Abstract
Purpose.
The lateral rectus (LR) and medial rectus (MR) extraocular muscles (EOMs) have largely nonoverlapping superior and inferior innervation territories, suggesting functional compartmental specialization. We used magnetic resonance imaging (MRI) in humans to investigate differential compartmental activity in the rectus EOMs during head tilt, which evokes ocular counter-rolling, a torsional vestibulo-ocular reflex (VOR).
Methods.
MRI in quasi-coronal planes was analyzed during target-controlled central gaze in 90° right and left head tilts in 12 normal adults. Cross sections and posterior partial volumes of the transverse portions of the four rectus EOMs were compared in contiguous image planes 2 mm thick spanning the orbit from origins to globe equator, and used as indicators of contractility.
Results.
Horizontal rectus EOMs had significantly greater posterior volumes and maximum cross sections in their inferior compartments (P < 10−8). In orbit tilt up (extorted) compared with orbit tilt down (intorted) head tilts, contractile changes in LR maximum cross section (P < 0.0001) and posterior partial volume (P < 0.05) were significantly greater in the inferior but not in the superior compartment. These changes were not explainable by horizontal or vertical eye position changes. A weaker compartmental effect was suggested for MR. The vertical rectus EOMs did not exhibit significant compartmental contractile changes during head tilt. Mechanical modeling suggests that differential LR contraction may contribute to physiological cyclovertical effects.
Conclusions.
Selective activation of the two LR, and possibly MR, compartments correlates with newly recognized segregation of intramuscular innervation into distinct compartments, and probably contributes to noncommutative torsion during the VOR.
Magnetic resonance imaging of extraocular muscles during ocular counter-rolling demonstrates selective activation of the lateral rectus inferior but not superior compartment. This is novel functional evidence that differential rectus compartmental activation contributes to a vestibulo-ocular reflex.
Introduction
The rectus extraocular muscles (EOMs) classically have been regarded as homogeneous actuators generating mechanical forces applied to their insertions on the eye.1 It has long been recognized that, because actual EOM tendons are fairly wide (approximately 10 mm in humans2), the functional insertion point of such idealized actuators is not always at the center point of the tendon's insertion on the globe, but rather shifts with eye orientation in a manner that depends upon factors such as differential stretching.3,4 However, rectus EOMs have features that would permit them a larger active repertoire than conventionally recognized, including more fibers than required for conventionally recognized eye movement mechanisms.5,6 If active rectus EOM force were applied focally at different points along the transverse dimension of a rectus EOM tendon, mechanical effects would vary correspondingly. This anatomical complexity was not considered in EOM biomechanics until the recent discovery, based upon three-dimensional histological reconstructions in humans and monkeys, of a nonoverlapping pattern of zonal innervation in the lateral rectus7 (LR) and medial rectus (MR) muscles.7,8 Proximal to entry into the corresponding horizontal EOM, its motor nerve divides into superior and inferior branches supplying essentially nonoverlapping arborizations that remain segregated into distinct neuromuscular compartments for the EOM's entire anteroposterior length.7,8 These neuromuscular compartments contain groups of parallel EOM fibers that have very few myomyous lateral interconnections.9,10 At the insertional tendon, regions of rectus EOMs may have considerable mechanical independence. As little as 5% of the passive tension imposed upon half of an isolated bovine rectus EOM tendon may be reflected in the loading of the other half of the tendon, suggesting substantial mechanical independence.11 If the anatomically distinct neuromuscular compartments of living horizontal rectus EOMs were to act in an at least partially independent mechanical fashion, this would permit an actively-modulated transverse force distribution surpassing that determined by purely elastic EOM properties, endowing the horizontal rectus EOMs with hitherto unsuspected capabilities.
There is less anatomical potential for selective compartmental control of vertical rectus EOMs. Motor nerves within the inferior rectus (IR) are “mixed” in the lateral portion of the EOM, but medial portion is exclusively innervated by the medial division of the motor nerve.8 A putative lateral IR compartment might, to a very limited degree, be controlled independently from the medial compartment. Compartmental control of the superior rectus (SR) seems anatomically unlikely, since intramuscular motor nerve arborizations within the SR are mixed.8
A troubling gap in our understanding of ocular kinematics raises the potential cyclovertical activity of rectus EOMs to a level of fundamental importance. It has long been recognized that when remote visual targets are fixated or pursued when the head is upright and stationary, Listing's law (LL) specifies unique eye torsion equal to that achieved by a single rotation from primary position about an axis lying in a unique plane, Listing's plane (LP).12 To keep orientation vectors in LP during an eye movement between a secondary and a tertiary position, the angular velocity vector about which the eye spins must tilt out of that plane.13 Tweed defined a necessary and sufficient condition for LL: That the ocular rotational velocity axis must shift continuously by half of the shift in ocular orientation.13 Since this velocity axis shift amounts to the introduction of torsional eye velocity that is not present in the visual stimulus, Ghasia and Angelaki have termed the torsional eye velocity “noncommutative torsion,” distinguishing it from “sensory torsion” that is present in a sensory stimulus such as head tilt.14 It was formerly believed that the brain explicitly computed all ocular torsion and commanded the cyclovertical (vertical rectus and oblique) EOMs to enforce LL.15–20 More recently, however, it has been demonstrated that motor neurons activating cyclovertical EOMs of monkeys do not encode noncommutative torsion during pursuit.14 Furthermore, electrical stimulation of the abducens nerve in monkeys evokes saccade-like movements that conform to LL whether the head is stationary,21 statically tilted in roll,22 or undergoing dynamic roll rotation.23 This means that the noncommutative torsion underlying LL is not neurally generated by classically cyclovertical EOMs, but that LL noncommutative torsion is generated by a peripheral mechanism in the orbit. The Active Pulley Hypothesis resolved the foregoing findings by proposing that the noncommutative torsion underlying LL is generated by gaze-related changes in rectus EOM pulling directions due to active positioning of their connective tissue pulleys.24–27
Little doubt now remains that the noncommutative torsion of LL is the peripheral oculomotor apparatus' kinematic default behavior. However, not all noncommutative torsion has the same half-angle dependence upon eye position as LL, engendering a serious paradox: The angular vestibulo-ocular reflex (VOR) violates LL such that its ocular velocity axis changes by one fourth of eye position.28 Yet, single-unit recordings in monkeys show that activity in cyclovertical motor neurons has the same dependence on eye position when the VOR violates LL, as during pursuit that complies with LL.14 Neither during the VOR nor during pursuit do the cyclovertical motor neurons encode noncommutative torsion. That finding leaves only two candidate EOMs that could encode noncommutative torsion: the MR and the LR. Therefore, the authors recently proposed that the noncommutative torsional violations of LL by the VOR must be executed by one or both horizontal rectus EOMs.7,8
Physiological activation of the angular VOR is impractical during magnetic resonance imaging (MRI). Nevertheless, head tilt relative to gravity evokes a static torsional VOR termed ocular counter-rolling (OCR), a form of vestibular sensory torsion. Roll head tilt of 90° evokes 3 to 7° OCR.29,30 An MRI study during static 90° head tilt demonstrated that the EOM pulley array rotates in the direction of OCR 31. Since MRI can also provide a functional indication of EOM contractility through changes in EOM cross section and volume31–37 (Clark RA, Demer JL, manuscript submitted, 2012), the authors sought evidence of selective compartmental activation of rectus EOMs during head tilt in normal subjects. The present paper also considers the possibility that horizontal rectus EOMs may be capable of generating cyclovertical movements, including sensory torsion, which would open the possibility that horizontal rectus EOMs might also be capable of generating noncommutative torsion.
Methods
Twenty-six subjects gave written informed consent according to a protocol conforming to the Declaration of Helsinki and approved by the Human Subject Protection Committee at the University of California, Los Angeles. Subjects underwent comprehensive eye examination of visual acuity, ocular motility, stereoacuity, and ocular anatomy.
High-resolution, T1 or T2 fast spin echo (T2FSE) MRI was performed using a 1,5-T scanner (Signa; General Electric, Milwaukee, WI). Technical details, which have been described in detail elsewhere,31 include use of a custom dual-phased surface coil array (Medical Advances, Milwaukee, WI) to improve signal-to-noise ratio and fixation targets to avoid motion artifacts. Technique for T2FSE has been published.38 Subjects were scanned in 90° right side down and left side down head tilt positions while lying on the scanner bed (Fig. 1). During imaging, the scanned eye monocularly fixated a fine optical fiber illuminated from its distal end by a red light-emitting diode in straight ahead gaze 2 cm distant. There was no stimulus to convergence.
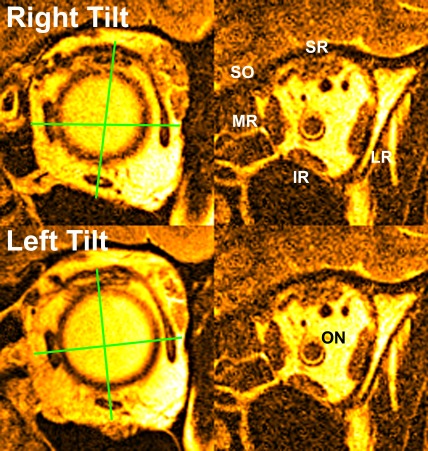
Figure 1.
Quasi-coronal MRI of left orbit in 90° right (top) and left head tilts. Right column is anterior view near rectus pulleys. Green lines through rectus EOM area centroids indicate pulley counter-rolling. Middle column is near maximum rectus cross section in midorbit. ON, optic nerve; SO, superior oblique.
A triplanar scan was obtained to verify correct head positioning relative to gravitational scanner coordinates, with head adjustment and repetition as necessary. Sets of 18 to 20 contiguous, 2-mm-thick quasi-coronal images were placed perpendicular to the long axis of each orbit using a 256 × 256 matrix over an 8-cm field of view (FOV), yielding a final pixel resolution of 312 microns. The paramagnetic MRI contrast agent gadodiamide (0.05 mM/kg) was given intravenously in some subjects before the first quasi-coronal scan in each tilt position to improve the contrast of EOMs against connective tissue in the anterior orbit. Imaging with T2FSE provides this contrast intrinsically without intravenous injection. Image sets were obtained first in the right and then in the left orbit.
Digital MRI images were quantified using image processing software (ImageJ64; W. Rasband, National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/) and customized image analysis programs (MatLab; MathWorks, Boston, MA). Eye position was determined by the location of the optic nerve centroid relative to the orbital centroid at the globe-optic nerve junction. Only orbits that were free of movement artifacts and that demonstrated less than 11° change in eye position for both head tilt positions were analyzed, to avoid confounding by large horizontal or vertical gaze shifts. Measurements indicated that between right side up and right side down head tilt positions, there was 3.8 ± 4.2° (SD) adduction, and 2.8 ± 4.9° supraduction. Since these values are significantly different from the ideal value of zero, the result of the gaze change was analyzed quantitatively.
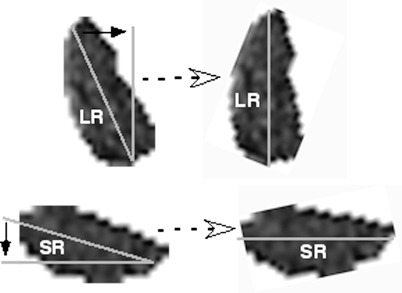
Beginning as far posteriorly within the orbit as individualized EOM bellies could be identified, each rectus EOM was manually outlined and cropped to include only the EOM belly. Each cropped image was first processed in image analysis software (Math Works) by calculating the angle of a linear best-fit line through the largest transverse dimension of the EOM. Then, for horizontal EOMs, the entire image was rotated to align that best-fit line with scanner vertical (Fig. 2, top), while for vertical EOMs the entire image was rotated to align that best-fit line with scanner horizontal (Fig. 2, bottom). For the horizontal EOMs, the superior and inferior EOM compartmental areas were calculated by separately counting the pixels above and below the 50% perpendicular to this best-fit line, while for the vertical EOMs, the medial and lateral compartments were calculated by separately counting the pixels medial and lateral to the 50% perpendicular to this best-fit line (Fig. 3). Then, the pixels were converted into mm2 to form the cross-sectional areas, and multiplied by the 2-mm slice thickness to form the partial volumes in mm3 for the respective compartments.
Figure 2.
A best-fit line was computed down the largest transverse dimension of each EOM to form the long axis of the cross section. Then, for horizontal EOMs (top), the image was rotated to align the long axis with scanner vertical, and to scanner horizontal for vertical EOMs (bottom).
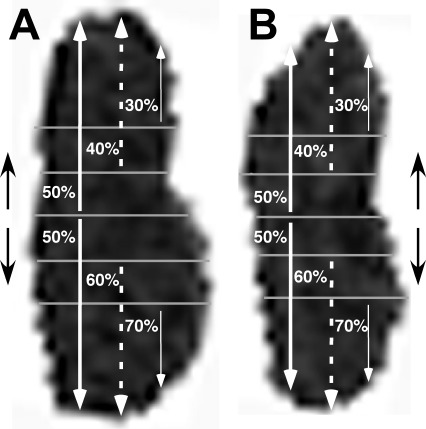
Figure 3.
Rotated images of LR cross sections in the same orbit in up (A, extorted) and orbit down (B, intorted) positions. In this subject, no visible feature demarcates segregation of LR compartments. An automated program calculated areas of the superior and inferior 50% splits of each EOM (thick white arrows). The program repeated the analysis using 40/60% splits (dashed white arrows), neglecting the middle 20%, and 30/70% splits (thin white arrows), neglecting the middle 40%, to exclude areas of potential mixed innervation. Similar analysis was performed on the vertical rectus EOMs using lateral and medial splits.
The authors recognized that segregating the EOM compartments into equal halves is a conservative assumption, since it likely includes central EOM areas with mixed compartmental innervation varying among individual subjects and perhaps the various EOMs. There are structural features sometimes visible in the LR of normal subjects that are believed to demarcate the borders between superior and inferior compartments, although in many subjects no structural demarcation could be visualized by MRI (Demer JL. In press. 2012:ARVO Abstract 2224). When structural demarcations were visible, the ratio of inferior compartment to total LR transverse width was 0.49 ± 0.10 (SD, implying 95% confidence limits for the compartmental border of 30% to 70% of transverse LR width (Demer JL. In press. 2012:ARVO Abstract). No structural MRI demarcations have been reported for MR compartments. In the present study, only a small minority of subjects had a visible demarcation of MR or LR compartments, so by way of sensitivity analysis, the same data reduction was repeated using 30% and 70% segregations (the 95% confidence intervals for the compartments), thereby excluding the middle 40% of the EOMs, and 40% and 60% segregation limits, thereby excluding the middle 20% of the EOM, to determine the effects of possible differences in compartmental segregation (Fig. 3).
Based upon a separate study optimizing measures of horizontal rectus EOM contractility, (Clark RA, Demer JL, submitted, 2012) two measures of compartmental contractility were analyzed for each gaze position: The single-plane maximum cross-sectional area, and the partial volume in four contiguous image planes beginning with the most posterior image plane that could be measured in every subject. Statistical comparisons were made for those values between head tilt up (extorted) and head tilt down (intorted) positions using paired t-tests. Differences in measures between head tilt up and head tilt down were also scaled as percentage changes from the values in the head tilt up position. The percentage changes in contractile measures between the two compartments for each EOM were compared using paired t-tests.
Results
Fixation Control
Twelve subjects of age 25.3 ± 5.2 (mean ± SD) years contributed data to the analysis. Gaze position was sufficiently repeatable that data could be analyzed for both orbits of six subjects, and one orbit each of six other subjects. Data from 14 other subjects, and from the second orbit of six subjects, were discarded because of movement artifacts, or large and potentially confounding changes in horizontal or vertical gaze position. For included subjects, the difference in vertical gaze position between right and left head tilts averaged 2.8 ± 4.9°, not significantly different from zero (P > 0.01). The difference in horizontal gaze position averaged 3.8 ± 4.2°, which was significantly different from zero (P < 0.002) but nevertheless a small variation relative to the ocular motor range testable during MRI of more than 50°. It is notable that average horizontal eye position with the orbit tilted up was 3.8° adducted compared with horizontal eye position with the orbit tilted down. The effect of this difference would be predicted to diminish, not increase, LR cross-sectional area and volume in the orbit-tilted-up position by approximately 2.5%, and correspondingly increase these values for the MR (Clark RA, Demer JL, submitted, 2012). Differential effects on horizontal rectus compartments would not be anticipated from small horizontal gaze changes, which would be assumed to produce similar effects in both compartments.
Size Distributions in Hemi-EOMs
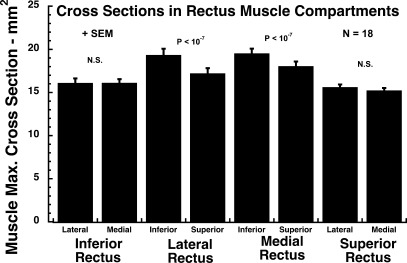
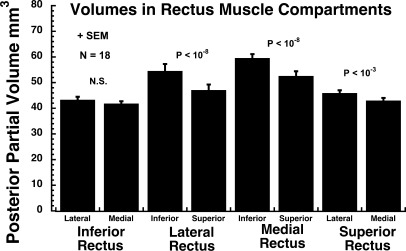
There were highly significant compartmental asymmetries in EOM size for the horizontal rectus EOMs, and one less significant difference for the SR. Based upon both maximum cross section (Fig. 4) and posterior partial volume (Fig. 5), the inferior compartment of the LR and MR was 8 to 14% larger than the superior compartment (P < 10−7). For the SR and IR, there were no significant differences between maximum cross sections of the medial and lateral compartments. There was no significant difference in posterior partial volume between the medial and lateral compartments for the IR. The posterior partial volume of the SR medial compartment was 7% larger than the lateral compartment (P < 0.001).
Figure 4.
Mean maximum cross section of each of the four rectus EOMs in 18 orbits, averaged between right and left head tilt positions. Only the horizontal rectus EOMs exhibited a significant (P < 10−7) asymmetry between their respective 50% compartments, as operationally defined by bisection of transverse EOM dimension.
Figure 5.
Mean posterior partial volume of each of the four rectus EOMs in 18 orbits, averaged between right and left head tilt positions. The horizontal rectus EOMs exhibited highly significant (P < 10−8) asymmetry between respective 50% compartments, as operationally defined by bisection of transverse EOM dimension. Less significant asymmetry was present in superior rectus (P < 10−3).
Contractility in Hemi-EOMs
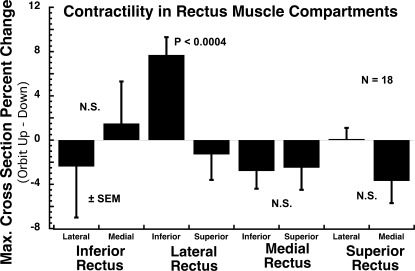
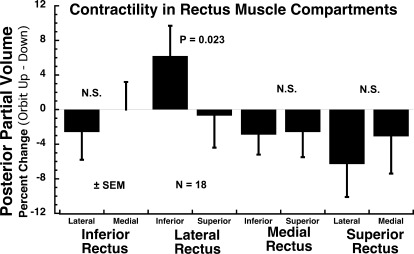
Since EOM bulk differed between compartments, contractile changes were normalized as percent changes in maximum area and posterior volume between the gaze positions to avoid confounding by the significant initial differences in cross section and volume between compartments. Contractile changes in the two compartments of each of the four rectus EOMs were assessed using both maximum cross section (Fig. 6) and posterior partial volume (Fig. 7) changes between the orbit up and the orbit down head tilts, with positive values defined as larger areas and posterior partial volumes in the orbit up position. Compartmental differences in maximum cross section were best demonstrated with the 50:50 split for the LR. The superior compartment LR cross section exhibited a 1.3% reduction in maximum cross section in orbit up minus down positions, while the corresponding value in the inferior compartment exhibited a 7.7% increase, such that the difference between compartments was highly significant (P < 0.0004; Fig. 6). Similar significant findings were also, albeit with greater P values, obtained with the 30:70 and 40:60 segmentations. Compartmental differences in posterior partial volume were best demonstrated with the 30:70 split for the LR. The superior LR compartment posterior partial volume exhibited a 0.7% reduction in maximum cross section in orbit up minus down positions, while the corresponding value in the inferior compartment exhibited a 6.2% increase, such that the difference between compartments was significant (P = 0.023; Fig. 7). The analysis was repeated using 30/70% and 40/60% splits in EOM cross-sectional areas with similar and significant results. The LR inferior compartment demonstrated a significant 7.5% (30/70 split; P < 0.01) and 7.8% (40/60 split; P < 0.0008) increase in maximum cross-sectional area and 6.9% (50/50 split; P < 0.03) and 6.3% (40/60 split; P < 0.03) increase in posterior partial volume in the orbit up position as compared with down. No other compartment demonstrated a significant change in contractile measures, regardless of how the EOMs were partitioned into compartments.
Figure 6.
Contractility, based upon percent change in mean maximum cross section from orbit up to down head tilt, of the two 50% compartments of each rectus EOM in 18 orbits. Significant differential contractility between compartments of the same EOM (P < 0.05) was observed only between the superior and inferior compartments of the lateral rectus muscle, for which the difference was highly significant (P = 0.0004).
Figure 7.
Contractility, based upon percent change in posterior partial volume from orbit up to down head tilt, of the extreme 30% compartments of each rectus EOM in 18 orbits. Significant differential contractility between compartments of the same EOM (P < 0.05) was observed only between the superior and inferior compartments of the lateral rectus muscle, for which the difference was significant (P = 0.023).
Correlations with Horizontal and Vertical Eye Position
Since the rectus EOMs contract and relax during horizontal and vertical ductions, changes in horizontal and vertical eye position would be expected to change the maximum cross sections and midorbital volumes of the rectus EOMs. While stringent effort was made to maintain unchanged central gaze position between right and left head tilts during MRI, some variation was unavoidable. This variation is due to imperfect horizontal and vertical target placement relative to the 2 cm distant eye, so tiny variations in head position relative to the target can lead to appreciable gaze direction differences.
The effect of horizontal gaze variation was explored by computing orbit-by-orbit linear correlations between gaze variations and contractility, as assessed both by change in eye position change between the orbit up and orbit down head tilts. There was a weak negative correlation of maximum cross-sectional area and posterior partial volume for both the superior and inferior LR compartments with degrees of abduction, opposite what would be predicted for passive changes in those contractile measures. This finding is consistent with the increase in those LR contractile measures despite an average slight bias towards adduction. The coefficient of determination R2 in the linear correlations indicates the fraction of variance in contractility due to change in horizontal eye position. The coefficient of determination suggests that less than 5% of the variance in maximum LR superior or inferior cross-sectional area was associated with the measured change in horizontal position. There was a larger variance with change in posterior partial volume, up to 35% for the inferior compartment, but once again, the change in volume was negatively correlated with abduction and cannot explain the finding of increased posterior partial volume in the orbit up position. There were smaller coefficients of determination linear fits for the LR compartments with infraduction, accounting for 6 to 16% of the variance in those measures.
Similar correlation analyses were performed for the remaining rectus EOMs between both horizontal and vertical eye position changes, versus both area and volume measures of contractility. Results were similar for both compartments of the IR, MR, and SR. Changes in horizontal and vertical eye position accounted for less than 35% of the variance in both measures of MR contractility, <21% of variance in the IR, and <12% of the variance in SR contractility.
A separate MRI study that systematically varied horizontal duction angle indicated that maximum whole LR cross section changes by 0.73% per degree of horizontal duction (Clark RA, Demer JL, submitted, 2012). The same study found that the maximum whole MR cross section changes by 0.82% per degree of horizontal duction. In both cases, duction angle accounted for about 80% of the variance in maximum horizontal rectus cross section (Clark RA, Demer JL, submitted, 2012). The effect of small horizontal eye position variations on contractile changes in horizontal rectus EOM compartments was examined by correcting the observed maximum cross section data using the foregoing linear model. From orbit up to orbit down head positions, the corrected maximum cross section of the LR superior compartment changed by −4.0 ± 2.3% (SEM), while the corresponding value of the LR inferior compartment changed by +5.0 ± 1.6%; the difference remained significant at the same P < 0.0004 level as depicted for the uncorrected data in Figure 6. Interestingly, when the corresponding correction was applied to the MR, maximum corrected cross section of the superior compartment changed by −5.2 ± 2.1%, while the corresponding value for the MR inferior compartment changed by 0.0 ± 1.8%; this difference became modestly significant at P = 0.011.
The separate MRI study also found that MR posterior partial volume changed by 3.1 ± 0.6% (SD) per degree of horizontal duction (Clark RA, Demer JL, submitted, 2012). The present study found that from orbit up to orbit down positions, the corrected posterior partial volume of the MR superior compartment changed by 5.0 ± 3.8% (SEM), while the corresponding value of the MR inferior compartment changed by 6.6 ± 3.4%; the difference between the two was not significant.
Discussion
While there have been intriguing anatomical suggestions that the horizontal rectus EOMs may be organized into selective neuromuscular compartments,7,8 the present MRI study provides the first functional data supporting differential compartmental function in a rectus EOM. All four rectus EOMs exhibited nonuniform mass distributions across their long transverse widths, approximating 7 to 15% asymmetry between the larger and smaller halves. This asymmetry was not inevitably associated with a difference in function in all rectus EOMs. Only the LR exhibited striking functional asymmetry, although behavior of the MR maximum cross section was also asymmetrical at a lower level of significance. While the OCR evoked by 90° head tilts is a small-amplitude and individually-variable torsional VOR of typically 3 to 7° magnitude,29,30 MRI demonstrated that OCR is associated with contractile activity in the inferior but not superior LR compartment. It is suggested that differential horizontal rectus contractility may contribute to OCR during head tilt. Analysis of small variations in horizontal and vertical eye position during OCR indicated that this activity in the LR inferior compartment is not attributable to changes in gaze direction, although there is weaker evidence for corresponding differential compartmental activity in the torsional antagonist, the MR superior compartment. Average horizontal eye position with the orbit tilted up was 3.7° adducted compared with horizontal eye position with the orbit tilted down, a difference that would have diminished, rather than increased, the effect observed here for the LR.
The finding of differential compartmental contractility in the LR implies differential neural control of the two EOM compartments evoked by torsional vestibular stimulation, and in this instance was not evoked by other factors. The present data provide some evidence for observed reciprocal behavior in the MR, although if MR reciprocity is weaker, there is some precedent. For example, activity of abducens motor neurons fails to account fully for LR force during convergence, because force in the relaxing primate LR decreases more than does pooled abducens motor neuron.39 As a result, the monkey MR and LR paradoxically corelax during convergence,39 corresponding to the absence of globe retraction demonstrated by MRI during human convergence.34
Could the present differential compartmental effects simply represent the passive effects of ocular torsion on the distributions of rectus EOM mass due to torsional changes in path length? This seems unlikely, since the effect was observed only in the horizontal rectus EOMs, and not in the vertical rectus EOMs, which should be equally subject to passive distribution effects. Compartmental differences in the horizontal rectus EOMs during OCR are therefore likely to be at least partial effectors of OCR, rather than the results of OCR.
It is likely that the MRI technique employed here underestimated the true difference in contractility between the superior and inferior LR zones. Histological data show, on average, about 50% of the abducens innervation is directed towards each zone,7,8 so division of the LR into two equally wide halves is a sensible anatomical approximation. The segregation of innervation, however, varies among a small number of histological samples from approximately 40% to 60% to favor one zone or the other.7,8 Larger MRI samples indicate that the average segregation is about 50%, but with a 95% confidence interval of 30 to 70% (Demer JL. In press. 2012:ARVO Abstract 2224). Re-analyzing the data using 30/70% and 40/60% splits resulted in identical findings for the LR, but each approximation does not actually segregate the activity of each anatomic compartment for each subject. Overlap might have accounted for the apparently absent differential compartmental activity in the MR during OCR (Figs. 6, 7), that was only significant after correction for small variations in horizontal eye position. It is possible that differential compartmental activity in MR might in reality be comparable to that of the LR, yet be more difficult to detect using MRI because of differing compartmental distribution, or greater innervational overlap at the compartmental boundary in MR than in LR.
Another separate MRI study of the effect of vertical duction on differential compartmental function of the MR has demonstrated significant superior compartment contraction from infraduction to infraduction, with no significant corresponding change in the inferior compartment (Clark RA, Demer JL, submitted, 2012). Since eye position was 2.8° supraducted from orbit up to orbit down position in the current study, target position might have accounted for slightly greater MR superior compartment size with the orbit down, perhaps cancelling some or all of the effect of OCR. Vertical duction was not observed to produce any compartmental effect in the LR, so data for LR were presumably not subject to this artifact.
Since the LR and MR form the principal antagonist EOM pair responsible for horizontal duction, contraction of either would ordinarily be expected to produce a change in eye position. Yet, in the present study, there was little or no change in horizontal and vertical eye position associated with inferior LR compartment contraction during OCR. This may be explained by reciprocal contractile activity in the MR superior compartment during OCR, cancelling horizontal rotation, yet summing the torsional effects of each horizontal rectus EOM during OCR.
A contribution to torsion by the horizontal rectus EOMs might be of general physiological importance. At least under some carefully controlled experimental conditions, Ghasia and Angelaki have demonstrated that the cyclovertical motor neurons do not carry the noncommutative torsional command for violations of LL during the angular VOR,14 raising the question of how non-LL torsion can be generated by “noncyclovertical” EOMs. The present study proposes differential compartmental activity in the horizontal rectus EOM as an answer to this conundrum. Torsion created by differential compartmental LR, and possibly MR, contraction would add no latency additional to the action of the horizontal rectus EOMs, so this mechanism has the potential to explain the otherwise mysterious quarter-angle violation of LL observed during the VOR that motivated the present study.40 It should be understood that the current study cannot confirm that differential compartmental contraction of one or both horizontal rectus EOMs actually implements the noncommutative torsion that permits the angular VOR to violate LL. Such confirmation would require dynamic VOR stimulation during functional monitoring of selective compartmental function in EOMs, a technical capability not yet available. The current study does, however, offer physiological evidence that differential compartmental LR contraction indeed occurs during one form of vestibular stimulation, static head tilt, and suggests that this mechanism could potentially account for quarter-angle violations of LL during rotational vestibular stimulation. Demonstration of even a potential mechanism is important, given that single unit recordings in monkeys by Ghasia and Angelaki have now excluded the only plausible alternative explanation, explicit commands to the cyclovertical EOMs.14 Ghasia and Angelaki found that while the motor neurons innervating all of the cyclovertical EOMs had sensitivities during horizontal and vertical VOR identical to those during pursuit, the noncommutative torsion during these behaviors differed: LL was violated during the VOR, but observed during corresponding pursuit.14 This behavioral difference thus cannot be attributable to the to noncommutative torsion during the VOR, yet could be consistent with the current finding of differential compartmental function of horizontal rectus EOMs.
A contribution to torsional VOR function by the LR implies a source of vestibular input to the abducens nucleus that was not classically considered, but recently documented by transneuronal transfer of rabies virus.41 Abundant cyclovertical premotor inputs, including vestibular inputs, are available to the abducens nucleus, derived from cell groups in vertical eye movement pathways, including the utricular otolith and superior vestibular nucleus, interstitial nucleus of Cajal, and rostral interstitial nucleus of the medial longitudinal fasciculus.36 While no anatomical data are available on possible topographic distribution of premotor inputs to compartmentally-projecting motor neuron pools, other segregation of the final LR nerve-muscle pathway is well-recognized. The LR belly originates from two myotomes42,43 as described in early studies, or in more recent terminology from both somitomeres 4 and 5.44,45 The abducens nucleus and nerve arise from both rhombomeres 5 and 6.44 In autopsies, 6 to 15% of humans are noted to have abducens nerves divided into parallel trunks46–50 that might subserve two functional compartments. The LR can sometimes be seen to have a dual-headed origin in the deep orbit.51,52 The LR belly often exhibits longitudinal splitting, sometimes in normal subjects, but more prominently in congenital cranial dysinnervation disorders such as congenital fibrosis of the extraocular muscles type 1,35 congenital oculomotor palsy,53 congenital trochlear palsy,53 and Duane syndrome.54–56 Intriguingly, the anomalous innervation of the LR by the oculomotor nerve in Duane syndrome appears to be predominantly or exclusively directed to the inferior compartment.54,55
So far, OCR is the only VOR that is suitable for study during MRI. Most physiological eye movements have substantially larger maximum amplitude than does OCR, and might involve greater differential compartmental rectus EOM activity. An additional candidate eye movement for involvement of differential compartmental activity in rectus EOMs would be convergence, which has larger amplitude than OCR and also includes noncommutative binocular extorsion.57–64 Preliminary MRI data suggest differential compartmental activity of the antagonist MR is involved in convergence.65 Perhaps differential contractility even occurs during normal ocular versions, in the absence of the VOR, vertically altering the effective origin of the LR and endowing this nominally “horizontal” rectus EOM with some vertical and torsional actions in addition to abduction. Possible differential compartmental rectus activity remains unexplored for most physiological eye movements.
Of course, differential LR compartmental activation requires differential transmission of force to the globe to allow each compartment to have a different effect on eye rotation. The broad LR insertion would allow independent compartments to generate force vectors displaced sufficiently from the globe center to impart substantial torsional and horizontal or vertical force, in much the same manner of action as surgical transposition of a rectus EOM insertion perpendicular to its usual path.66–68 Prior mechanical models of rectus EOM action supposed that the distribution of force across the width of an EOM tendon was nonuniform only because of differential stretch across the muscle width comprising a single compartment. The EOM has been effectively modeled as one thin string exerting neurally-modulated force inserted at a point on the globe whose location varies only under the influence of passive force distribution along the tendon width. The current finding leads to the proposition that force across an EOM tendon is also nonuniform because of differential innervation to upper and lower (for horizontal rectus) compartments. The simplest model of a dual-compartment EOM would be a pair of strings, one for each compartment, located at their individual force centroids (Fig. 8). The summed forces of the two compartment strings could still be reducible to a single net string, but its location could vary widely based upon differential generation of active tension in each string.
Figure 8.
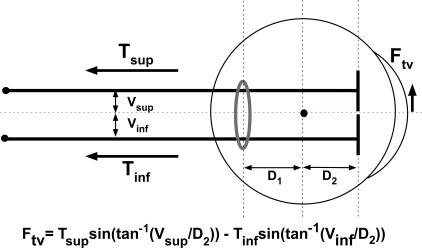
Simplified, dual-compartment model of LR action. Ftv, torsional-vertical force exerted by LR; Tsup, superior compartment tension; Tinf, inferior compartment tension; Vinf, displacement of superior compartment force centroid from globe center; Vinf, displacement of inferior compartment force centroid from globe center; Gray ring. pulley.
The terminal tendon is of roughly uniform thickness less than 1 mm, but 10 to 12 mm wide. It is assumed that the superior and inferior LR compartments transition anteriorly to tendons of equal width spanning an insertional width for the LR of 12 mm. The force centroid of each equal compartment would act on the sclera at the center of each compartment's width, or ±3 mm from the overall LR centroid by the foregoing assumption. In the extreme case, total relaxation to zero of one compartment, with tonus in the other compartment, would shift the LR active force centroid at the scleral insertion by 3 mm, one fourth of total tendon width, in the direction of the active compartment. The mechanical effect of such a shift in insertional force depends also upon the direction taken by each compartmental centroid farther posteriorly, at its pulley. The LR pulley is roughly elliptical, with a vertically-oriented major axis that the authors will assume, until data are available, to be equal to the LR insertion width. By this assumption, the centroids of the two LR compartments at the pulley would be offset vertically from the LR center in corresponding directions, but by distances dependent upon tension distributions within each compartment. This could be regarded as effectively shifting the vertical location of the aggregate LR pulley in the direction of the compartment exerting the greater tension. Once again considering the extreme case of total relaxation to zero tension in one compartment, with tonus in the other compartment, this would shift the LR pulley vertically by a maximum of ±3 mm. Since in young normal subjects, the LR pulley is about 0.5 mm inferior to globe center,69 this offset would be effectively 0.5 mm greater for the superior LR compartment, and 0.5 mm less for the inferior compartment. These distances are more significant they might at first appear. In central gaze, the LR insertion is only 8 mm anterior to globe center, and its pulley only 8 mm posterior to globe center.69 Small transverse shifts in either the LR pulley or insertion have large geometrical effects on LR path.
The foregoing assumptions are diagrammed in Figure 8. Let Tsup and Tinf be net tensions in the superior and inferior LR compartments, respectively. Let Vsup and Vinf be the vertical offsets of the force centroids of the superior and inferior LR compartments, respectively, relative to the position of the aggregate LR area centroid in its pulley. The authors assume that each LR compartment is homogeneously contractile, although contractility may differ between the two compartments. By this assumption, contractile force magnitudes would best be estimated by compartmental volumes throughout the orbit posterior to the pulley, while force locations at the pulley would be best estimated by vertical widths of the two LR compartments in the pulley. The torsional-vertical force Ftv exerted by tension in either LR compartment is therefore proportional to the sine of the angle whose tangent is the compartmental force offset Vi divided by the distance D2 from pulley to globe center, where i is “superior” or “inferior,” depending on the compartment under consideration (Fig. 8). Since Ftv for the two compartments is in opposite directions, overall Ftv is given by:
More rigorous numerical simulation with Orbit 1.81 was performed for assumed maximal vertical shift of the effective LR centroid of 3 mm, achieved by inhibition of one compartment, and activation of the other so that active tension substantially exceeds the passive tension of the inhibited compartment. Monocular implementation in central gaze results in 2.5Δ ipsilateral hypertropia with 1.2° excyclodeviation. Binocular implementation in central gaze of a mirror symmetric implementation (e.g., activation of right superior and left inferior compartments) results in 4.9Δ ipsilateral hypertropia without any excyclodeviation, since the torsional effects cancel.
The foregoing computations are for static eye position only. Dynamic effects in the velocity domain obviously depend strongly upon speed and frequency of the eye movement, and are considerable at high frequencies of rotational stimulation, even when torsional amplitudes are small. For example, 1.2° amplitude at 2.0 Hz was the vestibular stimulus employed in Ghasia and Angelaki's study of the motor neuron drive to the cyclovertical EOMs in the monkey.14 Since VOR peak velocity response was 15–16°/s at 2.0 Hz, the horizontal and VORs generated amplitude responses of only 1.27°. Since Ghasia and Angelaki's monkeys underwent rotational stimulation during eccentric fixations of up to 20°, the observed quarter-angle shift of VOR velocity axis was no more than 5°, representing a torsional velocity contribution of no more than 6°/s. The position amplitude of the noncommutative torsional violation of LL observed by Ghasia and Angelaki was thus less than 0.5°, appreciably less than the maximum capability of differential LR compartmental activation as estimated using Orbit 1.8 simulation, and much less than could be generated if the differential compartmental MR activity worked in concert with the LR. Obviously, the oblique EOMs generate most of the sensory ocular torsion during vestibular stimulation, and their roles are reflected in their motor neuron firing behavior.14 The present findings indicate, however, that differential LR compartmental activation is a quantitatively plausible effector for the VOR's noncommutative ocular torsion that violates LL, yet is not reflected in the firing behavior of the cyclovertical EOMs.
Whether the differential contractility of the LR compartments actually creates a differential oculorotary force, however, depends on incomplete mechanical force coupling between the parallel LR fibers that comprise the two neuromuscular zones. Parallel EOM fibers are likely to be neither totally coupled nor totally independent. Intramuscular coupling through endomysial fascia has been demonstrated in rat skeletal muscle.70 Nevertheless, sufficient slippage between the EOM fibers appears to occur in many types of split-tendon or partial EOM surgeries, allowing a separation of mechanical effects between the split EOMs.71,72 Simultaneous dual-channel load cell experiments in our laboratory suggest that the transverse tendon halves of each of the four bovine rectus EOMs may be as much 95% mechanically independent for small passive elongations11 (Shin A, et al. In press. 2012:ARVO Abstract 1008). The mechanical independence required for behavioral significance could be very much less than demonstrated in these preliminary observations. Given the bifid innervation and differential contractility observed during MRI, the two LR compartments are likely to behave in at least substantially independent fashion.
Histological data8 and weaker functional data from the present experiment suggest that the MR is compartmentally specialized similarly to the LR. If this is the case, then similar kinematic simulations could be made for simultaneous compartmental activation of both the superior MR and inferior LR compartments. Monocular implementation in central gaze results in 6.1Δ ipsilateral hypotropia with 2.1° incyclodeviation. Binocular implementation in central gaze results in 10Δ hypertropia with 2.6° excyclodeviation, since torsion is close to conjugate. These vertical effects are well within the range of clinical significance for cyclovertical strabismus, raising the possibility that horizontal rectus EOM dysinnervation might be a significant cause of vertical diplopia not previously considered. This may have implications for superior oblique palsy and masquerading conditions,73 and suggests that clinical patterns of incomitant strabismus traditionally interpreted as specific for the function of specific cyclovertical EOMs74 in fact may not be specific for cyclovertical EOMs at all.73,75
Footnotes
Supported by NEI Grants EY08313 and EY0331, the Shaw Family Endowment Fund (New York, NY), and by Research to Prevent Blindness (New York, NY). Joseph L. Demer is Leonard Apt Professor of Ophthalmology. The sponsor or funding organization had no role in the design or conduct of this research.
Disclosure: R.A. Clark, None; J.L. Demer, None
References
- 1. Miller JM, Pavlovski DS, Shaemeva I. Orbit 1.8 Gaze Mechanics Simulation. San Francisco, CA: Eidactics; 1999. [Google Scholar]
- 2. Demer JL. Extraocular Muscles. : Tasman W, Jaeger EA. Duane's Clinical Ophthalmology. Hagerstown, MD: Lipincott; 2009:1–30 [Google Scholar]
- 3. Robinson DA. A quantitative analysis of extraocular muscle cooperation and squint. Invest Ophthalmol. 1975;14:801–825 [PubMed] [Google Scholar]
- 4. Miller JM, Robinson DA. A model of the mechanics of binocular alignment. Comput Biomed Res. 1984;17:436–470 [DOI] [PubMed] [Google Scholar]
- 5. Goldberg SJ, Wilson KE, Shall MS. Summation of extraocular motor unit tensions in the lateral rectus muscle of the cat. Muscle Nerve. 1997;20:1229–1235 [DOI] [PubMed] [Google Scholar]
- 6. Goldberg SJ. Meredith MA, Shall MS. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J Neurosci. 1998;18:10629–10639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peng M, Poukens V, da Silva Costa RM, Yoo L, Tychsen L, Demer JL. Compartmentalized innervation of primate lateral rectus muscle. Invest Ophthalmol Vis Sci. 2010;51:4612–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. da Silva Costa RM, Kung J, Poukens V, Yoo L, Tychsen L, Demer JL. Intramuscular innervation of primate extraocular muscles: Unique compartmentalization in horizontal recti. Invest Ophtalmol Vis Sci. 2011;52:2830–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lim KH, Poukens V, Demer JL. Fascicular specialization in human and monkey rectus muscles: evidence for anatomic independence of global and orbital layers. Invest Ophthalmol Vis Sci. 2007;48:3089–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Demer JL, Poukens V, Ying H, Shan X, Tian J, Zee DS. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci. 2010;51:3485–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shin A, Yoo L, Demer JL. Mechanical study of compartmentalization in passive bovine extraocular muscles (EOMs). Abstract presented at: Annual American Association for Pediatric Ophthalmology and Strabismus Annual Meeting; March 27, 2012; San Antonio, TX: [Google Scholar]
- 12. Ruete CGT. Ocular physiology. Strabismus. 1999;7:43–60 [DOI] [PubMed] [Google Scholar]
- 13. Tweed D, Vilis T. Geometric relations of eye position and velocity vectors during saccades. Vision Res. 1990;30:111–127 [DOI] [PubMed] [Google Scholar]
- 14. Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293 [DOI] [PubMed] [Google Scholar]
- 15. Crawford JD, Vilis T. Symmetry of oculomotor burst neuron coordinates about Listing's plane. J Neurophysiol. 1992;68:432–448 [DOI] [PubMed] [Google Scholar]
- 16. Tweed D. Visual-motor optimization in binocular control. Vision Res. 1997;37:1939–1951 [DOI] [PubMed] [Google Scholar]
- 17. Misslisch H, Tweed D. Neural and mechanical factors in eye control. J Neurophysiol. 2001;86:1877–1883 [DOI] [PubMed] [Google Scholar]
- 18. Angelaki DE, Dickman DJ. Premotor neurons encode torsional eye velocity during smooth-pursuit eye movements. J Neurosci. 2003;23:2971–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angelaki DE. Three-dimensional ocular kinematics during eccentric rotations: Evidence for functional rather than mechanical constraints. J Neurophysiol. 2003;89:2685–2696 [DOI] [PubMed] [Google Scholar]
- 20. Angelaki DE, Hess BJ. Control of eye orientation: where does the brain's role end and the muscle's begin? Eur J Neurosci. 2004;19:1–10 [DOI] [PubMed] [Google Scholar]
- 21. Klier EM, Meng H, Angelaki DE. Three-dimensional kinematics at the level of the oculomotor plant. J Neurosci. 2006;26:2732–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klier EM, Meng H, Angelaki D. Revealing the kinematics of the oculomotor plant with tertiary eye positions and ocular counterroll. J Neurophysiol. 2011;105:640–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klier EM, Meng H, Angelaki DE. The half-angle rule is maintained for abducens nerve stimulation-evoked eye movements during roll VOR. Poster presented at: Society for Neuroscience Annual Meeting; November 15, 2011; Washington, DC: Abstract 699.07 [Google Scholar]
- 24. Demer JL. The orbital pulley system: a revolution in concepts of orbital anatomy. Ann N Y Acad Sci. 2002;956:17–32 [DOI] [PubMed] [Google Scholar]
- 25. Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738 [DOI] [PubMed] [Google Scholar]
- 26. Demer JL. Current concepts of mechanical and neural factors in ocular motility. Curr Opin Neurol. 2006;19:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crane BT, Tian J, Demer JL. Kinematics of vertical saccades during the yaw vestibulo-ocular reflex in humans. Invest Ophthalmol Vis Sci. 2005;46:2800–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bockisch CJ, Haslwanter T. Three-dimensional eye position during static roll and pitch in humans. Vision Res. 2001;41:2127–2137 [DOI] [PubMed] [Google Scholar]
- 30. Markham CH, Diamond SG. Ocular counterrolling in response to static and dynamic tilting: implications for human otolith function. J Vestib Res. 2002–2003;12:127–134 [PubMed] [Google Scholar]
- 31. Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302 [DOI] [PubMed] [Google Scholar]
- 32. Miller JM. Functional anatomy of normal human rectus muscles. Vision Res. 1989;29:223–240 [DOI] [PubMed] [Google Scholar]
- 33. Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36:906–913 [PubMed] [Google Scholar]
- 34. Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085 [DOI] [PubMed] [Google Scholar]
- 35. Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539 [DOI] [PubMed] [Google Scholar]
- 36. Demer JL, Miller JM, Koo EY, Rosenbaum AL. Quantitative magnetic resonance morphometry of extraocular muscles: a new diagnostic tool in paralytic strabismus. J Pediatr Ophthalmol Strabismus. 1994;31:177–188 [DOI] [PubMed] [Google Scholar]
- 37. Clark RA, Demer JL. Enhanced vertical rectus contractility by magnetic resonance imaging in superior oblique palsy. Arch Ophthalmol. 2011;129:904–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Demer JL, Dushyanth A. T2 fast spin echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011;15:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller JM, Davison RC, Gamlin PD. Motor nucleus activity fails to predict extraocular muscle forces in ocular convergence. J Neurophysiol. 2011;105:2863–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crane BT, Tian JR, Demer JL. Temporal dynamics of ocular position dependence of the initial human vestibulo-ocular reflex. Invest Ophthalmol Vis Sci. 2006;47:1426–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ugolini G, Klam F, Dans MD, et al. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: differences in monosynaptic input to “slow and “fast” abducens motoneurons.” J Comp Neurol. 2006;498:762–785 [DOI] [PubMed] [Google Scholar]
- 42. Gilbert PW. The origin and development of the extrinsic ocular muscles in the domestic cat. Contrib Embryol. 1957;36:61–78 [PubMed] [Google Scholar]
- 43. Neal HV. Ths history of the eye muscles. J Morphol. 1918;30:433–453 [Google Scholar]
- 44. Wahl CM, Noden DM, Baker R. Developmental relations between sixth nerve motor neurons and their targets in the chick embryo. Dev Dyn. 1994;20:191–202 [DOI] [PubMed] [Google Scholar]
- 45. Evans DJ, Noden DM. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dyn. 2006;235:1310–1325 [DOI] [PubMed] [Google Scholar]
- 46. Nathan H, Ouaknine G, Kosary IZ. The abducens nerve: Anatomical variations in its course. J Neurosurg. 1974;42:561–566 [DOI] [PubMed] [Google Scholar]
- 47. Jain KK. Aberrant roots of the abducens nerve. J Neurosurg. 1964;21:349–351 [DOI] [PubMed] [Google Scholar]
- 48. Iaconetta G, Tessitore E, Samii M. Duplicated abducent nerve and its course: microanatomical study and surgery-related considerations. J Neurosurg. 2001;95:853–858 [DOI] [PubMed] [Google Scholar]
- 49. Ozeren MF, Sam B, Akdemir I, Alkan A, Tekdemir I, Deda H. Duplication of the abducens nerve at the petroclival region: an anatomic study. Neurosurgery. 2003;52:645–651 [DOI] [PubMed] [Google Scholar]
- 50. Kilic C, Kirici Y. Location and course of the abducent nerve within the cavernous sinus. J Exp Integr Med. 2011;1:135–137 [PubMed] [Google Scholar]
- 51. Govsa F, Kayalioglu G, Erturk M, Ozgur T. The superior orbital fissure and its contents. Surg Radiol Anat. 1999;21:181–185 [DOI] [PubMed] [Google Scholar]
- 52. Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80 [DOI] [PubMed] [Google Scholar]
- 53. Okanobu H, Kono R, Miyake K, Ohtsuki H. Splitting of the extraocular horizontal rectus muscle in congenital cranial dysinnervation disorders. Am J Ophthalmol. 2009;147:550–556 [DOI] [PubMed] [Google Scholar]
- 54. Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane's retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging of innervational and extraocular muscle abnormalities in Duane-radial ray syndrome. Invest Ophthalmol Vis Sci. 2007;48:5505–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Okanobu H, Kono R, Ohtsuki H, Miyake K. Magnetic resonance imaging findings in Duane's retraction syndrome type III. Rinsho Ganka (Jpn Clin Ophthalmol). 2008;62:65–69 [Google Scholar]
- 57. Bruno P, van den Berg AV. Relative orientation of primary positions of the two eyes. Vision Res. 1997;37:935–947 [DOI] [PubMed] [Google Scholar]
- 58. Allen MJ. The dependence of cyclophoria on convergence elevation and the system of axes. Am J Optom. 1954;31:297–307 [DOI] [PubMed] [Google Scholar]
- 59. Allen MJ, Carter JH. The torsional component of the near reflex. Am J Optom. 1967;44:343–349 [PubMed] [Google Scholar]
- 60. Mikhael S, Nicolle D, Vilis T. Rotation of Listing's plane by horizontal, vertical and oblique prism-induced vergence. Vision Res. 1995;35:3243–3254 [DOI] [PubMed] [Google Scholar]
- 61. Minken AWH, Van Gisbergen JAM. A three-dimensional analysis of vergence movements at various levels of elevation. Exp Brain Res. 1994;101:331–345 [DOI] [PubMed] [Google Scholar]
- 62. Mok D, Ro A, Cadera W, Crawford JD, Vilis T. Rotation of Listing's plane during vergence. Vision Res. 1992;32:2055–2064 [DOI] [PubMed] [Google Scholar]
- 63. Somani RAB, Desouze JFX, Tweed D, Vilis T. Visual test of Listing's law during vergence. Vision Res. 1998;38:911–923 [DOI] [PubMed] [Google Scholar]
- 64. Steffen H, Walker MF, Zee DS. Rotation of Listing's plane with convergence: Independence from eye position. Invest Ophthalmol Vis Sci. 2000;41:715–721 [PubMed] [Google Scholar]
- 65. Demer JL, Clark RA. Differential compartmental activity in lateral (LR) rectus during convergence: Possible explanation of the force paradox? Poster presented at: Society for Neuroscience Annual Meeting; November 15, 2011; Washington, DC: Abstract 699.04 [Google Scholar]
- 66. von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. St. Louis, MO: Mosby; 1996;45–50 [Google Scholar]
- 67. Kono R, Ohtsuki H, Okanobu H, Kingugasa K. Displacement of rectus muscle pulleys by torsional muscle surgery for treatment of full macular translocation-induced incyclotropia. Am J Ophthalmol. 2005;140:144–146 [DOI] [PubMed] [Google Scholar]
- 68. Ohmi G, Fujikado T, Ohji M, Saito Y, Tano Y. Horizontal transposition of vertical rectus muscles for treatment of excyclotropia. Graefes Arch Clin Exp Ophthalmol. 1997;235:1–4 [DOI] [PubMed] [Google Scholar]
- 69. Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–3797 [PubMed] [Google Scholar]
- 70. Huijing PA, Baan GC, Rebel GT. Non-myotendinous force transmittion in rat extensor digitorum longus muscle. J Exp Biol. 1998;201:682–691 [PubMed] [Google Scholar]
- 71. Metz H, Lerner H. The adjustable Harada-ito procedure. Arch Ophthalmol. 1981:91 [DOI] [PubMed] [Google Scholar]
- 72. Paysse E, McCreery K, Ross A, Coats D. Use of augmented rectus muscle transposition surgery for complex strabismus. Ophthalmology. 2002;109:1309–1314 [DOI] [PubMed] [Google Scholar]
- 73. Demer JL, Clark RA, Kung J. Functional imaging of human extraocular muscles in head tilt dependent hypertropia. Invest Ophtalmol Vis Sci. 2011;52:3023–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mudgil AV, Walker M, Steffen H, Guyton DL, Zee DS. Motor mechanisms of vertical fusion in individuals with superior oblique paresis. J AAPOS. 2002;6:145–153 [DOI] [PubMed] [Google Scholar]
- 75. Demer JL. Clarity of words and thoughts about strabismus. Am J Ophthalmol. 2001;132:757–759 [DOI] [PubMed] [Google Scholar]