery. 2008;15(8):503–514. doi: 10.1080/10717540802321792. [DOI] [PubMed] [Google Scholar]
- Pouton CW. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. European Journal of Pharmaceutical Sciences. 2006;29(3-4):278–287. doi: 10.1016/j.ejps.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Advanced Drug Delivery Reviews. 2008;60(6):625–637. doi: 10.1016/j.addr.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Qian C, McClements D. Formation of Nanoemulsions stabilized by Model Food-Grade Emulsifiers using High Pressure Homogenization: Factors Affecting Particle Size. Food Hydrocolloids. 2010 [Google Scholar]
- Rhee YS, Mansour HM. Nanopharmaceuticals I: nanocarrier systems in drug delivery. International Journal of Nanotechnology. 2011;8(1-2):84–114. [Google Scholar]
- Rousseau D. Fat crystals and emulsion stability - a review. Food Research International. 2000;33(1):3–14. [Google Scholar]
- Sato K. Solidification and phase transformation behaviour of food fats - a review. Fett-Lipid. 1999;101(12):467–474. [Google Scholar]
- Sergeev IN, Ho C-T, Li S, Colby J, Dushenkov S. Apoptosis-inducing activity of hydroxylated polymethoxyflavones and polymethoxyflavones from orange peel in human breast cancer cells. Molecular Nutrition & Food Research. 2007;51(12):1478–1484. doi: 10.1002/mnfr.200700136. [DOI] [PubMed] [Google Scholar]
- Sergeev IN, Li S, Colby J, Ho C-T, Dushenkov S. Polymethoxylated flavones induce Ca2+- mediated apoptosis in breast cancer cells. Life Sciences. 2006;80(3):245–253. doi: 10.1016/j.lfs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. European Journal of Pharmaceutics and Biopharmaceutics. 2010;76(3):475–485. doi: 10.1016/j.ejpb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Sinha S, Baboota S, Ali M, Kumar A, Ali J. Solid Dispersion: An Alternative Technique for Bioavailability Enhancement of Poorly Soluble Drugs. Journal of Dispersion Science & Technology. 2009;30(10):1458–1473. [Google Scholar]
- Sjöström B, Bergenståhl B, Kronberg B. A method for the preparation of submicron particles of sparingly water-soluble drugs by precipitation in oil-in-water emulsions. II: Influence of the emulsifier, the solvent, and the drug substance. Journal of Pharmaceutical Sciences. 1993;82(6):584–589. doi: 10.1002/jps.2600820608. [DOI] [PubMed] [Google Scholar]
- Sjöstrom B, Kronberg B, Carlfors J. A method for the preparation of submicron particles of sparingly water-soluble drugs by precipitation in oil-in-water emulsions. I: Influence of emulsification and surfactant concentration. Journal of Pharmaceutical Sciences. 1993;82(6):579–583. doi: 10.1002/jps.2600820607. [DOI] [PubMed] [Google Scholar]
- Tsukayama M, Kusunoki E, Hossain MM, Kawamura Y, Hayashi S. Microwave-assisted efficient synthesis of polymethoxyacetophenones and natural polymethoxyflavones, and their inhibitory effects on melanogenesis. Heterocycles. 2007;71(7):1589–1600. [Google Scholar]
- Wang D, Wang J, Huang X, Tu Y, Ni K. Identification of polymethoxylated flavones from green tangerine peel (Pericarpium Citri Reticulatae Viride) by chromatographic and spectroscopic techniques. Journal of Pharmaceutical and Biomedical Analysis. 2007;44(1):63–69. doi: 10.1016/j.jpba.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Warren DB, Benameur H, Porter CJH, Pouton CW. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: A mechanistic basis for utility. Journal of Drug Targeting. 2010;18(10):704–731. doi: 10.3109/1061186X.2010.525652. [DOI] [PubMed] [Google Scholar]
- Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Advanced Drug Delivery Reviews. 2004;56(9):1257–1272. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Wooster T, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24(22):12758–12765. doi: 10.1021/la801685v. [DOI] [PubMed] [Google Scholar]
- Xiao H, Yang CS, Li S, Jin H, Ho C-T, Patel T. Monodemethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel Inhibit growth of human lung cancer cells by apoptosis. Molecular Nutrition & Food Research. 2009;53(3):398–406. doi: 10.1002/mnfr.200800057. [DOI] [PubMed] [Google Scholar]
- Yoo J, Shanmugam S, Thapa P, Lee E-S, Balakrishnan P, Baskaran R, Yoon S-K, Choi H-G, Yong C, Yoo B, Han K. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Archives of Pharmacal Research. 2010;33(3):417–426. doi: 10.1007/s12272-010-0311-5. [DOI] [PubMed] [Google Scholar]