Abstract
PlexinsA1–A4 participate in class 3 semaphorin signaling as co-receptors to neuropilin 1 and 2, PlexinA4 being the latest member of the PlexinA subfamily to be identified. Little is known about the cellular distribution of PlexinA4 in the spinal cord and dorsal root ganglion (DRG). Here, immunohistochemical studies using antibodies to PlexinA4 revealed immunolabeling in neurons in both dorsal and, to a greater extent, ventral horns of the spinal cord. Ventral horn PlexinA4 positive neurons exhibited morphology, size, and location consistent with both motor neurons and interneurons. Labeling was found in motor axons exiting through the ventral roots, and more widespread labeling was observed in ascending and descending white matter tracts. Within the DRG, immunostaining was observed in neuronal cell bodies as well as the central and peripheral processes of these cells. PlexinA4 is expressed in the peripheral nervous system where its expression is regulated upon nerve injury. This is the first detailed description of the cellular and subcellular distribution of PlexinA4 in the adult spinal cord and DRG, and it will set the basis for future studies on the potential role of PlexinA4 in regeneration and repair of the adult central and peripheral nervous system.
Keywords: Semaphorin, axon guidance molecule, Rattus norvegicus, spinal cord injury, protein distribution, plexin-a4, plxna4, plexa4
INTRODUCTION
Class 3 semaphorins are axon guidance molecules involved in diverse cellular processes including axon pruning and repulsion, dendritic attraction and branching, growth cone collapse, regulation of cell migration, and vascular remodelling (Bussolino et al., 2006; Potiron and Roche, 2005; Waimey et al., 2008). Further, class 3 semaphorins may be critical inhibitors of regeneration (Giger et al., 2010; Gross et al., 2007). For example, it was recently found that semaphorin 3A exerts an inhibitory effect on the regrowth of nociceptive axons into the adult rat spinal cord (Tang et al., 2007; Tang et al., 2004).
PlexinA4, the most recently identified member of the PlexinA family (currently known to consist of PlexinA1–4), is a crucial receptor mediating the effects of multiple semaphorins (Yazdani and Terman, 2006). In semaphorin 3 signalling, PlexinA4 acts as a critical signal transducer by way of its interaction with the ligand-binding class 3 semaphorin co-receptors neuropilin (Nrp) 1 and 2 (Yaron et al., 2005), that lack intracellular domains and thus the ability to solely transmit an extracellular signal into a cell (Nakamura et al., 1998). Further, PlexinA4 serves as the receptor and signal transducer for membrane-bound semaphorin 6A and 6B, which are particularly important in the development of the corticospinal tract and the layered organization of hippocampal mossy fibers (Faulkner et al., 2008b; Runker et al., 2008; Suto et al., 2005).
Mouse PlexinA4, originally characterized by Suto et al. (Suto et al., 2003), is a type 1 transmembrane protein consisting of 1890 amino acids (aa) and includes what is likely to be a signal sequence (aa 1–20), a transmembrane domain (aa 1230–1255), and 12 extracellular, N-linked glycosylation sites. There are also domains within the PlexinA4 protein that are common to all members of the PlexinA family: an extracellular Sema domain (aa 36–554), three extracellular “Met-related sequences” (MRS)/cysteine clusters, three extracellular glycine-proline repeats, intracellular SP domains, and a putative intracellular tyrosine kinase phosphorylation site (aa 1804–1811) (Suto et al., 2003). While the rat PlexinA4 protein has not been characterized in this much detail, a BLAST search with the sequence for rat PlexinA4 obtained from the Treefam database (Treefam ID: ENSRNOG00000013072) confirms that rat and mouse PlexinA4 share 100% sequence similarity.
In contrast to the moderate amount of functional information available on PlexinA4, little is known about the localization of PlexinA4 protein. Okada et al. observed its co-expression with NG-2 or O4 antigen in cells of primary mouse cortical cultures (Okada et al., 2007). Suto et al., as part of the initial characterization of PlexinA4, observed the protein in the hippocampi of P1 and P10 mice, particularly in different bundles of mossy fibers and in the dentate hilus (Suto et al., 2003). Additionally, in a previous study we characterized the distribution of PlexinA4 protein throughout the entire adult rat brain (Gutekunst et al., 2010a). All other studies that have addressed PlexinA4 localization have focused on the distribution of the mRNA (Faulkner et al., 2008b; Low et al., 2008; Perala et al., 2005; Runker et al., 2008; Schwarz et al., 2008; Spinelli et al., 2007).
Even less is known about the distribution of PlexinA4 in the spinal cord. Bron et al. has demonstrated the presence of PlexinA4 mRNA in the embryonic chicken spinal cord (Bron et al., 2007), and Suto et al. have observed high levels of PlexinA4 mRNA in the motor neurons and dorsal root ganglia (DRG) of embryonic mice, as well as moderate levels of the mRNA in the dorsal horn of the embryonic spinal cord (Suto et al., 2003). In the present study, we describe the spatial distribution of PlexinA4 in the rat spinal cord, as well as its co-expression with markers of motor neurons throughout the adult spinal cord. We also present evidence of PlexinA4 expression in peripheral nerve axons and at the neuromuscular junction as well as its regulation in response to femoral nerve cut injury.
MATERIALS AND METHODS
2.1 Animals
Adult (2 month old) Sprague–Dawley rats (200–250g) and timed-pregnant female rats were obtained from Charles River (Wilmington, MA). Adult SLICK-A mice were obtained from Jackson Laboratories (West Chester, PA). All animals were maintained in a 12/12 light/dark cycle with ad libitum access to food and water. All protocols involving animals were approved by the Emory University Institutional Animal Care and Use Committee (IACUC) and conform to NIH guidelines. Young adult (8–12 week old) C57Bl/6 mice were obtained from Charles River (Wilmington, MA). Mice were maintained in a 12/12 light/dark cycle with ad libitum access to food and water. All protocols involving animals were approved by the University of Calgary Animal Care Committee in accordance with the policies of the Canadian Council of Animal Care (CCAC).
2.2 Antibodies and plasmids
Rabbit polyclonal antibodies specific for PlexinA4 were used at 1:500 (ab39350-200; Abcam, Cambridge, MA) except when indicated otherwise. Mouse monoclonal antibodies specific for the neuronal marker NeuN were used at 1:100 (MAB377; Chemicon/Millipore, Billerica, MA). Mouse monoclonal specific for Myc-Tag (9B11) was used at 1:2000 (2276; Cell Signaling Technology, Danvers, MA). Mouse anti-Tuj1 (MMS-435P; Covance, Berkeley, CA) and anti-NF200 (NO142, Sigma Aldrich, Oakville, Canada) antibodies were used at 1:500. Mouse anti-glial fibrillary acidic protein (GFAP) antibodies were used at 1:500 (AB5804; Chemicon/Millipore). Goat anti-choline acetyl transferase (ChAT) antibodies were used at 1:100 (AB114P; Chemicon/Millipore). Plasmids pAG/mycPlexinA1(14-4-E) expressing mouse myc/His-PlexinA1 and pCAGGS/Sema3Ass-Myc-plxnA4 expressing mouse myc-PlexinA4 were generously provided by Dr. Jonathan Epstein and Dr. Fumikazu Suto respectively (Brown et al., 2001; Suto et al., 2003).
2.3 Immunoblots
Cervical spinal cord from rat and mouse were homogenized in lysis buffer (0.25M sucrose; 100 mM Tris-HCl) supplemented with protease inhibitor cocktail (11897100; Roche, Indianapolis, IN) followed by centrifugation at 600g and 4°C for 10 min. Supernatants were collected and protein content determined by BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) using a FL600 Microplate Fluorescence Reader (Bio-Tek, Winooski, VT). Samples and Kaleidoscope ladder (Bio-Rad, Hercules, CA) were separated on a 7.5% SDS-PAGE ReadyGel (Bio-Rad). Gels were electroblotted onto supported nitrocellulose membrane (Millipore, Billerica, MA). Membranes were then blocked in 5% non-fat dried milk in TBST (50 mM Tris buffered saline, 0.1% Tween 20) for 1 hr before being incubated overnight with PlexinA4 antibodies. The membranes were then rinsed and transferred into TBST with DyLight 800 goat anti-rabbit secondary antibody (1:2000; Thermo Scientific) for 1 hr. Blots were imaged using the Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). Controls included preabsorption of antibodies with excess PlexinA4 peptide (ab39349; Abcam) for 1hr at room temperature prior to use, as well as omission of primary antibody.
2.4 Cell culture, transfection and immunocytochemistry
Human embryonic kidney 293 cells (HEK293, American Type Culture Collection, Rockville MD, ATCC No. CRL1573) were grown in Minimal Essential Medium (Gibco BRL, Gaithersburg, MD), supplemented with 10% fetal bovine serum, 100 units /ml penicillin (Gibco BRL), and 100 units/ml streptomycin (Gibco BRL) in a 5% CO2 incubator. Exponentially growing cells were plated on plastic 24 well trays and transfected with PlexinA1 or PlexinA4 expressing plasmids using Lipofectamine 2000 following manufacturer’s instructions. Twenty four hrs post transfection, cells were fixed in 4% paraformaldehyde for 10 min, rinsed in PBS, and permebeali in 0.1% Triton X-100 for 5 min. After rinses in PBS, cells were incubated in 4% normal donkey serum (NDS) for 30 min at RT, then incubated in mouse anti-myc-tag and rabbit anti-PlexinA4 antibodies in PBS containing 2% NDS at 4°C overnight. After washing in PBS cells were incubated in Alexa 594 conjugated donkey anti-mouse and Alexa 488 conjugated donkey anti-rabbit (1:1000; Jackson Immunoresearch, West Grove, PA) for 1hr at RT then with biz-benzamide for 5 minutes. Controls included omission of one of both primary antibodies. Immunostained cells were visualized and imaged using an inverted Leica DMIRE2 (Leica Microsystems, Buffalo Grove, IL) equipped with 4 fluorescent cubes, a Retiga Exi camera (Qimaging, Surrey, BC, Canada) and Simple PCI imaging software.
2.5 Femoral nerve cut
Young adult mice (C57Bl/6) received a femoral nerve cut between the divergence points of the iliacus and pectineus nerve branches as previously described (Franz et al., 2005). The proximal nerve end was reattached to its distal stump with a single 11-0 nylon suture (FST, North Vancouver, Canada). Five days post surgery mice were deeply anesthetized with an overdose of Euthasol (5 ml/kg), injected intraperitoneally, and then perfused intracardially with 0.9% NaCl, followed by 4% paraformaldehyde in phosphate buffered saline at pH 7.4 for 5 min at a rate of 10 ml per min. The 3 mm segment of femoral nerve immediately proximal to the repair site was dissected free, cryoprotected in 30% sucrose at 4°C, then embedded in OCT compound (Sakura Finetechnical Co., Torrance, CA). Nerves were sectioned in the horizontal plane at 14 μm using a cryostat with sections from uninjured controls and 5 day post nerve cut conditions collected in adjacent rows on the same slide.
Slide mounted nerve cross-sections were incubated in 0.1% TritonX-100, rinsed, and preblocked in 2% bovine serum albumin in PBS for 60 min at room temperature (RT). Sections were incubated in rabbit anti-PlexinA4 (1:300) plus mouse anti-Tuj1 and mouse anti-NF200 or mouse anti-GFAP in PBS containing 2% BSA at 4°C for 24 hr, then rinsed in PBS, and incubated in Alexa 555 conjugated goat anti-rabbit and Alexa 488 conjugated goat anti-mouse (1:1000; Invitrogen, Grand Island, NY) in 2% BSA for 1 hr at RT. Sections were rinsed with PBS, then mounted on glass slides with FluorSave reagent (Calbiochem, San Diego, CA) for microscopy. Stained sections were visualized under an upright fluorescent microscope (Olympus BX51, Center Valley, PA) and images were captured with a Cool Snap Pro digital camera operated by Image Pro Plus software (Media Cybernetics, Silver Spring, MD). For these double-labeling experiments, controls included omission of one or both primary antibodies.
To compare changes in the expression of PlexinA4, two channels of fluorescence were captured and aligned as per the procedures of Hanson and Landmesser (Hanson and Landmesser, 2004). Images of sections stained with Anti-Tuj1+NF200 were acquired in the first channel and acted as control for potential variations in section thickness, nerve orientation, and the number of axons within each section. The second channel imaged was anti-PlexinA4. Pixel intensity (arbitrary units) was measured within, but not including, the outer epineurial layer that defined each nerve cross-section using Image J (National Institute of Mental Health, Bethesda, MD). The normalized pixel intensity was calculated by dividing the mean values of PlexinA4 by Tuj1+NF200 for uninjured controls (n=4) and 5 day post nerve cut (n=4) conditions. Images of representative nerve sections were acquired by a person blinded to treatment groups.
2.6 Immunohistochemistry and double immunofuorescence
Adult male Sprague Dawley rats (n = 8) were used for light microscopic immunohistochemistry. Each adult rat was deeply anesthetized with a lethal dose of Euthasol (130 mg/kg), injected intraperitoneally, and then perfused intracardially with 0.9% NaCl, followed by 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.2 (PB) for 15 min at a rate of 20 ml per min. Spinal cords were removed and cryoprotected in 30% sucrose at 4°C, sectioned in coronal and sagittal planes at 50 μm thickness using a freezing microtome, collected in PB, and rinsed in 0.1 M phosphate-buffered saline (PBS), pH 7.2. Dorsal root ganglia (DRGs) were sectioned at 10 μm using a cryostat and mounted onto gelatin coated slides. Spinal cord and brain sections were also obtained from perfusion fixed adult SLICK-A mice (Young et al., 2008). Brain sections were cut as described for rat tissue using a freezing microtome. Spinal sections were cut at 20 μm thickness in the coronal plane using a cryostat and mounted on gelatin coated glass slides prior to staining for PlexinA4.
Immunohistochemistry was performed as described previously (Gutekunst et al., 1995; Gutekunst et al., 1998; Gutekunst et al., 2010b). Free-floating (spinal cord) or slide mounted (DRGs) sections were incubated in 0.1% TritonX-100 and 3% hydrogen peroxide to eliminate endogenous peroxidase, rinsed in PBS, and preblocked in 4% normal goat serum (NGS) in PBS for 30 min at room temperature (RT). Sections were incubated in primary antibodies in PBS containing 2% NGS at 4°C for 48 hr, then rinsed and incubated for 1 hr at RT in biotinylated anti-rabbit antibody (ABC Elite; Vector Laboratories, Burlingame, CA) in PBS containing 2% NGS. After several rinses in PBS, the sections were incubated in avidin-biotin complex (ABC Elite; Vector) for 90 min at 4°C. Immunoreactivity was visualized by incubation in 0.05% 3,3'-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO) and 0.01% hydrogen peroxide in PBS, until a dark brown reaction product was evident (5–10 min). Sections were rinsed and mounted on gelatin coated glass slides, air dried and coverslipped. Controls included the omission of primary antibody and preabsorption of antibodies with excess PlexinA4 peptide (ab39349; Abcam) for 1hr at room temperature prior to use. Sections were visualized using either a Nikon eclipse E400 microscope and images captured using a color digital camera (Nikon Instruments Inc, Melville, NY).
Double immunofluorescence was used to co-localize PlexinA4, NeuN and GFAP. Free-floating spinal cord sections were rinsed in PBS, blocked in 5% normal NDS and 0.1% Triton-X for 30 min at RT and rinsed in PBS. After rinses in PBS, sections were incubated overnight at 4°C in rabbit anti-PleinxA4 and mouse anti NeuN or mouse anti GFAP in PBS containing 1% NDS. Sections were rinsed in PBS and incubated in Alexa 594 conjugated donkey anti-rabbit (1:1000; Jackson Immunoresearch) and Alexa 488 conjugated donkey anti-mouse (1:1000; Jackson Immunoresearch) in 1% NDS for 1 hr at RT. Sections were rinsed with PBS, then mounted on glass slides with Fluoromont-G mounting medium (SouthernBiotech) for fluorescence. For some sections, nuclear counterstaining was obtained by a short incubation of the sections in PBS containing bis-benzimide (Molecular Probes Inc., Eugene, OR) before mounting. For each double-label experiment, controls included omission of one or both primary antibodies. Sections were visualized using either a Nikon eclipse E400 microscope equipped with 4 fluorescent cubes, a monochrome and color digital camera and Nikon BR software (Nikon Instruments Inc, Melville, NY) or a confocal Zeiss 510 with META system equipped with Argon and HENE/3PMT lasers and a Ti-sapphire laser for two-photon excitation (Carl Zeiss MicroImaging Inc, Thornwood, NY).
2.7 Fluorogold injections
To identify motor efferents, 5 days old rat pups were injected intraperitoneally with 100 μl of Fluoro-Gold (2% in saline; Fluorochrome, Denver, CO) as described previously (Cui et al., 2006). On postnatal day 15, animals were anesthetized and perfused intracardially with 0.9% NaCl, followed by 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.2 (PB). By this age PlexinA4 distribution in the CNS appears similar to that seen in adult rats. Brains and spinal cord were removed, cryoprotected in 30% sucrose at 4°C and further processed for immunofluorecence. Free-floating sections were rinsed in PBS, blocked in 5% NDS and 0.1% Triton-X for 30 min at RT and rinsed in PBS. After rinses in PBS, sections were incubated overnight at 4°C in rabbit anti-PleinxA4 in PBS containing 1% NDS. Sections were rinsed in PBS and incubated in Alexa 488 conjugated donkey anti-rabbit (1:1000; Jackson Immunoresearch, West Grove, PA) in 1% NDS for 1 hr at RT. Sections were rinsed with PBS, then mounted on glass slides with Fluoromont-G mounting medium (SouthernBiotech, Birmingham, AL) for fluorescence.
2.8 Neuromuscular junction staining
The triceps surae muscles were isolated and dissected from young adult C57Bl/6 mice immediately after perfusion with 4% paraformaldehyde, immersed in 20% sucrose mixed with OCT compound (1:2), and then flash frozen in dry ice-cooled isopentane. The muscles were cut at their mid-belly with a cryostat at 20μm and the sections were collected on to glass slides. The tissue was air dried overnight, blocked for 1 hr at room temperature in PBS containing 0.3% triton x-100 and 2.5% bovine serum albumin (BSA), incubated overnight at 4ºC in rabbit anti-PlexinA4 (1:300) diluted in PBS containing 2% BSA, and washed several times with PBS. Goat anti rabbit IgG secondary antibody conjugated to Alexa Fluoro 488 (1:1000; Invitrogen) and rhodamine conjugated α-bungarotoxin (Btx, 1:100; Invitrogen) were diluted in blocking solution, applied to slides containing muscle sections for 60 min at room temperature, washed several times in PBS, and then mounted in glycerol-PBS (1:1) mixture. Stained sections were visualized under an upright fluorescent microscope (Olympus BX51) and images were captured at 40X with a Cool Snap Pro digital camera operated by Image Pro Plus software.
RESULTS
3.1 Specificity of antibodies
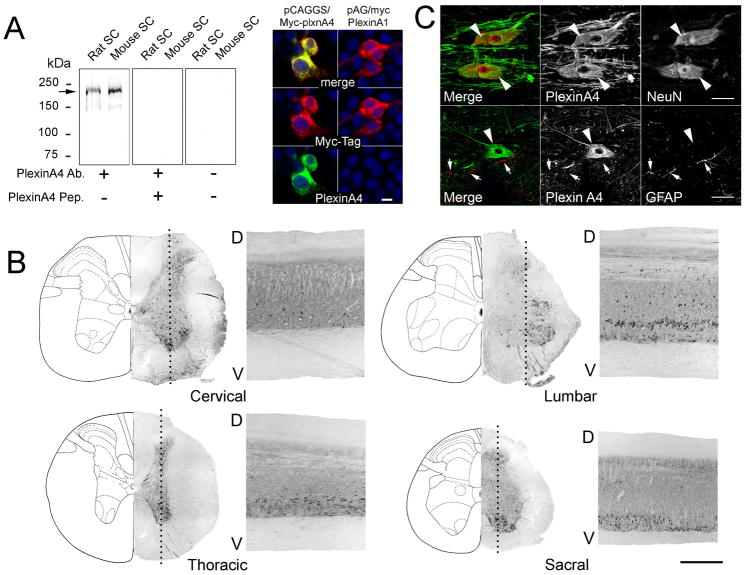
PlexinA4 was detected using a rabbit polyclonal antibody which was raised against a synthetic 16 amino acid peptide derived from within residues 500–600 of mouse PlexinA4 (Treefam gene ID:HENSMUSG00000029765H) conjugated to Keyhole limpet hemocyanin (KLH). This region is identical to that of rat PlexinA4 (Treefam gene ID:HENSRNOG00000013072H). We have previously demonstrated that on immunoblots of rat and mouse brain tissue these PlexinA4 antibodies detect a protein band with an approximate molecular mass of 210 kDa (Gutekunst et al., 2010b). Here we show that on immunoblots of rat and mouse spinal cord tissue, a similar band is detected (Figure 1A). In addition to the 210 kDa band, a fainter band was also visible around 150 kDa – most likely corresponding to degradation. In all cases, immunoreactivity was abolished when the antibodies were first preabsorbed with the PlexinA4 peptide or when the primary antibody was omitted (Figure 1A). Based on a BLAST search it is unlikely that the PlexinA4 antibody cross reacts with other members of the PlexinA family or other Plexin families. The 16 amino acid peptide sequence used to generate the PlexinA4 antibody showed no homology to rat Plexin A2 or 3 and only 62% identity to rat PlexinA1. To further confirm that the antibody did not immunoreact with PlexinA1, HEK293 cells were transfected with either PlexinA1 or PlexinA4 expressing plasmids and co-stained with anti-Myc-Tag and anti-PlexinA4 antibodies. HEK293 cells transfected with PlexinA4 immunoreacted with both anti-Myc-Tag and anti-PlexinA4 antibodies (Figure 1A). In contrast, HEK293 cells transfected with PlexinA1 immunoreacted with the anti-Myc-Tag but not with the anti-PlexinA4 antibodies (Figure 1A) further confirming the specificity of the latter for PlexinA4.
Figure 1. PlexinA4 in adult spinal cord.
A) Aliquots of rat and mouse spinal cord tissue were subjected to SDS/7.5% PAGE and immunoblotted with polyclonal anti-PlexinA4 antibodies. Molecular mass standards (in kDa) are indicated. A band of approximately 210 kDa is detected in both rat and mouse tissue (arrow). Immunoreactivity is abolished when antibodies are preabsorbed with corresponding peptide and when primary antibodies are omitted. Micrographs of HEK293 cells transfected with PlexinA4 or PlexinA1 expressing plasmids co-stained with myc-tag and PlexinA4 antibodies. The PlexinA4 antibodies only immunostained cells expressing PlexinA4. B) Coronal and sagittal sections from adult rat spinal cord immunolabelled with PlexinA4 antibodies. PlexinA4 immunostaining is visible at every spinal cord level in both the dorsal and ventral horns, as well as white matter tracts. PlexinA4 staining is visible in neurons and fibers in the dorsal and ventral horns. At each level, the placement of the sagittal section is indicated on the coronal sections by a dashed line. C) Representative micrographs from PlexinA4 (green) and NeuN (red) or GFAP (red) co-immunostained rat cervical ventral horn as indicated. All PlexinA4 positive cells are also NeuN positive suggesting that PlexinA4 is expressed by neurons (arrowheads). There is no overlap between PlexinA4 and GFAP staining suggesting that PlexinA4 is expressed by neurons (arrowheads) but not by astroglia (arrows). Abbreviations: D: dorsal, V: ventral. Scale bars: A: 10μm, B: 0.5 mm, C: 50 μm.
3.2 General distribution and neuron expression
PlexinA4 was found to label cells and fibers throughout the length of the spinal cord (Figure 1B). In the gray matter, PlexinA4 immunolabeling was found in neurons, in both dorsal and ventral horns. However, we consistently observed more PlexinA4 positive neurons in lamina 9 of the ventral horn, and their morphology, size, and location are consistent with both motor neurons and interneurons (Figure 1B). Although PlexinA4 immunoreactivity was widespread, regional differences could be observed, with the most intense labeling evident in large neurons and a subset of medium size neurons in both the ventral and dorsal horns (Figure 1B). In the white matter, large and small caliber fibers were PlexinA4 positive (Figure 1B). As observed in the brain (Gutekunst et al., 2010b), PlexinA4 immunoreactivity was confined to neurons only as observed in sections co-stained with PlexinA4 and NeuN, a marker of neurons, or GFAP, a marker of astroglial cells (Figure 1C). In all regions examined, every PlexinA4 immunoreactive cell was also NeuN positive. In contrast, there was no overlap between PlexinA4 and GFAP staining in any of the regions examined (Figure 1C). In the following sections, the distribution of PlexinA4 immunoreactivity throughout the major areas of the adult spinal cord will be described. The nomenclature used for spinal cord nuclei is based on a recently published rat spinal cord atlas (Anderson et al., 2008).
3.3 PlexinA4 in the dorsal horn
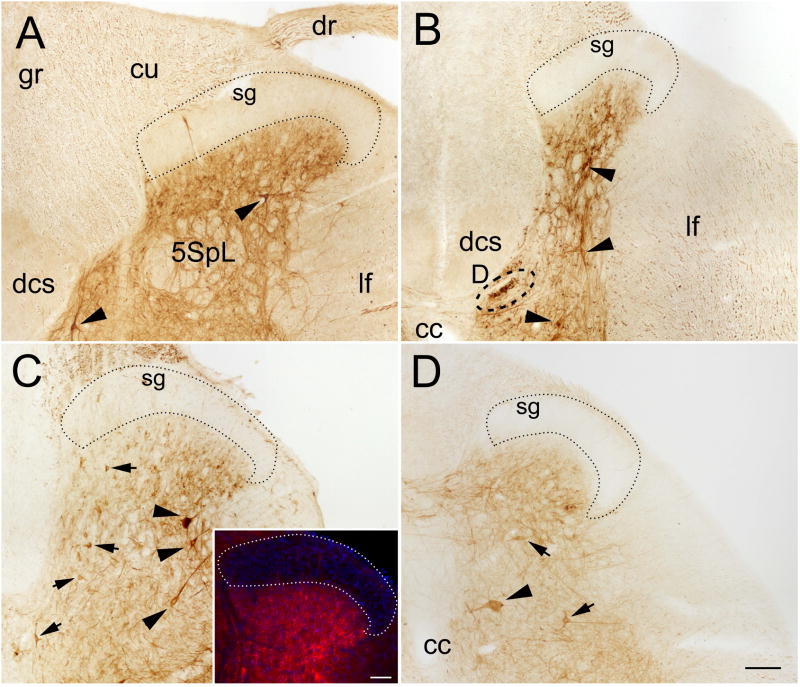
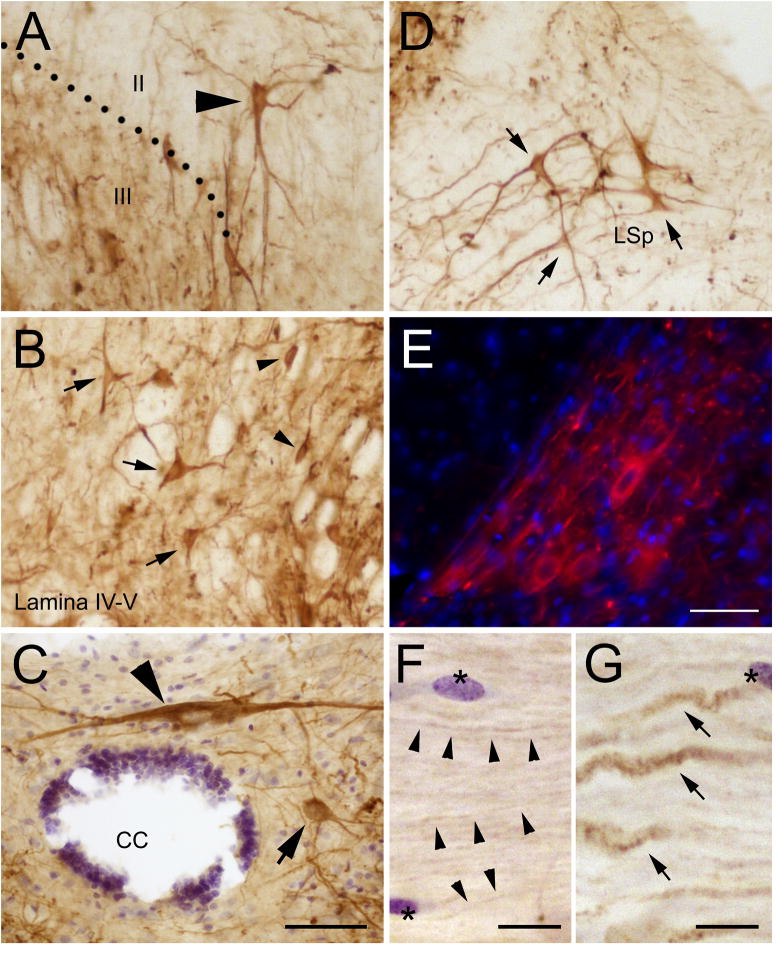
In the dorsal horn, PlexinA4 immunostaining was present in both gray and white matter with the noticeable exception of the substantia gelatinosa, which only rarely exhibited a few scattered immunoreactive fibers (sg; Figure 2A–D). In the gray matter, a few strongly immunostained neurons and a significant number of more weakly labeled neurons were observed scattered throughout lamina III to VI (Figure 2 and Figure 3B). PlexinA4 immunopositive neurons did not appear to belong to any particular lamina and their shape varied between multipolar, pyramidal, and fusiform (Figure 3A–B and D). Specific dorsal horn nuclei containing PlexinA4 labeled neurons included the lateral spinal nucleus (LSp; Figure 3D) and the dorsal nucleus (nucleus dorsalis of Clarke, D; Figure 2A–B and Figure 3E). PlexinA4 expressing neurons were also visible in lamina 10 surrounding the central canal (CC; Figure 3C). In the white matter, stained axons were present in the dorsal corticospinal tract (dcs), the gracilus (gr) and cuneate (cu) fasciculi and in the lateral fasciculi (lf; Figure 2A–B and Figure 3F–G)). The immunopositive axons of the dcs were much smaller diameter than those found in the gr and cu (Figure 3F–G).
Figure 2. PlexinA4 distribution in the dorsal horn of the spinal cord.
PlexinA4 distribution in the dorsal horn in the cervical (A), thoracic (B), lumbar (C), and sacral (D) regions. PlexinA4 is present in large (arrowhead) and medium size (arrows) neurons and their processes in the gray matter. It is also visible in cross sections of axons in the surrounding white matter. C inset: section stained with PlexinA4 (red) and bis-benzimide (blue) highlighting the lack of PlexinA4 immunoreactivity in the substantia gelatinosa. Abbreviations: 5SpL: lateral part of the lamina 5 of the spinal gray, cc: central canal, cf: cuneate fasciculus, D: dorsal nucleus of Clarke, dcs: dorsocorticospinal tract, dr: dorsal root, gf: gracilus fasciculus, lf: lateral fasciculus, sg: substantia gelatinosa. Scale bars: A–D: 100 μm (as shown in D); insert in C: 200 μm.
Figure 3. PlexinA4 in dorsal horn neurons and axons.
Micrographs of PlexinA4 stained sections in the gray (A–E) and the white (F–G) matter of the dorsal spinal cord. PlexinA4 is present in pools of large (arrowheads) and medium size (arrows) neurons in lamina II/III (A), lamina IV–V (B), the area dorsal to the central canal (C), the lateral spinal nucleus (D), and the dorsal nucleus of Clarke (E). In the white matter, PlexinA4 immunoreactivity was found in both small (arrowheads in F) and large (arrows in G) pools of axons. Sections were counterstained with cresyl violet, resulting in both neurons and glial cells (stars in F and G) staining light blue and purple respectively. Abbreviations: CC: central canal, LSp: lateral spinal nucleus. Scale bars: A–D: 100 μm (as shown in C), E: 100 μm, F–G: 20 μm.
3.4 PlexinA4 in the ventral horn
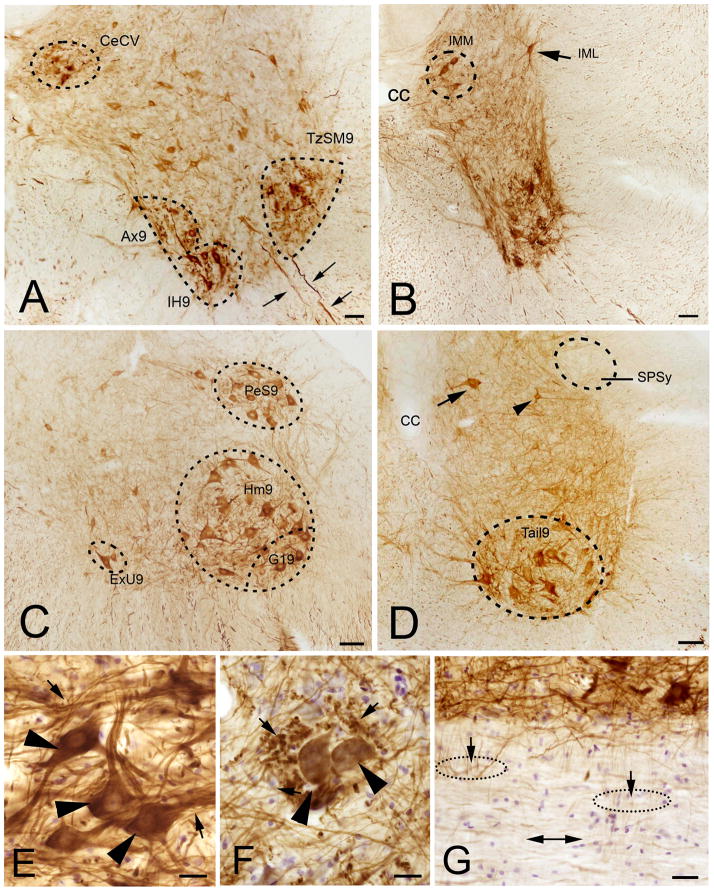
Throughout the spinal cord, large and medium size stained neurons were found in the ventral horn. Based on their location and size, the large PlexinA4 positive neurons in the ventral horn were hypothesized to be motor neurons (MNs). In the cervical region, PlexinA4 positive neurons were found in the trapezius and sternomastoid areas (TzSM9) and in the central cervical nucleus (CeCV; Figure 4A). In the thoracic region, PlexinA4 was present in MNs of the thoracoabdominal wall muscle, the intercostals muscle, the axial muscle, and in the preganglionic neurons of the intermediomedial (IMM) column and intermediolateral (IML) column (Figure 4B). In the lumbar region, PlexinA4 was present in many neurons including MNs in the pes (Pes9), hamstring (Hm9), and external urethral sphincter (ExU9; Figure 4C). In the sacral region, large PlexinA4 immunopositive neurons were found in the tail muscle MNs (Tail9) of lamina 9 and in neurons in more dorsal intercalated nuclei (Figure 4D). No labeled neurons were found in the sacral parasympathetic nucleus (SPSy; Figure 4D). Labeling was also present in MN somata, dendrites, and axons exiting through the ventral roots (Figure 4A and E). In the various MN pool areas, PlexinA4 was visible in tightly bundled large axons (Figure 4E and F). There was also widespread PlexinA4 labeling of ascending and descending white matter tracts travelling in the dorsoventral and anteroposterior axis (Figure 4G).
Figure 4. PlexinA4 distribution in the ventral horn of the spinal cord.
PlexinA4 distribution in the ventral horn in the cervical (A), thoracic (B), L2–L3 lumbar (C), and sacral (D) regions. PlexinA4 is present in pools of large (arrows in B and D) and medium (arrowhead in D) size neurons. PlexinA4 staining is present in motor neurons and their axons exiting the spine through their ventral roots (arrows in A). Higher magnification micrographs of ventral horn sections show large PlexinA4 stained neurons (arrowheads in E and F) and large axons bundles seen in longitudinal (arrows in E) and coronal sections (arrows in F). G) In sagittal sections, bundles of axons are seen coursing both in the dorsoventral (dashed lines) and antero-posterior (double headed arrow) directions. Abbreviations: Ax9: axial muscle motoneurons of lamina 9, CC: central canal, CeCV: central cervical nucleus, ExU9: external and sphincter motoneurons of lamina 9, Hm9: hamstring motor neurons of lamina 9, G19: gluteal motor neurons of lamina 9, IH9: infrahoyd muscle motor neurons of lamina 9, IML: intermediolateral column, IMM: intermediomedial column, PeS9: pes motor neurons of lamina 9, SPSy: sacral parasympathetic nucleus, Tail9: tail muscle motor neurons of lamina 9, TzSM9: trapezius and sternomastoid motor neurons of lamina 9. Scale bars: A–D: 100 μm, E–F: 30 μm, G: 50 μm.
3.5 PlexinA4 in motor neurons
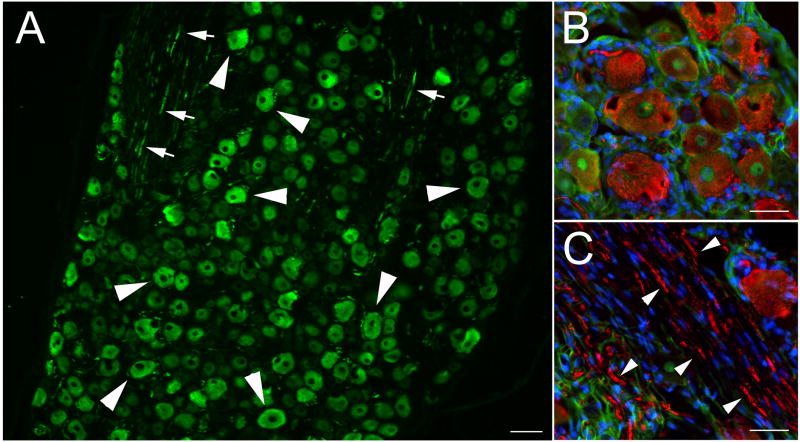
To confirm that the large PlexinA4 positive neurons found in the ventral horn were indeed MNs we labeled MNs by intraperitoneal injection of the tracer Fluoro-Gold (FG). FG is capable of labeling all MNs as it has access to their terminal fields via fenestrated capillaries (Cui et al., 2006). At every level of the spinal cord examined, FG labeling was confined to large and medium size MNs, and all ventral horn FG positive neurons were also PlexinA4 positive (cervical level shown in Figure 5A). Although most MNs appeared to express PlexinA4, not all PlexinA4 positive neurons were FG labeled suggesting that PlexinA4 might also be expressed in interneurons. Spinal cord sections from normal non - FG injected animals were also co-stained for PlexinA4 and ChAT, a marker of MNs (Figure 6). There is overlap of ChAT and PlexinA4 in a subset of MNs in the various MN pools examined. In the ventral horn, PlexinA4 was found predominantly in the large ChAT positive neurons (Figure 5B), which is consistent with observations from FG injected animals (Figure 5A).
Figure 5. PlexinA4 is found in motor neurons.
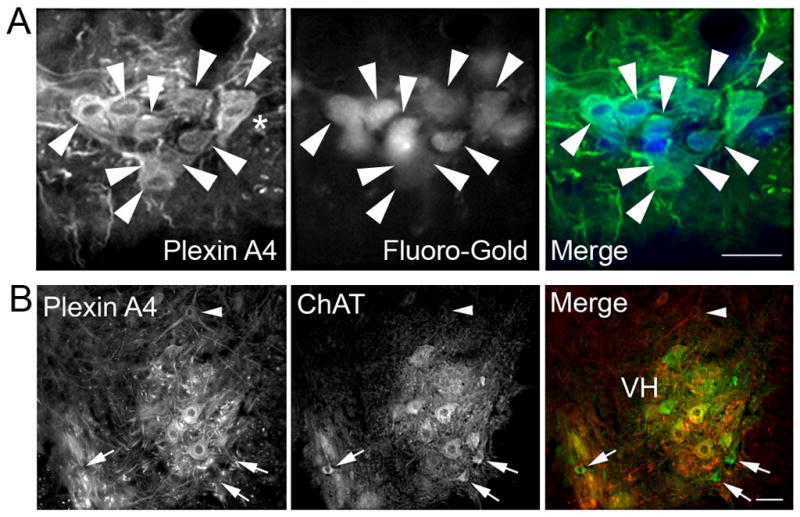
A). Micrographs of PlexinA4 stained sagittal sections through the ventral horn of the cervical spinal cord from FG injected P15 rat pups. Almost all PlexinA4 positive neurons are filled with FG (arrowheads). The smaller FG filled motor neurons (small arrows) do not appear to be stained with PlexinA4. B) Micrographs of spinal cord sections co-stained for PlexinA4 (red) and ChAT (green). PlexinA4 is found in a subset of motor neurons in the ventral horn. Some smaller size ChAT positive motor neurons (arrows) did not express PlexinA4 and not all PlexinA4 immunostained neurons (arrowhead) were motor neurons. Abbreviations: VH: ventral horn. Scale bars: 100 μm.
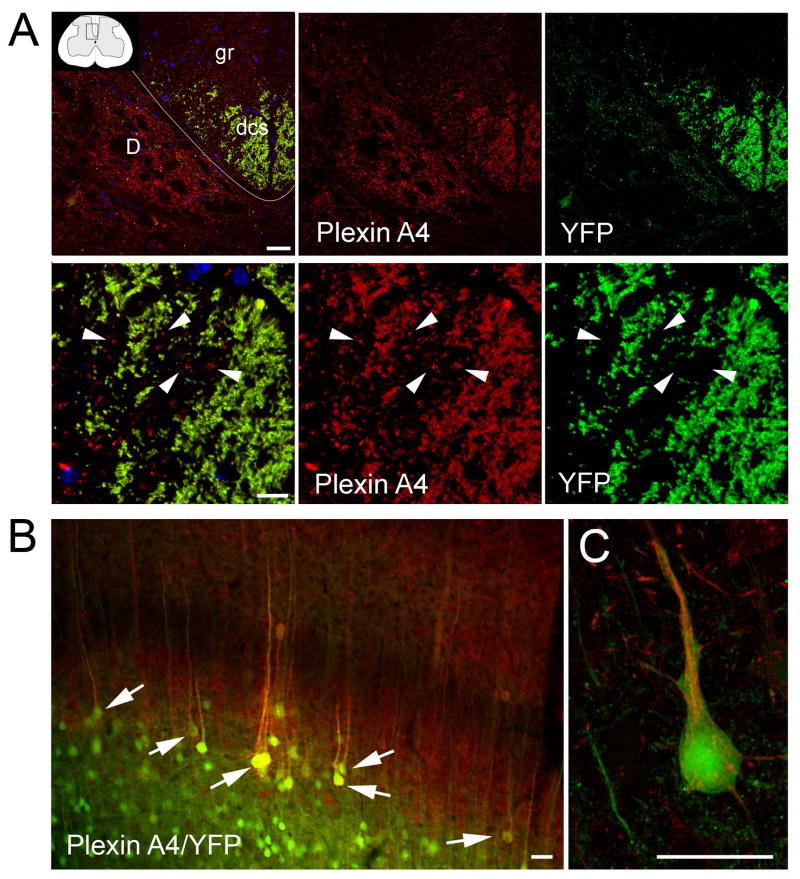
Figure 6. PlexinA4 in the dorsal corticospinal tract.
A) Confocal micrographs of coronal sections through the dorsal region of the spinal cord of SLICKA mice stained for PlexinA4 (red). Whereas YFP (green) is mostly confined to the dorsal corticospinal tract, PlexinA4 (red) is found in additional areas, including the dorsal nucleus of Clarke and the gracilus fasciculus. Although every YFP containing fiber is also PlexinA4 labeled, some PlexinA4 axons are devoid of YFP (arrowheads). B) Micrograph of section through the motor cortex of SLICKA mice stained for PlexinA4 (red). YFP positive large pyramidal neurons in layer V are stained for PlexinA4 (arrows). C) Higher magnification of YFP positive cortical pyramidal neurons positive for PlexinA4. Abbreviations: D: dorsal nucleus or Clarke’s column; dcs: dorsocorticospinal tract; gr: gracile fasciculus. Scale bars: A top panel: 100 μm, A bottom panel: 30 μm, B: 100 μm.
3.6 PlexinA4 in the intermediate zone
In the intermediate zone between the ventral and dorsal horns, PlexinA4 expressing neurons were found in the preganglionic cell columns of the autonomic nervous system including the intermediolateral nucleus (IML), the paraependymal part of the intercalated nucleus and the intercalated nucleus (ICI; Figure 4A). Retrograde labeling using the horseradish peroxidase method has identified MNs in the intermediate zone of the gray matter and occasionally even in the most basal parts of the dorsal horns (Hashizume et al., 1988). Most but not all of the ChAT positive neurons in the intermediolateral columns were PlexinA4 positive (data not shown).
3.7 PlexinA4 in the descending motor tracts
In the rat, the descending motor tracts have distinct positioning in the spinal cord (Watson and Kayalioglu, 2008). The corticospinal tract is localized to the ventromedial part of the dorsal funiculus, whereas the rubrospinal and tectospinal fibers are found in the ventral and lateral funiculi respectively (Waldron and Gwyn, 1969). PlexinA4 staining was present in fibers in the areas of white matter known to contain these descending pathways. In the dorsal funiculus, numerous small caliber fibers and occasional large myelinated fibers could be seen in the dorsal corticospinal tract (dcs; Figure 6). Large PlexinA4 positive myelinated fibers were also present in the lateral and ventral funiculi.
To further confirm the presence of PlexinA4 in the corticospinal tract we stained spinal cord sections from SLICK-A transgenic mice with PlexinA4 (Young et al., 2008). These mice express YFP under the control of the thy-1 promoter in a subset of layer V pyramidal cortical neurons. As the corticospinal tract is the only direct projection from cortex to spinal cord, this tract is specifically labeled with YFP, and it can be followed as it courses from the cortex to its spinal cord destination (Young et al., 2008). In coronal sections of the lumbar region of the SLICK-A mouse spinal cord, where YFP is mostly concentrated in the dcs, PlexinA4 can be seen in the dcs, the gr, and the dorsal nucleus of Clarke (D; Figure 6A). In the dcs, every YFP positive corticospinal fiber is also PlexinA4 positive whereas some PlexinA4 stained fibers do not co-localize with YFP (Figure 6A). These YFP negative PlexinA4 axons are most likely also corticospinal axons, as YFP is not expressed in all axons or even in all thy-1 expressing axons. PlexinA4 was also present in YFP positive pyramidal neurons in layers III and V of cortex where the corticospinal tract originates (Figure 6B).
3.8 PlexinA4 in the dorsal root ganglion
The expression of PlexinA4 was examined by immunofluorescence in the rat sciatic nerve DRGs (L4, L5 and L6). PlexinA4 staining was present in neurons and processes in every DRG examined (Figure 7). These peripheral sensory neurons are classified as small, unmyelinated C-fiber or thinly myelinated Aδ-fiber neurons that transmit pain and thermal information, and large myelinated non-nociceptive Aβ-fiber neurons that transmit proprioceptive and tactile information from low threshold mechanoreceptors (Baba et al., 1999; Lawson, 2002; Willis, 2007). Both small and large DRG neurons were noted to contain PlexinA4 (Figure 7B).
Figure 7. PlexinA4 in sciatic nerve dorsal root ganglion.
A) Micrographs of sections through DRG stained for PlexinA4 showing immunoreactive neurons (arrowheads) and processes (arrows). B–C) Higher magnification micrographs of sections co-stained with PlexinA4 and NeuN antibodies showing co-localization of PlexinA4 (red) and NeuN (green) in most neurons (B). In C, arrowheads indicate PlexinA4 immunoreactive axons. Hoescht staining (blue) is used as a nuclear marker (B–C). Scale bars: A: 50 μm, B–C: 20 μm.
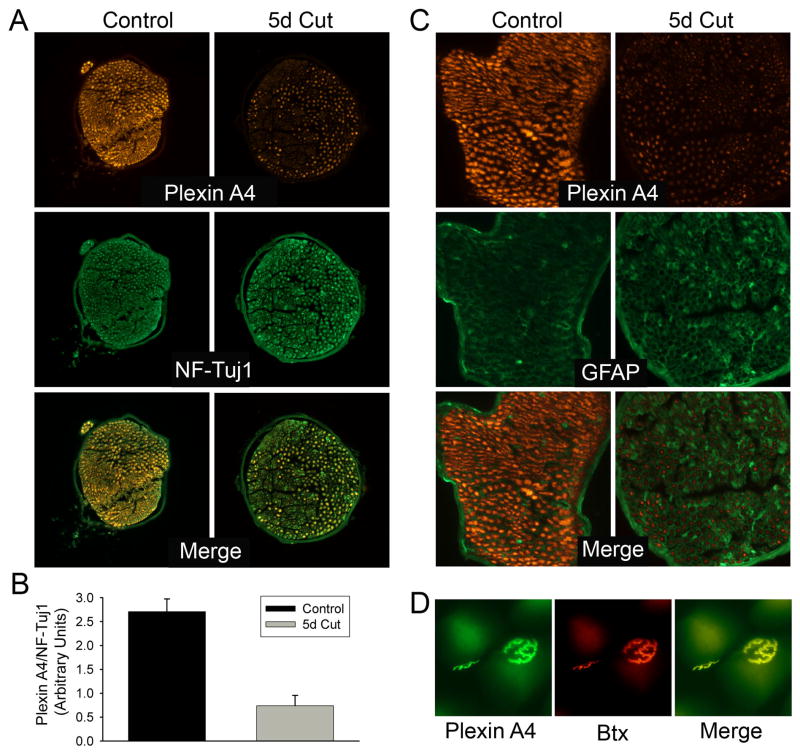
3.9 PlexinA4 is downregulated in the regenerating femoral nerve
The expression of PlexinA4 was examined by immunofluorescence in the mouse proximal femoral nerve and compared to that of Tuj1/NF200 and GFAP five days following a complete nerve cut between the divergence points of the iliacus and pectineus nerve branches, a site previously used to study the specificity of motor axon regeneration in mice (Franz et al., 2005; 2008) and rats (Madison et al., 1996). Following transection, the proximal nerve end was sutured to its distal stump to offer a permissive environment for axonal regeneration. However, the majority of axons do not cross the nerve repair site until after at least 1 week post transection (Brushart et al., 2002). In the controls, Tuj1 and NF200 but not GFAP were found to co-localize with PlexinA4 in axons throughout the nerve (Figure 8A, B). PlexinA4 was significantly decreased in a pool of axons following nerve transection, with no significant difference between expression of Tuj1, NF200 and GFAP in control and cut nerve (Figure 8A). PlexinA4 intensity levels normalized to Tuj1/NF200 was significantly decreased by 76% in injured nerve compared to control (T-test, p=0.000657921, Fig. 8C).
Figure 8. Downregulation of PlexinA4 following femoral cut injury.
A) Micrographs of sections through an uninjured control femoral nerve and 5 day post cut femoral nerve stained for PlexinA4 (red) and NF200+Tuj1 (green). B) Graph comparing the mean intensity of PlexinA4 expression normalized over that of NF200-Tuj1 between control and 5 day post cut. (p=0.000657921). C) Micrographs of sections through an uninjured control and 5 day post cut femoral nerve stained for PlexinA4 (red) and GFAP (green). D) Micrographs of neuromuscular junction from control mice stained for PlexinA4 (red) and Btx (green). Scale bars: A,C: 100 μm, :D: 40 μm.
3.10 PlexinA4 at the Neuromuscular Junction
The expression of PlexinA4 was examined at the neuromuscular junction (NMJ) in mice. PlexinA4 immunostaining co-localized with the neuromuscular marker α-bungarotoxin (Btx) implying a potential role in synaptic function (Figure 8D). The absence of clear PlexinA4 positive axonal processes extending to NMJs and the finding of PlexinA4 in muscle fibers (data not shown) suggest that the localization is post-synaptic but this would need to be further confirmed using specific markers and confocal microscopy.
DISCUSSION
Most studies of PlexinA4 have been performed at the embryonic level revealing its mRNA expression in both CNS and PNS as well as its participation in axon guidance and the development of various circuits. The present study provides the first description of PlexinA4 distribution in the adult rat spinal cord using a recently developed polyclonal antibody, the specificity of which has been described previously (Gutekunst et al., 2010b). By Western blot, the antibody detects a band of predicted molecular weight in both spinal cord and brain lysate from rat and mouse. As we previously described, PlexinA4 is expressed in neurons but not detected in glia (Gutekunst et al., 2010b). At the subcellular level, PlexinA4 is found in the soma and in both dendrites and axons, but it is not present in nuclei. In the cytoplasm it strongly associates with perinuclear rough endoplasmic- and Golgi-like structures as well as small puncta consistent with the localization of a membrane protein.
4.1 Comparison with previous PlexinA4 mRNA distribution
In the spinal cord, PlexinA4 immunolabeling was found in neurons in both dorsal and ventral horns. However, we consistently observed more PlexinA4 labeling in the ventral horn where it is expressed in motor neurons and some interneurons throughout the length of the spinal cord. PlexinA4 was also found in virtually all DRG neurons. The distribution of PlexinA4 protein in the spinal cord and DRGs is consistent with that of its mRNA (Bron et al., 2007; Haklai-Topper et al., 2010; Mauti et al., 2006; Perala et al., 2005; Suto et al., 2003). In developing chicks, PlexinA4 mRNA becomes visible by stage 22 in the ventral horn and in the dorsal commissural neurons (Bron et al., 2007; Mauti et al., 2006). In mouse embryos, PlexinA4 mRNA is found in the spinal cord where it is highly expressed in the ventral horn with more moderate expression in the dorsal horn (Perala et al., 2005; Suto et al., 2003). PlexinA4 was expressed in both somatic motor neurons in the gray matter of the ventral horn and autonomic motor neurons in the intermediate zone. PlexinA4 was not exclusively expressed in MNs but was also found in some interneurons in both the ventral and dorsal horn. Further characterization of the types of interneurons expressing PlexinA4, using markers specific to homeodomain transcription factors or neurotransmitters, could identify more specific pathways in which the protein is involved (Helms and Johnson, 2003).
The nearly complete absence of PlexinA4 staining in the substantia gelatinosa was striking and it suggests that the protein is not expressed in the sensory pathways involved in pain and temperature modulation, as the substantia gelatinosa is made up of finely myelinated and unmyelinated sensory fibers originating in the DRGs. The lack of PlexinA4 staining is consistent with some DRG neurons being devoid of PlexinA4 staining or faintly immunopositive. Another possibility is that the protein remains localized close to the cell body and might not be transported to the most distal portion of the sensory projections in the substantia gelatinosa. PlexinA4 was also not seen in neurons in the sacral parasympathetic nucleus, which consists of a group of preganglionic neurons that innervate the large intestine, sphincters, bladder and reproductive organs (Dorofeeva et al., 2009; Im et al., 2008). The absence of PlexinA4 in this nucleus may not extend to the rest of the parasympathetic nuclei since we and others previously have found PlexinA4 expression in the oculomotor and facial nerves as well as in neurons in the dorsal motor nucleus of the vagus and the nucleus ambiguus (Gai and Blessing, 1996; Gutekunst et al., 2010b; Tayo and Williams, 1988).
In the white matter, we found widespread PlexinA4 labeling in regions containing ascending and descending myelinated and unmyelinated fibers, suggesting that PlexinA4 might participate in mechanisms at work in both motor and sensory pathways. As we have previously shown, labeling was also seen in the large caliber axons of motor neurons as they are making their way to the ventral roots (Gutekunst et al., 2010b).
4.2 PlexinA4 in the corticospinal tract
PlexinA4 has recently been implicated in the guidance of cortical neurons during development of visual and motor pathways (Faulkner et al., 2008b; Low et al., 2008; Runker et al., 2008). In mice lacking PlexinA4 and in PlexinA4/PlexinA3 double knockout mice, half of the motor corticospinal axons are guided to the ventral spinal cord resulting in an abnormal ipsilateral ventrolateral tract (Faulkner et al., 2008a). In situ hybridization has revealed the presence of PlexinA4 mRNA in the developing neocortex immediately after layer V neurons are born and migrate to their appropriate layer in the cortex (Faulkner et al., 2008b; Runker et al., 2008). Here we confirm the presence of PlexinA4 protein in the cortex and show its expression in the layer V pyramidal neurons of the adult rat motor cortex that give rise to the CST. PlexinA4 is not only seen in the cortical pyramidal neurons, it is also visible in the tract itself at various levels of the spinal cord. The persistent expression of PlexinA4 in the adult CST suggests that PlexinA4 plays a role beyond the guidance of the tract during development and that it could possibly participate in maintenance and the regenerative properties of the pathway. Other proteins involved in CST development and maintenance include Nrp1 and L1-CAM. The presence of these proteins is consistent with the observation that Nrp1-expressing cortical projections are defective in mice lacking PlexinA4 (Bechara et al., 2008). Aside from the CST, the most prominent descending tracts are those that originate in the red nucleus, the vestibular nuclei, and the hindbrain reticular formation. Other sites of origin include the paraventricular nucleus of the hypothalamus, the zona incerta, the superior colliculi, the periaqueductal gray, and the supraoculomotor nucleus. We have previously shown PlexinA4 expression in some but not all of these nuclei (Gutekunst et al., 2010b).
4.3 PlexinA4 interacting partners in the spinal cord and DRG
Several studies have demonstrated the cooperation of Sema3A and its receptor Nrp1 and co-receptor PlexinA4 in the axon guidance and normal development of spinal motor and peripheral sensory projections in mice (Huber et al., 2005; Suto et al., 2005; Yaron et al., 2005). We found the distribution of PlexinA4 protein in the adult spinal cord to be comparable to that of the known distribution of its co-receptor Nrp1 at least during development. Nrp1 mRNA is highly expressed in the MNs of developing chicks and rats, and it is also present in rat DRG neurons (Mauti et al., 2006; Takahashi et al., 1999). In addition, Nrp1 protein has been detected in the corticospinal tract of mice (Mire et al., 2008). Co-culture assays with DRG explants from mice lacking PlexinA3 and PlexinA4 have shown that Sema3A - responsive axons primarily utilize PlexinA4 for their repulsive response to Sema3A (Suto et al, 2005; Yaron et al, 2005). However, cooperative effects exist between different PlexinAs as some DRG axons from PlexinA4−/−mice exhibited reduced sensitivity to Sema3A but were fully desensitized by additional genetic removal of PlexinA3 (Yaron et al, 2005).
4.4 A role for semaphorins and their receptors and co-receptors in the adult nervous system
PlexinA4 protein levels were significantly decreased in regenerating femoral axons following nerve transection. This is in contrast with a previous study showing upregulation of PlexinA4 mRNA levels in the facial motor neurons following facial nerve transection (Spinelli et al., 2007). There are many parameters that could account for the discrepancy between the two studies including species difference, analysis of mRNA versus protein levels, time point difference post-injury, distinct neuronal sub-populations (i.e. DRG and spinal motor neurons versus cranial motor neurons), and examination of different cellular compartments, i.e. cell bodies versus axons. In addition it is likely that the type of injury also plays an important role in the response. As opposed to the facial nerve study, the femoral nerve was reattached to its distal portion allowing regeneration to take place. Therefore, the downregulation of PlexinA4 observed in the femoral nerve is possibly associated with a regenerative mechanism whereas the upregulation in the facial nerve might have been part of a degenerative response (Spinelli et al., 2007). Examining PlexinA4 levels at multiple time points could help better identify potential factors regulating its expression in the context of degeneration and regeneration. Finally, PlexinA2 appears to be downregulated post facial nerve injury and it is conceivable that different plexins participate differently in the response to injury in different systems (Spinelli et al., 2007).
4.5 Conclusions
Semaphorin family members and their receptors and co-receptors continue to be expressed in the adult CNS. Based on studies showing continuous expression of these molecules in adulthood along with changes in expression levels following injury, in the context of neurological diseases, and in response to physiological or pharmacological manipulation (Barnes et al., 2003; Holtmaat et al., 2002; Jassen et al., 2006; Mann et al., 2007), it has been suggested that these molecules contribute to the maintenance and stability of neuronal networks, as well as repair and remodeling of such networks (De Winter et al., 2002; de Wit and Verhaagen, 2003; Pasterkamp and Verhaagen, 2006). A more detailed analysis of adult PlexinA4 knockout animals, including histological and behavioral assessment, could further clarify the role of PlexinA4 in the mature brain and spinal cord. In addition, genetic manipulation of PlexinA4 using lentiviral or adenoviral delivery of PlexinA4 shRNA in precise CNS regions where PlexinA4 has been observed should provide a deeper insight into the mechanisms by which PlexinA4 signaling contributes to structural plasticity and regeneration in the adult nervous system.
HIGHLIGHTS.
We examine the distribution of PlexinA4 in adult rat spinal cord and DRGs.
PlexinA4 is expressed by motor neurons and interneurons along the cord.
PlexinA4 is expressed in the corticospinal tract.
In the DRGs, PlexinA4 is expressed in both nociceptive and proprioceptive neurons.
PlexinA4 levels decrease in femoral nerve 5 days post nerve cut.
Acknowledgments
This work was supported by a grant from NIH (K08 NS46322-01A1) to REG. CAG is funded in part by NIH (R03 NS58376-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson C, Ashwell K, Collewijn H, Conta A, Harvey A, Heise C, Hodgetts S, Holstege G, Layalioglu G, Keast J, McHanwell S, McLachlan E, Paxinos G, Plant G, Scremin O, Sidhu A, Stelzner D, Watson C. In: The Spinal Cord, A Christopher and Dana Reeves Foundation Text and Atlas. Charles Watson GPaGL., editor. San Diego CA: Elsevier; 2008. [Google Scholar]
- Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Abeta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 1999;19(2):859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G, Puranam RS, Luo Y, McNamara JO. Temporal specific patterns of semaphorin gene expression in rat brain after kainic acid-induced status epilepticus. Hippocampus. 2003;13(1):1–20. doi: 10.1002/hipo.10041. [DOI] [PubMed] [Google Scholar]
- Bechara A, Nawabi H, Moret F, Yaron A, Weaver E, Bozon M, Abouzid K, Guan JL, Tessier-Lavigne M, Lemmon V, Castellani V. FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27(11):1549–1562. doi: 10.1038/emboj.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev. 2007;2:21. doi: 10.1186/1749-8104-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128(16):3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22(15):6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F, Valdembri D, Caccavari F, Serini G. Semaphoring vascular morphogenesis. Endothelium. 2006;13(2):81–91. doi: 10.1080/10623320600698003. [DOI] [PubMed] [Google Scholar]
- Cui D, Dougherty KJ, Machacek DW, Sawchuk M, Hochman S, Baro DJ. Divergence between motoneurons: gene expression profiling provides a molecular characterization of functionally discrete somatic and autonomic motoneurons. Physiol Genomics. 2006;24(3):276–289. doi: 10.1152/physiolgenomics.00109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter F, Holtmaat AJ, Verhaagen J. Neuropilin and class 3 semaphorins in nervous system regeneration. Adv Exp Med Biol. 2002;515:115–139. doi: 10.1007/978-1-4615-0119-0_10. [DOI] [PubMed] [Google Scholar]
- de Wit J, Verhaagen J. Role of semaphorins in the adult nervous system. Prog Neurobiol. 2003;71(2–3):249–267. doi: 10.1016/j.pneurobio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Dorofeeva AA, Panteleev SS, Makarov FN. Involvement of the sacral parasympathetic nucleus in the innervation of the descending colon and rectum in cats. Neurosci Behav Physiol. 2009;39(2):207–210. doi: 10.1007/s11055-009-9104-z. [DOI] [PubMed] [Google Scholar]
- Faulkner RL, Low LK, Liu XB, Coble J, Jones EG, Cheng HJ. Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling. Neural Develop. 2008a;3:21. doi: 10.1186/1749-8104-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner RL, Low LK, Liu XB, Coble J, Jones EG, Cheng HJ. Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling. Neural Dev. 2008b;3:21. doi: 10.1186/1749-8104-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CK, Rutishauser U, Rafuse VF. Polysialylated neural cell adhesion molecule is necessary for selective targeting of regenerating motor neurons. J Neurosci. 2005;25(8):2081–2091. doi: 10.1523/JNEUROSCI.4880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CK, Rutishauser U, Rafuse VF. Intrinsic neuronal properties control selective targeting of regenerating motoneurons. Brain. 2008;131(Pt 6):1492–1505. doi: 10.1093/brain/awn039. [DOI] [PubMed] [Google Scholar]
- Gai WP, Blessing WW. Human brainstem preganglionic parasympathetic neurons localized by markers for nitric oxide synthesis. Brain. 1996;119 ( Pt 4):1145–1152. doi: 10.1093/brain/119.4.1145. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2(7):a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RE, Mei Q, Gutekunst CA, Torre E. The pivotal role of RhoA GTPase in the molecular signaling of axon growth inhibition after CNS injury and targeted therapeutic strategies. Cell Transplant. 2007;16(3):245–262. doi: 10.3727/000000007783464740. [DOI] [PubMed] [Google Scholar]
- Gutekunst CA, Levey AI, Heilman CJ, Whaley WL, Yi H, Nash NR, Rees HD, Madden JJ, Hersch SM. Identification and localization of huntingtin in brain and human lymphoblastoid cell lines with anti-fusion protein antibodies. Proc Natl Acad Sci U S A. 1995;92(19):8710–8714. doi: 10.1073/pnas.92.19.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst CA, Li SH, Yi H, Ferrante RJ, Li XJ, Hersch SM. The cellular and subcellular localization of huntingtin-associated protein 1 (HAP1): comparison with huntingtin in rat and human. J Neurosci. 1998;18(19):7674–7686. doi: 10.1523/JNEUROSCI.18-19-07674.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst CA, Stewart EN, Gross RE. Immunohistochemical distribution of Plexin A4 in the adult rat central nervous system. Front Neuroanat. 2010a;4(25) doi: 10.3389/fnana.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutekunst CA, Stewart EN, Gross RE. Immunohistochemical distribution of Plexin A4 in the adult rat central nervous system. Front Neuroanat. 2010b;4(25):1–17. doi: 10.3389/fnana.2010.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haklai-Topper L, Mlechkovich G, Savariego D, Gokhman I, Yaron A. Cis interaction between Semaphorin6A and Plexin-A4 modulates the repulsive response to Sema6A. EMBO J. 2010;29(15):2635–2645. doi: 10.1038/emboj.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43(5):687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Hashizume K, Kanda K, Burke RE. Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol. 1988;269(3):425–430. doi: 10.1002/cne.902690309. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13(1):42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, De Winter F, De Wit J, Gorter JA, da Silva FH, Verhaagen J. Semaphorins: contributors to structural stability of hippocampal networks? Prog Brain Res. 2002;138:17–38. doi: 10.1016/s0079-6123(02)38068-3. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kania A, Tran TS, Gu C, De Marco Garcia N, Lieberam I, Johnson D, Jessell TM, Ginty DD, Kolodkin AL. Distinct roles for secreted semaphorin signaling in spinal motor axon guidance. Neuron. 2005;48(6):949–964. doi: 10.1016/j.neuron.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Im YJ, Hong CH, Jin MH, Lee BH, Han SW. c-fos expression in bladder-specific spinal neurons after spinal cord injury using pseudorabies virus. Yonsei Med J. 2008;49(3):479–485. doi: 10.3349/ymj.2008.49.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassen AK, Yang H, Miller GM, Calder E, Madras BK. Receptor regulation of gene expression of axon guidance molecules: implications for adaptation. Mol Pharmacol. 2006;70(1):71–77. doi: 10.1124/mol.105.021998. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol. 2002;87(2):239–244. [PubMed] [Google Scholar]
- Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc Natl Acad Sci U S A. 2008;105(23):8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison RD, Archibald SJ, Brushart TM. Reinnervation accuracy of the rat femoral nerve by motor and sensory neurons. J Neurosci. 1996;16(18):5698–5703. doi: 10.1523/JNEUROSCI.16-18-05698.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Chauvet S, Rougon G. Semaphorins in development and adult brain: Implication for neurological diseases. Prog Neurobiol. 2007;82(2):57–79. doi: 10.1016/j.pneurobio.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Mauti O, Sadhu R, Gemayel J, Gesemann M, Stoeckli ET. Expression patterns of plexins and neuropilins are consistent with cooperative and separate functions during neural development. BMC Dev Biol. 2006;6:32. doi: 10.1186/1471-213X-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire E, Thomasset N, Jakeman LB, Rougon G. Modulating Sema3A signal with a L1 mimetic peptide is not sufficient to promote motor recovery and axon regeneration after spinal cord injury. Mol Cell Neurosci. 2008;37(2):222–235. doi: 10.1016/j.mcn.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Tanaka M, Takahashi T, Kalb RG, Strittmatter SM. Neuropilin-1 extracellular domains mediate semaphorin D/III-induced growth cone collapse. Neuron. 1998;21(5):1093–1100. doi: 10.1016/s0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- Okada A, Tominaga M, Horiuchi M, Tomooka Y. Plexin-A4 is expressed in oligodendrocyte precursor cells and acts as a mediator of semaphorin signals. Biochemical and biophysical research communications. 2007;352(1):158–163. doi: 10.1016/j.bbrc.2006.10.176. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J. Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1499–1511. doi: 10.1098/rstb.2006.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perala NM, Immonen T, Sariola H. The expression of plexins during mouse embryogenesis. Gene Expr Patterns. 2005;5(3):355–362. doi: 10.1016/j.modgep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Potiron V, Roche J. Class 3 semaphorin signaling: the end of a dogma. Sci STKE. 2005;(285):pe24. doi: 10.1126/stke.2852005pe24. [DOI] [PubMed] [Google Scholar]
- Runker AE, Little GE, Suto F, Fujisawa H, Mitchell KJ. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev. 2008;3:34. doi: 10.1186/1749-8104-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Waimey KE, Golding M, Takamatsu H, Kumanogoh A, Fujisawa H, Cheng HJ, Ruhrberg C. Plexin A3 and plexin A4 convey semaphorin signals during facial nerve development. Dev Biol. 2008;324(1):1–9. doi: 10.1016/j.ydbio.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli ED, McPhail LT, Oschipok LW, Teh J, Tetzlaff W. Class A plexin expression in axotomized rubrospinal and facial motoneurons. Neuroscience. 2007;144(4):1266–1277. doi: 10.1016/j.neuroscience.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci. 2005;25(14):3628–3637. doi: 10.1523/JNEUROSCI.4480-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Murakami Y, Nakamura F, Goshima Y, Fujisawa H. Identification and characterization of a novel mouse plexin, plexin-A4. Mech Dev. 2003;120(3):385–396. doi: 10.1016/s0925-4773(02)00421-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99(1):59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Heron P, Mashburn C, Smith GM. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27(22):6068–6078. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24(4):819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayo EK, Williams RG. Catecholaminergic parasympathetic efferents within the dorsal motor nucleus of the vagus in the rat: a quantitative analysis. Neurosci Lett. 1988;90(1–2):1–5. doi: 10.1016/0304-3940(88)90776-8. [DOI] [PubMed] [Google Scholar]
- Waimey KE, Huang PH, Chen M, Cheng HJ. Plexin-A3 and plexin-A4 restrict the migration of sympathetic neurons but not their neural crest precursors. Dev Biol. 2008;315(2):448–458. doi: 10.1016/j.ydbio.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron HA, Gwyn DG. Descending nerve tracts in the spinal cord of the rat. I. Fibers from the midbrain. J Comp Neurol. 1969;137(2):143–153. doi: 10.1002/cne.901370203. [DOI] [PubMed] [Google Scholar]
- Watson C, Kayalioglu G. The Organization of the Spinal Cord. In: Charles Watson GPaGL., editor. The Spinal Cord: Christopher and Dana Reeves Foundation. 2008. pp. 1–7. [Google Scholar]
- Willis WD., Jr The somatosensory system, with emphasis on structures important for pain. Brain Res Rev. 2007;55(2):297–313. doi: 10.1016/j.brainresrev.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron. 2005;45(4):513–523. doi: 10.1016/j.neuron.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome biology. 2006;7(3):211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Qiu L, Wang D, Zhao S, Gross J, Feng G. Single-neuron labeling with inducible Cre-mediated knockout in transgenic mice. Nat Neurosci. 2008;11(6):721–728. doi: 10.1038/nn.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]