Abstract
Purpose.
To evaluate the effect of location and size of biopsy on phenotype and proliferative capacity of cultured rat conjunctival epithelial cells.
Methods.
Pieces of conjunctiva were used from six areas: superior and inferior areas of bulbus, fornix, and tarsus of male Sprague-Dawley rats (n = 6). Explants were grown in RPMI 1640 with 10% fetal bovine serum on coverslips for 8 days or assayed for colony-forming efficiency (n = 9). Analysis included immunofluorescence microscopy and outgrowth measurements with ImageJ software. The Mann-Whitney test and Spearman's rank-order correlation test were used.
Results.
Superior (23.9 ± 2.9-fold growth) and inferior (22.4 ± 1.2-fold growth) forniceal tissues yielded significantly more outgrowth with respect to explant size than superior bulbar (13.4 ± 1.9-fold growth; P < 0.05 and P < 0.01, respectively), inferior bulbar (13.6 ± 1.6-fold growth; P = 0.01 and P < 0.01, respectively), and inferior tarsal tissues (14.0 ± 1.3-fold growth; P = 0.01). Outgrowth size correlated positively with explant size (rs = 0.54; P < 0.001), whereas explant size correlated negatively with fold growth (rs = 0.36; P < 0.001). Superior forniceal cells displayed higher colony-forming efficiency (3.6% ± 0.9%) than superior bulbar (1.1% ± 0.3%; P < 0.05) and inferior bulbar cells (1.6% ± 0.8%; P < 0.05). Percentage of p63+ and PCNA+ cells correlated positively with explant and outgrowth size.
Conclusions.
Small forniceal conjunctival explants grow the most effectively; however, for transplantation purposes, the loss of p63+ and PCNA+ cells with small explants must be considered.
Small forniceal conjunctival explants grow the most effectively for transplantation purposes.
Introduction
The ocular surface is covered by corneal and conjunctival epithelia; the latter also covers the posterior surface of the eyelids. The conjunctival epithelium secrets the mucin component of the tear film and protects the ocular surface through mechanical and immunological properties.1 Numerous ocular surface disorders can damage the conjunctiva, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and chemical/thermal burns. Depending on the severity of the conjunctival damage, a range of complications may develop that could culminate in corneal blindness if left untreated.2,3 A damaged conjunctiva can be restored by transplanting ex vivo expanded conjunctival epithelium,4–6 thereby avoiding the need for harvesting of a large conjunctival autograft. Cultured conjunctival epithelium also represents a treatment option in limbal stem cell deficiency.7
Several culture variables affect the phenotype of ex vivo expanded epithelial cells, such as the time in culture,8 culture media composition,9,10 the use of feeder layer,9,11 and airlifting.12 Even though the size of the biopsy used for culture is easily controlled, no studies have investigated the effect of biopsy size on the phenotype and proliferative ability of cultured conjunctival epithelial transplants. This issue is important, as harvested conjunctival tissue ideally should include only the smallest number of cells necessary to culture a clinically successful conjunctival transplant, thereby keeping the donor site damage to a minimum. The choice of biopsy size is especially relevant in cases where there already is a large loss of conjunctiva (e.g., owing to tumors, pterygium, or infectious diseases). Furthermore, the issue of biopsy size becomes increasingly important in cases where repeated harvesting of conjunctival tissue is warranted, as in chronic inflammatory ocular surface disease where the transplantations are more prone to fail.1
The location of the conjunctival stem cells has been a matter of controversy. Conjunctival epithelial stem cells have been suggested to reside in the limbus (rat13), bulbar conjunctiva (mouse14 and human15,16), forniceal conjunctiva (rabbit,17 mouse,18,19 and human15), palpebral conjunctiva (rat20), and mucocutaneous junction (rat13 and rabbit21). Because of the wide discrepancy between these proposed locations for the conjunctival stem cells, it seems evident that to locate the best biopsy harvesting site, all conjunctival regions (i.e. bulbus, fornix, and tarsus/palpebra in both the superior and inferior conjunctiva) must be taken into consideration; however, to our knowledge, no studies have compared all the conjunctival regions.
In the present study, we hypothesized that biopsy size and harvesting site affect the proliferative ability, clonal growth capacity, and phenotype of cultured conjunctival epithelial cells.
Materials and Methods
RPMI-1640 culture medium, L-glutamine, penicillin-streptomycin, Hanks' balanced salt solution, and trypsin-EDTA solution were obtained from BioWhittaker (Walkersville, MD). Fetal bovine serum (FBS) was from HyClone Laboratories (Logan, UT). Falcon tissue culture plates, pipettes, and other routine plastics were obtained from Becton Dickson Labware (Franklin Lakes, NJ); glass coverslips were from VWR Scientific (San Francisco, CA); and Laboratory Tek chamber slides were from Nunc, Inc. (Naperville, IL). Antibodies against p63 and proliferating cell nuclear antigen (PCNA) were from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies were either Cy2 or 3 conjugated to mouse or rabbit IgG from Jackson Immuno Research Laboratories, Inc. (West Grove, PA). For colony-forming efficiency (CFE) assay, 3T3 fibroblasts, kindly provided by Ula Jurkunas, MD, Schepens Eye Research Institute, were used. Mitomycin C was purchased from Sigma-Aldrich (St. Louis, MO).
Explant Culture
All removal of tissue and subsequent manipulations of animals used in this study conformed to the guidelines established by the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee. Male Sprague-Dawley rats were used as previously described.22 Conjunctival tissue was excised at the 12 and 6 o'clock positions and immediately placed into PBS (145 mM NaCl, 7.3 mM Na2HPO4, 2.7 mM NaH2PO4 [pH 7.2]). Tissue from each eye was divided into six pieces: Superior and inferior bulbus, fornix, and tarsus. The fornix was identified as the band running along the most posterior part of the fold at the junction of the bulbar and tarsal conjunctiva. The superior fornix was grasped and lifted, and then excised from the conjunctiva. Thereafter, the superior tarsal and bulbar tissue were collected in similar fashion. Finally, the inferior tissues were excised accordingly after first identifying the inferior fornix. A total of six pieces, each measuring about 1 to 2 mm × 4 mm, were collected from each rat. The tissue was further divided into 0.5- to 1.0-mm2 pieces that were anchored onto glass coverslips placed within six-well culture dishes. With a yield of six explants from each conjunctival region, a total of 36 explants were obtained from each animal. One explant was anchored in each tissue culture well. The culture dishes contained just enough medium to cover the bottom of the dish so that the tissue would receive nutrients through surface tension. The cell medium used to feed explants consisted exclusively of RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 2 mM L-glutamine, and 100 μg/mL penicillin-streptomycin. Explants were refed every 2 days with this medium and were grown for 8 days under routine culture conditions of 95% air–5% CO2 at 37°C.
Outgrowth Measurements
Nuclei of epithelial cells in primary culture grown from explants from the superior and inferior bulbar, forniceal, and tarsal areas of the conjunctiva were stained with DAPI and outgrowth was visualized with a fluorescence microscope (Eclipse E 800; Nikon, Tokyo, Japan) at a magnification of ×40 (n = 6). Occasional fibroblasts were recognized by morphology and excluded. After 8 days in culture, outgrowth size and explant size were quantified using ImageJ software (National Institutes of Health, Bethesda, MD). Fold growth in each primary culture was quantified as follows: outgrowth size/explant size.
CFE Assay
The clonal growth capacity of epithelial cells from the superior and inferior bulbar, forniceal, and tarsal membrane of the conjunctiva was determined by CFE (n = 9). Conjunctival tissues from six regions were left in Dispase II overnight at 4°C, thereafter trypsinized for 10 minutes at 37°C to achieve single cells. Mitomycin C–treated 3T3 feeder cells were seeded onto 6- and 12-well plates at a density of 1 × 105/cm2 24 hours before plating conjunctival cells at a clonal density of 50 cells/cm2. A colony was defined as a group of eight or more contiguous cells.23 Colonies were fixed on day 8, stained with Rhodamine B Fluka (ready-to-use solution, Sigma) and counted independently by two investigators; data were then averaged. CFE was defined as follows: CFE (%) = Total number of colonies formed at the end of growth period/Total number of cells seeded × 100 %.
Immunocytochemistry
Coverslips with methanol-fixed cells were incubated for 1 hour at room temperature in blocking buffer that consisted of 1% BSA and 0.2% Triton X-100 in PBS. Cells were then incubated with the following dilutions of primary antibodies overnight at 4°C: Antibody to p63, a marker for undifferentiated and highly proliferative cells,24 was diluted 1:100 in PBS. Antibody to PCNA, which reacts with nuclei in proliferating cells, was diluted 1:200 in PBS. The secondary antibodies, conjugated to either Cy2 or 3, were diluted 1:100 or 1:300, respectively, in PBS and incubated for 1 hour at room temperature. Coverslips were washed three times in PBS, after which coverslips were mounted on microscope slides with mounting media containing 100 mM Tris (pH 8.5), 25% glycerol, 10% polyvinyl alcohol, and 2.5% 1,4-diazobicyclo-[2.2.2]-octane. Cell cultures adherent to glass coverslips were visualized with an epifluorescence microscope (Eclipse E 800; Nikon). Negative controls consisted of substituting PBS for the primary antibody. Positive controls included fixed sections of whole rat eyes with eyelids containing structures with known positive staining for each of the antibodies used. Expression of the markers was assessed at a magnification of ×630 and 100 cells in six fields were counted in each culture by two independent investigators. The number of positive cells/total number of cells × 100% was calculated.
Comparison of Explant and Outgrowth Size with Phenotype
To investigate whether the proliferative state of the cells was affected by the size of the explant or the outgrowth, phenotypic data from primary cultures from all six conjunctival regions were combined and correlated with the explant and outgrowth measurements, respectively.
Statistical Analysis
The Mann-Whitney test was used to compare measurements of fold growth among all six conjunctival regions. In contrast to these measurements, all the CFE measurements were paired, hence the Wilcoxon test was used for comparison of CFE among the six conjunctival regions. To analyze the relationship among fold growth, outgrowth size, and explant size, as well as among outgrowth size, explant size, and phenotypic data, the Spearman's rank-order correlation test was used. Data were expressed as mean ± SEM. A significance level of 5% was used throughout the study (SPSS version 18.0; SPSS Inc., Chicago, IL).
Results
Outgrowth Comparisons
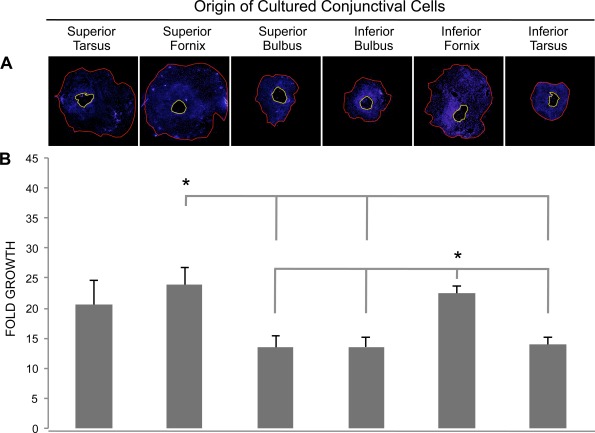
The amount of outgrowth from explants from the six conjunctival areas was defined as shown in Figure 1A after 8 days in culture (n = 6). Tissue from the superior (23.9 ± 2.9-fold growth) and inferior (22.4 ± 1.2-fold growth) fornix yielded a significantly larger outgrowth size with respect to explant size than the superior bulbus (13.4 ± 1.9-fold growth; P < 0.05 and P < 0.01, respectively), inferior bulbus (13.6 ± 1.6-fold growth; P = 0.01 and P < 0.01, respectively), and the inferior tarsus (14.0 ± 1.3-fold growth; P = 0.01; Fig. 1). Thus, the forniceal areas provide the optimal outgrowth of conjunctival epithelial cells.
Figure 1.
Outgrowth in primary cultures from six conjunctival regions was compared. (A) Photomicrograph montages showing the extent of outgrowth from different conjunctival regions. Cell nuclei were visualized with DAPI (blue). The area encircled by the yellow line reflects the explant size, whereas the red line indicates the size of the cell outgrowth area. Explant size and outgrowth size were measured with ImageJ software and fold growth was defined as outgrowth size/explant size (magnification ×40). (B) Bar chart displaying fold growth (±SEM) obtained in cultures from the six conjunctival regions. *P < 0.05 compared with superior bulbus, inferior bulbus, and inferior tarsus (n = 6).
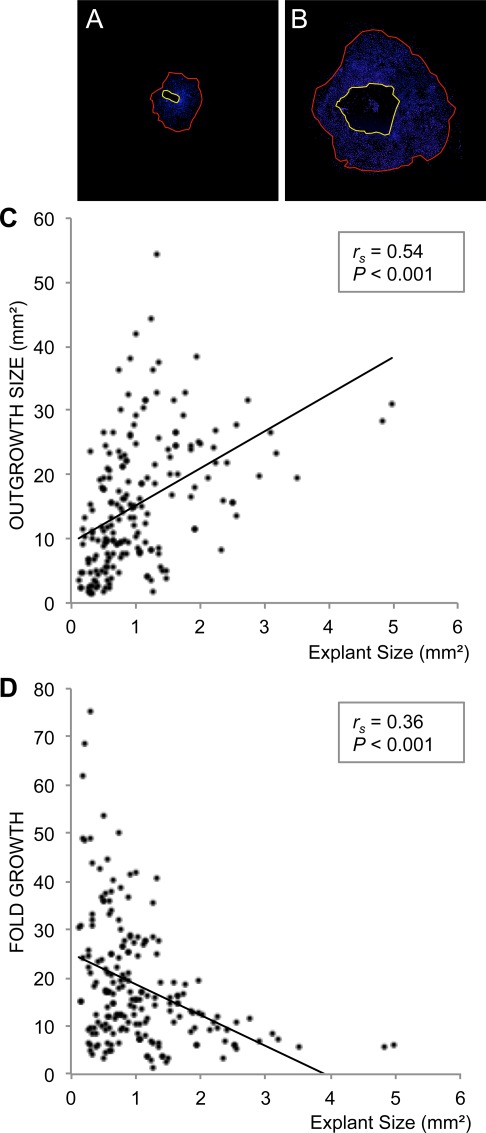
We then combined the primary culture measurements from all six conjunctival regions to assess whether the explant size affects outgrowth size or fold growth, irrespective of harvesting site (Figs. 2A, 2B). The explant size was positively correlated with outgrowth size (rs = 0.54; P < 0.001; 196 paired observations; Fig. 2C). On the other hand, the explant size was negatively correlated with fold growth (rs = 0.36; P < 0.001; 196 paired observations; Fig. 2D). These results suggest that independent of location in the conjunctiva, the larger the explants, the more extensive outgrowth; however, when correcting for the explant size, small explants yield relatively more outgrowth than large ones, thus small explants may be more effective for the culture of conjunctival transplants.
Figure 2.
The relationship between the size of the conjunctival explants and outgrowth size or fold growth were investigated. Photomicrograph montages show examples of a small (A) and large (B) primary culture grown from a small (A) and large (B) explant. Nuclei were stained with DAPI (blue). The area encircled by the yellow line reflects the explant size, whereas the red line indicates the size of the cell outgrowth area. Explant size and outgrowth size were measured with ImageJ software and fold growth was defined as outgrowth size/explant size (magnification ×40). (C) Bivariate scattergram illustrating the positive correlation between outgrowth size and explant size (196 paired observations). (D) Bivariate scattergram illustrating the negative correlation between fold growth and explant size (196 paired observations). The Spearman's correlation test was applied. rs = Spearman's correlation coefficient.
Clonal Growth Assessment of Six Conjunctival Regions
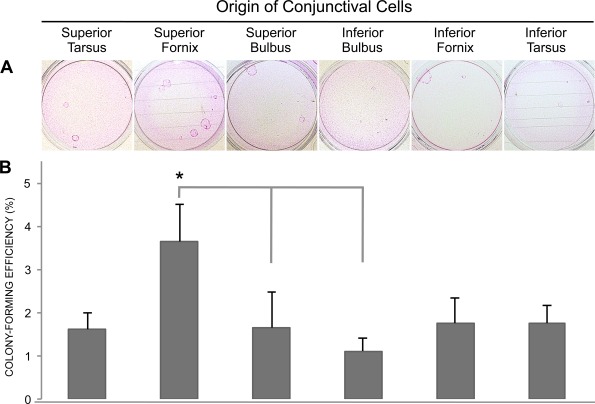
To further compare the growth capacity of the six conjunctival regions, a CFE assay was performed (n = 9; Fig. 3A). Cells obtained from the superior forniceal regions showed higher CFE (3.6% ± 0.9%) compared with cells originating from the superior bulbar (1.1% ± 0.3%; P < 0.05) and inferior bulbar (1.6% ± 0.8%; P < 0.05) regions (Fig. 3B). In contrast to outgrowth, the inferior forniceal region did not support high clonal growth.
Figure 3.
Colony-forming efficiency was determined for cells from six conjunctival regions. (A) Photographs of conjunctival colonies stained with Rhodamine B Fluka. A colony was defined as eight or more contiguous cells. (B) Bar chart showing the colony-forming efficiency (percentage ±SEM) achieved with cells from six conjunctival regions. *P < 0.05 compared with superior bulbus and inferior bulbus (n = 9).
Explant and Outgrowth Size Compared with Phenotype
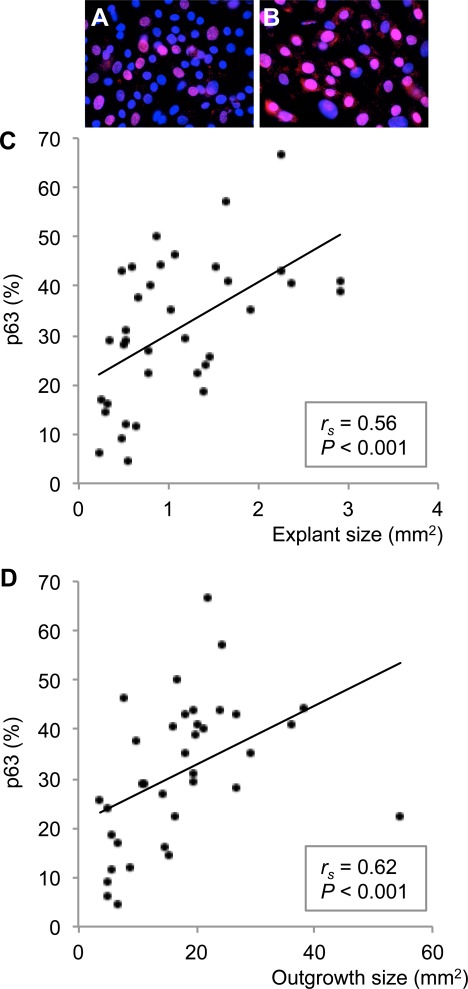
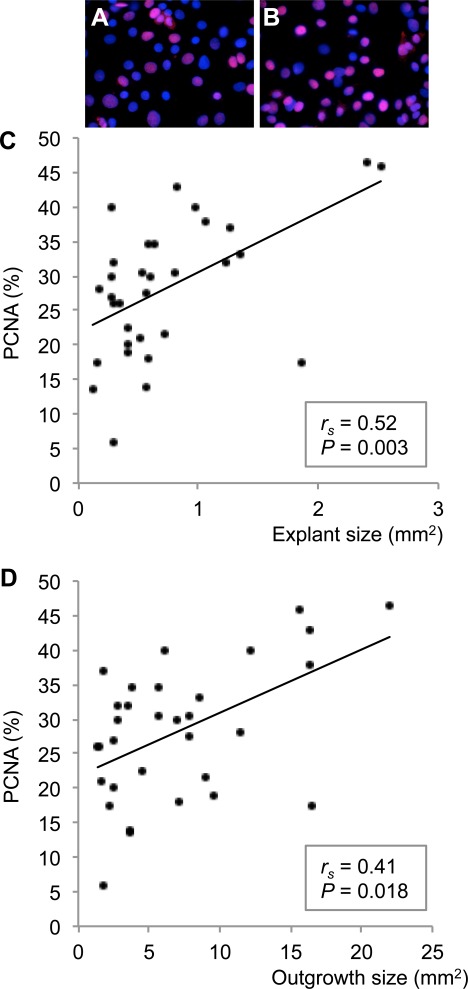
As the size of the explant was related to both outgrowth size and fold growth, we hypothesized that the explant size would also correlate with the percentage of proliferating, undifferentiated cells (p63+/PCNA+) in the cultures. Indeed, independent of the conjunctival origin, the explant size was positively correlated with the percentage of p63+ (rs = 0.56; P < 0.001; 36 paired observations; Figs. 4A–C) and PCNA+ cells in the cultures (rs = 0.52; P = 0.003; 32 paired observations; Figs. 5A–C). Thus, the larger the original explants, the more p63+/PCNA+ cells were present in the outgrowth. Furthermore, outgrowth size was also positively correlated with the percentage of p63+ (rs = 0.62; P < 0.001; 36 paired observations; Figs. 4A, 4B, 4D) and PCNA+ cells in the cultures (rs = 0.41; P = 0.018; 32 paired observations; Figs. 5A, 5B, 5D), meaning that the larger the culture, the more p63+/PCNA+ cells were present. These data suggest that highly proliferating undifferentiated cells probably were responsible for the cellular growth and that the use of large explants might increase the percentage of such cells in the cultures.
Figure 4.
The percentage of p63+ undifferentiated, proliferating cells in conjunctival primary cultures was correlated with explant and outgrowth size. The photomicrographs illustrate that small explants and cultures yielded fewer p63+ (red; A) cells than large explants and cultures (B). Nuclei were stained with DAPI (blue) (magnification ×630). (C) Bivariate scattergram illustrating the positive correlation between explant size and percentage of p63+ cells in the culture (36 paired observations). (D) Bivariate scattergram illustrating the positive correlation between outgrowth size and percentage of p63+ cells in the culture (36 paired observations). The Spearman's correlation test was applied. rs = Spearman's correlation coefficient.
Figure 5.
The percentage of PCNA+ proliferating cells in conjunctival primary cultures was correlated with explant and outgrowth size. The photomicrographs illustrate that small explants and cultures yielded fewer PCNA+ (red; A) cells than large explants and cultures (B). Nuclei were stained with DAPI (blue) (magnification ×630). (C) Bivariate scattergram illustrating the positive correlation between explant size and percentage of PCNA+ cells in the culture (32 paired observations). (D) Bivariate scattergram illustrating the positive correlation between outgrowth size and percentage of PCNA+ cells in the culture (32 paired observations). The Spearman's correlation test was applied. rs = Spearman's correlation coefficient.
Discussion
In the present study, we demonstrated that rat forniceal conjunctiva divided into small pieces has the highest proliferative capacity ex vivo. Several studies have shown that conjunctival stem cells are located in the forniceal region. Thus, activation of stem cells could have accounted for the high proliferation ability of the fornix.
Our results showing the highest outgrowth relative to explant size in forniceal cultures correspond to previous studies.17,25 The finding that smaller explants yielded relatively more growth ex vivo may be of importance in improving culture protocols for conjunctival transplantation, as well as decreasing the donor site defects by necessitating less donor tissue. Ang et al.4,5 described an explant culture technique where they minced the conjunctival biopsies into small pieces (about 0.5 × 0.5 mm) before culturing. As those authors did not investigate the significance of explant size on the cultured cells, our study could supplement theirs in this regard. The principle of dividing biopsy tissue to cover a larger wound area has long been used in split-skin transplantation.26 Using the mesh split-skin technique, between 1.5:1.0- to 6.0:1.0-fold expansion has been possible. This has been increased to a 20:1 expansion by mincing the epidermal biopsy into numerous smaller fragments (40 × 40 μm).27 The reason why small biopsies give relatively larger outgrowth may be hypothesized to result partly from the shorter average distance from the cut edge of the biopsy to the cells. Cytokine discharge from damaged epithelial cells can induce growth factor release from stromal fibroblasts.28 In skin biopsies, keratinocytes close to the cut edge show a shift toward the hyperproliferative phenotype.29
Stem cells are surrounded by a particular microenvironment known as a niche,30 hence one benefit of using explants for ex vivo expansion is that the stem cells are not removed from their niche. There is increasing evidence that neighboring cells, intercellular interactions, and other local environmental factors, such as extracellular matrix (ECM) and signaling molecules, control stem cell function.31 The specific composition of the ECM also shows topographical variations throughout the ocular surface.32 Thus, as the outgrowth measurements in the current study were performed on explant cultures, those measurements may have been affected by differences in the composition of the ECM in each conjunctival origin. Similarly, the outgrowth obtained from explants of different sizes may also have been related to the amount of growth-stimulating ECM components in each explant. A cell suspension approach could have normalized for such effects; however, we used the explant method because it has been used in clinical studies for culturing conjunctival epithelial transplants for use in humans.4,5,33
We also showed greater clonal growth capacity of the superior fornix. Few studies have investigated the difference between the superior and inferior conjunctiva with respect to proliferative or clonal growth capacity.15 In contrast to our results, Pellegrini et al.15 concluded with similar CFE in conjunctiva from the superior and inferior fornix and the four bulbar quadrants. Their results were based on only one experiment using biopsies from a single human organ donor, however, and the CFE was higher in the superior fornix (18%) than in the inferior fornix (10%). There were no clear differences in the number of aborted and growing colonies in each conjunctival origin. In the current study, we used a CFE protocol involving only 8 days of culture, as previously described by Nakamura et al.23 Thus, this may have been too short a time to detect any differences in the type of colonies.
The higher number of p63+ and PCNA+ (proliferating) cells in cultures from large explants may be related to the finding that small explants yielded a relatively larger outgrowth area after 8 days in culture. In split-skin transplantation, mincing of the split-skin into smaller fragments has been shown to increase the fold growth without necessitating a longer growth period,27 thus suggesting that smaller explants lead to faster outgrowth. As a result of a higher relative growth yield, cultured cells from small explants in our study may have gone through more cell divisions at 8 days in culture, compared with the large explants. Kolli et al.34 reported a loss of p63+ cells with increasing distance to the explants; in addition, the number of p63+ cells in the explant falls as the culture reaches confluence.8 By day 8, culture cells from the small explants in our study might have gone through more cell divisions than cells from large explants, thereby reaching senescence earlier and becoming p63–/PCNA–. We also reported higher p63, as well as PCNA, expression in larger cultures. This could be explained by the finding of a positive correlation between explant size and culture size and that large explants yield less fold growth after 8 days ex vivo.
In conclusion, we have shown that forniceal explants appeared the most proliferative and thereby seem to be optimal for conjunctival epithelial cell transplantation. The use of small, rather than large, explants is most effective for ex vivo expansion, potentially resulting in smaller donor site defects. The loss of p63+ and PCNA+ cells when using small explants must be taken into consideration, however, favoring the use of larger explants.
Acknowledgments
The authors thank Robin R. Hodges, Donald Pottle, and Ula Jurkunas at the Schepens Eye Research Institute, Harvard Medical School, Boston, MA; Torstein Lyberg and Leiv Sandvik at the Center for Clinical Research, Oslo University Hospital, Oslo, Norway; and Astrid Østerud at the Department of Ophthalmology, Oslo University Hospital, Oslo, Norway, for excellent assistance and support.
Footnotes
Supported by National Institutes of Health Grants EY009057 and EY019470, in addition to the Norwegian Research Council and Eastern Norway Regional Health Authority.
Disclosure: J.R. Eidet, None; I.G. Fostad, None; M.A. Shatos, None; T.P. Utheim, None; Ø.A. Utheim, None; S. Raeder, None; D.A. Dartt, None
References
- 1. Schrader S, Notara M, Beaconsfield M, Tuft SJ, Daniels JT, Geerling G. Tissue engineering for conjunctival reconstruction: established methods and future outlooks. Curr Eye Res. 2009;34:913–924 [DOI] [PubMed] [Google Scholar]
- 2. Dua HS, Forrester JV. The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol. 1990;110:646–656 [DOI] [PubMed] [Google Scholar]
- 3. Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Invest Ophthalmol Vis Sci. 1981;21:135–142 [PubMed] [Google Scholar]
- 4. Ang LP, Tan DT, Cajucom-Uy H, Beuerman RW. Autologous cultivated conjunctival transplantation for pterygium surgery. Am J Ophthalmol. 2005;139:611–619 [DOI] [PubMed] [Google Scholar]
- 5. Tan DT, Ang LP, Beuerman RW. Reconstruction of the ocular surface by transplantation of a serum-free derived cultivated conjunctival epithelial equivalent. Transplantation. 2004;77:1729–1734 [DOI] [PubMed] [Google Scholar]
- 6. Scuderi N, Alfano C, Paolini G, Marchese C, Scuderi G. Transplantation of autologous cultivated conjunctival epithelium for the restoration of defects in the ocular surface. Scand J Plast Reconstr Surg Hand Surg. 2002;36:340–348 [DOI] [PubMed] [Google Scholar]
- 7. Tanioka H, Kawasaki S, Yamasaki K, et al. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Invest Ophthalmol Vis Sci. 2006;47:3820–3827 [DOI] [PubMed] [Google Scholar]
- 8. Joseph A, Powell-Richards AO, Shanmuganathan VA, Dua HS. Epithelial cell characteristics of cultured human limbal explants. Br J Ophthalmol. 2004;88:393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ang LP, Tan DT, Phan TT, Li J, Beuerman R, Lavker RM. The in vitro and in vivo proliferative capacity of serum-free cultivated human conjunctival epithelial cells. Curr Eye Res. 2004;28:307–317 [DOI] [PubMed] [Google Scholar]
- 10. Meyer-Blazejewska EA, Kruse FE, Bitterer K, et al. Preservation of the limbal stem cell phenotype by appropriate culture techniques. Invest Ophthalmol Vis Sci. 2010;51:765–774 [DOI] [PubMed] [Google Scholar]
- 11. Kim HS. Jun Song X, de Paiva CS, Chen Z, Pflugfelder SC, Li DQ. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ban Y, Cooper LJ, Fullwood NJ, et al. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res. 2003;76:735–743 [DOI] [PubMed] [Google Scholar]
- 13. Pe'er J, Zajicek G, Greifner H, Kogan M. Streaming conjunctiva. Anat Rec. 1996;245:36–40 [DOI] [PubMed] [Google Scholar]
- 14. Nagasaki T, Zhao J. Uniform distribution of epithelial stem cells in the bulbar conjunctiva. Invest Ophthalmol Vis Sci. 2005;46:126–132 [DOI] [PubMed] [Google Scholar]
- 15. Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi H, Zheng X, Yuan X, Pflugfelder SC, Li DQ. Potential localization of putative stem/progenitor cells in human bulbar conjunctival epithelium. J Cell Physiol. 2010;225:180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei ZG, Wu RL, Lavker RM, Sun TT. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993;34:1814–1828 [PubMed] [Google Scholar]
- 18. Lavker RM, Wei ZG, Sun TT. Phorbol ester preferentially stimulates mouse fornical conjunctival and limbal epithelial cells to proliferate in vivo. Invest Ophthalmol Vis Sci. 1998;39:301–307 [PubMed] [Google Scholar]
- 19. Wei ZG, Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells are preferentially located in fornical epithelium: implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci. 1995;36:236–246 [PubMed] [Google Scholar]
- 20. Chen W, Ishikawa M, Yamaki K, Sakuragi S. Wistar rat palpebral conjunctiva contains more slow-cycling stem cells that have larger proliferative capacity: implication for conjunctival epithelial homeostasis. Jpn J Ophthalmol. 2003;47:119–128 [DOI] [PubMed] [Google Scholar]
- 21. Wirtschafter JD, Ketcham JM, Weinstock RJ, Tabesh T, McLoon LK. Mucocutaneous junction as the major source of replacement palpebral conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:3138–3146 [PubMed] [Google Scholar]
- 22. Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001;42:1455–1464 [PubMed] [Google Scholar]
- 23. Nakamura T, Ang LP, Rigby H, et al. The use of autologous serum in the development of corneal and oral epithelial equivalents in patients with Stevens-Johnson syndrome. Invest Ophthalmol Vis Sci. 2006;47:909–916 [DOI] [PubMed] [Google Scholar]
- 24. Hsueh YJ, Wang DY, Cheng CC, Chen JK. Age-related expressions of p63 and other keratinocyte stem cell markers in rat cornea. J Biomed Sci. 2004;11:641–651 [DOI] [PubMed] [Google Scholar]
- 25. Nizam MH, Ruszymah BH, Chua KH, Ghafar NA, Hamzah JC. Ex vivo growth of rabbit bulbar, fornix and palpebral conjunctival epithelia in a serum-free and feeder layer-free culture system. Med J Malaysia. 2008;63:111–112 [PubMed] [Google Scholar]
- 26. Tanner JC, Jr, Vandeput J, Olley JF. The mesh skin graft. Plast Reconstr Surg. 1964;34:287–292 [PubMed] [Google Scholar]
- 27. Nanchahal J. Stretching skin to the limit: a novel technique for split skin graft expansion. Br J Plast Surg. 1989;42:88–91 [DOI] [PubMed] [Google Scholar]
- 28. Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79 [DOI] [PubMed] [Google Scholar]
- 29. Komine M, Rao LS, Freedberg IM, Simon M, Milisavljevic V, Blumenberg M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. J Invest Dermatol. 2001;116:330–338 [DOI] [PubMed] [Google Scholar]
- 30. Schofield R. The stem cell system. Biomed Pharmacother. 1983;37:375–380 [PubMed] [Google Scholar]
- 31. Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430 [DOI] [PubMed] [Google Scholar]
- 32. Schlotzer-Schrehardt U, Dietrich T, Saito K, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845–860 [DOI] [PubMed] [Google Scholar]
- 33. Di Girolamo N, Bosch M, Zamora K, Coroneo MT, Wakefield D, Watson SL. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009;87:1571–1578 [DOI] [PubMed] [Google Scholar]
- 34. Kolli S, Lako M, Figueiredo F, Mudhar H, Ahmad S. Loss of corneal epithelial stem cell properties in outgrowths from human limbal explants cultured on intact amniotic membrane. Regen Med. 2008;3:329–342 [DOI] [PubMed] [Google Scholar]