Abstract
Background:
Little is known about the association between left ventricular (LV) diastolic dysfunction and outcomes in patients with idiopathic or heritable pulmonary arterial hypertension (PAH). Our rationale was to investigate the prevalence of LV diastolic dysfunction, and its association with disease severity and outcomes, in patients with idiopathic or heritable PAH.
Methods:
Using the Cleveland Clinic Pulmonary Hypertension Registry, we identified subjects with heritable or idiopathic PAH who underwent Doppler echocardiography and right-sided heart catheterization. Echocardiographic diastolic parameters were assessed in each patient.
Results:
A total of 61 patients met the inclusion criteria (idiopathic 85%, heritable 15%). The age at the time of echocardiography was 48.3 ± 18 years, 84% of the subjects were women, and 48% were on PAH-targeted therapies. Normal LV diastolic function, impaired relaxation, and pseudonormalization were seen in 10%, 88%, and 2% of the patients, respectively. Peak early diastolic (peak E) velocity was directly associated with LV end-diastolic volume and cardiac index and inversely associated with the degree of right ventricular dilation, right atrial pressure, and pulmonary vascular resistance. Peak E velocity was associated with mortality adjusted for age and sex (hazard ratio [HR], 1.5; 95% CI, 1.1-2 per 10 cm/s decrease; P = .015) and age, sex, 6-min walk distance, and cardiac output (HR, 1.8; 95% CI, 1.2-2.9 per 10 cm/s decrease; P = .01).
Conclusions:
LV diastolic dysfunction of the impaired relaxation type is observed in the majority of patients with advanced idiopathic or heritable PAH. A decrease in transmitral flow peak E velocity is associated with worse hemodynamics and outcome.
Several authors have reported a high prevalence of impaired left ventricular (LV) relaxation (diastolic dysfunction grade I) in patients with pulmonary arterial hypertension (PAH).1‐3 Little is known, however, about its association with severity of disease and outcomes in patients with idiopathic or heritable PAH, since most of the studies were done on subjects with chronic thromboembolic disease or included a heterogeneous group of patients with PAH.
Impaired LV relaxation in PAH is commonly attributed to decreased LV compliance as a result of right-to-left interventricular septal displacement1,2 or reduced LV preload.4,5 Studies comparing echocardiographic with hemodynamic data have noted an association between the degree of PAH and the presence of LV diastolic dysfunction,3,6,7 but limited information exists about the prognostic value of parameters that measure LV diastolic function.8
We sought to investigate the LV diastolic function in patients with idiopathic or heritable PAH, using Doppler echocardiography, and compare the results with hemodynamic data obtained by right-sided heart catheterization (RHC). Our hypothesis is that LV diastolic dysfunction is common and correlates with severity of disease and outcomes in patients with idiopathic or heritable PAH.
Materials and Methods
Study Design and Inclusion Criteria
This study was approved by the Cleveland Clinic institutional review board (protocol approval number: 10-1127). Informed consent was waived. We identified subjects by using the Cleveland Clinic Pulmonary Hypertension Registry. A total of 1,329 individuals underwent RHC. We selected patients with hemodynamic criteria for PAH (pulmonary artery occlusion pressure [PAOP] ≤ 15 mm Hg, pulmonary vascular resistance [PVR] ≥ 3 Wood units)9 who also had FEV1/FVC ≥ 0.6 and total lung capacity ≥ 60%. Of those, we only included patients with either idiopathic or heritable PAH and no other medical conditions known to be associated with PAH (n = 140). Each of these patients underwent a careful selection process that included extensive testing to exclude other causes of pulmonary hypertension (additional data in e-Appendix 1). No patient had evidence of coronary artery disease or LV systolic, mitral, or aortic valvular dysfunction. We only included patients who had Doppler echocardiography performed within 2 months of the RHC and after the year 2004, to be sure that the echocardiography studies were accessible for off-line review. In addition, each patient was independently reviewed by two pulmonary hypertension specialists. We excluded all patients in whom both physicians were not in complete agreement about the diagnosis. All selected patients were in sinus rhythm at the time of the echocardiography. The presence or absence of LV diastolic dysfunction on echocardiography was not part of the inclusion or exclusion criteria.
Measurements and Calculations
We selected the RHC and Doppler echocardiogram pair that was first performed at our institution. Echocardiographic measurements were obtained by experienced observers unaware of the patients’ clinical status. All echocardiographic determinations were compared with the ones originally reported at the time of the study. In case of discrepancies, another physician reviewed the echocardiographic images and a consensus was obtained. We obtained all the traditional echocardiographic (two-dimensional [2D] and Doppler) measurements following American Society of Echocardiography recommendations.10
Transmitral flow measurements included peak early diastolic velocity (peak E velocity), peak late diastolic velocity (peak A velocity), E/A ratio, and deceleration time of the E velocity. Pulmonary vein flow determinations (feasible in 46 patients) consisted of peak systolic velocity (peak S velocity), peak anterograde diastolic velocity (peak D velocity), S/D ratio, and peak velocity in late diastole (peak Ar velocity). Tissue Doppler measurements (available in 25 patients) were early (e′) and late (a′) diastolic velocities and E/e′ and e′/a′ ratios at the septal and lateral areas of the mitral annulus.
Abnormal function was divided in three grades, following recommendations from the American Society and European Association of Echocardiography (grade I: impaired LV relaxation; grade II: pseudonormal LV filling; and grade III: restrictive LV filling).6 When indicated, Valsalva maneuver was performed to distinguish a “true-normal” from a pseudonormal diastolic filling pattern. A detailed description of the methodology used to obtain echocardiographic parameters is in e-Appendix 1.
Statistics
Two-group comparisons were performed by Student t test or Mann-Whitney U test when appropriate. For three or more groups comparison we used one-way analysis of variance with Bonferroni correction. Categorical data were compared using Fisher exact test. We used linear regression analysis to compare continuous variables. Survival was assessed by Kaplan-Meier methodology. Time 0 was the date at the time of the initial echocardiogram. The end of follow-up was December 2009 or the time of the recipient’s death (or transplantation, when a combined end point was used). Survival differences between patients with peak E wave ≥ 60 cm/s or < 60 m/s was assessed using log-rank test. A cutoff of 60 cm/s was chosen as it was the closest round value from the peak E wave median. Cox proportional hazards modeling, adjusted by age and sex, was used for survival analysis of echocardiographic variables as predictors of death. When appropriate, we also adjusted the analyses for other previously identified predictors of survival.11,12 Death of the study participants was ascertained by reviewing our records and querying the US Social Security Death Index. All P values reported are two-tailed. A P value of < .05 was considered significant. The statistical analyses were performed using the statistical package SPSS, version 17 (SPSS Inc).
Results
Overall Characteristics of the Patients
A total of 61 patients with either idiopathic (n = 52, 85.2%) or heritable (n = 9, 14.8%) PAH were included in the study. New York Heart Association (NYHA) functional class was II, III, and IV in 31%, 49%, and 20% of the patients, respectively. The age (mean ± SD) at the time of the echocardiography was 48 ± 18 years, and 84% of the subjects were women. Forty-eight percent of the patients were on PAH-targeted therapies at the time of echocardiography (phosphodiesterase 5 inhibitors, nine patients [15%]; endothelin receptor antagonists, five patients [8%]; prostacyclin analogs, 15 patients [25%]). Hemodynamic characteristics are shown in Table 1.
Table 1.
—Hemodynamic Parameters in All Patients (N = 61) and in Those With (n = 55) or Without (n = 6) LV Diastolic Dysfunction
| Parameter | All Patients (mean ± SD) | Normal Diastolic Function (mean ± SD) | Diastolic Dysfunction Grade ≥ I (mean ± SD) | P Value (Mann-Whitney Test) |
| HR, beats/min | 86 ± 14 | 82 ± 19 | 85 ± 13 | .66 |
| Systolic ABP, mm Hg | 125 ± 20 | 128 ± 12 | 125 ± 21 | .71 |
| Diastolic ABP, mm Hg | 79 ± 12 | 75 ± 9 | 80 ± 13 | .28 |
| RA, mm Hg | 11 ± 6 | 6 ± 3 | 12 ± 6 | .04 |
| Systolic PAP, mm Hg | 86 ± 19 | 84 ± 12 | 86 ± 19 | .95 |
| Diastolic PAP, mm Hg | 38 ± 13 | 34 ± 9 | 39 ± 13 | .34 |
| Mean PAP, mm Hg | 54 ± 14 | 51 ± 9 | 54 ± 14 | .77 |
| PAOP, mm Hg | 10 ± 4 | 13 ± 4 | 9 ± 4 | .04 |
| Cardiac index, L/m/m2 | 2.1 ± 0.7 | 2.9 ± 0.4 | 2 ± 0.6 | .001 |
| TPG, mm Hg | 44 ± 15 | 38 ± 7 | 45 ± 15 | .22 |
| PVR, Wood units | 13 ± 6 | 7 ± 1 | 13 ± 6 | .002 |
ABP = arterial BP; HR = heart rate; LV = left ventricular; PAOP = pulmonary artery occlusion pressure; PAP = pulmonary artery pressure; PVR = pulmonary vascular resistance; RA = right atrial; TPG = transpulmonary gradient.
Echocardiographic Evaluation
General Measurements:
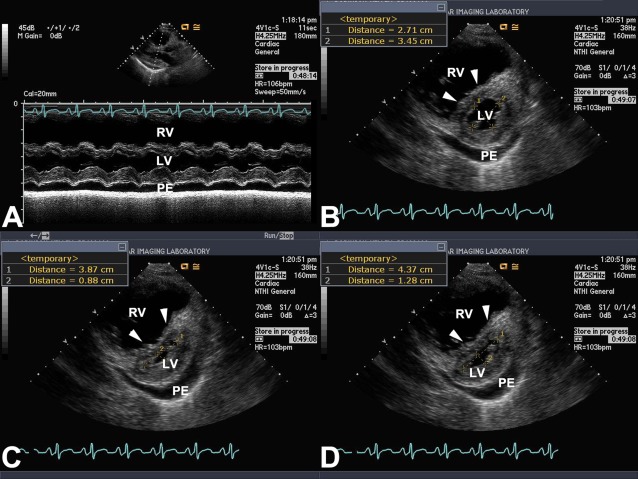
Echocardiography was performed 1.5 ± 7 weeks from the RHC. Left heart cavities were small, with biplane right-over-left atrial and ventricular volume ratios of 3.3 ± 3.4 and 2.3 ± 1.4, respectively. Eccentricity index was higher in early diastole than in end diastole (1.8 ± 0.6 vs 1.3 ± 0.2, P < .001) (Fig 1). Right ventricle was dilated in 98% of the patients (mild, 15%; moderate, 28%; moderate to severe, 20%; severe, 37%) and the same percentage had right ventricular (RV) dysfunction (mild, 15%; moderate, 27%; moderate to severe, 27%; and severe, 31%). Tricuspid regurgitation was present in 98% of the patients (trivial, 7%; mild, 29%; moderate, 32%; and severe, 32%). Tricuspid jet velocity and estimated RV systolic pressure (n = 59) were 4.4 ± 0.7 m/s and 86 ± 24.1 mm Hg, respectively. Other measured echocardiographic parameters are shown in Table 2.
Figure 1.
Eccentricity index. A, M mode at the level of LV papillary muscles in parasternal long axis view. There is marked RV dilation, early diastolic septal flattening, and PE. B-D, Measurements of eccentricity index in the parasternal short axis view at the level of the LV papillary muscles. B, End diastole; C, end systole; D, early diastole. Arrowheads in each panel point the interventricular septum. bpm = beats/min; HR = heart rate; LV = left ventricular; PE = pericardial effusion; RV = right ventricular.
Table 2.
—Two-Dimensional Echocardiographic Measurements in All Patients (N = 61) and in Those With (n = 55) or Without (n = 6) LV Diastolic Dysfunction
| Measurement | All Patients | Normal Diastolic Function (mean ± SD) | Diastolic Dysfunction Grade ≥ I (mean ± SD) | P Value (Mann-Whitney Test) |
| Interventricular septum, cm | 1.2 ± 0.9 | 1.1 ± 0.3 | 1.3 ± 1 | .72 |
| Posterior wall septum, cm | 1.2 ± 0.9 | 1.1 ± 0.3 | 1.2 ± 1 | .72 |
| LA diameter, cm | 3.5 ± 0.7 | 3.8 ± 0.5 | 3.4 ± 0.7 | .26 |
| LA volume index, mL/m2 | 18 ± 9 | 26 ± 12 | 18 ± 8 | .026 |
| RA volume index, mL/m2 | 48 ± 26 | 40 ± 12 | 49 ± 27 | .51 |
| RA/LA ratio | 3.3 ± 3.4 | 1.7 ± 0.7 | 3.5 ± 3.6 | .17 |
| LV EDV, mL | 48 ± 19 | 62 ± 21 | 47 ± 18 | .07 |
| RV EDV, mL | 94 ± 40 | 85 ± 19 | 95 ± 42 | .83 |
| RV/LV EDV ratio | 2.3 ± 1.4 | 1.6 ± 0.8 | 2.4 ± 1.5 | .12 |
| Eccentricity index (end systole) | 1.3 ± 0.2 | 2.2 ± 1.1 | 2.3 ± 0.9 | .65 |
| Eccentricity index (early diastole) | 2.3 ± 0.9 | 1.9 ± 0.8 | 1.8 ± 0.5 | .97 |
| Eccentricity index (end diastole) | 1.8 ± 0.6 | 1.2 ± 0.1 | 1.3 ± 0.3 | .20 |
Pericardial effusion was observed in eight patients (mild in five and moderate in three subjects). EDV = end-diastolic volume; LA = left atrium; RV = right ventricular. See Table 1 legend for expansion of other abbreviations.
LV Diastolic Dysfunction Evaluation:
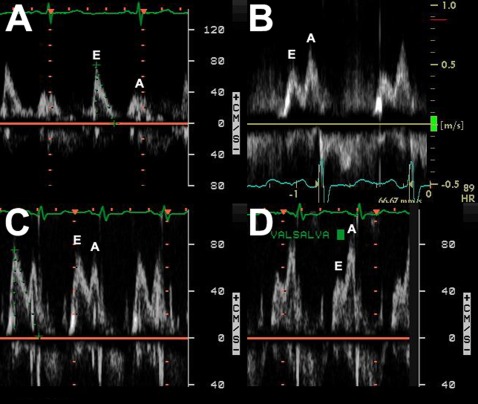
We observed normal diastolic function, impaired relaxation, and pseudonormalization pattern in 10%, 88%, and 2% of the patients, respectively (Fig 2). Diastolic parameters measured are shown in Table 3. There were no differences in age (P = .16), sex (P = .58), race (P = .8), or heart rate (P = .66) between patients with or without LV diastolic dysfunction.
Figure 2.
Diastolic function evaluated with pulse Doppler of the mitral inflow. A, Normal diastolic function (peak E wave velocity [E] over peak A wave velocity [A] ratio > 1). B, Impaired LV relaxation (E/A ratio < 1). C, Pseudonormal LV filling pattern (during normal respiration E/A ratio > 1). D, During Valsalva maneuver there is reversal of the ratio (E/A ratio < 1) due to LV preload reduction. See Figure 1 legend for expansion of abbreviations.
Table 3.
—Echocardiographic Evaluation of Diastolic Parameters
| Location | Measurement | Mean ± SD |
| Transmitral flow (n = 61) | Peak E velocity, cm/s | 58 ± 17 |
| Peak A velocity, cm/s | 74 ± 20 | |
| E/A ratio | 0.83 ± 0.3 | |
| E deceleration time, ms | 225 ± 55 | |
| Pulmonary vein flow (n = 46) | Peak S velocity, cm/s | 50 ± 10.3 |
| Peak D velocity, cm/s | 38 ± 10.5 | |
| S/D ratio | 1.4 ± 0.3 | |
| Peak A velocity | 29 ± 5.8 | |
| Tissue Doppler (n = 25) | Lateral e′ velocity, cm/s | 11 ± 4.5 |
| Lateral a′ velocity, cm/s | 12 ± 4.3 | |
| Lateral e′/a′ ratio | 1 ± 0.5 | |
| Lateral E/e′ ratio | 6.7 ± 3.9 | |
| Septal e′ velocity, cm/s | 8.1 ± 4.1 | |
| Septal a′ velocity, cm/s | 8.8 ± 3.1 | |
| Septal e′/a′ ratio | 1 ± 0.4 | |
| Septal E/e′ ratio | 9.7 ± 6.5 |
Few patients had mitral color M mode to measure propagation velocity or reliable echocardiographic evaluation to precisely measure intraventricular relaxation time. A = late diastolic; D = anterograde diastolic; E = early diastolic; S = systolic.
Comparison of LV Diastolic Function With 2D Echocardiographic Parameters:
Patients with LV diastolic dysfunction had significantly smaller left atrial (LA) volume index (P = .03) and a trend toward a lower LV end-diastolic volume (P = .07) than subjects with normal diastolic function (Table 2). No correlation was found between diastolic LV wall thickness and mitral flow velocities. Peak E velocity was directly associated with the LV end-diastolic volume (R = 0.37, P = .004), LA volume index (R = 0.49, P < .001), and inversely associated with RV over LV volume ratio (R = 0.35, P = .007) and right atrial (RA) over LA volume ratio (R = 0.37, P = .005). Peak A velocity was directly associated with LA volume index (R = 0.29, P = .03) and inversely associated with RA over LA volume ratio (R = 0.31, P = .02). E/A ratio directly related to LV end-diastolic volume (R = 0.3, P = .02).
Eccentricity index at late systole, early diastole, and late diastole did not vary significantly in patients with or without LV diastolic dysfunction. In addition, the eccentricity indexes were not associated with peak E velocity, peak A velocity, or E/A ratio. Patients with normal or mild RV dysfunction had a trend to have higher peak E velocity than subjects with more severe degrees of dysfunction (70 ± 22 vs 56.1 ± 15 cm/s, P = .06). Patients with normal to mild RV dilation had higher peak E velocities than patients with more severe degrees of dilation (73 ± 19 vs 56 ± 15, P = .02).
Comparison of LV Diastolic Parameters With NYHA Functional Class and Use of PAH-Targeted Therapies
When patients were divided by NYHA functional class (II-IV), no statistical difference was noted in diastolic parameters assessed by transmitral flow, pulmonary vein flow, or tissue Doppler (data not shown). The E/A ratio was 0.97, 0.81, and 0.79 for NYHA functional class II, III, and IV, respectively (P = .32).
Individuals receiving PAH-targeted therapy covered more distance on 6-min walk test than patients on no therapy (375 m vs 283 m, P = .003), suggesting less severe disease. Hemodynamic and echocardiographic variables did not significantly differ between the groups. Patients on PAH-targeted therapy had higher E/A ratio than patients on no therapy (0.91 vs 0.75, P = .04); nevertheless peak E velocity did not vary between the groups (no treatment, 54.4 cm/s vs on treatment, 60.6 cm/s; P = .15).
Relationship of LV Diastolic Function With Hemodynamics Parameters
Patients with LV diastolic dysfunction had worse hemodynamic profile than patients with normal diastolic function (lower cardiac index and higher RA pressure, transpulmonary gradient, and PVR) (Table 1). Peak E velocity was inversely associated with PVR (R = 0.26, P = .05), RA pressure (R = 0.27, P = .04), and RA minus PAOP (R = 0.34, P = .009). Peak E velocity was directly associated with cardiac index (R = 0.35, P = .007) but not with PAOP, mean pulmonary artery pressure (PAP), or transpulmonary gradient. Peak A velocity was not significantly associated with any hemodynamic parameter. E/A ratio was inversely associated with RA pressure (R = 0.31, P = .02) and RA pressure minus PAOP (R = 0.38, P = .003) and directly associated with cardiac index (R = 0.32, P = .014). Septal e′, septal and lateral a′, e′/a′, and E/e′ were not associated with RA pressure, mean PAP, cardiac index, transpulmonary gradient, or PVR.
Outcomes
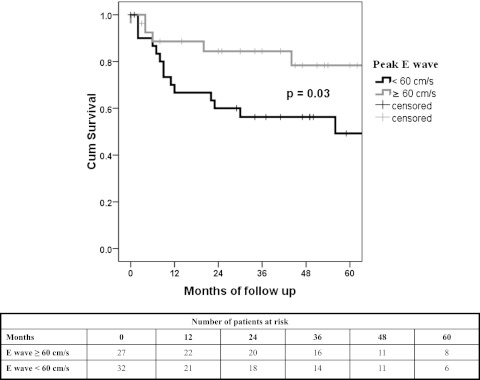
Mean survival (censored for lung transplantation, n = 5) was 54 (95% CI, 45.9-62.9) months. At 1, 2, 3, and 5 years the survival was 78%, 70%, 66%, and 60%, respectively. When adjusted by age and sex, we did not identify a difference in survival in patients with (any degree) or without LV diastolic dysfunction (P = .62). Transmitral flow E/A ratio showed a nonsignificant trend for predicting adjusted mortality (HR, 7.7; 95% CI, 0.92-50) per 0.1 decrease, P = .06). Peak E velocity was associated with adjusted mortality (HR, 1.5; 95% CI, 1.1-2 per 10 cm/s decrease; P = .015). When peak E velocity was dichotomized in ≥ 60 and < 60 cm/s, a peak E velocity < 60 cm/s was associated with adjusted mortality (HR, 2.7; 95% CI, 1.1-6.7; P = .028). Kaplan-Meier analysis with log-rank test is shown in Figure 3. Peak E velocity < 60 cm/s continued to be predictive of mortality after adjusting for RV function (normal, mild, moderate, moderate to severe, and severe impairment) (HR, 3.85; 95% CI, 1.3-11.3; P = .014).
Figure 3.
Kaplan-Meier survival analysis. Kaplan-Meier survival analysis using peak E wave velocity of < 60 and ≥ 60 cm/s. Log rank statistics showed P = .03. Cum = cumulative.
When adjusted for mean PAP, RA pressure, and cardiac index,13 E/A ratio was a significant predictor of mortality (P = .03). When adjusted by age, sex, 6-min walk distance, and cardiac output,11 E/A ratio and peak E velocity were associated with mortality (P = .04 and P = .01, respectively). None of the variables measured by tissue Doppler predicted adjusted mortality.
Discussion
In the present study we demonstrated that the majority of patients with severe idiopathic or heritable PAH have grade I (impaired LV relaxation) diastolic dysfunction. Other grades of LV diastolic dysfunction were very rare and a few patients with less advanced PAH disease have normal diastolic function. Transmitral flow peak E velocity, which is a simple echocardiographic measure to obtain, was inversely associated with the ratio of right-to-left atrial and ventricular chambers, PVR, and RA pressure. More importantly, peak E velocity was associated with mortality (whether lung transplantation was included as end point) even when adjusted for several variables known to predict survival in patients with PAH.
Several authors have previously noted the high prevalence of LV diastolic dysfunction in patients with PAH.1‐3,14 However, most of these studies included patients with chronic thromboembolic disease or a heterogenous group of patients with PAH. In our cohort, which consisted of carefully selected patients with hemodynamic criteria for PAH,9 we observed that nine out of 10 patients had impaired LV relaxation (grade I diastolic dysfunction) by echocardiographic criteria, with redistribution of the LV filling from early to late diastole. Only one patient had grade II (pseudonormal filling) LV diastolic dysfunction.
Impaired relaxation has been predominantly attributed to displacement of the interventricular septum toward the left ventricle, predominantly during early diastole, due to negative left-to-right transseptal pressure gradient,15,16 leading to decrease in LV compliance.1,2,14,17‐19 Other causes include an increase in RV tension that leads to prolonged RV myocardial shortening with abnormal relaxation of the interventricular septum that impairs RV systole and LV diastole,1,20 decrease in LV torsion,21 delay in early diastolic LV untwisting,22 reduction in the preload,4,5,21 diastolic asynchrony in the anterior-lateral or apical regions,23,24 and intrinsic disease of the left ventricle.25‐27
In our study, as well as others,2,14 septal displacement (measured as eccentricity index) was predominantly observed in systole and early diastole, possible decreasing LV compliance and explaining impaired LV relaxation. However, unlike other investigations,7 we did not observe a relationship between eccentricity indices and parameters of LV diastolic dysfunction. Interestingly, tissue Doppler analysis provided no data to suggest a myocardial abnormality.
In the present study, patients with normal diastolic function had less severe PAH, supported by a higher cardiac index and lower RA pressure and PVR. Moustapha et al2 showed that impaired LV relaxation was more commonly observed in patients with estimated RV systolic pressure by echocardiography of ≥ 60 mm Hg. The authors also reported a negative correlation between estimated RV systolic pressure and E/A ratio.2 Other studies have shown an inverse relationship between diastolic parameter and PAH severity.7 More importantly, this ratio was found to track with the hemodynamic improvements observed after pulmonary thromboendarterectomy or initiation of PAH-targeted therapy.18,28,29
A variety of clinical, echocardiographic, and hemodynamic variables with prognostic value have been identified, but very limited information exists about the usefulness of diastolic function parameters to predict outcomes in patients with PAH. Galiè et al29 found that patients with PAH treated with the endothelin receptor antagonist bosentan had improvement in mitral flow peak E velocity and E/A ratio. Ruan and Naguen3 described an increase in mitral E/A ratio, PV systolic filling fraction, septal e′, and lateral E/e′ ratio with PAH-targeted therapies. Eysmann et al8 found that mitral E/A ratio ≤ 1 correlated with poor survival in a small number of patients with relatively short follow-up. In our study, we found that E/A ratio was higher in patients receiving PAH-targeted therapy and that peak E velocity significantly predicted mortality when adjusted by age and sex and RV function. E/A ratio remained an independent predictor of mortality even when adjusted by variables suggested by the National Institutes of Health13 and Humbert et al.11
Strengths of this study are (1) rigorous selection of study participants to form a homogenous group of patients with advanced PAH diagnosed by RHC, (2) meticulous review of echocardiographic studies, and (3) precise data on outcome. Limitations include: (1) Doppler echocardiography was not performed simultaneously with RHC but within 2 months; (2) few Tricuspid Annular Plane Systolic Excursion (TAPSE)30 measurements were available for analysis; (3) the use of diuretics and/or vasodilators in a few patients could have temporally reduced the value of the PAOP, thus meeting the inclusion criteria; and (4) the study group consisted only of patients with advanced idiopathic or heritable PAH, thus, the results might not be generalizable to other pulmonary hypertension causes or milder disease. It is likely that a less severely affected cohort of patients with idiopathic or heritable PAH might have a lower prevalence of impaired relaxation.
In summary, impaired relaxation is expected in patients with advanced idiopathic and heritable PAH and should not motivate the diagnosis of left-sided heart disease (diastolic dysfunction). The observations noted in our study are clearly different from those observed in individuals with LV diastolic dysfunction, elevated PAOP, and enlarged left atrium, in whom pulmonary hypertension is likely a passive or reactive response to the elevated LV filling pressures. In patients with advanced idiopathic and heritable PAH, abnormal LV filling is more apt to be related to other mechanisms. The presence of grade II or III LV diastolic dysfunction is seldom seen in idiopathic and heritable PAH; thus, a diagnosis other than idiopathic PAH should be considered. The traditional belief that the LV diastolic dysfunction is associated with LA enlargement is not upheld by our observations in patients with idiopathic and heritable PAH, in whom a smaller LA is associated with impaired relaxation of the LV.
Conclusions
LV diastolic dysfunction of the impaired relaxation type is observed in the majority of patients with advanced idiopathic or heritable PAH. A decrease in transmitral flow peak E velocity is associated with worse hemodynamics and outcome.
Supplementary Material
Acknowledgments
Author contributions: Drs Tonelli and Dweik are guarantors of the paper, taking responsibility for the integrity of the work as a whole.
Dr Tonelli: contributed to the study design, data collection, institutional review board application, statistical analysis, interpretation of data, and writing and revision of the manuscript.
Dr Plana: contributed to the study design, data collection, interpretation of data, and writing and revision of the manuscript.
Dr Heresi: contributed to the study design, interpretation of data, and writing and revision of the manuscript.
Dr Dweik: contributed to the study design, institutional review board application, data collection, interpretation of data, and revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Plana is part of the speakers’ bureau of General Electric. Dr Heresi has received a board fee from Lung Rx United Therapeutics; his institution has received grants from Gilead Sciences Research Scholars Program and from Bayer HealthCare Pharmaceuticals. Drs Tonelli and Dweik have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- A
late diastolic
- D
anterograde diastolic
- E
early diastolic
- HR
hazard ratio
- LA
left atrial
- LV
left ventricular
- NYHA
New York Heart Association
- PAH
pulmonary arterial hypertension
- PAOP
pulmonary artery occlusion pressure
- PAP
pulmonary artery pressure
- PVR
pulmonary vascular resistance
- RA
right atrial
- RHC
right-sided heart catheterization
- RV
right ventricular
- S
systolic
Footnotes
Funding/Support: This study was funded by CTSA KL2 [Grant RR024990] (salary support to Dr Tonelli) from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Dr Dweik receives salary support from the NIH [Grants HL081064, HL107147, HL095181, and RR026231] and a Biomedical Research and Commercialization Program 08-049 Third Frontier Program grant from the Ohio Department of Development.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Stojnic BB, Brecker SJ, Xiao HB, Helmy SM, Mbaissouroum M, Gibson DG. Left ventricular filling characteristics in pulmonary hypertension: a new mode of ventricular interaction. Br Heart J. 1992;68(1):16–20. doi: 10.1136/hrt.68.7.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moustapha A, Kaushik V, Diaz S, Kang SH, Barasch E. Echocardiographic evaluation of left-ventricular diastolic function in patients with chronic pulmonary hypertension. Cardiology. 2001;95(2):96–100. doi: 10.1159/000047353. [DOI] [PubMed] [Google Scholar]
- 3.Ruan Q, Nagueh SF. Clinical application of tissue Doppler imaging in patients with idiopathic pulmonary hypertension. Chest. 2007;131(2):395–401. doi: 10.1378/chest.06-1556. [DOI] [PubMed] [Google Scholar]
- 4.Gurudevan SV, Malouf PJ, Auger WR, et al. Abnormal left ventricular diastolic filling in chronic thromboembolic pulmonary hypertension: true diastolic dysfunction or left ventricular underfilling? J Am Coll Cardiol. 2007;49(12):1334–1339. doi: 10.1016/j.jacc.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Marcus JT, Vonk Noordegraaf A, Roeleveld RJ, et al. Impaired left ventricular filling due to right ventricular pressure overload in primary pulmonary hypertension: noninvasive monitoring using MRI. Chest. 2001;119(6):1761–1765. doi: 10.1378/chest.119.6.1761. [DOI] [PubMed] [Google Scholar]
- 6.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22(2):107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Nagaya N, Satoh T, Uematsu M, et al. Shortening of Doppler-derived deceleration time of early diastolic transmitral flow in the presence of pulmonary hypertension through ventricular interaction. Am J Cardiol. 1997;79(11):1502–1506. doi: 10.1016/s0002-9149(97)00179-3. [DOI] [PubMed] [Google Scholar]
- 8.Eysmann SB, Palevsky HI, Reichek N, Hackney K, Douglas PS. Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation. 1989;80(2):353–360. doi: 10.1161/01.cir.80.2.353. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin VV, Archer SL, Badesch DB, et al. American College of Cardiology Foundation Task Force on Expert Consensus Documents American Heart Association American College of Chest Physicians American Thoracic Society, Inc Pulmonary Hypertension Association ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group American Society of Echocardiography’s Guidelines and Standards Committee European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 12.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 13.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 14.Louie EK, Rich S, Brundage BH. Doppler echocardiographic assessment of impaired left ventricular filling in patients with right ventricular pressure overload due to primary pulmonary hypertension. J Am Coll Cardiol. 1986;8(6):1298–1306. doi: 10.1016/s0735-1097(86)80300-x. [DOI] [PubMed] [Google Scholar]
- 15.Dong SJ, Smith ER, Tyberg JV. Changes in the radius of curvature of the ventricular septum at end diastole during pulmonary arterial and aortic constrictions in the dog. Circulation. 1992;86(4):1280–1290. doi: 10.1161/01.cir.86.4.1280. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka H, Tei C, Nakao S, et al. Diastolic bulging of the interventricular septum toward the left ventricle. An echocardiographic manifestation of negative interventricular pressure gradient between left and right ventricles during diastole. Circulation. 1980;62(3):558–563. doi: 10.1161/01.cir.62.3.558. [DOI] [PubMed] [Google Scholar]
- 17.Krayenbuehl HP, Turina J, Hess O. Left ventricular function in chronic pulmonary hypertension. Am J Cardiol. 1978;41(7):1150–1158. doi: 10.1016/0002-9149(78)90872-x. [DOI] [PubMed] [Google Scholar]
- 18.Dittrich HC, Chow LC, Nicod PH. Early improvement in left ventricular diastolic function after relief of chronic right ventricular pressure overload. Circulation. 1989;80(4):823–830. doi: 10.1161/01.cir.80.4.823. [DOI] [PubMed] [Google Scholar]
- 19.Gan CT, Lankhaar JW, Marcus JT, et al. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2006;290(4):H1528–H1533. doi: 10.1152/ajpheart.01031.2005. [DOI] [PubMed] [Google Scholar]
- 20.Marcus JT, Gan CT, Zwanenburg JJ, et al. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol. 2008;51(7):750–757. doi: 10.1016/j.jacc.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Puwanant S, Park M, Popović ZB, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation. 2010;121(2):259–266. doi: 10.1161/CIRCULATIONAHA.108.844340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi M, Borden WB, Nakai H, et al. Reduced and delayed untwisting of the left ventricle in patients with hypertension and left ventricular hypertrophy: a study using two-dimensional speckle tracking imaging. Eur Heart J. 2007;28(22):2756–2762. doi: 10.1093/eurheartj/ehm440. [DOI] [PubMed] [Google Scholar]
- 23.Bhargava V, Sunnerhagen KS. Left ventricular asynchrony in patients with pulmonary hypertension. J Appl Physiol. 1990;69(2):517–522. doi: 10.1152/jappl.1990.69.2.517. [DOI] [PubMed] [Google Scholar]
- 24.López-Candales A, Dohi K, Rajagopalan N, et al. Right ventricular dyssynchrony in patients with pulmonary hypertension is associated with disease severity and functional class. Cardiovasc Ultrasound. 2005;3:23. doi: 10.1186/1476-7120-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez A, Unruh H, Mink SN. Altered left ventricular chamber stiffness and isovolumic relaxation in dogs with chronic pulmonary hypertension caused by emphysema. Circulation. 1993;87(1):247–260. doi: 10.1161/01.cir.87.1.247. [DOI] [PubMed] [Google Scholar]
- 26.Davis KL, Mehlhorn U, Laine GA, Allen SJ. Myocardial edema, left ventricular function, and pulmonary hypertension. J Appl Physiol. 1995;78(1):132–137. doi: 10.1152/jappl.1995.78.1.132. [DOI] [PubMed] [Google Scholar]
- 27.Xie GY, Lin CS, Preston HM, et al. Assessment of left ventricular diastolic function after single lung transplantation in patients with severe pulmonary hypertension. Chest. 1998;114(2):477–481. doi: 10.1378/chest.114.2.477. [DOI] [PubMed] [Google Scholar]
- 28.Menzel T, Wagner S, Kramm T, et al. Pathophysiology of impaired right and left ventricular function in chronic embolic pulmonary hypertension: changes after pulmonary thromboendarterectomy. Chest. 2000;118(4):897–903. doi: 10.1378/chest.118.4.897. [DOI] [PubMed] [Google Scholar]
- 29.Galiè N, Hinderliter AL, Torbicki A, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41(8):1380–1386. doi: 10.1016/s0735-1097(03)00121-9. [DOI] [PubMed] [Google Scholar]
- 30.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.