Abstract
Identifying patients with impaired renal function is crucial in the setting of critical illness. Serum creatinine serves as the gold standard for assessing steady-state renal function, helping to define those with chronic kidney disease (CKD). Although these baseline creatinine values are often not available in the setting of critical illness, CKD, whether defined by serum creatinine or proteinuria, increases the risk of developing acute kidney injury (AKI). Despite delays in elevations following renal insults, serum creatinine remains the standard for assessing acute changes in renal function. Standardized definitions of AKI, using changes in serum creatinine and urine output, have informed the epidemiology of ICU-acquired AKI and have helped define the long-term outcomes in patients who experience AKI. A complex cyclical interplay exists between AKI and CKD, in which CKD predisposes patients to an increased risk of AKI, whereas those with AKI, regardless of baseline renal function, are more likely to suffer from post-AKI CKD. The clarification of the AKI-CKD dynamic remains a work in progress and will be aided by the implementation of novel measures of renal function. Several novel biomarkers of renal function have been proposed to augment serum creatinine in the diagnosis of AKI and CKD. These biomarkers, taken with recent clinical investigations, have laid the groundwork for the impending paradigm shift in risk stratifying and in diagnosing changes in renal function in the ICU.
Physicians and scientists have been measuring creatinine concentrations in the blood for well over a century, and this simple and aged clinical test remains firmly entrenched in modern-day patient care.1 Creatinine has long served as a biomarker of glomerular filtration and, thus, a proxy for renal function. However, physicians are no longer solely reliant on imperfect creatinine as their only method of assessing renal function, because other markers of renal function have been developed and validated. In this review, I outline the current methods for assessing renal function, in both steady state and during acute kidney injury (AKI), and discuss their usefulness in caring for the critically ill.
Steady State: Defining Chronic Kidney Disease
Estimating the glomerular filtration rate (GFR) via serum creatinine allows us to assess the degree of kidney impairment and track the course of chronic kidney disease (CKD). CKD has been defined by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-K/DOQI) work group as either2
(1) the presence of markers of kidney damage for ≥ 3 months, as defined by structural or functional abnormalities of the kidney with or without decreased GFR, manifest by either pathologic abnormalities or other markers of kidney damage, including abnormalities in the composition of blood or urine or abnormalities in imaging tests or
(2) GFR < 60 mL/min/1.73 m2 for ≥ 3 months, with or without other signs of kidney damage as described previously.
The classification of CKD is described in Table 1.
Table 1.
— National Kidney Foundation Kidney Disease Outcomes Quality Initiative Definitions of CKD
| CKD Stage | Definition |
| 1 | Normal GFR (> 90 mL/min/1.73 m2) with persistent kidney damage (eg, albuminuria) |
| 2 | GFR between 60 and 89 mL/min/1.73 m2 with persistent kidney damage (eg, albuminuria) |
| 3 | GFR between 30 and 59 mL/min/1.73 m2 |
| 4 | GFR between 15 and 29 mL/min/1.73 m2 |
| 5 | GFR < 15 mL/min/1.73 m2 or end-stage renal disease |
The table displays the consensus classification of CKD, in which CKD is defined by either a decrease in GFR or the presence of kidney damage typically quantified as abnormal protein excretion. CKD = chronic kidney disease; GFR = glomerular filtration rate.
Despite providing no information on the source of the kidney disease, estimates of GFR remain ubiquitous, with creatinine-based equations being the preferred method. The Cockcroft and Gault and the Modification of Diet in Renal Disease (MDRD) equations are flawed in that they do not accurately estimate renal function in those with normal GFRs, the obese, or older adults (> 70 years).3,4 In 2009, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was developed and is as accurate as the MDRD in those with preexisting CKD (estimated GFR [eGFR] < 60 mL/min) and more accurate in those without CKD (≥ 60 mL/min).5 Compared with other equations, the CKD-EPI equation results in a lower prevalence estimate of CKD and a more accurate risk prediction for adverse patient outcomes.6 As such, we anticipate increased reporting of the CKD-EPI eGFR in the near future. Finally, the US Food and Drug Administration recommends the use of the Cockcroft and Gault equation when looking to estimate GFR for drug dosing; however, recent studies have demonstrated excellent concordance rates with the MDRD equation.7
Proteinuria
Abnormal protein excretion is often present in the setting of CKD, with urine protein being measured semiquantitatively with a dipstick or quantitatively through urine protein to creatinine ratios. Dipsticks are flawed in that they detect protein concentration as a function of urine volume. Urine protein (or urine albumin) to creatinine ratios are the preferred method of quantification but are predicated on the assumption that an individual excretes 1 g of creatinine per day and that creatinine excretion is in steady state. For adults < 50 years of age, creatinine excretion should be 20 to 25 mg/kg of lean body weight for men and 15 to 20 mg/kg for women, with estimates decreasing with age.8 Persistent albumin excretion between 30 and 300 mg/dL is considered to be microalbuminuria (1-2+ on dipstick). Values > 300 mg/dL are considered to be macroalbuminuria (3-4+ on dipstick). There are limited standards for 24-h protein excretion, but values < 150 mg/d are considered normal, whereas values between 150 and 3,500 mg are labeled “subnephrotic,” and values > 3,500 mg are considered to be in the nephrotic range. Individuals with subnephrotic- and nephrotic-range proteinuria will invariably have underlying renal disease, which places them at increased risk of AKI (as discussed following). Because 24-h collections are cumbersome and difficult to carry out in hospitalized patients, 8- or 12-h collections with extrapolation and random (spot) ratios have become an accepted alternative.
Novel Biomarkers of GFR/CKD
Increasingly, investigators have sought novel markers of renal function that will provide an accurate estimation of renal function without the foibles of serum creatinine (variation with muscle mass/age and tubular secretion). Serum cystatin C (SCyC) may be more sensitive than serum creatinine in detecting mild reductions in GFR.9 Additionally, SCyC is associated with cardiovascular disease (CVD) outcomes and patient mortality.10‐12 After a mean of 10 years of follow-up, in nondiabetic subjects (eGFR, 15-60 mL/min), baseline SCyC was strongly associated with both all-cause and CVD mortality. In multivariate-adjusted models, both creatinine and SCyC were associated with increased risks of all-cause mortality (1.27 [95% CI, 1.06-1.49] and 1.41 [95% CI, 1.18 -1.67]), respectively. For CVD mortality, the risks were 1.32 (95% CI, 1.05-1.64) and 1.64 (95% CI, 1.28-2.08), respectively.12
Despite this, the mainstream use of SCyC has been hindered by data demonstrating that SCyC is affected by non-GFR factors, including diabetes, thyroid dysfunction, corticosteroids, sex, and age.13,14 Additionally, the acceptance of SCyC has been limited by the lack of standardized reference material and by the assay drift that impacts interlaboratory extrapolation.15 As such, no single eGFR-SCyC equation has been uniformly validated or accepted.
Several other biomarkers of CKD are under investigation, including urinary neutrophil gelatinase-associated lipocalin (NGAL), cystatin C, and N-acetyl-β-D-glucosaminidase. Many of these candidates function as biomarkers of tubular function and are being studied as biomarkers of AKI (see “Novel Biomarkers of AKI” section).16‐18 Promising preliminary data display the importance of quantifying renal tubular function and reinforce the notion that renal function is more than just glomerular filtration.
CKD as a Risk Factor for AKI
The importance of estimating renal function goes beyond a more precise assessment of GFR. CKD has been increasingly validated as an important risk factor for the development of AKI. In the past, absolute elevations in baseline serum creatinine have been included in AKI-specific risk stratification systems; however, recent systems have used standard CKD definitions.19‐23
In a nested case-controlled study of > 600,000 hospitalized adults, those who developed AKI (n = 1,764), defined as the need for renal replacement therapy (RRT), were more likely to have baseline CKD, compared with those without AKI.24 There was a dose response, with increasing risk of AKI with decreasing eGFR (MDRD), which persisted after controlling for clinical risk (diabetes, sepsis, cardiac surgery, and so forth). Individuals with a baseline GFR of 30 to 45 mL/min were nearly five times as likely to develop AKI compared with those without CKD (GFR > 60 mL/min), whereas those with a baseline of 15 to 29 mL/min were 20 times as likely to be hospitalized with AKI. This International Classification of Diseases, Ninth Revision code-reliant retrospective study has been corroborated by other investigations25,26 and supports the role of baseline GFR as a major risk factor for AKI.
Proteinuria is also recognized increasingly as an important risk factor for the development of AKI.27‐29 In a study of 1,051 subjects undergoing cardiac surgery, heavy proteinuria (2+ to 4+) was associated with a sevenfold-increased risk of postoperative RRT compared with those without proteinuria (OR, 7.29; 95% CI, 3.0-17.7).27 This study did not attempt to quantify the proteinuria beyond the dipstick but demonstrates the importance of AKI risk stratification via proteinuria.
In 11,200 subjects from the Atherosclerosis Risk in Communities (ARIC) study, proteinuria correlated with an increased future risk of AKI. Excluding patients with an eGFR < 15 mL/min, the hazard of developing AKI displayed a stepwise increase according to the albumin to creatinine ratio. Individuals with microalbuminuria (30-299 mg/g) and macroalbuminuria (> 300 mg/g) were 2.2 times (95% CI, 1.6-3.0) and 4.8 times (95% CI, 3.2.-7.2) as likely to be hospitalized for AKI compared with those with a normal albumin to creatinine ratio (< 10 mg/g).30 Similarly, in a cohort of 920,985 Canadian adults who had at least one measurement of serum creatinine and proteinuria over a 5-year period, there was a stepwise increase in the risk of AKI and need for RRT across proteinuria strata.31 Over the median 35-month follow-up, > 6,500 adults were admitted with AKI and 516 (< 0.01%) required RRT. Subjects with normal eGFR (> 60 m/min) and mild proteinuria (dipstick, trace and 1+) were hospitalized with AKI 2.5 times more often than were individuals without proteinuria. These odds increased to 4.4 times (95% CI, 3.7-5.2) in those with similar GFR and ≥ 2+ proteinuria. The adjusted rates of death were higher in participants admitted with AKI, although the rise associated with this injury was attenuated in those with low baseline eGFR and heavy proteinuria. Despite relying on laboratory tests that were drawn up as part of clinical care and administrative codes, these studies still demonstrate that proteinuria is an emerging risk factor for AKI.
Other Risk Factors for AKI
The 2010 United States Renal Data System demonstrated that over the past decade, the incidence of AKI has been increasing and that AKI is associated with older age and black race.32 Several scoring systems have been constructed to reliably forecast AKI and the need for RRT following cardiac surgery.20‐22 Although there are several similarities among these risk-assessment models (baseline renal function, presence of diabetes, and type of surgery), there are also differences. Similarly, in the setting of radiocontrast administration, several risk factors have been shown to increase the risk of AKI, including preexisting CKD, diabetes, congestive heart failure, anemia, hypotension, and contrast-load volume.33 In the setting of the critically ill, AKI-specific risk-stratification systems have been proposed but they remain underused in clinical practice.19,23 Finally, it deserves noting that the avoidance of nephrotoxins (radiocontrast, aminoglycosides, nonsteroidal antiinflammatory drugs, and so forth) is of the utmost importance in vulnerable patient populations.
AKI as a Risk Factor for CKD
CKD is a risk factor for AKI; however, there is a growing body of evidence to support the bidirectionality of this dynamic. Studies of the long-term outcomes of AKI suggest that episodes of AKI, regardless of severity, are associated with adverse CKD-related outcomes.
In a large, community-based, retrospective, matched-cohort analysis (N = 556,090), the impact of AKI requiring RRT was assessed in those with baseline eGFRs > 45 mL/min who did not initially develop end-stage renal disease (ESRD). After controlling for potential confounders, AKI was independently associated with a 28.1-fold increased risk of developing stage IV or V CKD (95% CI, 21.1-37.6) and a 2.3-fold increased risk of death (95% CI, 1.8-30).34
In a population-based cohort study investigating the long-tem risk of an episode of AKI requiring RRT, 3,769 individuals who were RRT independent at the time of discharge, following a hospitalization that required transient RRT, were compared with 13,598 matched control subjects who did not have AKI.35 Over a median follow-up period of 3 years, the incidence of ESRD was 2.63 per 100 person-years in patients with AKI and 0.91 per 100 person-years in control subjects (hazard ratio, 3.23; 95% CI, 2.70-3.86). Surprisingly, there was no difference in mortality, which may have been because of the inability to match a cohort of the 297 sickest patients with AKI, the exclusion of those still dependent on RRT at the time of hospital discharge, and the exclusion of those who died within the first 30 postdischarge days. Regardless, a hospital-limited episode of AKI requiring RRT increases the long-term risk of ESRD.
Increasingly, investigators have sought long-term outcomes of AKI that have not required RRT. Using a multivariable model in a cohort of 233,803 Medicare recipients (7,197 who had AKI), ESRD was found to be associated with the presence of both CKD and AKI. The hazard ratio for developing ESRD was 41.2 (95% CI, 34.6-49.1) for patients who had both CKD and AKI compared with those without kidney disease. Similarly, those with AKI (HR, 13.0; 95% CI, 10.6-16.0) and those with CKD (HR, 8.4; 95% CI, 7.4-9.6) were at increased risk of developing ESRD compared with those without kidney disease.36 In an analysis of 5 years of data from the US Department of Veterans Affairs, subjects without preexisting CKD who were hospitalized with AKI (n = 5,058) and its most severe form, acute tubular necrosis (ATN) (n = 346), were compared with 63,491 subjects who were hospitalized with pneumonia and acute myocardial infarction without AKI. Subjects with AKI and ATN were more likely to develop CKD of any stage compared with control subjects, and their progression to stage IV CKD was similar to that of individuals from a separate cohort with known preexisting CKD.37 These studies cement the role of AKI as a risk factor for the development of CKD.
This recent literature supports the bidirectional relationship of the AKI and CKD, but it also demonstrates that the development of post-AKI CKD is perhaps malleable. Not all subjects with AKI go on to develop CKD, just as not all hospitalized CKD patients develop AKI. Although large numbers of patients with AKI do not return to normal renal function, our ability to forecast who will recover and who will develop CKD and progress to ESRD is limited.38‐43
AKI: Diagnostic Criteria
For decades, clinical investigation into AKI was hampered by the lack of a consensus definition of AKI, with studies using nonstandardized medical coding or arbitrary serum creatinine cutoffs.44 Consensus criteria for AKI have been established through the collaboration of intensivists and nephrologists, and these projects have led to the concomitant shift in nomenclature from acute renal failure to AKI. The Acute Dialysis Quality Initiative (ADQI) created a graded definition of AKI called the RIFLE criteria, where RIFLE stands for risk, injury, failure, loss, and end-stage renal disease (Table 2).45
Table 2.
—RIFLE Criteria for AKI
| Category | GFR Criteria | Urine Output Criteria |
| Risk | 1.5-fold increase in serum creatinine or > 25% decrease in GFR | < 0.5 mL/kg/h for 6 h |
| Injury | 2-fold increase in serum creatinine or > 50% decrease in GFR | < 0.5 mL/kg/h for 12 h |
| Failure | 3-fold increase in serum creatinine or > 75% decrease in GFR or serum creatinine > 4.0 mg/dL in the setting of an absolute increase of 0.5 mg/dL | < 0.3 mL/kg/h for 24 h or anuria for 12 h |
| Loss | Persistent AKI (on RRT > 4 w) | |
| ESRD | ESRD (on RRT > 3 mo) | |
This was the first consensus classification schema and accounted for changes in both creatinine and GFR, as well as changes in urine output. AKI = acute kidney injury; ESRD = end-stage renal disease; RIFLE = risk, injury, failure, loss, and end-stage renal disease; RRT = renal replacement therapy. See Table 1 for expansion of other abbreviations.
Recognizing the potential limitations of RIFLE, the Acute Kidney Injury Network (AKIN) published their own criteria (Table 3).46 Included in these RIFLE criticisms were the use of eGFR (eg, an individual with severe nonoliguric AKI may have a GFR of < 15 mL/min upon arrival to the ICU but it may take days for him/her to progress through the criteria before getting to “failure”). This critique displays the pitfalls of diagnosing AKI via serum creatinine. The latter two stages of AKIN are similar to RIFLE, whereas AKIN stage 1 accounts for the absolute change in serum creatinine. This departure from RIFLE is based on the evidence that small changes in serum creatinine over discrete time periods (< 48 h) are associated with adverse patient outcomes in a variety of settings of critical illness.39,47,48
Table 3.
—AKIN Criteria for AKI
| Stage | Serum Creatinine Criteria | Urine Output Criteria |
| 1 | Increase in serum creatinine ≥ 0.3 mg/dL or > 1.5- to 2-fold increase from baseline serum creatinine | < 0.5 mL/kg/h for > 6 h |
| 2 | > 2- to 3-fold increase from baseline serum creatinine | < 0.5 mL/kg/h for > 12 h |
| 3 | > 3-fold increase from baseline serum creatinine or an absolute increase of ≥ 0.5 mg/dL in serum creatinine or the need for RRT | < 0.3 mL/kg for 24 h or anuria for 12 h |
This classification schema, which followed the RIFLE system, is to be applied over a discrete 48-h period and accounts for an absolute change in serum creatinine rather than relying solely on percentage change. The AKIN criteria also permit the evaluation of urine output but do not include GFR in their definitions. AKIN = Acute Kidney Injury Network. See Table 1 and 2 legends for expansion of other abbreviations.
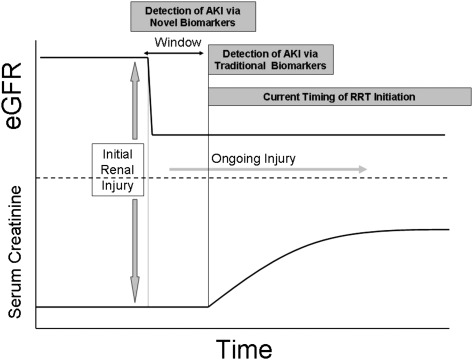
Both of these AKI classification schemas have been shown to be associated with length of ICU and hospital stay, as well as mortality.49‐51 However, the use of AKIN and RIFLE as diagnostic tools remain limited given their reliance on serum creatinine and the absence of effective therapeutic interventions for the treatment of AKI. Creatinine kinetics are such that serum creatinine often does not increase until 24 or 48 h after a renal injury; thus, by the time a patient reaches AKIN stage 1 or RIFLE “risk,” the “window” for a potential therapeutic intervention may be closed (Fig 1). Future iterations of AKI diagnostic schema will include biomarkers of renal tubular injury/AKI (discussed later), which increase within minutes to hours of renal injury, thus obviating the need to rely on creatinine, an imperfect, time-delayed marker of filtration.
Figure 1.
AKI timing and changes in creatinine/glomerular filtration. The figure demonstrates the timing among severe renal injury, the drop in glomerular filtration rate, and the time-delayed rise in serum creatinine and other traditional markers of renal function. Several novel biomarker are able to forecast AKI prior to changes in traditional markers (creatinine and urine output). This early diagnosis could potentially provide a window to treat/intervene with RRT or other novel therapeutics and possibly improve outcomes in the setting of AKI. AKI = acute kidney injury; eGFR = estimated glomerular filtration rate; RRT = renal replacement therapy.
Regardless of these limitations, the current diagnostic criteria have informed and advanced the epidemiology of AKI and permit consistent AKI definitions in clinical investigations. Studies that have specifically compared RIFLE and AKIN have demonstrated that neither criteria offer a clear advantage and both remain hindered by the need to establish a “baseline,” which is often impossible in the ICU.52
These criteria have furthered our understanding of the link between AKI and mortality.39 In 71,486 veterans with AKI (22% of a large ICU cohort), after adjusting for severity of illness, the odds of death were 2.2 (95% CI, 2.17-2.3) for AKIN stage 1, 6.1 (95% CI, 5.74-6.44) for stage 2, and 8.6 (95% CI, 8.07-9.15) for stage 3.53 The recent focus on small changes in serum creatinine (AKIN stage 1 or RIFLE “risk”) impacting adverse patient outcomes should not take away from the extremely high mortality rate in patients with AKI requiring RRT.48
In two recent large randomized trials of RRT dose (ATN trial [N = 1,124] and RENAL study [N = 1,465]), the in-hospital mortality rates were 49.6% and 44.3%, respectively.38,41 These data are limited in that RRT is often reserved for the most severely ill patients and that the initiation RRT, which automatically constitutes AKIN stage 3, is highly variable across physicians.
In the setting of CKD, the traditional teaching had always been to initiate RRT when the GFR reached 10-15 mL/min because this is when patients began to experience complications related to abnormal electrolytes, acidosis, uremia, and fluid overload (CKD stage V, Table 1). However, a recent randomized controlled trial demonstrated that the proactive initiation of RRT at higher eGFRs (10-14 mL/min) provided no benefit in terms of long-term mortality or other adverse patient outcomes compared with waiting for a definitive indication at lower GFRs, (5-7 mL/min).54
In the setting of AKI, the established “indications” for RRT (Table 4) are severely limited and are reactive in nature. These criteria aim only to prevent the complications of acute renal dysfunction just prior to the point where they become life threatening. Although some of the indications are objective and readily apparent (ie, hyperkalemia with ECG changes or pulmonary edema requiring maximum ventilatory support), others are subjective and nonspecific (the clinical diagnosis of uremia). Levels of certain serum chemistries (eg, potassium, phosphorus, bicarbonate) are affected by issues not directly related to the severity of AKI, including dietary intake, choice of fluid administration, and medication use; thus, the use of electrolyte concentrations as thresholds for initiation of RRT is not always appropriate.
Table 4.
—Five Most Commonly Cited Indications for Acute Initiation of RRT
| Severe acidemia (pH < 7.1) secondary to metabolic acidosis refractory to medical care |
| Severe hyperkalemia (K > 6.5 mM) or rapidly rising K refractory to medical care |
| Ingestion of dialyzable toxins |
| Volume overload impairing cardiopulmonary function refractory to medical care |
| Uremic complications of renal dysfunction |
See Table 2 legend for expansion of abbreviations.
Trials investigating the timing of RRT initiation have failed to provide definitive clinical answers. Data supporting the early initiation of RRT come from retrospective, observational studies examining the use of RRT in a variety of clinical settings (sepsis, cardiac surgery, and liver failure).55‐58 However, the retrospective nature of these studies, the variable criteria used to define “early” vs “late,” and the diversity of patient populations make it impossible to craft strong, evidence-based recommendations for the early initiation of RRT. Although the early initiation of RRT may be associated with improved survival and outcome, an adequately powered randomized controlled trial to address this question is needed.
Urine output can be measured inexpensively and easily, and is an important and equally weighted portion of AKIN and RIFLE. However, it is also impacted by a variety of clinical factors, including a patient’s intravascular volume status and diuretics. The accuracy of the urine output criteria has not been as widely studied as that of creatinine. In studies that included both creatinine- and urine-output-based definitions, AKI rates were higher with lower overall mortality compared with creatinine-based AKI.49,59 This finding suggests that urine output may overestimate the severity of AKI, compared with creatinine alone. Relative oliguria is often an appropriate clinical response in the setting of hypovolemia, and temporary drops in urine output, which may be overcome with fluid administration or diuretics, may not correlate with eventual rises in serum creatinine.
Although further investigation of the urine output criteria seems needed, the AKIN and RIFLE criteria have reshaped how clinicians and investigators have defined and studied AKI. Despite international acceptance, these schemas remain, on some level, imperfect, given their reliance on serum creatinine and the need for an established baseline value. These schemas will continue to be validated and may some day evolve to include biomarkers of AKI besides serum creatinine and urine output.
Diagnostics of AKI: Traditional and Novel Methods
Urine Microscopy
The development of RIFLE and AKIN has led to a more rigorous examination of the diagnostic capabilities of urine microscopy, with several recent articles investigating the ability of microscopy to reliably detect the presence and severity of AKI.60‐63 Urinary sediment analysis can detect glomerulonephritis (erythrocyte casts), pyelonephritis and acute interstitial nephritis (leukocyte casts), and crystal- and renal-stone-induced disease. Additionally, urinalysis can demonstrate the pathognomonic muddy brown casts and renal tubule epithelial cells in the sediment associated with the most severe form of AKI, ATN. In the critically ill, the most common use of microscopy is to differentiate the featureless sediment of prerenal azotemia from that of ATN. Recently, two distinct scoring systems that rely on counting renal tubule epithelial cells and granular casts for the diagnosis of ATN have been validated in a formal and rigorous evaluation which was long overdue.60,63 Despite strides to formalize and modernize urinalysis, investigations of the interobserver reliability of microscopy demonstrate that nephrologists achieve only slight to moderate agreement and, as such, urine microscopy remains a good but imperfect diagnostic tool for AKI.61
Novel Biomarkers of AKI
The past several years have seen a flurry, nay blizzard, of investigations of novel biomarkers of AKI and several in-depth reviews have been published recently.64,65 Table 5, which is by no means exhaustive, represents a selected summary of the characteristics and the clinical settings in which the top four most widely investigated biomarkers have been studied: NGAL, kidney injury molecule-1, IL-18, and cystatin C. The majority of articles cited in Table 5 are limited in that they are predominantly single-center prospective investigations with low numbers of severe AKI/RRT events. Additionally, there is an inherent bias in this table because negative studies often go unpublished. Finally, Table 5 makes no attempt to quantify the results of these investigations.
Table 5.
—Novel Biomarkers of AKI: Clinical Settings of Investigation
| Biomarker | Biologic Function and Link to AKI | Source | Clinical Settings in Which Biomarkers Detected AKI | Clinical Settings in Which Biomarkers Detected CKD |
| NGAL | Protein expressed in neutrophils and proximal, distal renal tubule cells; binds and traffics free iron and is upregulated in the setting of renal tubular ischemia | Blood | Adult cardiac surgery66,67 | Adult CKD17,68 |
| Pediatric cardiac surgery69 | Pediatric CKD70 | |||
| Contrast nephropathy71 | ||||
| General ICU72 | ||||
| Urine | Adult cardiac surgery67,73‐75 | Adult CKD17 | ||
| Pediatric cardiac surgery69 | ||||
| Liver transplant76 | ||||
| General ICU77 | ||||
| ED78 | ||||
| Pediatric ICU79 | ||||
| Contrast nephropathy71 | ||||
| Distinguishes prerenal from ATN80 | ||||
| Delayed graft function following renal transplant81,82 | ||||
| IL-18 | Inflammatory cytokine found in macrophages and proximal tubule cells that is upregulated in the setting of renal ischemic injury | Urine | Adult cardiac surgery75,83,84 | |
| General ICU85 | ||||
| Sepsis-AKI86 | ||||
| ARDS87 | ||||
| Contrast nephropathy88 | ||||
| Delayed graft function following renal transplant81,82 | ||||
| Cystatin C | Cysteine protease inhibitor freely filtered at glomerulus (serum marker of GFR) that is normally reabsorbed by proximal tubule cells and appears in the urine in the setting of tubular injury | Blood | Adult cardiac surgery66,67,89 | Adult CKD10‐12 |
| Pediatric cardiac surgery90 | Pediatric CKD70 | |||
| General ICU91 | ||||
| ED92 | ||||
| Contrast nephropathy93 | ||||
| Urine | Adult cardiac surgery73,75 | Adult CKD18 | ||
| General ICU85 | ||||
| Sepsis-AKI86,94 | ||||
| ED92 | ||||
| Delayed graft function following renal transplant95 | ||||
| KIM-1 | Type 1 cell membrane glycoprotein that is shed into the urine following proximal tubule injury | Urine | Adult cardiac surgery73,75 | Adult CKD96 |
| Pediatric cardiac surgery97 | ||||
| General ICU85 | ||||
| Contrast nephropathy88 | ||||
| Delayed graft function following renal transplant81 |
Summary of data on the four most frequently investigated biomarkers of AKI. The table, which is not exhaustive, notes the biologic function of the biomarkers as well as the variety of clinical settings (AKI and CKD) in which the biomarkers have been investigated. ATN = acute tubular necrosis; KIM-1 = kidney injury molecule-1; NGAL = neutrophil gelatinase-associated lipocalin. See Table 1 and 2 legends for expansion of other abbreviations.
It is our opinion that no single biomarker will be able to predict early AKI (prior to changes in serum creatinine) as well as AKI severity and duration in all clinical settings. The pathophysiology of ischemia-reperfusion-AKI is different from that of sepsis-AKI, which is different from radiocontrast nephropathy. As such, each of these clinical entities may be best detected by a different set of biomarkers at different clinical time points.
Increasingly, biomarkers are being investigated for their ability to complement, rather than replace, creatinine. In a pooled prospective study (n = 2,322) that designated subjects as NGAL(+) or NGAL(−) and creatinine(+) or creatinine(−) (AKI was defined as RIFLE “risk”), individuals who were NGAL(+) but creatinine(−) needed more RRT (OR, 16.4; 95% CI, 3.6-76.9; P < .001) compared with those who were NGAL(−) and creatinine(−).98 There was a stepwise increase in ICU stay, hospital stay, and mortality across the four study groups:
This ability to detect adverse patient outcomes in the absence of significant changes in creatinine is a paradigm shift for physicians and will certainly need to be validated in follow-up studies.
There is growing evidence to support the role of biomarkers at the time of AKI, with two separate publications showing that urine NGAL at the time of clinical creatinine increase (AKIN stage 1 or RIFLE “risk”) can predict the severity of AKI (who will progress to AKIN stage 2 or 3 or RIFLE “injury” or “failure”).72,80 If validated, routine measurement of NGAL in those with newly diagnosed AKI may identify those at highest risk of the most adverse patient outcomes and those who would be ideal participants in future interventional trials.
Novel biomarkers are inching closer toward clinical relevance, but there is still much we do not know about their usefulness. Several investigations have recently shown that the ability of a biomarker to detect AKI may be impacted by underlying CKD.73,74,85 Additionally, the impact of medications (diuretics, pressors, angiotensin-converting enzyme inhibitors) and clinical care on biomarker values is unknown. Until these issues are clarified and biomarkers are uniformly validated, we will remain reliant on our current “bronze standard” serum creatinine.
Conclusions
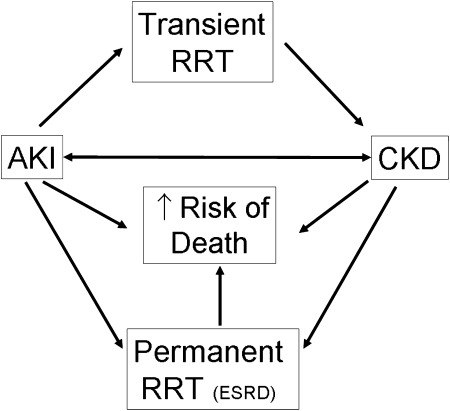
In the setting of critical illness, AKI and CKD are major players in a vicious cycle that leads to adverse patient outcomes (Fig 2). Recent data have confirmed that CKD, defined via serum creatinine or proteinuria, is a primary risk factor for AKI. AKI is associated with increased mortality and can also lead to the transient or permanent need for RRT. Even when not requiring RRT, patients can develop post-AKI CKD, which carries an increased risk of ESRD and death. This complex interplay of AKI and CKD will continue to be defined as nephrologists and intensivists further explore novel methods to assess renal function in the ICU.
Figure 2.
This diagram displays the complex dynamic among AKI, CKD, and adverse patient outcomes. AKI and CKD are known to cross-react and augment each other in a vicious cycle that leads to increased need for RRT, either transient or permanent (ESRD), as well as increased mortality. CKD = chronic kidney disease; ESRD = end-stage renal disease. See Figure 1 legend for expansion of other abbreviations.
Acknowledgments
Financial/nonfinancial disclosures: The author has reported to CHEST the following conflicts of interest: Dr Koyner has received money from Abbott Laboratories for patient enrollment for research conducted investigating urine NGAL as a biomarker of acute kidney injury following adult cardiac surgery.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: The author expresses gratitude to Jason Poston, MD, University of Chicago, for his invaluable assistance in preparing this manuscript.
Abbreviations
- AKI
acute kidney injury
- AKIN
Acute Kidney Injury Network
- ATN
acute tubular necrosis
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- GFR
glomerular filtration rate
- MDRD
Modification of Diet in Renal Disease
- NGAL
neutrophil gelatinase-associated lipocalin
- RIFLE
risk, injury, failure, loss, and end-stage renal disease
- RRT
renal replacement therapy
- SCyC
serum cystatin C
Footnotes
Funding/Support: Dr Koyner is supported by the National Institutes of Health-National Institute of Diabetes and Digestive and Kidney Diseases [Grant K23 DK081616].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Colls PC. Notes on creatinine. J Physiol. 1896;20(2-3):107–111. doi: 10.1113/jphysiol.1896.sp000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2) suppl 1:S1–S266. [PubMed] [Google Scholar]
- 3.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens LA, Nolin TD, Richardson MM, et al. Chronic Kidney Disease Epidemiology Collaboration Comparison of drug dosing recommendations based on measured GFR and kidney function estimating equations. Am J Kidney Dis. 2009;54(1):33–42. doi: 10.1053/j.ajkd.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walser M. Creatinine excretion as a measure of protein nutrition in adults of varying age. JPEN J Parenter Enteral Nutr. 1987;11(suppl 5):73S–78S. doi: 10.1177/014860718701100510. [DOI] [PubMed] [Google Scholar]
- 9.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 10.Mathisen UD, Melsom T, Ingebretsen OC, et al. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol. 2011;22(5):927–937. doi: 10.1681/ASN.2010050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 12.Menon V, Shlipak MG, Wang X, et al. Cystatin C as a risk factor for outcomes in chronic kidney disease. Ann Intern Med. 2007;147(1):19–27. doi: 10.7326/0003-4819-147-1-200707030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63(5):1944–1947. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 15.Maahs DM, Jalal D, McFann K, Rewers M, Snell-Bergeon JK. Systematic shifts in cystatin C between 2006 and 2010. Clin J Am Soc Nephrol. 2011;6(8):1952–1955. doi: 10.2215/CJN.11271210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devarajan P. The use of targeted biomarkers for chronic kidney disease. Adv Chronic Kidney Dis. 2010;17(6):469–479. doi: 10.1053/j.ackd.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh-Asahara N, Suganami T, Majima T, et al. Japan Obesity and Metabolic Syndrome Study (JOMS) Group Urinary cystatin C as a potential risk marker for cardiovascular disease and chronic kidney disease in patients with obesity and metabolic syndrome. Clin J Am Soc Nephrol. 2011;6(2):265–273. doi: 10.2215/CJN.04830610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liaño F, Gallego A, Pascual J, et al. Prognosis of acute tubular necrosis: an extended prospectively contrasted study. Nephron. 1993;63(1):21–31. doi: 10.1159/000187139. [DOI] [PubMed] [Google Scholar]
- 20.Mehta RH, Grab JD, O’Brien SM, et al. Society of Thoracic Surgeons National Cardiac Surgery Database Investigators Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114(21):2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 21.Thakar CVAS, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16(1):162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 22.Wijeysundera DN, Karkouti K, Dupuis JY, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297(16):1801–1809. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 23.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13(5):1350–1357. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18(4):1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 26.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang TM, Wu VC, Young GH, et al. National Taiwan University Hospital Study Group of Acute Renal Failure Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol. 2011;22(1):156–163. doi: 10.1681/ASN.2010050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu RK, Hsu CY. Proteinuria and reduced glomerular filtration rate as risk factors for acute kidney injury. Curr Opin Nephrol Hypertens. 2011;20(3):211–217. doi: 10.1097/MNH.0b013e3283454f8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73(4):465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 30.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21(10):1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James MT, Hemmelgarn BR, Wiebe N, et al. Alberta Kidney Disease Network Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376(9758):2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 32. Chapter eight: acute kidney injury. United States Renal Data System website. http://www.usrds.org/2010/pdf/v1_08.pdf. Accessed May 25, 2010.
- 33.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 34.Lo LJ, Go AS, Chertow GM, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76(8):893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wald R, Quinn RR, Luo J, et al. University of Toronto Acute Kidney Injury Research Group Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 36.Ishani A, Xue JL, Himmelfarb J, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. 2009;76(10):1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 38.Bellomo R, Cass A, Cole L, et al. RENAL Replacement Therapy Study Investigators Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 39.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 40.Liaño F, Pascual J. Madrid Acute Renal Failure Study Group Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Kidney Int. 1996;50(3):811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 41.Palevsky PM, Zhang JH, O’Connor TZ, et al. VA/NIH Acute Renal Failure Trial Network Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srisawat N, Murugan R, Wen X, et al. Recovery from acute kidney injury: determinants and predictors. Contrib Nephrol. 2010;165:284–291. doi: 10.1159/000313768. [DOI] [PubMed] [Google Scholar]
- 44.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 45.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molitoris BA, Levin A, Warnock DG, et al. Acute Kidney Injury Network working group Improving outcomes of acute kidney injury: report of an initiative. Nat Clin Pract Nephrol. 2007;3(8):439–442. doi: 10.1038/ncpneph0551. [DOI] [PubMed] [Google Scholar]
- 47.Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. 2005;33(10):2194–2201. doi: 10.1097/01.ccm.0000182798.39709.84. [DOI] [PubMed] [Google Scholar]
- 48.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 49.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73(5):538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 50.Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(4):1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 51.Cruz DN, Ricci Z, Ronco C. Clinical review: RIFLE and AKIN—time for reappraisal. Crit Care. 2009;13(3):211–219. doi: 10.1186/cc7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bagshaw SM. Acute kidney injury: diagnosis and classification of AKI: AKIN or RIFLE? Nat Rev Nephrol. 2010;6(2):71–73. doi: 10.1038/nrneph.2009.225. [DOI] [PubMed] [Google Scholar]
- 53.Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 54.Cooper BA, Branley P, Bulfone L, et al. IDEAL Study A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 55.Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Med. 1999;25(8):805–813. doi: 10.1007/s001340050956. [DOI] [PubMed] [Google Scholar]
- 56.Demirkiliç U, Kuralay E, Yenicesu M, et al. Timing of replacement therapy for acute renal failure after cardiac surgery. J Card Surg. 2004;19(1):17–20. doi: 10.1111/j.0886-0440.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 57.Elahi MM, Lim MY, Joseph RN, Dhannapuneni RR, Spyt TJ. Early hemofiltration improves survival in post-cardiotomy patients with acute renal failure. Eur J Cardiothorac Surg. 2004;26(5):1027–1031. doi: 10.1016/j.ejcts.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 58.Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis. 2008;52(2):272–284. doi: 10.1053/j.ajkd.2008.02.371. [DOI] [PubMed] [Google Scholar]
- 59.Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35(10):1692–1702. doi: 10.1007/s00134-009-1530-4. [DOI] [PubMed] [Google Scholar]
- 60.Chawla LS, Dommu A, Berger A, Shih S, Patel SS. Urinary sediment cast scoring index for acute kidney injury: a pilot study. Nephron Clin Pract. 2008;110(3):c145–c150. doi: 10.1159/000166605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wald R, Bell CM, Nisenbaum R, et al. Interobserver reliability of urine sediment interpretation. Clin J Am Soc Nephrol. 2009;4(3):567–571. doi: 10.2215/CJN.05331008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408. doi: 10.2215/CJN.06960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615–1619. doi: 10.2215/CJN.02860608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siew ED, Ware LB, Ikizler TA. Biological markers of acute kidney injury. J Am Soc Nephrol. 2011;22(5):810–820. doi: 10.1681/ASN.2010080796. [DOI] [PubMed] [Google Scholar]
- 65.Parikh CR, Lu JC, Coca SG, Devarajan P. Tubular proteinuria in acute kidney injury: a critical evaluation of current status and future promise. Ann Clin Biochem. 2010;47(Pt 4):301–312. doi: 10.1258/acb.2010.010076. [DOI] [PubMed] [Google Scholar]
- 66.Haase-Fielitz A, Bellomo R, Devarajan P, et al. Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery—a prospective cohort study. Crit Care Med. 2009;37(2):553–560. doi: 10.1097/CCM.0b013e318195846e. [DOI] [PubMed] [Google Scholar]
- 67.Koyner JL, Bennett MR, Worcester EM, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74(8):1059–1069. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malyszko J, Malyszko JS, Bachorzewska-Gajewska H, Poniatowski B, Dobrzycki S, Mysliwiec M. Neutrophil gelatinase-associated lipocalin is a new and sensitive marker of kidney function in chronic kidney disease patients and renal allograft recipients. Transplant Proc. 2009;41(1):158–161. doi: 10.1016/j.transproceed.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 69.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 158(6):1009–1015. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 70.Mitsnefes MM, Kathman TS, Mishra J, et al. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in children with chronic kidney disease. Pediatr Nephrol. 2007;22(1):101–108. doi: 10.1007/s00467-006-0244-x. [DOI] [PubMed] [Google Scholar]
- 71.Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007;22(12):2089–2095. doi: 10.1007/s00467-007-0601-4. [DOI] [PubMed] [Google Scholar]
- 72.Cruz DN, de Cal M, Garzotto F, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36(3):444–451. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koyner JL, Vaidya VS, Bennett MR, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5(12):2154–2165. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McIlroy DR, Wagener G, Lee HT. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: the effect of baseline renal function on diagnostic performance. Clin J Am Soc Nephrol. 2010;5(2):211–219. doi: 10.2215/CJN.04240609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liangos O, Tighiouart H, Perianayagam MC, et al. Comparative analysis of urinary biomarkers for early detection of acute kidney injury following cardiopulmonary bypass. Biomarkers. 2009;14(6):423–431. doi: 10.1080/13547500903067744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagener G, Minhaz M, Mattis FA, Kim M, Emond JC, Lee HT. Urinary neutrophil gelatinase-associated lipocalin as a marker of acute kidney injury after orthotopic liver transplantation. Nephrol Dial Transplant. 2011;26(5):1717–1723. doi: 10.1093/ndt/gfq770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–1832. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zappitelli M, Washburn KK, Arikan AA, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11(4):R84–R95. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singer E, Elger A, Elitok S, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80(4):405–414. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2009;21(1):189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parikh CR, Jani A, Mishra J, et al. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6(7):1639–1645. doi: 10.1111/j.1600-6143.2006.01352.x. [DOI] [PubMed] [Google Scholar]
- 83.Haase M, Bellomo R, Story D, Davenport P, Haase-Fielitz A. Urinary interleukin-18 does not predict acute kidney injury after adult cardiac surgery: a prospective observational cohort study. Crit Care. 2008;12(4):R96–R104. doi: 10.1186/cc6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70(1):199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 85.Endre ZH, Pickering JW, Walker RJ, et al. Improved performance of urinary biomarkers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney Int. 2011;79(10):1119–1130. doi: 10.1038/ki.2010.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bagshaw SM, Langenberg C, Haase M, Wan L, May CN, Bellomo R. Urinary biomarkers in septic acute kidney injury. Intensive Care Med. 2007;33(7):1285–1296. doi: 10.1007/s00134-007-0656-5. [DOI] [PubMed] [Google Scholar]
- 87.Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 88.Malyszko J, Bachorzewska-Gajewska H, Poniatowski B, Malyszko JS, Dobrzycki S. Urinary and serum biomarkers after cardiac catheterization in diabetic patients with stable angina and without severe chronic kidney disease. Ren Fail. 2009;31(10):910–919. doi: 10.3109/08860220903216113. [DOI] [PubMed] [Google Scholar]
- 89.Wald R, Liangos O, Perianayagam MC, et al. Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5(8):1373–1379. doi: 10.2215/CJN.06350909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zappitelli M, Krawczeski CD, Devarajan P, et al. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80(6):655–662. doi: 10.1038/ki.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115–1122. doi: 10.1111/j.1523-1755.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 92.Soto K, Coelho S, Rodrigues B, et al. Cystatin C as a marker of acute kidney injury in the emergency department. Clin J Am Soc Nephrol. 2010;5(10):1745–1754. doi: 10.2215/CJN.00690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Briguori C, Visconti G, Rivera NV, et al. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121(19):2117–2122. doi: 10.1161/CIRCULATIONAHA.109.919639. [DOI] [PubMed] [Google Scholar]
- 94.Nejat M, Pickering JW, Walker RJ, et al. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care. 2010;14(3):R85–R98. doi: 10.1186/cc9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hall IE, Koyner JL, Doshi MD, Marcus RJ, Parikh CR. Urine cystatin C as a biomarker of proximal tubular function immediately after kidney transplantation. Am J Nephrol. 2011;33(5):407–413. doi: 10.1159/000326753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Waanders F, Vaidya VS, van Goor H, et al. Effect of renin-angiotensin-aldosterone system inhibition, dietary sodium restriction, and/or diuretics on urinary kidney injury molecule 1 excretion in nondiabetic proteinuric kidney disease: a post hoc analysis of a randomized controlled trial. Am J Kidney Dis. 2009;53(1):16–25. doi: 10.1053/j.ajkd.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2007;73(7):863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57(17):1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]