Abstract
Mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome) is one of approximately 50 known lysosomal storage disorders. MPS VI is characterized by an absence or deficiency of N-acetylgalactosamine 4-sulfatase (arylsulfatase B) resulting in accumulation of dermatan sulfate. Prior to the availability of enzyme replacement therapy (ERT) the clinical management of MPS VI was limited to supportive care and allogeneic hematopoietic stem cell transplantation (HSCT); however, due to the rarity of this disease, little is known about the long-term outcomes of HSCT for MPS VI. The following retrospective study was performed using aggregate data gathered by the Center for International Blood and Marrow Transplant Research (CIBMTR) between 1982 and 2007 to determine survival probability for patients with MPS VI following allogeneic HSCT. This analysis identified 45 MPS VI patients with a median age of 5 years (range, 1-22 years) at the time they received an allogeneic HSCT. Cumulative incidence (95% CI) of acute graft vs. host disease at 100 days was 36% (21-53%). Probability of survival was 78% (65-89%) at 100 days and 66% (52-79%) at 1 and 3 years. While these data are based upon small numbers of recipients, they represent the largest series to date and may help clinicians assess the relative risks and benefits of currently available therapies.
Keywords: mucopolysaccharidosis VI, hematopoietic stem cell transplantation, enzyme replacement therapy, mortality, arylsulfatase B, galsulfase
1. INTRODUCTION
Mucopolysaccharidosis VI (MPS VI) or Maroteaux-Lamy syndrome (MIM # 253200) is one of approximately 50 known congenital lysosomal storage disorders. It is an autosomal recessive disorder caused by the absence or deficiency of N-acetylgalactosamine 4-sulfatase (arylsulfatase B) [1]. This enzyme is responsible for one in a series of steps involving the catabolism of glycosylaminoglycan dermatan sulfate. As glycosylaminoglycans (GAGs) are precursor components of connective tissue, they are widely distributed throughout the body. Diminished arylsulfatase B activity results in progressive accumulation of GAG dermatan sulfate with serious clinical consequences, particularly in connective tissues of the skin, heart valves, airway, and skeleton of these patients, [2, 3]. The incidence of MPS VI is highly variable among different populations, ranging from 1 in 43,261 live births in Turkish immigrants living in Germany [4] to 1 in 1,505,160 live births in Sweden [5]. About 1,100 individuals may be affected worldwide although far fewer are diagnosed [6].
1.1 Clinical Course
Similar to other lysosomal storage diseases, untreated MPS VI is a progressive disease. Symptoms worsen as GAG’s continues to accumulate in affected tissues. As patients with MPS VI have dissimilar amounts of residual arylsulfatase B activity, the age of onset of symptoms as well as the rate and severity of disease progression can vary widely. In patients with rapidly progressive disease, clinical manifestations, such as severe dysostosis multiplex, short stature, and respiratory complications generally become apparent during early childhood while patients with a less severe phenotype may not develop signs or symptoms until early adolescence or even adulthood [2]. As the effects of MPS VI are irreversible [7], it is generally accepted that clinical outcomes are better when diagnosis is early; and treatment is initiated earlier in the course of disease [8-10].
1.2 Treatment
Prior to the availability of enzyme replacement therapy (ERT) [11], the clinical management of MPS VI was limited to supportive care and allogeneic hematopoietic stem cell transplantation (HSCT) when a suitable donor was available; however, due to the rarity of this disease, little is known about the long-term outcomes of HSCT for the treatment of MPS VI. Morbidity and mortality estimates are derived from studies of patients with MPS I, a lysosomal storage disorder caused by the deficiency of alpha-L-iduronidase [12]. It is now estimated that over 400 patients with MPS I have undergone HSCT worldwide and a recent report described the clinical outcome over a 10-year period for 146 of these patients who were registered with the European Blood and Marrow Transplantation [12]. Six months following an initial transplant, the rate of ‘survival’ and ‘alive and engrafted’ status among MPS I patients was reported to be 85% and 56%, respectively. Similar to solid organ transplants, infection and rejection remain the major causes of morbidity and mortality following HSCT [13]. Other reports have also provided HSCT outcome data for large cohorts of MPS I patients suggesting improved outcomes, particularly with the use of cord blood grafts [14-16]. These studies further suggest that the current transplantation experience with MPS I is more favorable with up to 85% of patients alive and engrafted following transplantation [17, 18].
In contrast, a review of the literature revealed only 10 published reports that described the use of HSCT to treat 18 patients with MSP VI (Table 1). The first of these was a 13-year-old girl with severe MPS VI who was transplanted with bone marrow from an identical HLA-matched sibling in 1984 [19]. Following engraftment, arylsulfatase B activity1 in peripheral lymphocytes and granulocytes increased from 0.023 to 14.3 nmol/hr/mg protein after 600 days. The arylsulfatase B activity of the donor sibling was 12.5 nmol/hr/mg protein. Two years after HSCT, accumulated urinary dermatan sulfate GAG was no longer detectable hepatosplenomegaly was substantially decreased and cardiopulmonary function was normal. Visual acuity and joint mobility were also improved. The patient returned to school and continued to perform well in academic studies.
Table 1. Published Reports of Hematopoietic Stem Cell Transplantation for the Treatment of Mucopolysaccaroidosis VI.
| Author | Patients (N) |
Age at HSCT Mean (Range) |
Clinical Outcomes |
|---|---|---|---|
| Krivit et al., 1984. | 1 | 13 | Normal enzyme activity was reported after 2 years. Normal cardiopulmonary function, reduced hepatosplenomegaly, improved visual acuity and joint mobility, good academic performance. |
| Krivit, 1992. | 6 | 7.4 (1.6-13.8) | After a mean of 4.9 years (range, 2-12), improvements in hepatosplenomegaly, cardiovascular manifestations and coarse facial features were reported; however, skeletal defects remained. |
| Imaizumi et al., 1994. | 1 | 13.75 | After 47 months, improvements in hepatosplenomegaly, joint contractures, short stature, tight skin and quality of life were reported; however, no change in corneal clouding of dysostosis multiplex. |
| Alvaro et al., 1998. | 1 | 12 | Improvements in enzyme activity, airway disease, skin coarseness, hepatosplenomegaly, and chronic otorrhea were reported after 207 days. |
| Herskhovitz et al., 1999. | 4 | 6.5 (3-9.5) | After a mean of 4.5 years (range, 1-9), improved or stable cardiac manifestations, improved facial feature coarseness, joint mobility and posture were reported; however, skeletal abnormalities persisted or advanced. |
| Lee et al., 2000. | 1 | 5 | Normal enzyme activity, improved hepatosplenomegaly, facial and skin features, motor abilities, middle ear effusion, height; good academic performance after 15 months. |
| Krivit, 2004. | 1 | 13 | This is the same patient reported by Krivit in 1984. After 20 years, the patient enjoyed very good quality of life but skeletal abnormalities persist. |
| Lange et al., 2006. | 2 | 4.5 (2-7) | One patient died of graft-vs.-host disease, the other survived with unspecified disease improvements after 151 days. |
| Chen et al., 2008. | 1 | 1.5 | Enzyme activity increased and good general condition was reported after 28 months. |
| Wang et al., 2008. | 1 | 10 | After 12 years, the patient maintained enzyme activity, normal urinary GAG secretion, improvements in motor function, dyspnea, vertigo, snoring. |
For each report, the conditioning regimen was busulfan and cyclophosphamide except Alvaro et al., 1998 was busulfan, cyclophosphamide melphalan and antithymocyte globulin and Krivit et al., 1992, not stated.
The patient described above by Krivit et al. (1984) is known to have survived for at least 20 years with a productive life despite persistent skeletal abnormalities [20] and another patient with MPS VI demonstrated a good clinical outcome 12 years following HSCT [21]. Still, much less is known about the long-term outcomes of HSCT for the treatment of MPS VI when compared to MPS I. Although most reports describe satisfactory outcomes, it is difficult to draw conclusions due to the small number of transplanted patients with MPS VI and the limited amount of time these patients have been followed post transplant. The current analysis described here was performed using aggregate data collected by the Center for International Blood and Marrow Transplant Research (CIBMTR) to determine the probability of overall survival and incidence of morbidity of patients with MPS VI following allogeneic HSCT.
2. METHODS
2.1 Data Source
A retrospective analysis was performed using data submitted to the CIBMTR, a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP). Established in 2004, CIBMTR comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous HSCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis, Minnesota. Participating centers are required to report all transplants consecutively and compliance is monitored by on-site audits. Patients are followed longitudinally with annual follow-up. Computerized checks for discrepancies, physician review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule HIPAA (Health Insurance Portability and Accountability Act) as a Public Health Authority and in compliance with all applicable regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Boards of the National Marrow Donor Program and the Medical College of Wisconsin since 1985.
CIBMTR collects data at two levels: Transplant Essential Data (TED) and Comprehensive Report Form (CRF) data. TED data include disease type, age, sex, pre-transplant disease stage and chemotherapy-responsiveness, date of diagnosis, graft type (bone marrow-derived stem cells and/or blood-derived stem cells), high-dose conditioning regimen, post-transplant disease progression and survival, development of a new malignancy and cause of death. All CIBMTR teams contribute TED data. More detailed disease and pre- and post-transplant clinical information are collected on a subset of registered patients selected for CRF data by a weighted randomization scheme. TED and CRF level data are collected pre-transplant, 100 days and six months post-transplant and annually thereafter or until patient death.
2.2 Patient Selection
All MPS VI patients who underwent an allogeneic HSCT and voluntarily submitted data to the CIBMTR between 1982 and 2007 were included in this analysis.
2.3 Outcomes and Definitions
Primary outcomes were 1) overall survival, 2) neutrophil recovery defined as time to achieving an absolute neutrophil count of ≥ 500 neutrophils/mL sustained for 3 consecutive days, 3) incidence of grade 2 to 4 acute graft vs. host disease (GvHD) [22] and 4) presence or absence of chronic GvHD [23].
2.4 Statistical Analysis
Descriptive statistics were used to summarize patient-, disease-, and transplantation-related variables. Univariate probabilities of overall survival were calculated using the Kaplan-Meier estimator with variance estimated by Greenwood’s formula [24]. Probabilities of neutrophil recovery and acute and chronic GvHD were generated using cumulative incidence curves to accommodate competing risks [24]. All analyses were performed by the Statistical Center of the CIBMTR using SAS Version 9.1 statistical package (SAS Institute, Cary, NC).
3. RESULTS
3.1 Patient Characteristics
The analysis identified 45 patients with MPS VI who received an allogeneic HSCT. The country of origin for these patients was the United States (N=27; 60%), Saudi Arabia (N=8; 18%), Brazil (N=3; 7%), England (N=3; 7%), China (N=2; 4%), Australia (N=1; 2%) and Japan (N=1; 2%). The median age at HSCT transplantation was 5 years (range, 1-22 years). Thirty-nine patients (87%) were between the ages of 0-9 years at the time of their transplant (Table 2).
Table 2. Characteristics of 45 Patients with MPS VI Undergoing Hematopoietic Stem Cell Transplantation at 19 Transplant Centers.
| Patient characteristic | N | (Median, range) (%) |
|---|---|---|
| Median Age at diagnosis (years) | 44 | (1, <1-9) |
| 0 – 2 | 30 | 68% |
| 3 – 5 | 10 | 23% |
| 6 – 9 | 4 | 9% |
| Median Age at Transplant (years) | 45 | (5, 1-22) |
| 0 – 9 | 39 | 87% |
| 10 – 19 | 4 | 9% |
| 20 – 29 | 2 | 4% |
| Gender | 45 | |
| Male | 24 | 53% |
| Female | 21 | 47% |
| Year of transplant | 45 | |
| 1982-1989 | 3 | 7% |
| 1990-1994 | 5 | 11% |
| 1995-1999 | 21 | 47% |
| 2000-2007 | 16 | 36% |
| Graft type | 45 | |
| Bone marrow | 34 | 76% |
| Peripheral blood | 1 | 2% |
| Cord blood | 10 | 22% |
| Graft type, Unrelated donors | 27 | |
| Bone marrow | 18 | 67% |
| Cord blood | 9 | 33% |
| Conditioning regimen | 44 | |
| Cy + TBI* ± other | 6 | 13% |
| Bu + Cy ± other | 30 | 67% |
| Flu + Mel** | 1 | 2% |
| Other | 7 | 16% |
| Donor | 45 | |
| HLA-identical sibling | 15 | 33% |
| Other related | 3 | 7% |
| Unrelated | 27 | 60% |
| Country | 45 | |
| United States | 27 | 60% |
| Australia | 1 | 2% |
| Brazil | 3 | 7% |
| England | 3 | 7% |
| Japan | 1 | 2% |
| Saudi Arabia | 8 | 18% |
| China | 2 | 4% |
| Median Follow-up of Survivors, Months (range), | 29 | (52, 3-267) |
TBI, Total Body Irradiation.
Fludarabine + Melphalan.
The most commonly reported conditioning regimen was cyclophosphamide + busulfan (N=30; 67%). Bone marrow was the most common graft type (N=31; 74%) followed by cord (N=10; 24%) and peripheral blood (N=1; 2%). Most patients received an HSCT from an unrelated donor (N=27; 60%) while 15 (33%) were from an HLA-identical sibling (Table 2). Among the 21 unrelated donors with comprehensive research data available, 20 were T-cell depleted and one was undetermined.
3.2 Overall Survival
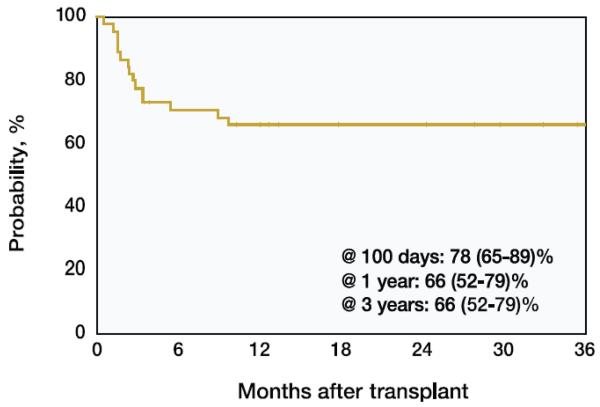
The probability of survival (95% CI) was 78% (65-89%) at 100 days, 66% (52-79%) at 1 year and remained 66% (52-79%) at 3 years post-transplant (Table 3; Figure 1). Although the sample size was limited, survival rates appeared to be unaffected by the year of transplantation. Due to the relatively small number of patients, the survival rate prior to 1995 was numerically but not significantly greater than that after 1995. The most common cause of death was infection (44%) and organ failure (31%) (Table 4).
Table 3. Overall survival of MPS VI patients receiving allogeneic hematopoietic stem cell transplantation.
| Outcome Event | N | Probability (95% CI) |
|---|---|---|
| Overall Survival a | ||
| 100 days | 45 | 78% (65-89) |
| 1 yr | 45 | 66% (52-79) |
| 3 yrs | 45 | 66% (52-79) |
| ≤ 1995 | 14 | |
| 100 days | 93% (74-100) | |
| 1 yr | 79% (54-95) | |
| 3 yrs | 79% (54-95) | |
| ≥1996 | 31 | |
| 100 days | 71% (54-85) | |
| 1 yr | 61% (43-77) | |
| 3 yrs | 61% (43-77) | |
| Acute GvHD Grades 2-4 b | ||
| 100 days | 34 | 36% (21-53) |
| Acute GvHD Grades 3-4 b | ||
| 100 days | 34 | 21% (9-36) |
| Chronic GvHDb | ||
| 1 yr | 34 | 13% (4-28) |
| 2 yrs | 34 | 17% (6-33) |
| Absolute Neutrophil Count Recovery b | ||
| 28 days | 33 | 88% (75-97) |
| 35 days | 33 | 91% (79-98) |
Analysis from registration
Analysis from research
Figure 1. Probability of Overall Survival for Parents with MPS VI Receiving Allogeneic HSCT and Registered with the CIBMTR since 1982.
Table 4. Cause of death.
| Cause | N (%) |
|---|---|
| Graft Rejection/Graft Failure | 1 ( 6) |
| Infection | 7 (44) |
| Acute GvHD | 1 ( 6) |
| Secondary Malignancy | 1 ( 6) |
| Organ Failure | 5 (31) |
| Other Cause | 1 ( 6) |
3.3 Neutrophil Recovery
Thirty-three patients had absolute neutrophil count recovery information available. The cumulative incidence of recovery (95% CI) at 28 and 35 days were 88% (75-97%) and 91% (79-98%), respectively.
3.4 Acute and Chronic Graft vs. Host Disease
Information was available for 34 patients that developed acute Grades 2-4 (N=15), and Grades 3-4 (N=34) and chronic (N=19) GvHD. Cumulative incidence (95% CI) of acute Grades 2-4 and Grades 3-4 at 100 days was 36% (21-53%) and 21% (9-36%), respectively. The organs most commonly affected by GvHD were the skin (93%) followed by the liver (73%) and gut (47%). Cumulative incidence of chronic GvHD at 2 years was 17% (6%-33%).
4. DISCUSSION
This analysis represents the largest experience to date of the use of HSCT as therapy to prevent the clinical manifestations of MPS VI. A survey of 121 untreated patients with MPS VI older than 4 years of age revealed a broad range of symptoms including short stature, large head with coarse facial features, flat nasal bridge, enlarged tongue, several skeletal and joint abnormalities, valvular heart disease, hernias, weakness, hepatosplenomegaly, airway obstruction, chronic ear and respiratory infections, carpal tunnel syndrome, corneal clouding, poor vision and blindness [6]. Consequently, patients born with rapidly progressing MPS VI usually suffer severe disability and diminished life expectancy. In the absence of ERT or HSCT, life expectancy for rapidly progressing patients is limited to the 2nd or 3rd decades [2] while slowly progressing patients have a life expectancy into the 5th or 6th decade of life [7, 25]. Following engraftment of HSCT, increased arylsulfatase B activity can be demonstrated and there is evidence that the overall clinical condition of these patients improves. Long-term improvements in facial dysmorphism, hepatosplenomegaly, joint mobility, and cardiac manifestations has been demonstrated in MPS VI patients following HSCT [26-28]; however, MPS VI patients are not known to have primary cognitive impairment due to central nervous system GAG storage, skeletal disease known as dysostosis multiplex tends to persist or progress despite HSCT and visual manifestations show only limited beneficial effects [21, 29-31].
As complications may include infections, GvHD, rejection or low donor chimerism, the risks and benefit of HSCT must be carefully weighed for each disease. For patients with MPS I, HCST is the only means to deliver enzyme into the brain to prevent the neurologic manifestations of the disease. In contrast, HSCT does not ensure enzyme penetration into distal tissues such as the bone and eye of patients with MPS VI [21, 29-32].
The 45 patients described in this report represent the largest available cohort of MPS VI patients undergoing HSCT. The overall 3-year survival rate in these patients was 66% which is comparable with survival outcomes in MPS I patients treated with HSCT [14, 16, 33]; however, the results presented here are only representative of those patients selected for HSCT and it is possible that there is a selection bias for patients undergoing transplantation. For example, survival rates may be increased by choosing patients who are healthier and more likely to tolerate HCST or decreased by selecting patients who are poor candidates but lack other treatment alternatives. It is also feasible that the use of enzyme replacement prior to transplantation could decrease the morbidity and/or mortality of transplant, as is being explored for transplantation for MPS I [17, 18]. However, this issue will currently be difficult to explore in MPS VI, as few transplants are being done at this time due to availability of enzyme replacement as therapy. Other study limitations include a lack of information regarding post-transplant chimerism or subsequent therapy after HSCT. In addition, as 64% of patients in this analysis were transplanted prior to 2000, it is possible that the reported outcomes may be less advantageous than would be expected in a more recent cohort.
This analysis only includes patients whose data have been voluntarily submitted to the CIBMTR and although it is the largest report of its kind to date, it may not fully represent the HSCT experience in the entire patient population. Data available at the time of analysis did not include information about the use of ERT before or after HSCT for those patients to whom it may have been available. Pre-transplant ERT may be important for patients waiting for suitable marrow donors [31, 32] while some patients receive ERT in addition to HSCT [17, 34]. As no studies have compared the efficacy of HSCT to ERT, further research is needed to assist clinicians who must weigh the risks and benefits of all treatment approaches and determine best therapy for individual patients [21, 29-32].
5. CONCLUSION
Forty-five (45) patients with mucopolysaccharidosis VI received an allogeneic HSCT over a 25-year period. Acute graft vs. host disease occurred in 44% of patients with evaluable data. The probability of survival (95% CI) was 78% (65-89%) at 100 days, 66% (52-79%) at 1 and 3 years. While limited, these data may help clinicians assess the relative risks and benefits of currently available therapies.
6. ACKNOWLEDGEMENT
The data analysis described in this report was funded by BioMarin Pharmaceutical Inc. Sean Turbeville and Helen Nicely are employees and stockholders of BioMarin Pharmaceutical Inc. The authors acknowledge the assistance of Dr. Carl S. Hornfeldt during the preparation of this manuscript. The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute, the National Heart, Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases; a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
4-methylumbelliferyl sulfate used as the assay substrate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conception and design: Turbeville, Rizzo
Provision of study materials or patients: Orchard, Bonfim, Al-Seraihy
Collection and assembly of data: Pedersen
Data analysis and interpretation: Turbeville, Nicely, Rizzo, Pedersen, Orchard, Bonfim, Al-Seraihy
Manuscript writing: Turbeville, Rizzo, Pedersen
Final approval of manuscript: Turbeville, Nicely, Rizzo, Pedersen, Orchard, Bonfim, Al-Seraihy
REFERENCES
- [1].Litjens T, Baker E, Beckmann K, Morris C, Hopwood J, Callen D. Chromosomal localization of ARSB, the gene for human N-acetylgalactosamine-4-sulphatase. Hum Genet. 1989;82:67–68. doi: 10.1007/BF00288275. [DOI] [PubMed] [Google Scholar]
- [2].Neufeld E, Muenzer J. The mucopolysaccharidoses. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York, NY: 2001. pp. 3421–3452. [Google Scholar]
- [3].Staretz-Chacham O, Lang T, LaMarca M, Krasnewich D, Sidransky E. Lysosomal storage disorders in the newborn. Pediatrics. 2009;123:1191–1207. doi: 10.1542/peds.2008-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baehner F, Schmiedeskamp C, Krummenauer F, Miebach E, Bajbouj M, Whybra C, Kohlschutter A, Kampmann C, Beck M. Cumulative incidence rates of the mucopolysaccharidoses in Germany. J Inherit Metab Dis. 2005;28:1011–1017. doi: 10.1007/s10545-005-0112-z. [DOI] [PubMed] [Google Scholar]
- [5].Malm G, Lund A, Mansson J, Heiberg A. Mucopolysaccharidoses in the Scandinavian countries: incidence and prevalence. Acta Paediatr. 2008;97:1577–1581. doi: 10.1111/j.1651-2227.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- [6].Swiedler S, Beck M, Bajbouj M, Giugliani R, Schwartz I, Harmatz P, Wraith J, Roberts J, Ketteridge D, Hopwood J, Guffon N, Sá Miranda M, Teles E, Berger K, Piscia-Nichols C. Threshold effect of urinary glycosaminoglycans and the walk test as indicators of disease progression in a survey of subjects with Mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) Am J Med Genet. 2005;134:144–150. doi: 10.1002/ajmg.a.30579. [DOI] [PubMed] [Google Scholar]
- [7].Valayannopoulos V, Nicely H, Harmatz P, Turbeville S. Mucopolysaccharidosis VI Orphanet. J Rare Dis. 2010;5:1–20. doi: 10.1186/1750-1172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meikle P, Hopwood J. Lysosomal storage disorders: emerging therapeutic options require early diagnosis. Eur J Pediatr. 2003;162:S34–37. doi: 10.1007/s00431-003-1348-y. [DOI] [PubMed] [Google Scholar]
- [9].Wenger D, Coppola S, Liu S. Insights into the diagnosis and treatment of lysosomal storage diseases. Arch Neurol. 2003;60:322–328. doi: 10.1001/archneur.60.3.322. [DOI] [PubMed] [Google Scholar]
- [10].Giugliani R, Harmatz P, Wraith J. Management guidelines for mucopolysaccharidosis VI. Pediatrics. 2007;120:405–418. doi: 10.1542/peds.2006-2184. [DOI] [PubMed] [Google Scholar]
- [11].BioMarin Pharmaceutical Naglazyme® (galsulfase) Solution for Intravenous Infusion Prescribing Information. 2005 [Google Scholar]
- [12].Boelens J, Wynn R, O’Meara A, Veys P, Bertrand Y, Souillet G, Wraith J, Fischer A, Cavazzana-Calvo M, Sykora K, Sedlacek P, Rovelli A, Uiterwaal C, Wulffraat N. Outcomes of hematopoietic stem cell transplantation for Hurler’s syndrome in Europe: a risk factor analysis for graft failure Bone Marrow. Transplant. 2007;40:225–233. doi: 10.1038/sj.bmt.1705718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].del Pozo J. Update and actual trends on bacterial infections following liver transplantation World. J Gastroenterol. 2008;14:4977–4983. doi: 10.3748/wjg.14.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Staba S, Escolar M, Poe M, Kim Y, Martin P, Szabolcs P, Allison-Thacker J, Wood S, Wenger D, Rubinstein P, Hopwood J, Krivit W, Kurtzberg J. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- [15].Pastores G, Arn P, Beck M, Clarke J, Guffon N, Kaplan P, Muenzer J, Norato D, Shapiro E, Thomas J, Viskochil D, Wraith J. The MPS I registry: design, methodology, and early findings of a global disease registry for monitoring patients with Mucopolysaccharidosis Type I. Mol Genet Metab. 2007;91:37–47. doi: 10.1016/j.ymgme.2007.01.011. [DOI] [PubMed] [Google Scholar]
- [16].Boelens J, Rocha V, Aldenhoven M, Wynn R, O’Meara A, Michel G, Ionescu I, Parikh S, Prasad V, Szabolcs P, Escolar M, Gluckman E, Cavazzana-Calvo M, Kurtzberg J. Risk factor analysis of outcomes after unrelated cord blood transplantation in patients with Hurler syndrome Biol Blood Marrow. Transplant. 2009;15:618–625. doi: 10.1016/j.bbmt.2009.01.020. [DOI] [PubMed] [Google Scholar]
- [17].Tolar J, Grewal S, Bjoraker K, Whitley C, Shapiro E, Charnas L, Orchard P. Combination of enzyme replacement and hematopoietic stem cell transplantation as therapy for Hurler syndrome Bone Marrow. Transplant. 2008;41:531–535. doi: 10.1038/sj.bmt.1705934. [DOI] [PubMed] [Google Scholar]
- [18].Wynn R, Wraith J, Mercer J, O’Meara A, Tylee K, Thornley M, Church H, Bigger B. Improved metabolic correction in patients with lysosomal storage disease treated with hematopoietic stem cell transplant compared with enzyme replacement therapy. J Pediatr. 2009;154:609–611. doi: 10.1016/j.jpeds.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [19].Krivit W, Pierpont M, Ayaz K, Tsai M, Ramsay N, Kersey J, Weisdorf S, Sibley R, Snover D, McGovern M. Bone-marrow transplantation in the Maroteaux-Lamy syndrome (mucopolysaccharidosis type VI). Biochemical and clinical status 24 months after transplantation. N Engl J Med. 1984;311:1606–1611. doi: 10.1056/NEJM198412203112504. [DOI] [PubMed] [Google Scholar]
- [20].Krivit W. Allogeneic stem cell transplantation for the treatment of lysosomal and peroxisomal metabolic diseases Springer Semin. Immunopathol. 2004;26:119–132. doi: 10.1007/s00281-004-0166-2. [DOI] [PubMed] [Google Scholar]
- [21].Wang C, Hwu W, Lin K. Long-term follow-up of a girl with Maroteaux-Lamy syndrome after bone marrow transplantation World. J Pediatr. 2008;4:152–154. doi: 10.1007/s12519-008-0031-9. [DOI] [PubMed] [Google Scholar]
- [22].Przepiorka D, Weisdorf D, Martin P, Klingemann H, Beatty P, Hows J, Thomas E. 1994 Consensus Conference on Acute GVHD Grading Bone Marrow. Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- [23].Filipovich A, Weisdorf D, Pavletic S, Socie G, Wingard J, Lee S, Martin P, Chien J, Przepiorka D, Couriel D, Cowen E, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo J, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers M. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report Biol Blood Marrow. Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [24].Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. Springer-Verlag; 2003. [Google Scholar]
- [25].Scarpa M, R B, Fiumara A, Astarita L, Parenti G, Rampazzo A, Sala S, Sorge G, Parini R. Mucopolysaccharidosis VI: The Italian experience. Eur J Pediatr. 2009;168:1203–1206. doi: 10.1007/s00431-008-0910-z. [DOI] [PubMed] [Google Scholar]
- [26].Herskhovitz E, Young E, Rainer J, Hall C, Lidchi V, Chong K, Vellodi A. Bone marrow transplantation for Maroteaux-Lamy syndrome (MPS VI): long-term follow-up. J Inherit Metab Dis. 1999;22:50–62. doi: 10.1023/a:1005447232027. [DOI] [PubMed] [Google Scholar]
- [27].Malatack J, Consolini D, Bayever E. The status of hematopoietic stem cell transplantation in lysosomal storage disease. Pediatr Neurol. 2003;29:391–403. doi: 10.1016/j.pediatrneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [28].Beck M. New therapeutic options for lysosomal storage disorders: enzyme replacement, small molecules and gene therapy. Hum Genet. 2007;121:1–22. doi: 10.1007/s00439-006-0280-4. [DOI] [PubMed] [Google Scholar]
- [29].Boelens J. Trends in haematopoietic cell transplantation for inborn errors of metabolism. J Inherit Metab Dis. 2006;29:413–420. doi: 10.1007/s10545-005-0258-8. [DOI] [PubMed] [Google Scholar]
- [30].Orchard P, Blazar B, Wagner J, Charnas L, Krivit W, Tolar J. Hematopoietic cell therapy for metabolic disease. J Pediatr. 2007;151:340–346. doi: 10.1016/j.jpeds.2007.04.054. [DOI] [PubMed] [Google Scholar]
- [31].Rovelli A. The controversial and changing role of haematopoietic cell transplantation for lysosomal storage disorders: an update Bone Marrow. Transplant. 2008;41:S87–89. doi: 10.1038/bmt.2008.62. [DOI] [PubMed] [Google Scholar]
- [32].Prasad V, Kurtzberg J. Transplant outcomes in mucopolysaccharidoses Semin. Hematol. 2010;47:59–69. doi: 10.1053/j.seminhematol.2009.10.008. [DOI] [PubMed] [Google Scholar]
- [33].Peters C, Balthazor M, Shapiro E, King R, Kollman C, Hegland J, Henslee-Downey J, Trigg M, Cowan M, Sanders J, Bunin N, Weinstein H, Lenarsky C, Falk P, Harris R, Bowen T, Williams T, Grayson G, Warkentin P, Sender L, Cool V, Crittenden M, Packman S, Kaplan P, Lockman L, Anderson J, Krivit W, Dusenbery K, Wagner J. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- [34].Grewal S, Wynn R, Abdenur J, Burton B, Gharib M, Haase C, Hayashi R, Shenoy S, Sillence D, Tiller G, Dudek M, van Royen-Kerkhof A, Wraith J, Woodard P, Young G, Wulffraat N, Whitley C, Peters C. Safety and efficacy of enzyme replacement therapy in combination with hematopoietic stem cell transplantation in Hurler syndrome. Genet Med. 2005;7:143–146. doi: 10.1097/01.gim.0000154299.22120.6a. [DOI] [PubMed] [Google Scholar]